Abstract
Blood-borne angiotensin II (ANG II) can upregulate p44/42 mitogen-activated protein kinase (MAPK) signaling and ANG II type-1 receptors (AT1R) in the hypothalamic paraventricular nucleus (PVN), a critical cardiovascular and autonomic center. We tested the hypothesis that brain p44/42 MAPK signaling contributes to the development of ANG II-induced hypertension. ANG II infusion (120 ng/kg/min, SC) induced increases in phosphorylated p44/42 MAPK and AT1R in the PVN after 1 week, before the onset of hypertension, that were sustained as hypertension developed during a 2- or 3-week infusion protocol. Bilateral PVN microinjections of small interfering RNAs (siRNA) for p44/42 MAPK, at the onset of the ANG II infusion or one week later, prevented the early increase in p44/42 MAPK activity. The early treatment normalized AT1R expression in the PVN and attenuated the hypertensive response to the 2-week infusion of ANG II. The later siRNA microinjections had a transient effect on AT1R expression in PVN and no effect on the hypertensive response to the 3-week infusion of ANG II. The early treatment normalized the pressure response to ganglionic blockade. ANG II also induced increases in mRNA for pro-inflammatory cytokines that were not affected by either siRNA treatment. These results suggest that the full expression of ANG II-induced hypertension depends upon p44/42 MAPK-mediated effects. A potential role for p44/42 MAPK in modulating the ANG II-induced central inflammatory response might also be considered. MAPK signaling in PVN may be a novel target for early intervention in the progression of ANG II-dependent hypertension.
Keywords: brain, renin-angiotensin system, pro-inflammatory cytokines, autonomic regulation
INTRODUCTION
Hypertension is associated with augmented renin-angiotensin system activity (RAS) and an increase in proinflammatory cytokines (PICs) in the periphery and in the brain.1-5 Overactivity of the brain RAS and PICs has been implicated in the development and the maintenance of hypertension in multiple experimental and genetic animal models, via alterations in body fluid homeostasis, neurohormonal release, and sympathetic outflow.2, 4, 6, 7 Interventions that reduce the expression of RAS or PICs in the brain can significantly ameliorate these effects and attenuate hypertension. 2, 4, 6, 8
Recent studies from our laboratory and others have demonstrated that p44/42 mitogen-activated protein kinase (MAPK) signaling regulates the expression of RAS and PICs in the brain.9-13 p44/42 MAPK is expressed in several brain regions associated with cardiovascular and autonomic regulation, including the paraventricular nucleus of hypothalamus (PVN) and the subfornical organ (SFO).9, 10 Blood-borne angiotensin II (ANG II), which is increased in heart failure and many forms of hypertension, increases p44/42 MAPK activity in the PVN and SFO.11 Activation of p44/42 MAPK can upregulate the PVN expression of ANG II type-1 receptor (AT1R) 9-11 and of PICs.12, 13 Pharmacological inhibition of p44/42 MAPK signaling in the brain can reduce AT1R expression in the PVN of normal rats subjected to a continuous low dose of ANG II, sympathetic activity in rats with heart failure induced by myocardial infarction and the pressor response to acute central administration of ANG II in normal rats.9-11 The prominent involvement of brain p44/42 MAPK signaling in ANG II-mediated cardiovascular and sympathetic responses led us to hypothesize an important role for p44/42 MAPK signaling in the PVN in the development of ANG II-induced hypertension.
METHODS
Experimental Protocols
The slow ANG II-infusion protocol was used to induce hypertension in adult Sprague-Dawley rats as previously described. 2 Some animals (n=33) underwent continuous monitoring of mean blood pressure (MBP) and heart rate (HR) by telemetry. These rats were anesthetized with ketamine-xylazine (100 and 10 mg/kg), and under sterile conditions a telemetry probe (TA11PA-C40, Data Science International) was implanted in a femoral artery. After a one-week recovery period, baseline MBP and HR were recorded for 5 days. They were then re-anesthetized with ketamine-xylazine, and under sterile conditions an osmotic minipump (model 2002 for 2-week infusion; model 2004 for 3-week infusion, Alzet) was implanted subcutaneously to deliver ANG II (120 ng/kg per minute). Others (n=84) received the ANG II infusion without telemetry monitoring.
To test the role of p44/42 MAPK signaling in the PVN in the development of ANG II-induced hypertension, rats received bilateral PVN microinjections of p44/42 siRNA, scrambled siRNA or vehicle, at one of two time points during the ANG II infusion. The early treatment group (n=60, including 18 rats with telemetry probes), received the PVN microinjections during the surgery to implant the osmotic minipump. The duration of the ANG II infusion in this group was 2 weeks. The late treatment groups (n=57, including 15 rats with telemetry probes) received the PVN microinjections in a separate sterile surgical procedure under ketamine-xylazine anesthesia one week after starting the ANG II infusion. The duration of the ANG II infusion in this group was three weeks. In animals with telemetry probes, sympathetic tone was assessed at baseline and 2 weeks after the PVN microinjections by examining the MBP response to ganglionic blockade with hexamethonium bromide (30 mg/kg, ip).
