Abstract
The glucose transporter, GLUT4, redistributes to the plasma membrane (PM) upon insulin stimulation, but also recycles through endosomal compartments. Different Rab proteins control these transport itineraries of GLUT4. However, the specific roles played by different Rab proteins in GLUT4 trafficking has been difficult to assess, primarily due to the complexity of endomembrane organization and trafficking. To address this problem, we recently performed advanced live cell imaging using total internal reflection fluorescence (TIRF) microscopy, which images objects ~150 nm from the PM, directly visualizing GLUT4 trafficking in response to insulin stimulation. Using IRAP-pHluorin to selectively label GSVs undergoing PM fusion in response to insulin, we identified Rab10 as the only Rab protein that binds this compartment. Rab14 was found to label transferrin-positive, endosomal compartments containing GLUT4. These also could fuse with the PM in response to insulin, albeit more slowly. Several other Rab proteins, including Rab4A, 4B and 8A, were found to mediate GLUT4 intra-endosomal recycling, serving to internalize surface-bound GLUT4 into endosomal compartments for ultimate delivery to GSVs. Thus, multiple Rab proteins regulate the circulation of GLUT4 molecules within the endomembrane system, maintaining optimal insulin responsiveness within cells.
Keywords: GLUT4, IRAP, Rab10, Rab14, adipocytes, TIRF
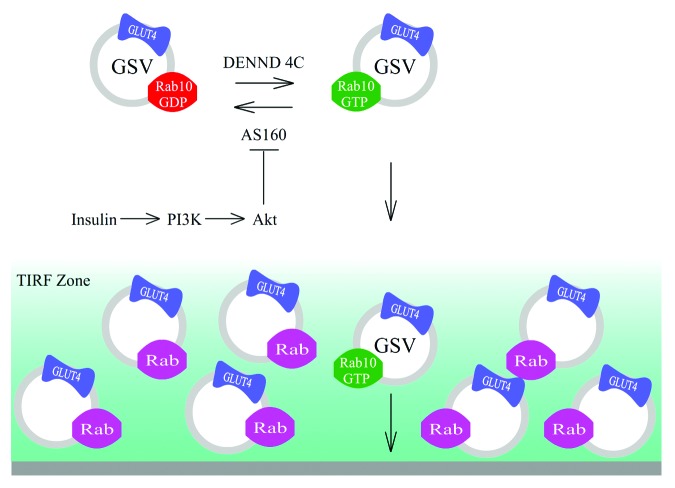
Insulin stimulates GLUT4 redistribution to the plasma membrane (PM) in adipocytes and muscle cells. This results in increased glucose influx into these cells, leading to reduction of circulating glucose level in the blood. GLUT4 redistribution to the PM involves physical trafficking of GLUT4 storage vesicles (GSVs) to the PM and is regulated by a signaling cascade of PI3K, AKT/PKB and AS160.1-3 In this cascade, PI3K and AKT/PKB are activated in response to insulin stimulation, causing phosphorylation of the Rab GTPase activation protein (GAP) AS160 by AKT.4 Akt phosphorylation inactivates the AS160 GAP domain, making it unable to stimulate hydrolysis of GTP on the Rab.5-7 In response, GSVs redistribute from internal sites to the PM, where fusion finally occurs. The negative regulatory role of AS160 Rab GAP domain in insulin-stimulated GSV delivery to the PM has implicated Rab proteins as key regulators (downstream of AS160) for PM delivery of GSVs.8,9
After being delivered to the PM during insulin stimulation, GLUT4 is endocytosed into the endosomal system and recycles through early endosomes, recycling endosomes and the trans-Golgi network before being reloaded into GSVs.10,11 This complex intracellular trafficking itinerary results in GLUT4 having a broad distribution pattern, with steady-state localization in many intracellular compartments. As these different compartments are characterized by having different Rab proteins associated with them,5,12,13 it has been difficult to determine which compartment(s) and its associated Rab protein(s) specifically responds to insulin through inactivation of AS160 by AKT.14-16
To identify the specific Rab protein(s) mediating delivery of GSVs to the PM after insulin stimulation, we employed insulin-responsive aminopeptidase (IRAP, which has the same intracellular distribution as GLUT4 in the cell) tagged with pHluorin (IRAP-pHluorin) to visualize GSV fusion at the PM after insulin stimulation.17-19 IRAP-pHluorin is a pH sensitive probe that only fluoresces when exposed to a nonacidic environment. Because the pH inside GSVs is acidic and that outside of cells is neutral, when a GSV fuses with the PM, exposing IRAP-pHluorin extracellularly, the probe now fluoresces brightly. We first characterized the identities of insulin-responsive IRAP-pHluorin vesicles by monitoring the presence of GLUT4 and transferrin receptor (TfR) on these vesicles under insulin stimulation in adipocytes. The majority of insulin-responsive IRAP-pHluorin vesicles contained GLUT4 but not TfR. This suggested the insulin-responsive IRAP-pHluorin vesicles were bona fide GSVs rather than recycling endosomes, as the latter are known to contain TfR whereas GSVs do not. With IRAP-pHluorin established as a reliable tool for selectively visualizing GSVs, we determined the association of candidate Rab proteins with IRAP-pHluorin fusing vesicles. Among 25 candidates, only Rab10 and Rab14 were observed to be specifically associated with IRAP-pHluorin fusing vesicles. Other Rab proteins, including Rab4A, Rab4B and Rab8A, resided in GLUT4-containing compartments but were not observed on IRAP-pHluorin fusing vesicles. This indicated that while they were not involved in GLUT4 exocytosis, they could possibly be mediating GLUT4 trafficking in the endosomal system.
