Abstract
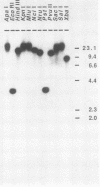
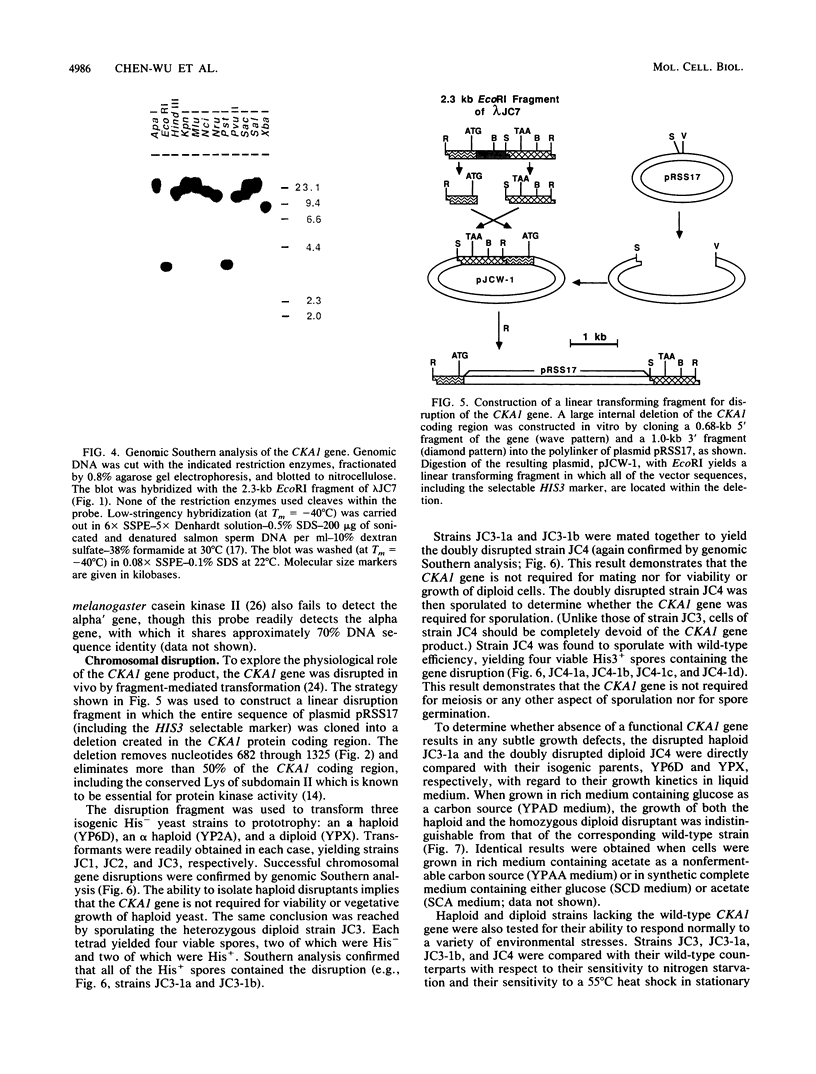
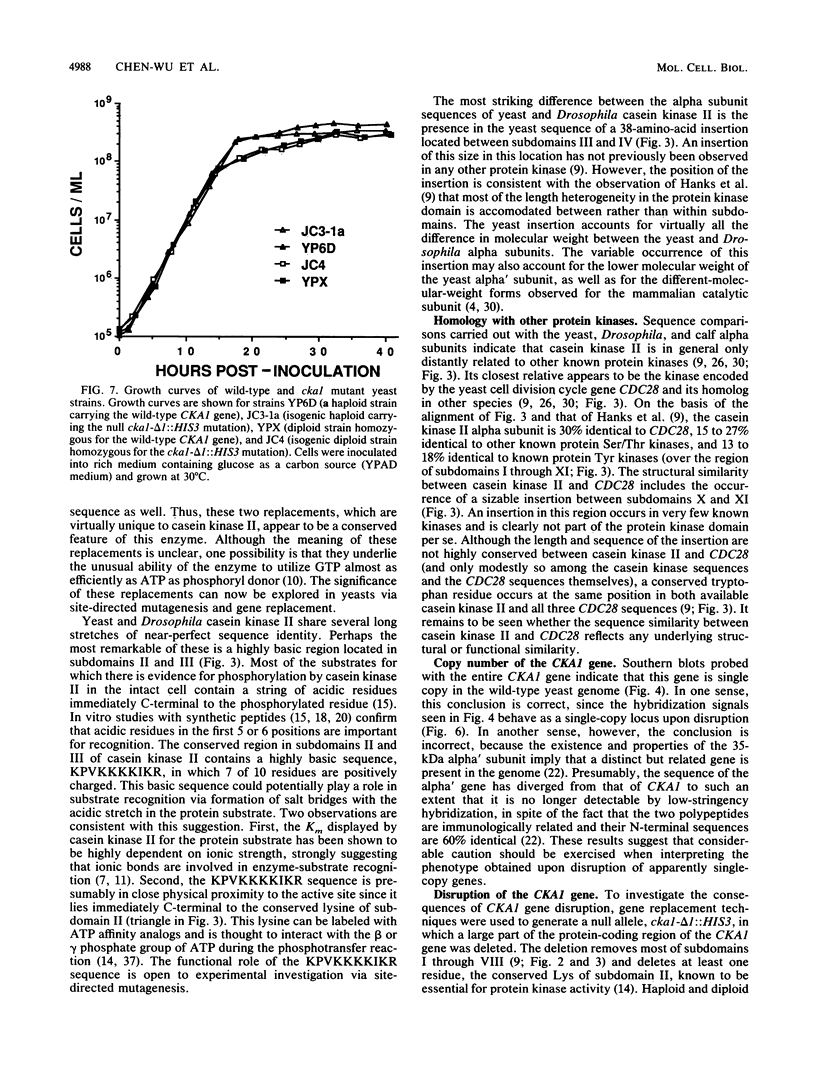
Casein kinase II of Saccharomyces cerevisiae contains two distinct catalytic subunits, alpha and alpha', which must be encoded by separate genes (R. Padmanabha and C. V. C. Glover, J. Biol. Chem. 262:1829-1835, 1987). The gene encoding the 42-kilodalton alpha subunit has been isolated by screening a yeast genomic library with oligonucleotide probes synthesized on the basis of the N-terminal amino acid sequence of the polypeptide. This gene (designated CKA1) contains an intron-free open reading frame of 372 amino acid residues. The deduced amino acid sequence is 67% identical to the alpha subunit of Drosophila melanogaster casein kinase II. The CKA1 gene product appears to be distantly related to other known protein kinases but exhibits highest similarity to the CDC28 gene product and its homolog in other species. Gene replacement techniques have been used to generate a null cka1 mutant allele. Haploid and diploid strains lacking a functional CKA1 gene appear to be phenotypically wild type, presumably because of the presence of the alpha' gene. Interestingly, the CKA1 gene appears to be single copy in the yeast genome; i.e., the alpha' gene, whose existence is known from biochemical studies and protein sequencing, cannot be detected by low-stringency hybridization.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Dahmus G. K., Glover C. V., Brutlag D. L., Dahmus M. E. Similarities in structure and function of calf thymus and Drosophila casein kinase II. J Biol Chem. 1984 Jul 25;259(14):9001–9006. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glover C. V. A filamentous form of Drosophila casein kinase II. J Biol Chem. 1986 Oct 25;261(30):14349–14354. [PubMed] [Google Scholar]