One week or two weeks after the PVN microinjections, animals in both groups were euthanized with an overdose of urethane to collect brain tissue for molecular studies. The thoracic aorta and heart tissues were also collected for molecular or anatomical studies in some animals after 2 or 3 weeks of ANG II infusion. Untreated rats (n=22) were used as controls.
Additional animals were used in control studies to determine the specificity and optimal dose of the p44/42 siRNA (n=15), the effects of the siRNA microinjections on central indicators of inflammation (n=12), and the accuracy of the PVN microinjections (n=6).
Additional Methods
Please see online data supplement at http://hyper.ahajournals.org
RESULTS
Hemodynamic and PVN molecular effects of the slow pressor ANG II infusion
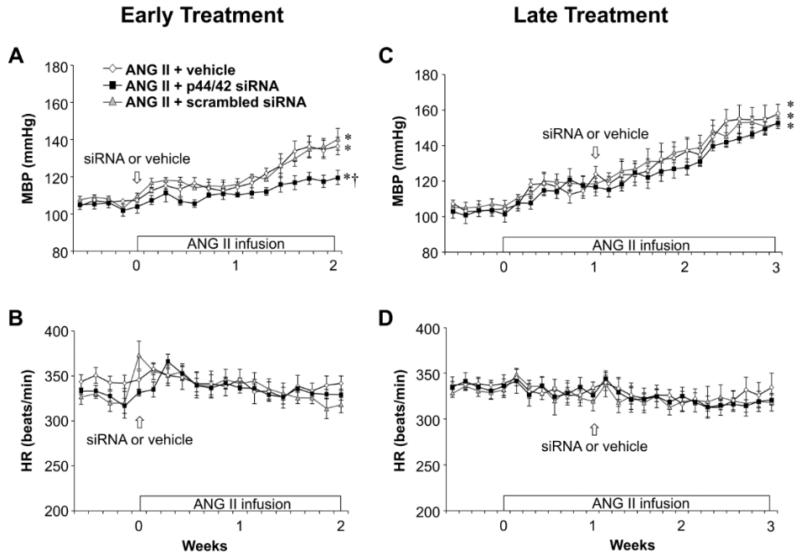
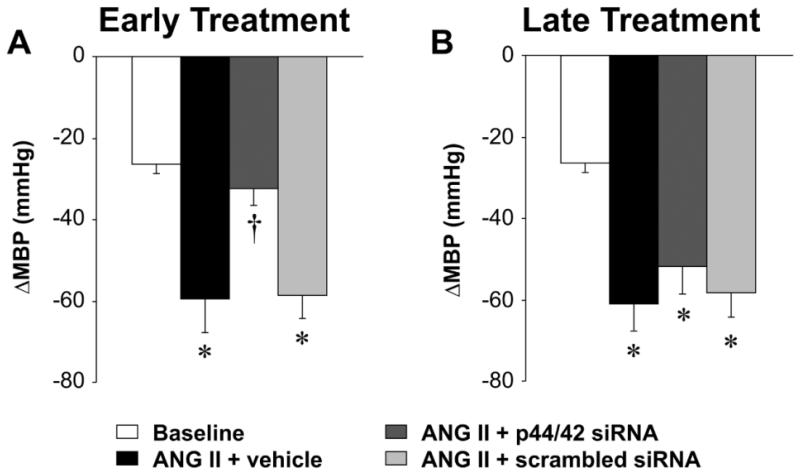
In rats receiving the 2-week infusion of ANG II + vehicle (n=6), in the early treatment protocol, MBP increased slightly but not significantly during the first week of the ANG II infusion, but increased dramatically during week 2, peaking at 136 ± 6 mmHg (Figure 1A). Rats receiving the 3-week infusion of ANG II + vehicle (n=5), in the later treatment protocol, had a similar rise in MBP in week 2 and a further increase in week 3, peaking at 159 ± 5 mmHg (Figure 1C). There were no significant changes in HR in either protocol. Hexamethonium bromide administered at week 2 (Figure 2A) or week 3 (Figure 2B) to assess sympathetic tone elicited a ~60 mmHg drop in MBP, substantially larger than that observed at baseline.
Figure 1.
The effect of bilateral PVN microinjections of p44/42 MAPK siRNA on ANG II-induced hypertension in rats. Daily mean blood pressure (MBP) and heart rate (HR) before and during systemic infusion of ANG II in rats treated early (A and B) or late (C and D) with p44/42 siRNA, a scrambled siRNA, or vehicle. Values are mean ± SEM (n = 5-6 for each group). *P< 0.05, vs. baseline; †P< 0.05, ANG II + p44/42 siRNA vs. ANG II + vehicle or ANG II + scrambled siRNA.
Figure 2.
Peak changes (Δ) in MBP in response to ganglionic blockade at baseline and 2 weeks after early (A) or late (B) PVN microinjections of p44/42 siRNA, a scrambled siRNA, or vehicle in ANG II-infused rats. Values are mean ± SEM (n = 5-6 for each group). *P< 0.05, vs. baseline; †P< 0.05, ANG II + p44/42 siRNA vs. ANG II + vehicle or ANG II + scrambled siRNA.
