Abstract
In Pseudomonas aeruginosa eDNA is a crucial component essential for biofilm formation and stability. In this study we report that release of eDNA is influenced by the production of phenazine in P. aeruginosa. A ∆phzA-G mutant of P. aeruginosa PA14 deficient in phenazine production generated significantly less eDNA in comparison with the phenazine producing strains. The relationship between eDNA release and phenazine production is bridged via hydrogen peroxide (H2O2) generation and subsequent H2O2 mediated cell lysis and ultimately release of chromosomal DNA into the extracellular environment as eDNA.
Keywords: Pseudomonas aeruginosa, phenazines, hydrogen peroxide, extracellular DNA
Introduction
Pseudomonas aeruginosa is an opportunistic human pathogenic bacterium well known for causing chronic lung infections in cystic fibrosis patients.1 Similar to many other bacterial species, biofilm formation of P. aeruginosa is facilitated by self-produced extracellular polymeric substances primarily composed of polysaccharides, proteins, lipids and extracellular DNA (eDNA).2,3 eDNA is crucial in various bacterial species for adhesion, cell-to-cell interaction/aggregation, biofilm formation and stability and protection of biofilms against antibiotics and detergents.4-8 In Gram positive bacteria release of eDNA involves lysis of a small population of bacterial cells, mediated through various autolysin proteins such as AtlE in S. epidermidis9 and gelatinase and serine protease by Enterococcus faecalis.10 Additionally phage and hydrogen peroxide (H2O2) mediated eDNA release at the cost of cell lysis have also been reported in diverse Streptococcus species.11,12
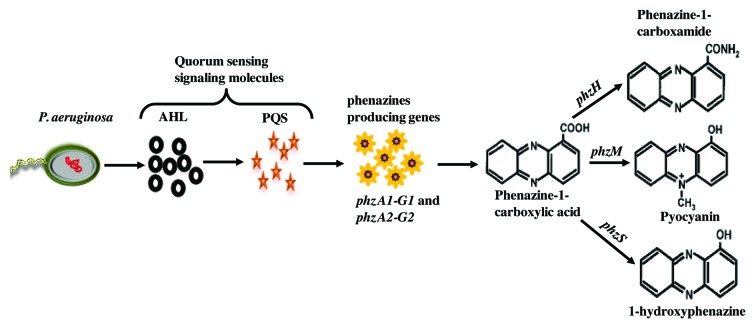
In P. aeruginosa, eDNA release is mediated through quorum sensing (QS) molecules such as N-acyl-L-homoserine lactones and the Pseudomonas quinolone signal by inducing phage production.13 eDNA release via non-QS pathways has also been documented in P. aeruginosa biofilms involving flagella and type IV pili mediated cell lysis.13,14 Recently Das and Manefield exposed that eDNA release in P. aeruginosa also happens through production of the phenazine pyocyanin.15 Phenazine production is mediated via QS controlled expression of phzA-G operons resulting in production of the primary phenazine phenazine-1-carboxylic acid (PCA). PCA is then modified to produce a variety of secondary phenazine molecules such as pyocyanin (PYO) through action of the phzM gene, phenazine-1-carboxamide (PCN), encoded by phzH and 1-hydroxy phenazine (1-OHPHZ) encoded by phzS as shown in Figure 1.16,17 In relation to our recently published paper,15 where we showed pyocyanin enhances eDNA release, in this addendum we demonstrated that like pyocyanin other phenazine molecules also promote eDNA production in P. aeruginosa.
Figure 1. Schematic represents quorum sensing controlled expression of genes for phenazine production. P. aeruginosa synthesizes acylated homoserine lactones (AHLs) and Pseudomonas quinone signaling (PQS) as their primary and secondary quorum sensing signaling molecules. PQS regulates the synthesis of phenazine-1-carboxylic acid (PCA) through a set of primary phenazine producing genes phzA1-G1 and phzA2-G2. PCA then converts into various kinds of specific phenazine encoded by specific genes.
Materials and Methods
Bacterial species, culture conditions and quantification of eDNA
All P. aeruginosa strains used in this study are listed in Table 1. Strains were plated onto LB agar plates and incubated overnight under aerobic conditions at 37°C. Single colonies from the agar plates were used to inoculate 20 ml cultures in LB medium (ph 7) for 0, 1, 3 and 5 d at 30°C, 150 rpm. After growth, the P. aeruginosa strains were harvested and pelleted out by centrifugation at 6,500 rpm (4,912 × g) for 5 min at 10°C. After centrifugation, supernatants were separated from bacterial pellets. In order to remove remaining bacteria, supernatants were again filtered using 0.22 µm Millipore filter units (Millipore). To further ensure the filtered supernatants were free of bacterial cells, 50 µl of filtered supernatant was used to inoculate LB agar plates and incubated aerobically for 48 h at 37°C. No bacterial colony formation on LB agar plates was observed (Fig. 2A). The concentration of eDNA present in the filtered supernatant of various P. aeruginosa strains at various growth days was quantified by using Qubit 2.0 Fluorometer (Invitrogen, Life Technologies).15
Table 1.P. aeruginosa strains used in this study and their relevant phenazine producing characteristics.