- Glover C. V., Shelton E. R., Brutlag D. L. Purification and characterization of a type II casein kinase from Drosophila melanogaster. J Biol Chem. 1983 Mar 10;258(5):3258–3265. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Regulation of casein kinase II by 2,3-bisphosphoglycerate in erythroid cells. J Biol Chem. 1984 Mar 10;259(5):2850–2855. [PubMed] [Google Scholar]
- Hathaway G. M., Zoller M. J., Traugh J. A. Identification of the catalytic subunit of casein kinase II by affinity labeling with 5'-p-fluorosulfonylbenzoyl adenosine. J Biol Chem. 1981 Nov 25;256(22):11442–11446. [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Marin O., Meggio F., Marchiori F., Borin G., Pinna L. A. Site specificity of casein kinase-2 (TS) from rat liver cytosol. A study with model peptide substrates. Eur J Biochem. 1986 Oct 15;160(2):239–244. doi: 10.1111/j.1432-1033.1986.tb09962.x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Grankowski N., Kudlicki W., Szyszka R., Gasior E., Pinna L. A. Structure and properties of casein kinase-2 from Saccharomyces cerevisiae. A comparison with the liver enzyme. Eur J Biochem. 1986 Aug 15;159(1):31–38. doi: 10.1111/j.1432-1033.1986.tb09829.x. [DOI] [PubMed] [Google Scholar]
- Meggio F., Marchiori F., Borin G., Chessa G., Pinna L. A. Synthetic peptides including acidic clusters as substrates and inhibitors of rat liver casein kinase TS (type-2). J Biol Chem. 1984 Dec 10;259(23):14576–14579. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Glover C. V. Casein kinase II of yeast contains two distinct alpha polypeptides and an unusually large beta subunit. J Biol Chem. 1987 Feb 5;262(4):1829–1835. [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Padmanabha R., Glover C. V. Isolation and sequencing of cDNA clones encoding alpha and beta subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987 Oct;7(10):3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takio K., Kuenzel E. A., Walsh K. A., Krebs E. G. Amino acid sequence of the beta subunit of bovine lung casein kinase II. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4851–4855. doi: 10.1073/pnas.84.14.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]