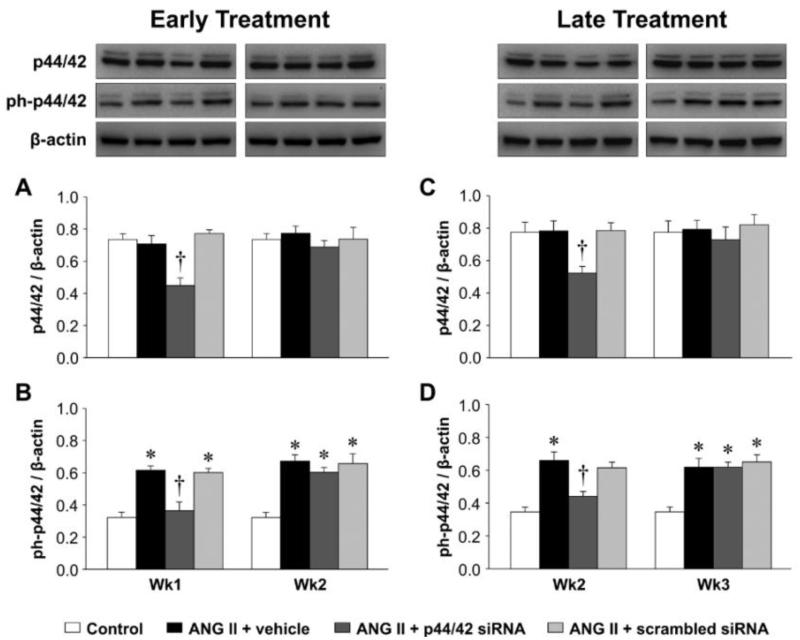
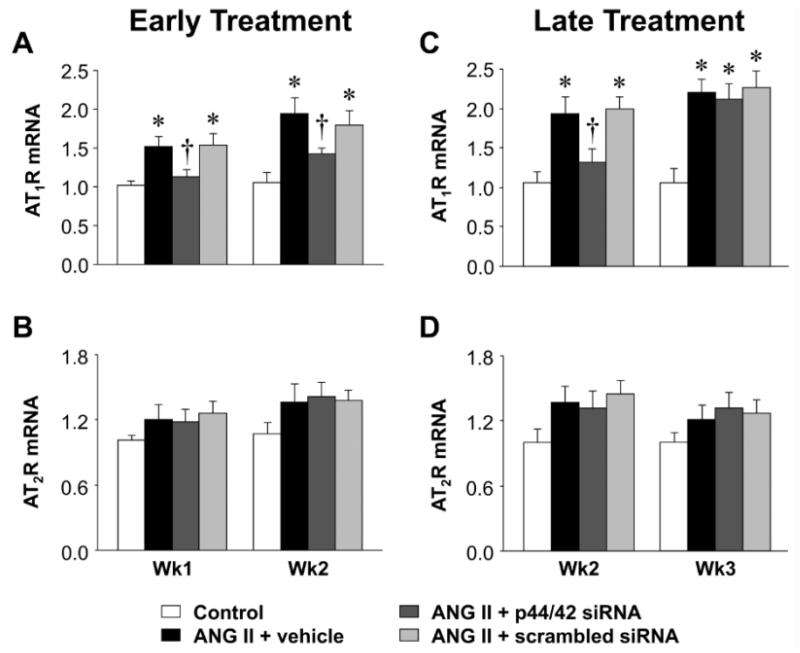
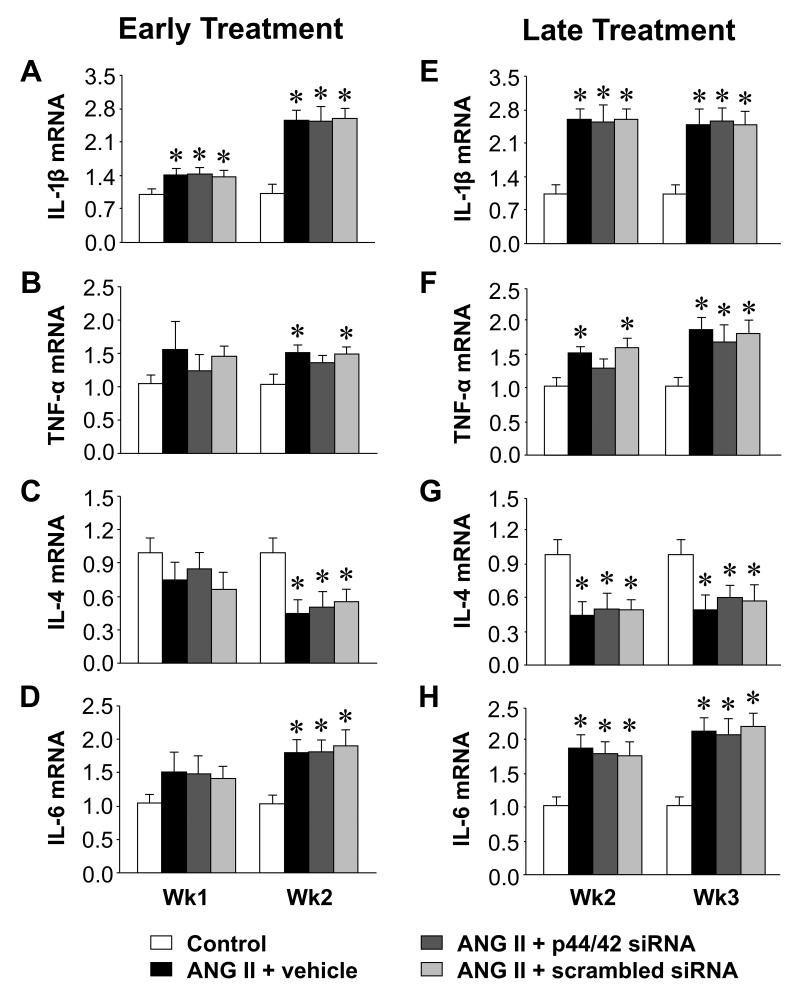
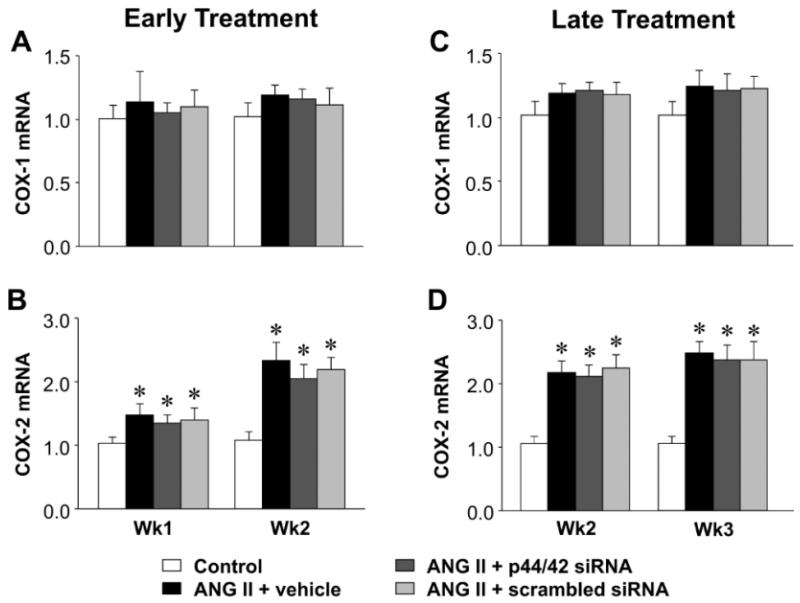
In the PVN of rats infused with ANG II + vehicle, compared with control rats, phosphorylated (ph-) p44/42 MAPK (Figure 3) and AT1R (Figure 4, 5) expression had increased at the end of week 1, prior to the rise in MBP, and there were small but significant increases in mRNA for interleukin (IL)-1β (Figure 6) and cyclooxygenase (COX)-2 (Figure 7). At that time point, there were no changes in mRNA expression of AT2R or mRNA for tumor necrosis factor (TNF)-α, IL-6, IL-4 or COX-1. After 2 or 3 weeks of ANG II + vehicle infusion, as pressure was rising, the increases in ph-p44/42 and AT1R were sustained, the increases in mRNA for IL-1β and COX-2 were larger, mRNA for TNF-α and IL-6 had also increased, and mRNA for IL-4 had decreased. There were no changes in mRNA for AT2R or COX-1.
