Abstract
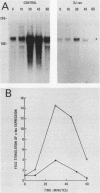
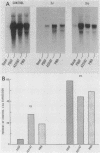
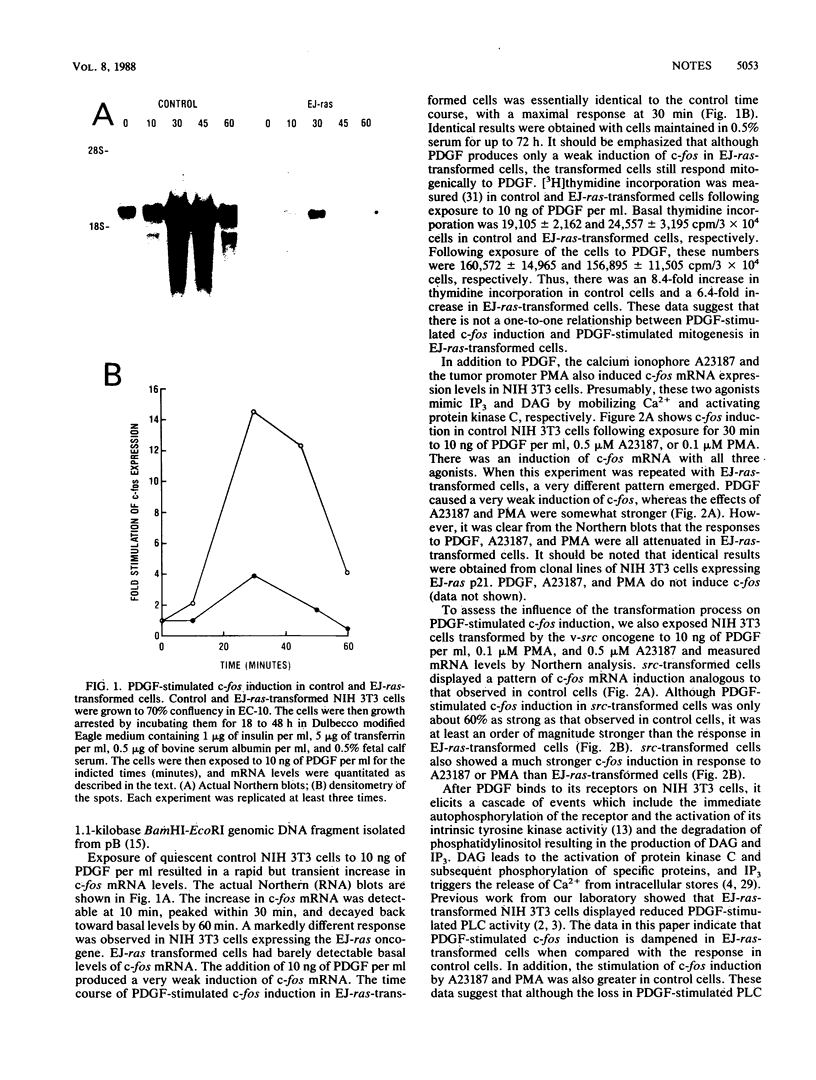
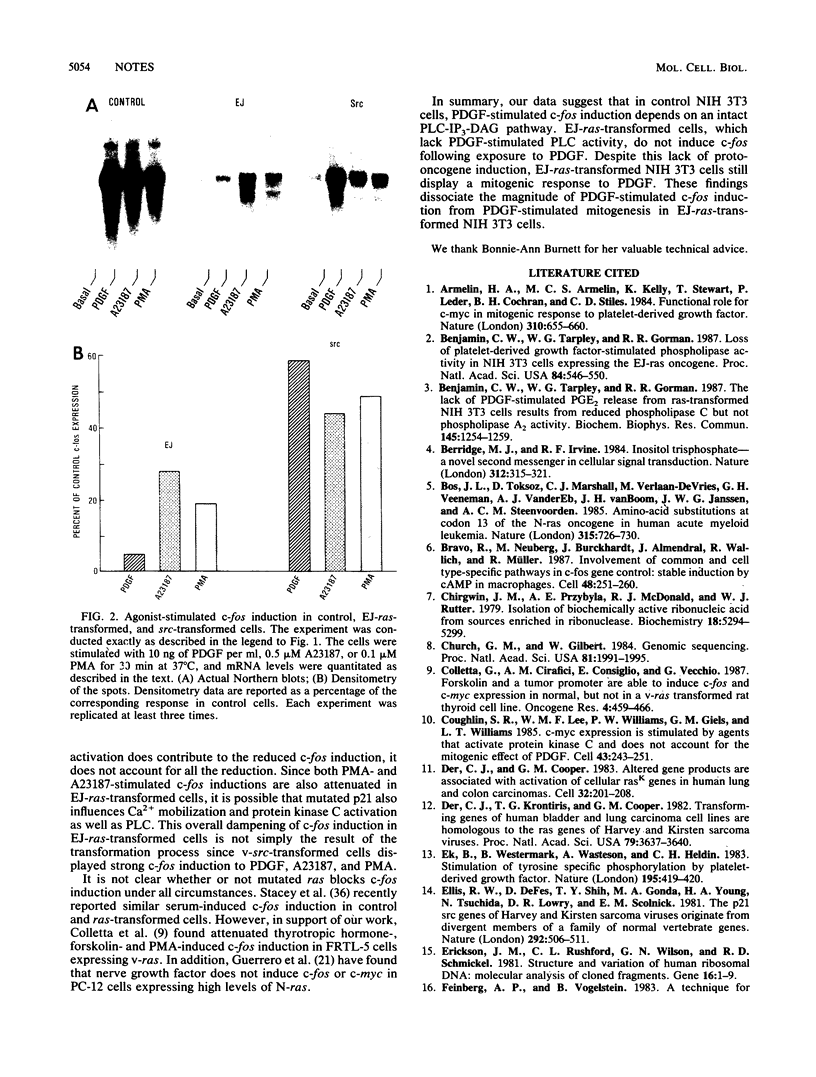
Platelet-derived growth factor (PDGF), the calcium ionophore A23187, and the tumor promoter phorbol myristate acetate stimulated c-fos mRNA levels in control NIH 3T3 cells. However, NIH 3T3 cells transformed by EJ-ras DNA transfection, which have diminished PDGF-stimulated phospholipase C activity, showed a 95% reduction in PDGF-stimulated c-fos mRNA levels. The responses to A23187 and phorbol myristate acetate were also attenuated, but not as severely as the PDGF-mediated induction. The reduction in PDGF-stimulated c-fos induction did not appear to be a general result of cellular transformation, since src-transformed NIH 3T3 cells displayed a strong PDGF-stimulated c-fos induction. Despite the reduction in PDGF-stimulated c-fos induction, EJ-ras-transformed cells still responded mitogenically to PDGF. These data suggest that the magnitude of c-fos induction cannot be directly correlated with PDGF-stimulated mitogenesis in EJ-ras-transformed NIH 3T3 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. Loss of platelet-derived growth factor-stimulated phospholipase activity in NIH-3T3 cells expressing the EJ-ras oncogene. Proc Natl Acad Sci U S A. 1987 Jan;84(2):546–550. doi: 10.1073/pnas.84.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. The lack of PDGE-stimulated PGE2 release from ras-transformed NIH-3T3 cells results from reduced phospholipase C but not phospholipase A2 activity. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1254–1259. doi: 10.1016/0006-291x(87)91572-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta G., Cirafici A. M., Consiglio E., Vecchio G. Forskolin and a tumor promoter are able to induce c-fos and c-myc expression in normal, but not in a v-ras-transformed rat thyroid cell line. Oncogene Res. 1987 