| P.aeruginosa strains | Phenazine production | Source | ||||
|---|---|---|---|---|---|---|
| |
PCA |
PCN |
5-MCA |
1-OHPHZ |
PYO |
|
| PAO1 Wildtype |
+ |
+ |
+ |
+ |
+ |
15 |
|
∆phzS |
+ |
+ |
+ |
- |
- |
18 |
|
∆phzH |
+ |
- |
+ |
+ |
+ |
18 |
|
∆phzM |
+ |
+ |
- |
+ |
- |
18 |
|
phzSH |
+ |
- |
- |
- |
++ |
15 |
| PA14 Wildtype |
+ |
+ |
+ |
+ |
+ |
15 |
| PA14 ∆phzM |
+ |
+ |
- |
+ |
- |
D.K. Newman lab |
| PA14 ∆phzA-G | - | - | - | - | - | 19 |
Note: +, produce basal level of phenazine; ++, produce elevated amount of phenazine.
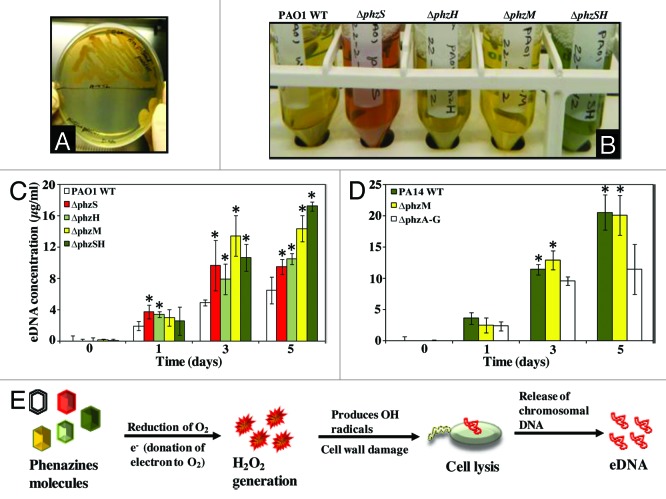
Figure 2. Different kinds of phenazine production and its influence in eDNA release. Comparison of bacterial colony formation on LB agar plate after 48 h incubation with non-filtered (upper half) and filtered (bottom half) bacterial supernatant (A). Production of various kinds of phenazine molecules by P. aeruginosa PAO1 strains (B). Quantification of eDNA release in supernatants of various kinds of phenazine producing P. aeruginosa PAO1 (C) and PA14 (D) strains. Error bars represents standard deviations from the mean (n = 3). Asterisks indicate statistically significant (p < 0.05) differences in eDNA concentration in comparison to the PAO1 wildtype (C) and mutant strain ∆phzA-G (D). Schematic represents the relationship between eDNA release and phenazine production is bridged via H2O2 generation and subsequent H2O2 mediated cell lysis (E).
Statistical analysis
The amount of eDNA release by various phenazine producing P. aeruginosa strains was analyzed using a two-tailed Student’s t-test. Differences were considered significant if p < 0.05.
Results and Discussion
By disrupting the activity of specific phenazine genes in wildtype PA01 the ratio of production of the various phenazine molecules is altered as indicated by the change in bacterial cell free supernatant color (Fig. 2B). For instance the mutant ∆phzS appears red because it lacks the gene responsible for 1-OHPHZ production and also responsible for conversion of 5-methylphenazine-1-carboxylic acid (5-MCA), an immediate precursor of PYO (which is red colored compound), into PYO.18 The ∆phzH mutant appears light green because it produces 1-OHPHZ and PYO but lacks production of PCN.18 The double mutant ∆phzSH is unable to produce PCN or 1-OHPHZ and consequently overproduces PYO giving the supernatant a dark green appearance.15
Figure 2C demonstrates that mutant strains over-producing specific phenazines released significantly more eDNA than the PAO1 wildtype strain especially after 3 and 5 d of batch culture growth. In support of this observation it has been reported previously that altering the proportion of specific phenazine production, via activating or deleting specific phz genes, has significant impacts on Pseudomonas adhesion, biofilm formation and biocontrol activity.19-21 Similar observations were made using a ∆phzA-G mutant of P. aeruginosa strain PA14 incapable of producing phenazines. In this case, significantly lower eDNA production was observed in comparison to PA14 wildtype that predominantly produces pyocyanin15 and a PA14 ∆phzM mutant that is deficient in pyocyanin production but producing PCN and 1-OHPHZ (Fig. 2D).16
The relationship between eDNA release and phenazine production is bridged via H2O2 generation as an intermediate agent. H2O2 generation occurs when phenazines, which are electrochemically active, accept electrons from NADH in the biofilm or cell culture and subsequently transfers that electrons it to molecular oxygen. Phenazines are thus involved in reduction of molecular oxygen to form reactive oxygen species like O2-, H2O2.1 H2O2 can react with metals to produce highly reactive hydroxyl radicals that damage bacterial cell walls resulting in lysis of cells22 and ultimately release of chromosomal DNA forming eDNA (Fig. 2E).15 Linking the results from the previous and current study, we propose that phenazines may have significant ecological impact not only on P. aeruginosa biofilm formation but also on other bacterial species that persist in mixed biofilms along with P. aeruginosa. Phenazine influenced H2O2 generation and lysis of competing bacterial cells in mixed biofilms and subsequent eDNA release may give it a competitive edge.