Figure 3.
Change of PVN p44/42 MAPK activity at 1 week and 2 weeks after early (A and B) or late (C and D) PVN microinjections of p44/42 siRNA, a scrambled siRNA, or vehicle in ANG II-infused rats. Untreated rats served as Control. Representative Western blots are aligned with the matching grouped data. Values are corrected by β-actin and expressed as mean ± SEM (n=4 for each group). *P< 0.05, vs. Control; †P< 0.05, ANG II + p44/42 siRNA vs. ANG II + vehicle or ANG II + scrambled siRNA.
Figure 4.
Quantitative comparison of the mRNA expression of AT1R and AT2R in the PVN of ANG II-infused rats treated early (A and B) or late (C and D) with PVN microinjections of p44/42 MAPK siRNA, a scrambled siRNA, or vehicle. Untreated rats served as Control. Values are mean ± SEM (n = 5-8 for each group). *P< 0.05, vs. Control; †P< 0.05, ANG II + p44/42 siRNA vs. ANG II + vehicle or ANG II + scrambled siRNA.
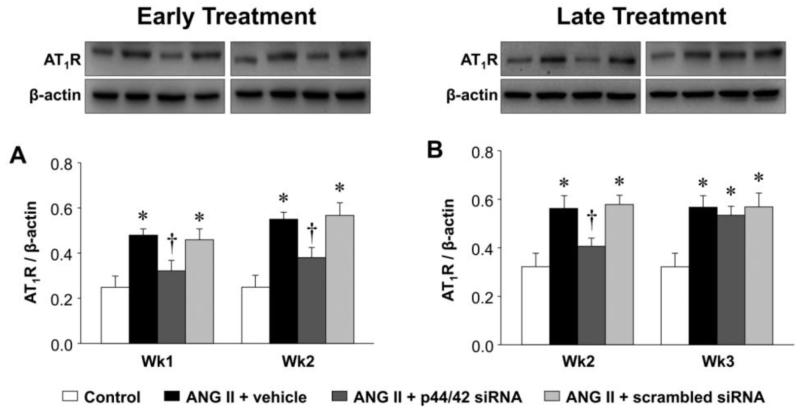
Figure 5.
