Abstract
The molecular mechanism of action of presynaptically neurotoxic secreted phospholipases A2 (sPLA2s) has not been fully elucidated. We have recently proposed a model to explain one of the hallmarks of their action – the reduction in endocytosis leading to synaptic vesicle depletion in nerve terminals. Our results speak strongly in favor of a mechanism in which both specific protein-protein interactions and enzymatic activity of the neurotoxic sPLA2 ammodytoxin A (AtxA) are necessary for impairment of clathrin-dependent endocytosis in yeast cells. The reduction of endocytosis was strictly dependent on the enzymatic activity of sPLA2s expressed ectopically in our yeast model cells and was not observed with the catalytically inactive, non-neurotoxic AtxA-homolog, ammodytin L (AtnL). Here we confirm the validity of the model in mammalian cells also, by demonstrating that the enzymatically active mutant of AtnL, shown to inhibit endocytosis in yeast, acts as a presynaptically neurotoxic sPLA2 at the mammalian neuromuscular junction.
Keywords: ammodytoxin, ammodytin, secreted phospholipase A2, presynaptic neurotoxicity, myotoxicity, endocytosis, neuromuscular activity
Secreted phospholipases A2 (sPLA2s) are pharmacologically important components of several animal venoms. Their neurotoxic action on presynaptic neurons involves a reduction in endocytosis, leading to synaptic vesicle depletion. This results in suppression of the release of acetylcholine from neurons into the synaptic cleft, causing irreversible blockade of neurotransmission to the postsynaptic muscle cell.1 We have recently proposed a model of the molecular mechanism of action of the neurotoxic sPLA2 ammodytoxin A (AtxA).2 It is based on the experimental demonstration that the AtxA-induced reduction in endocytosis is a consequence of: (1) the specific intracellular interaction of AtxA with 14-3-3 proteins, which enables the toxin molecules to concentrate on the plasma membrane near the endocytic vesicles; and of (2) the PLA2 enzymatic activity of AtxA, which changes the shape and/or composition of the presynaptic membrane, thus inhibiting the activity of the endocytic protein amphiphysin.2 The study was, however, performed in yeast, making the validity of the proposed model for mammalian cells a pivotal question.
The reduction of endocytosis in yeast was not observed with the catalytically inactive structural paralog of AtxA, ammodytin L (AtnL), although it was capable of binding to 14–3-3 proteins. A critical control in establishing the model in yeast was the demonstration that the introduction, by genetic engineering, of PLA2 enzymatic activity into AtnL generated a protein capable of reducing endocytosis in the same way as AtxA.2 This experiment provided the crucial proof for the indispensable role of PLA2 activity in the reduction of endocytosis by AtxA in yeast. In contrast to AtxA, AtnL is not neurotoxic in mammals and is a member of a large group of myotoxic snake venom sPLA2-homologs that have lost their enzymatic activity during evolution.3,4 While PLA2 enzymatic activity is necessary for the presynaptic neurotoxicity of AtxA and of other sPLA2s,1 the myotoxic action of AtnL is based on a direct interaction with the cell membrane that is calcium- and PLA2 activity-independent. This action leads to sustained membrane damage and, eventually, cell lysis.4 Restoration of the calcium-dependent PLA2 activity in AtnL was achieved by introducing several residues from the calcium-binding network of AtxA. The enzymatically active mutant thus produced—AtnL(H28Y/L31W/N33G/S49D), abbreviated as LW—displayed several important characteristics typical of the neurotoxic AtxA: the enzymatic activity-dependent membrane-damaging activity, a higher cytotoxicity than that of AtnL in cell culture in vitro and, importantly, a significant increase in lethal potency in vivo.3 The LD50 of the LW mutant in mice (2.2 mg/kg), although still approximately 100-fold higher than that of AtxA, was at least 5-fold lower than that of AtnL (> 10 mg/kg). This offered the possibility that, with this acquired enzymatic activity, the LW mutant has also acquired the ability to act as a presynaptically neurotoxic sPLA2. The fact that the LW mutant and AtxA, but not AtnL, reduced endocytosis in the yeast model further strengthened such an explanation. To test the hypothesis that the mutations in AtnL that endowed it with PLA2 activity and the ability to impair endocytosis in yeast would also render it neurotoxic for mammals, we determined the effect of recombinant AtnL and its LW mutant on an isolated mouse phrenic nerve-hemidiaphragm preparation.