Moreover, large quantities of pyocyanin as well as DNA are known to exist in the sputum of cystic fibrosis (CF) patients1,23 and these factors could promote biofilm formation and subsequently enhance mortality in CF patients through deterioration of the host immune system. Host immune cells called neutrophils kill infecting microbes by trapping them in the neutrophil extracellular matrix which mainly consists of DNA and antimicrobial peptides.24 However, killing of microbes may further encourage release of microbial eDNA and excess of DNA is responsible for failure of host immune systems by inciting autoimmune diseases (systemic lupus erythematosus).25 This makes the biological significance of eDNA of greater interest for further research since it not only encourages biofilm formation but also damages host immune systems.
Acknowledgments
We thank Professor Dianne K. Newman, Department of Biology, California Institute of Technology for providing us with P. aeruginosa ∆phzA-G and ∆phzM strains. We thank Professor Korneel Rabaey, Laboratory of Microbial Ecology and Technology (LabMET), Ghent University for providing us with ∆phzS, ∆phzH and ∆phzM strains.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by Australian Research Council (ARC) Future Fellowship Project ID FT100100078 (www.arc.gov.au). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23570
References
- 1.Price-Whelan A, Dietrich LEP, Newman DK. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol. 2006;2:71–8. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 2.Tsuneda S, Aikawa H, Hayashi H, Yuasa A, Hirata A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol Lett. 2003;223:287–92. doi: 10.1016/S0378-1097(03)00399-9. [DOI] [PubMed] [Google Scholar]
- 3.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–33. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 4.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 5.Das T, Sharma PK, Busscher HJ, van der Mei HC, Krom BP. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl Environ Microbiol. 2010;76:3405–8. doi: 10.1128/AEM.03119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das T, Krom BP, Van der Mei HC, Busscher HJ, Sharma PK. DNA-mediated bacterial aggregation is dictated by acid-base interactions. Soft Matter. 2011;7:2927–35. doi: 10.1039/c0sm01142h. [DOI] [Google Scholar]
- 7.Das T, Sharma PK, Krom BP, van der Mei HC, Busscher HJ. Role of eDNA on the adhesion forces between Streptococcus mutans and substratum surfaces: influence of ionic strength and substratum hydrophobicity. Langmuir. 2011;27:10113–8. doi: 10.1021/la202013m. [DOI] [PubMed] [Google Scholar]
- 8.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–92. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 10.Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–8. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrolo M, Frias MJ, Pinto FR, Melo-Cristino J, Ramirez M. Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS ONE. 2010;5:e15678. doi: 10.1371/journal.pone.0015678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol. 2011;193:516–26. doi: 10.1128/JB.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol. 2006;59:1114–28. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 14.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, et al. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 2003;185:4585–92. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das T, Manefield M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE. 2012;7:e46718. doi: 10.1371/journal.pone.0046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkataraman A, Rosenbaum M. Arends jan BA, Halitschke R, Angenent LT. Quorum sensing regulates electric current generations of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem Commun. 2010;12:459–62. doi: 10.1016/j.elecom.2010.01.019. [DOI] [Google Scholar]
- 17.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6454–65. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabaey K, Boon N, Höfte M, Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol. 2005;39:3401–8. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich LEP, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–6. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddula VSRK, Pierson EA, Pierson LS., 3rd Altering the ratio of phenazines in Pseudomonas chlororaphis (aureofaciens) strain 30-84: effects on biofilm formation and pathogen inhibition. J Bacteriol. 2008;190:2759–66. doi: 10.1128/JB.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin-A-Woeng TFC, Thomas-Oates JE, Lugtenberg BJJ, Bloemberg GV. Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp. strains. Mol Plant Microbe Interact. 2001;14:1006–15. doi: 10.1094/MPMI.2001.14.8.1006. [DOI] [PubMed] [Google Scholar]
- 22.Infection Control Today website. Available: http://www.infectioncontroltoday.com/articles/2001/09/infection-control-today-09-2001-the-effects-of-ge.aspx Accessed 2012 October 30.
- 23.Bakker EM, Tiddens HAWM. Pharmacology, clinical efficacy and safety of recombinant human DNase in cystic fibrosis. Expert Rev Respir Med. 2007;1:317–29. doi: 10.1586/17476348.1.3.317. [DOI] [PubMed] [Google Scholar]
- 24.Medina E. Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J Innate Immun. 2009;1:176–80. doi: 10.1159/000203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su K-Y, Pisetsky DS. The role of extracellular DNA in autoimmunity in SLE. Scand J Immunol. 2009;70:175–83. doi: 10.1111/j.1365-3083.2009.02300.x. [DOI] [PubMed] [Google Scholar]