Quantitative comparison of protein level of AT1R in the PVN of ANG II-infused rats treated early (A) or late (B) with PVN microinjections of p44/42 MAPK siRNA, a scrambled siRNA, or vehicle. Untreated rats served as Control. Representative Western blots are aligned with the matching grouped data. Values are corrected by β-actin and expressed as mean ± SEM (n = 4 for each group). *P< 0.05, vs. Control; †P< 0.05, ANG II + p44/42 siRNA vs. ANG II + vehicle or ANG II + scrambled siRNA.
Figure 6.
Quantitative comparison of the mRNA expression for IL-1β, TNF-α, IL-4 and IL-6 in the PVN of ANG II-infused rats treated early (A, B, C and D) or late (E, F, G and H) with PVN microinjections of p44/42 MAPK siRNA, a scrambled siRNA, or vehicle. Untreated rats served as Control. Values are mean ± SEM (n = 6-8 for each group). *P< 0.05, vs. Control.
Figure 7.
Quantitative comparison of the mRNA expression for COX-1 and COX-2 in the PVN of ANG II-infused rats treated early (A and B) or late (C and D) with PVN microinjections of p44/42 MAPK siRNA, a scrambled siRNA, or vehicle. Untreated rats served as Control. Values are mean ± SEM (n = 6-8 for each group). *P< 0.05, vs. Control.
Effects of PVN microinjections of p44/42 siRNA on responses to the ANG II infusion
Early treatment with bilateral PVN microinjections of p44/42 siRNA, delivered at the onset of the ANG II infusion, had no effect on MBP in week 1 but significantly attenuated the rise in MBP in week 2 (Figure 1A). Sympathetic tone, as measured by the response to hexamethonium bromide (Figure 2A), was normalized by the p44/42 siRNA. Later treatment with p44/42 siRNA, at the end of week 1 of ANG II infusion but still in the pre-hypertensive phase, had no significant effect on the subsequent ANG II-induced rise in MBP (Figure 1C) or the response to hexamethonium bromide (Figure 2B). Neither treatment affected HR. Treatment with a scrambled siRNA control had no effect on ANG II-induced increases in MBP or HR.
At the end of week 1, the ANG II infused rats treated early with p44/42 siRNA rats had reduced total p44/42 MAPK and normal ph-p44/42 MAPK (Figure 3A and 3B) and AT1R levels (Figure 4A and 5A) in the PVN, compared with normal control rats. At the end of week 2, the reductions in p44/42 MAPK and ph-p44/42 MAPK were no longer present, but AT1R mRNA and protein remained at normal levels. The early p44/42 siRNA treatment had no effect on mRNA for AT2R, IL-1β, TNF-α, IL-6, IL-4, COX-1 or COX-2 (Figure 4B, Figure 6 and 7).
The ANG II-infused rats that were treated later with p44/42 siRNA had similar reductions in total p44/42 and ph-p44/42 (Figure 3C and 3D) and in AT1R (Figure 4C and 5B) expression in the PVN one week after receiving the PVN microinjections. However, two weeks after the PVN microinjections, both p44/42 and AT1R expression in these rats was similar to that in the ANG II-infused rats treated with vehicle or a scrambled siRNA. The later p44/42 siRNA treatment also had no effect on the mRNA for AT2R or the inflammatory mediators (Figure 4D, Figure 6 and 7).
Effects of ANG II infusion and PVN microinjections of p44/42 siRNA on heart and vascular tissues
Two weeks of ANG II infusion significantly increased mRNA expression of IL-1β, TNF-α and IL-6 and decreased mRNA expression of IL-4 in the thoracic aorta of rats receiving PVN microinjections of vehicle or scrambled siRNA, compared with control rats. These changes were similar to those observed in the brain of ANG II-infused rats (Figure S2). The early treatment with PVN microinjections of p44/42 siRNA had no effect on these ANG II-induced inflammatory changes.
As seen in Table S2, the heart weight (HW) and HW/body weight (BW) ratio were significantly higher in the ANG II-infused rats treated with vehicle or scrambled siRNA. The ANG II-infused rats that received early PVN microinjections of p44/42 siRNA had significantly lower HW/BW ratio. The later PVN microinjections of p44/42 siRNA had no effect on the HW/BW ratio. There was no significant difference in BW across the experimental groups.
Control Studies
In the absence of ANG II-infusion, PVN microinjections of p44/42 siRNA, scrambled siRNA or vehicle alone had no effect on the expression of inflammatory mediators in the PVN (Figure S3). The average cycle threshold values for each gene in control rats were similar at the 2- and 3-week time points (data not shown).