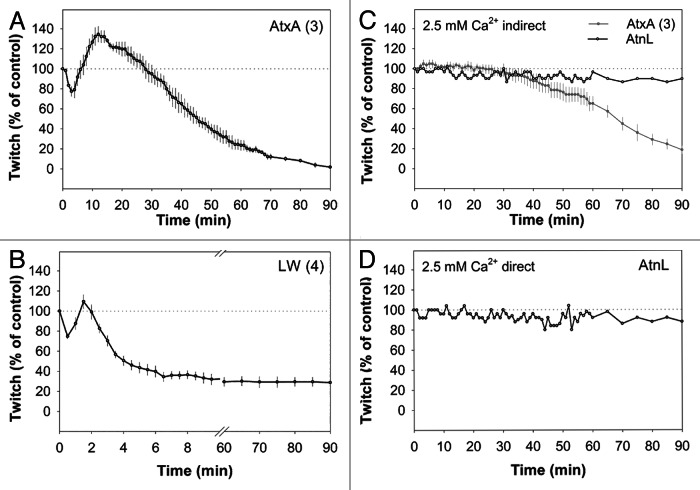
A hallmark of the action of presynaptically neurotoxic sPLA2s, including AtxA (Fig. 1A), is a triphasic effect on indirectly elicited muscle twitch tension of partially paralyzed neuromuscular (NM) preparations.5,6 The modulation of twitch tension induced by the LW mutant conformed to the characteristic triphasic action of presynaptically neurotoxic sPLA2s—a short initial depression of muscle contraction followed by a transient increase in twitch tension and, third, a progressive decline in muscle contractility within 10 min after the application of the mutant (Fig. 1B). The triphasic effect occurred almost 10-fold faster than in the case of AtxA. In contrast, recombinant AtnL did not trigger the triphasic response and had only a minor inhibitory effect on the twitch tension of a partly paralyzed NM preparation (data not shown). In experiments performed in standard Krebs solution with 2.5 mM Ca2+, in which the majority of motor end-plates within the NM preparation are sensitive to neuronal stimulation, AtxA inhibited muscle contractions in a manner consistent with our previous results.7 However, muscle contraction in standard Krebs solution was not affected by the addition of AtnL, at least not for up to 90 min, in the case of either indirect (Fig. 1C) or direct (Fig. 1D) stimulation of the muscle. This indicates that AtnL had no effect on acetylcholine release from the presynaptic neurons or on the functionality of the postsynaptic muscle cell. Furthermore, addition of a very potent presynaptically neurotoxic sPLA2, taipoxin, to an NM preparation pre-treated with AtnL, triggered the characteristic triphasic change of muscle contractility, demonstrating that this preparation retained the ability to respond to neurotoxic sPLA2s (data not shown). These results show clearly that, by switching on calcium-binding and thus the PLA2 activity of AtnL, by replacing amino acid residues at four positions,3 this presynaptically inactive protein has been converted into a presynaptically active sPLA2 in mammalian cells.
Figure 1. AtxA and the LW mutant, but not the enzymatically inactive AtnL, display the triphasic modulation of twitch tension characteristic of presynaptically neurotoxic sPLA2s. The effects of recombinant AtxA (10 µg/ml; 0.725 µM), the LW mutant (6 µg/ml; 0.430 µM) and AtnL (6 µg/ml; 0.433 µM) on muscle twitch tension were determined at 37°C. (A and B). The NM preparation was partially paralyzed by a low Ca2+ concentration (0.38–0.50 mM) Krebs solution and stimulated indirectly. Note that, upon addition of LW, the triphasic muscle twitch response occurred within 10 min (B). (C and D) The NM preparation was stimulated indirectly (C) or directly (D) in standard Krebs solution containing 2.5 mM Ca2+ as described in materials and methods. Note that AtxA, but not AtnL (C), inhibited muscle contractions. Proteins were added to the bath at time 0. Twitch tension is expressed as a percentage of the control value. The number of repeats of experiments is shown in brackets. Each point on the diagrams represents the mean ± SE. Standard errors are indicated by bars unless smaller than symbols.
The progressive decline of NM activity, along with other characteristic morphological changes induced by presynaptically neurotoxic sPLA2s at the NM junction (i.e., appearance of omega-shaped invaginations in the plasma membrane and synaptic vesicle depletion), has been ascribed to an irreversible blockade of synaptic vesicle retrieval from the plasma membrane.1,7,8 This parallels the impairment of the endocytic machinery observed on expression of AtxA and LW in yeast cells.2 To summarize, the reduction in endocytosis previously observed in yeast cells, coupled with the neurotoxic action at the mouse NM junction by AtxA and the enzymatically active mutant of AtnL, but not by AtnL itself, confirm that the proposed model of sPLA2 toxicity in yeast is applicable to the molecular mechanism of action of neurotoxic sPLA2s in mammals.
Materials and Methods
Recombinant proteins and chemicals
Recombinant AtxA, AtnL and its quadruple H28Y/L31V/N33G/S49D mutant LW were prepared by in vitro refolding following expression in E. coli as described.3,9 All other chemicals were from Sigma Aldrich or Gibco Life Technologies.