Sep-Oct;1(4):459–466. [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Der C. J., Cooper G. M. Altered gene products are associated with activation of cellular rasK genes in human lung and colon carcinomas. Cell. 1983 Jan;32(1):201–208. doi: 10.1016/0092-8674(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rushford C. L., Dorney D. J., Wilson G. N., Schmickel R. D. Structure and variation of human ribosomal DNA: molecular analysis of cloned fragments. Gene. 1981 Dec;16(1-3):1–9. doi: 10.1016/0378-1119(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Pellicer A., Burstein D. E. Dissociation of c-fos from ODC expression and neuronal differentiation in a PC12 subline stably transfected with an inducible N-ras oncogene. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1185–1192. doi: 10.1016/0006-291x(88)90754-1. [DOI] [PubMed] [Google Scholar]
- Hall A., Marshall C. J., Spurr N. K., Weiss R. A. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature. 1983 Jun 2;303(5916):396–400. doi: 10.1038/303396a0. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- McCoy M. S., Toole J. J., Cunningham J. M., Chang E. H., Lowy D. R., Weinberg R. A. Characterization of a human colon/lung carcinoma oncogene. Nature. 1983 Mar 3;302(5903):79–81. doi: 10.1038/302079a0. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Tabin C. J., Shih C., Weinberg R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982 Jun 10;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Watson T., Kung H. F., Curran T. Microinjection of transforming ras protein induces c-fos expression. Mol Cell Biol. 1987 Jan;7(1):523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Tarpley W. G., Hopkins N. K., Gorman R. R. Reduced hormone-stimulated adenylate cyclase activity in NIH-3T3 cells expressing the EJ human bladder ras oncogene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3703–3707. doi: 10.1073/pnas.83.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]