In a separate group of rats (n=6), microinjections of sky blue dye validated the coordinates used for the PVN microinjections of siRNA (Figure S4).
DISCUSSION
The novel finding of this study is that the full expression of ANG II-induced hypertension requires the early engagement of p44/42 MAPK signaling in the PVN. A previous study from our laboratory demonstrated that a chronic (4-wk) subcutaneous administration of a low dose of ANG II increased both ph-p44/42 MAPK and AT1R expression in PVN and SFO.11 Intracerebroventricular (ICV) administration of the AT1R blocker losartan prevented the phosphorylation of p44/42 MAPK, and ICV administration of either losartan or the p44/42 MAPK inhibitor PD98059 prevented the increase in AT1R. Those findings demonstrated that systemically administered ANG II upregulates AT1R expression in PVN and SFO in a p44/42 MAPK dependent manner. In the present study, a “slow pressor” dose of ANG II that induced hypertension increased ph-p44/42 MAPK and AT1R mRNA and protein in the PVN, and early intervention to reduce p44/42 MAPK activity in PVN significantly attenuated the ANG II-induced increases in AT1R expression, sympathetic nerve activity, blood pressure and indices of cardiac remodeling. The ANG II infusion also upregulated mRNAs for the inflammatory mediators IL-1β, IL-6, TNF-α, and COX-2, but these were not affected by interrupting p44/42 MAPK signaling. There was no apparent effect of the ANG II infusion or of p44/42 MAPK activity on AT2R expression in the PVN. These results demonstrate that p44/42 MAPK signaling in the PVN is a pivotal mechanism in the pre-hypertensive phase of ANG II-induced hypertension.
Considering the ability of ANG II to upregulate its own receptors in cardiovascular regions of the brain11, 14 and the well-recognized role of the brain RAS in hypertension, the most likely explanation for the salutary effect of early intervention in PVN p44/42 MAPK signaling is the observed reduction in AT1R expression. The early siRNA treatment was more effective in that regard, reducing AT1R at week 1 and week 2, during which MBP remained significantly lower than expected. This is particularly interesting because the effect of the early treatment on AT1R expression outlasted the transient effect of the siRNA to suppress of ph-p44/42 levels. siRNA treatment a week after starting the ANG II infusion also reduced AT1R expression in the PVN, when measured at ANG II infusion week 2, but did not attenuate the ANG II-induced rise in blood pressure. These findings suggest that there is a narrow therapeutic window for effective intervention in the upregulation of brain RAS activity in the pre-hypertensive phase of ANG II hypertension. Notably, at the 2-week time point the ANG II infusion had induced a broader and more vigorous inflammatory response in the PVN that was unaffected by the p44/42 siRNA treatment and was sustained for the remainder of the 3-week infusion protocol. This ANG II-induced rise in pro-inflammatory cytokines may explain the failure of later intervention to affect the rise in MBP or the expression of AT1R at week 3.
The contribution of central interactions between ANG II and the pro-inflammatory cytokines to the pathophysiology of hypertension is well described in the extant literature. Microglial cells express AT1R receptors,15 and chronic ANG II infusion stimulates the production of inflammatory mediators in PVN – including TNF-α, IL-1β, and IL6, the pro-inflammatory cytokines measured in the present study.4, 5 Reducing the expression of these mediators by inhibiting microglial activation or by overexpressing the anti-inflammatory cytokine IL-10 significantly attenuates the blood pressure response.5 In the present study, the more substantial expression of TNF-α, IL-1β, and IL-6 at 2 and 3 weeks may well have contributed to the continued rise in MBP. The associated increase in the expression of COX-2, which is induced by the pro-inflammatory cytokines, may also be a contributing factor. COX-2 is the limiting enzyme in the synthesis of prostaglandin E2 (PGE2), which is known to disinhibit parvocellular PVN neurons.16 We have demonstrated a role for cytokine-induced COX-2 activity in sympathetic activation in heart failure.17 And although the present study provided no evidence for upregulation of COX-1 activity in PVN, a role for constitutively expressed COX-1, an alternative route for PGE2 production that has been reported to contribute to hypertension in this model,18 might also be considered.
The increase in inflammatory mediators may have contributed to upregulation of AT1R at the later time points, independent of ph-p44/442 MAPK activity. The tight link between inflammatory mediators and the brain RAS is emphasized by recent work in the heart failure model demonstrating that expression of pro-inflammatory cytokines and AT1R is affected by agents that block either pathway.19 More pertinent to the present study is the observation that blocking TNF-α in the PVN of rats with heart failure reduced the PVN expression of AT1R.20 The crucial link between these two systems appears to be nuclear factor kappa B (NF-kB) - the pro-inflammatory cytokines, acting through NF-kB, are known to upregulate the expression of AT1R21 and angiotensinogen,22 and ANG II apparently upregulates both brain RAS components and pro-inflammatory cytokines via this transcription factor.4
Finally, a potential role for inflammation as an alternative stimulus to MAPK activity deserves mention. Although the pro-inflammatory cytokines are more commonly associated with activation of p38 MAPK,23 in unpublished work24 we have found that an acute intracarotid artery injection of TNF-α can activate p44/42 MAPK signaling in PVN, and that ICV administration of a p44/42 MAPK inhibitor reduces the associated sympathetic response. It is therefore conceivable that at least some of the observed effect of the p44/42 siRNA to reduce ANG II-induced increases in blood pressure and sympathetic activity may be attributed to inhibiting the downstream effects of pro-inflammatory cytokines.