Neuromuscular activity
Adult male BALB/c mice (20–25 g) were maintained and humanely killed according to the Guiding Principles in the Use of Animals in Toxicology (Society of Toxicology, 1999, Guidelines are available at www.toxicology.org) and the Animals (Scientific Procedures) Act 1986. Hemidiaphragms and accompanying phrenic nerves were dissected and placed into 10 ml tissue baths containing Krebs solution (118.4 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.4 mM MgSO4 and 2.5 mM CaCl2), maintained at 37°C and oxygenated with a mixture 95% O2 and 5% CO2. Muscle contractions were evoked indirectly or directly. In the case of indirect neurally-evoked twitches, the attached phrenic nerve was stimulated at a frequency of 0.2 Hz with rectangular pulses of 0.2 ms duration and a supramaximal voltage (< 15 V) from a Grass force displacement transducer. Muscle twitches were recorded on a Grass polygraph model 79 (Grass Instruments). In order to reveal the triphasic effect of recombinant proteins on NM transmission, in some experiments the preparation was partially paralyzed (to 15–20% of control) by reducing the concentration of Ca2+ to 0.38–0.50 mM and allowing the tissues to equilibrate for 30–45 min. In these experiments only a reduced number of motor end-plates were sensitive to stimulation. Recombinant proteins were added to the tissue bath and changes in the amplitude of twitch tension responses were followed continuously. The functionality of the muscle in the preparation at the end of the recordings was checked directly by acetylcholine stimulation or indirectly by 2.5 mM Ca2+ stimulation.
In experiments with directly-evoked twitches the preparation was set up as described for the phrenic nerve-hemidiaphragm for recording neurally-evoked twitches. In addition, one end of a bipolar stimulating electrode was sutured into the diaphragm muscle near the costal margin and the other one was attached to the base of the hemidiaphragm. The directly-evoked twitches were recorded by supramaximal voltage stimulation (50 V) at a frequency of 0.2 Hz and 2 ms duration. To eliminate NM transmission in the preparation, d-tubocurarine (10 µM), an antagonist of the nicotinic acetylcholine receptors, was added into the organ bath 5 min prior to the beginning of direct stimulation. Positive controls for the neurotoxic triphasic effect were performed by adding taipoxin (0.5–1 µg/ml) after reducing the concentration of Ca2+ in order to partially paralyze the NM preparation as described above.
Acknowledgments
We sincerely thank Dr. Roger H. Pain for critical reading of the manuscript. This work was supported by grants P1-0207, Z1-4468 and J1-6507 from the Slovenian Research Agency and by NATO Collaborative Linkage Grant No. 980899.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23600
References
- 1.Pungerčar J, Križaj I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon. 2007;50:871–92. doi: 10.1016/j.toxicon.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Mattiazzi M, Sun Y, Wolinski H, Bavdek A, Petan T, Anderluh G, et al. A neurotoxic phospholipase A2 impairs yeast amphiphysin activity and reduces endocytosis. PLoS ONE. 2012;7:e40931. doi: 10.1371/journal.pone.0040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petan T, Križaj I, Pungerčar J. Restoration of enzymatic activity in a Ser-49 phospholipase A2 homologue decreases its Ca(2+)-independent membrane-damaging activity and increases its toxicity. Biochemistry. 2007;46:12795–809. doi: 10.1021/bi701304e. [DOI] [PubMed] [Google Scholar]
- 4.Lomonte B, Rangel J. Snake venom Lys49 myotoxins: From phospholipases A(2) to non-enzymatic membrane disruptors. Toxicon. 2012;60:520–30. doi: 10.1016/j.toxicon.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Lee CY, Tsai MC, Chen YM, Ritonja A, Gubenšek F. Mode of neuromuscular blocking action of toxic phospholipases A2 from Vipera ammodytes venom. Arch Int Pharmacodyn Ther. 1984;268:313–24. [PubMed] [Google Scholar]
- 6.Prijatelj P, Vardjan N, Rowan EG, Križaj I, Pungerčar J. Binding to the high-affinity M-type receptor for secreted phospholipases A(2) is not obligatory for the presynaptic neurotoxicity of ammodytoxin A. Biochimie. 2006;88:1425–33. doi: 10.1016/j.biochi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Logonder U, Križaj I, Rowan EG, Harris JB. Neurotoxicity of ammodytoxin A in the envenoming bites of Vipera ammodytes ammodytes. J Neuropathol Exp Neurol. 2008;67:1011–9. doi: 10.1097/NEN.0b013e318188c2d7. [DOI] [PubMed] [Google Scholar]
- 8.Dixon RW, Harris JB. Nerve terminal damage by beta-bungarotoxin: its clinical significance. Am J Pathol. 1999;154:447–55. doi: 10.1016/S0002-9440(10)65291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petan T, Križaj I, Gelb MH, Pungerčar J. Ammodytoxins, potent presynaptic neurotoxins, are also highly efficient phospholipase A2 enzymes. Biochemistry. 2005;44:12535–45. doi: 10.1021/bi051024r. [DOI] [PubMed] [Google Scholar]