The ANG II infusion induced peripheral cardiovascular effects, including vascular inflammation and cardiac remodeling. The vascular inflammatory effects were unaffected by a reduction in PVN ph-p44/42 MAPK, suggesting their dependence on the local effects of circulating ANG II. In contrast, the increase in HW/BW ratio was significantly reduced by the PVN microinjections of p44/42 siRNA. Since that central intervention reduced both MBP and sympathetic drive, as indicated by the response to hexamethonium bromide, the present study cannot determine the relative influence of these two factors that can independently influence cardiac remodeling. In addition, a role for local effects of circulating ANG II on cardiac and vascular AT1R cannot be excluded.
Limitations of the Study
The present study did not determine the extent to which ANG II-induced hypertension is dependent upon brain p44/42 MAPK signaling. A partial reduction in p44/42 activity in PVN attenuated but did not prevent the ANG II-induced hypertensive response. The residual blood pressure response might be explained by the incomplete knockdown of p44/42 MAPK. The effectiveness of microinjected siRNA is limited, in our hands reducing p44/42 MAPK by only about 38 %, and the reduction occurs in close proximity to the injection site.25 Moreover, in the present study the effect of the p44/42 siRNA to silence p44/42 expression was no longer present at week 2 after the microinjections, consistent with a previous report indicating that the maximal silencing effect of siRNA transfection typically occurs 5-7 days after injection.26 Viral transfection would likely produce a more widespread and sustained reduction in p44/42 MAPK, with greater impact on the measured variables.
Other possible explanations for the residual ANG II-induced rise in blood pressure might also be entertained, including the potential involvement of MAPK signaling and AT1R upregulation in other central cardiovascular nuclei (e.g., SFO, rostral ventrolateral medulla) that contribute to sympathetic activation in hypertension. The PVN was singled out for these microinjections, but the effects of MAPK on angiotensinergic signaling in other key regions in which it has been identified (e.g, in SFO) have yet to be studied. In addition, while p44/42 MAPK signaling was the focus of this investigation, a role for other MAPK signaling pathways (e.g., p38 MAPK, JNK) cannot be excluded.
Finally, the influence of other excitatory mediators (e.g., aldosterone, pro-inflammatory cytokines) that may be present in the PVN and/or in other cardiovascular-related regions of the brain must also be considered. We recently demonstrated that aldosterone activates p44/42 MAPK in PVN via an interaction with AT1R that apparently mediates its central effects on sympathetic drive.27 Other recent studies have shown that ANG II-induced hypertension can be largely blocked by a mineralocorticoid receptor antagonist,2 reinforcing the concept of a central interdependence of ANG II and aldosterone effects.
Perspectives
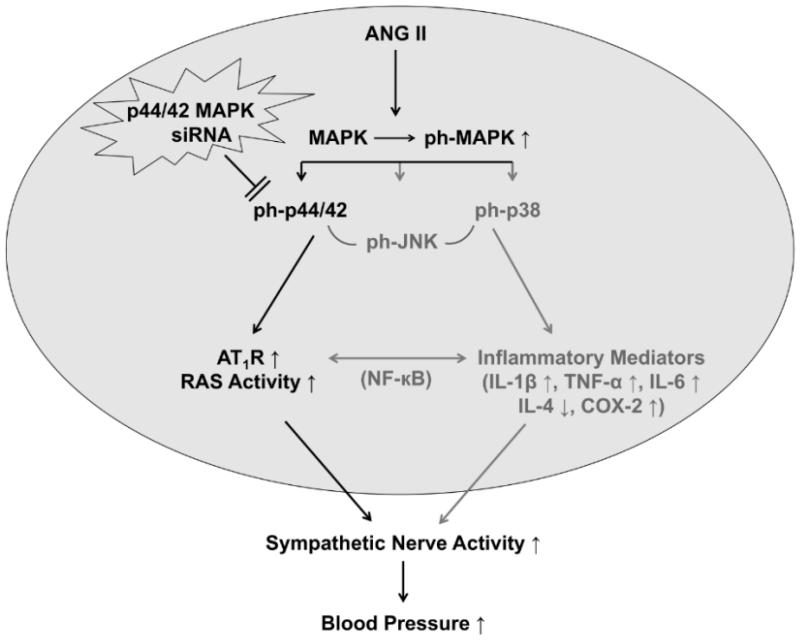
This study identifies an intracellular signaling mechanism in the PVN that is activated in the pre-hypertensive phase of ANG II-induced hypertension and that can be manipulated early to prevent the full expression of the hypertensive state. Blocking brain p44/42 MAPK signaling has also been shown to ameliorate sympathetic activation in an animal model of systolic heart failure.10 While the present study focused on the effects of ANG II-induced p44/42 MAPK signaling in the upregulation of the AT1R and brain RAS activity, as illustrated in Figure 8, this mechanism can also be activated by aldosterone and the pro-inflammatory cytokines, either directly or indirectly via their effects on RAS. As an inducible downstream signaling pathway for several key sympatho-excitatory mediators present in the brain in heart failure and hypertension, p44/42 MAPK signaling seems an ideal target for central intervention in cardiovascular disease states.
Figure 8.
Schematic diagram showing possible mechanisms by which p44/42 MAPK activity in the PVN might contribute to ANG II-induced hypertension. Early PVN microinjections of p44/42 MAPK siRNA inhibit ANG II-induced p44/42 MAPK activity to reduce upregulation of AT1R in the PVN, resulting in decreased blood pressure mediated by sympathetic excitation during the development of hypertension. ANG II also induces upregulation of inflammatory mediators, independent of p44/42 MAPK, and these may contribute to the subsequent progression of hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
Activation of the p44/42 mitogen-activated protein kinase (MAPK) pathway in the hypothalamic paraventricular nucleus (PVN) is necessary for the full expression of the slow pressor response to angiotensin-II (ANG II).
What Is Relevant?
In ANG II-induced hypertension, p44/42 MAPK signaling upregulates ANG II type-1 receptor (AT1R) expression in PVN.
ANG II-induced upregulation of inflammatory mediators in PVN is independent of p44/42 MAPK signaling.
Early interference with PVN p44/42 MAPK signaling ameliorates ANG II-induced hypertension.
Summary of Conclusions.
Pretreatment of PVN with p44/42 siRNA reduces the ANG II-induced increase in AT1R expression in the PVN and the full expression of ANG II-induced hypertension.
Pretreatment with p44/42 siRNA does not affect the PVN expression of inflammatory mediators, but may reduce their downstream influences on blood pressure and sympathetic activity.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, and by Award Numbers HL-096671 (to RBF), HL-073986 (to RBF), HL-14388 (to AKJ), HL-98207 (to AKJ) and MH-80241 (to AKJ) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
DISCLOSURES
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 2.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H555–H564. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marc Y, Gao J, Balavoine F, Michaud A, Roques BP, Llorens-Cortes C. Central antihypertensive effects of orally active aminopeptidase a inhibitors in spontaneously hypertensive rats. Hypertension. 2012;60:411–418. doi: 10.1161/HYPERTENSIONAHA.112.190942. [DOI] [PubMed] [Google Scholar]
- 7.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northcott CA, Watts S, Chen Y, Morris M, Chen A, Haywood JR. Adenoviral inhibition of AT1a receptors in the paraventricular nucleus inhibits acute increases in mean arterial blood pressure in the rat. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1202–R1211. doi: 10.1152/ajpregu.00764.2009. [DOI] [PubMed] [Google Scholar]
- 9.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension. 2008;52:679–686. doi: 10.1161/HYPERTENSIONAHA.108.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension. 2008;52:342–350. doi: 10.1161/HYPERTENSIONAHA.108.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1425–H1433. doi: 10.1152/ajpheart.00942.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- 13.Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;299:R343–R351. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappaB and activator protein-1 activation. Eur J Neurosci. 2008;27:343–351. doi: 10.1111/j.1460-9568.2007.06014.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1beta on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2005;17:498–508. doi: 10.1111/j.1365-2826.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–659. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension. 2012;59:869–876. doi: 10.1161/HYPERTENSIONAHA.111.182071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, Zhao XF, Zhang J, Zhang LH, Yang ZM, Francis J. TNF-alpha in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. The Tohoku Journal of Experimental Medicine. 2010;222:251–263. doi: 10.1620/tjem.222.251. [DOI] [PubMed] [Google Scholar]
- 21.Cowling RT, Gurantz D, Peng J, Dillmann WH, Greenberg BH. Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta. J Biol Chem. 2002;277:5719–5724. doi: 10.1074/jbc.M107515200. [DOI] [PubMed] [Google Scholar]
- 22.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Molecular and Cellular Biochemistry. 2000;212:155–169. [PubMed] [Google Scholar]
- 23.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZH, Yu Y, Wei SG, Felder RB. Brain p44/42 mitogen-activated protein kinase contributes to the sympathetic response to blood-borne TNF-{alpha} in rats (Abstract) The FASEB Journal. 2010;24:1050.4. [Google Scholar]
- 25.Yu Y, Zhang ZH, Wei SG, Weiss RM, Felder RB. Silencing p44/42 Mitogen-Activated Protein Kinase in the Paraventricular Nucleus of Hypothalamus Ameliorates Sympathetic Excitation in Rats after Myocardial Infarction (Abstract) Hypertension. 2010;56:E101–E102. [Google Scholar]
- 26.Ashfaq UA, Yousaf MZ, Aslam M, Ejaz R, Jahan S, Ullah O. siRNAs: potential therapeutic agents against hepatitis C virus. Virology Journal. 2011;8:276. doi: 10.1186/1743-422X-8-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZH, Yu Y, Wei SG, Felder RB. Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am J Physiol Heart Circ Physiol. 2012;302:H742–H751. doi: 10.1152/ajpheart.00856.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.