We next investigated whether it is Rab10 or Rab14 that directly mediates GSV translocation to the PM in response to insulin. Rab14 was localized on compartments containing GLUT4 and TfR, suggesting it regulated movement of GLUT4 within endosomes. On the other hand, Rab10 was only localized on TfR negative structures that contained GLUT4. This suggested it was responsible for GSV delivery to the PM. Trafficking of GLUT4 through both Rab10 and Rab 14 compartments appeared to be important under insulin stimulation since loss of Rab10 and Rab14 additively inhibited GLUT4 delivery to the PM, and re-addition of either Rab10 or Rab14 partially restored GLUT4 translocation. Given Rab10’s role in mediating GSV delivery to the PM, its regulatory role was further explored. Rab10 was associated with GSVs as the vesicles moved into the TIRF zone under insulin stimulation. This indicated its association with GSVs occurs early during insulin signaling and deep inside the cells. A Rab10 constitutively active mutant was found to be associated with GSVs and induced GSV recruitment to the TIRF zone in the absence of insulin stimulation. This indicated Rab10 activation is sufficient to recruit GSVs to the cell periphery.
These findings have helped clarify the exact roles played by different Rab proteins in trafficking of GLUT4 proteins during insulin stimulation. They have further emphasized the heterogeneity of GLUT4 compartments at the cell periphery. Indeed, due to the proximity of endosomal GLUT4 compartments to the PM, most GLUT4 compartments observed by TIRF microscopy are endosomes rather than GSVs.12,20 After insulin stimulation, however, Rab10-labeled GSVs begin to enter into the TIRF zone and fuse at the PM. Because they fuse efficiently with the PM upon entry into the TIRF zone, GSVs are only transiently observed in the TIRF zone before disappearing. This contrasts with peripheral endosomal GLUT4 compartments, which fuse with the PM less frequently. Therefore, GSVs comprise only a small percentage of the total GLUT4 compartments in the TIRF zone, even after insulin stimulation. Consequently, GLUT4 vesicle density in the TIRF zone21 should not be taken as a direct measurement of the delivery of GSVs to the cell periphery.
We are only at the very beginning of understanding the intricacies of GSV formation, translocation and fusion at the PM. Although our studies revealed Rab10 serves as a key component in mobilizing GSVs so that they reach the cell periphery in response to insulin stimulation, further studies are needed to determine what molecules in addition to Rab10 participate in subsequent steps, such as GSV docking and/or fusion at the PM.22-24 The IRAP-pHluorin labeling strategy for monitoring GSV dynamics described in our study should be helpful in revealing these molecular machineries, which include those for anchoring GSVs at the PM25 and mediating their final fusion.26 (Fig. 1)
Figure 1. Heterogeneity of GLUT4 vesicles in the TIRF zone. Endosomal GLUT4 vesicles associated by endocytic Rab proteins (Rab4A, Rab4B and Rab8A) are abundant in the TIRF zone. After insulin stimulation, GSVs labeled by Rab10 start to move into the TIRF zone and fuse at the PM. Because of their efficient fusion, each GSV only appears transiently in the TIRF zone, leading to GSVs making up a very small fraction of GLUT4 vesicles in the TIRF zone during insulin stimulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23779
References
- 1.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–77. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 2.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–61. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 3.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 4.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–8. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 5.Mîinea CP, Sano H, Kane S, Sano E, Fukuda M, Peränen J, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 7.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–72. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:E29–37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 10.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic. 2011;12:672–81. doi: 10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 11.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–42. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaddai V, Gonzalez T, Keslair F, Grémeaux T, Bonnafous S, Gugenheim J, et al. Rab4b is a small GTPase involved in the control of the glucose transporter GLUT4 localization in adipocyte. PLoS ONE. 2009;4:e5257. doi: 10.1371/journal.pone.0005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–13. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Bilan PJ, Liu Z, Klip A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci USA. 2010;107:19909–14. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 16.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Fan J, Bai L, Wang Y, Chen Y, Yang L, et al. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J Biol Chem. 2008;283:8508–16. doi: 10.1074/jbc.M708688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–5. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Rubin BR, Orme CM, Karpikov A, Yu C, Bogan JS, et al. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J Cell Biol. 2011;193:643–53. doi: 10.1083/jcb.201008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–93. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, et al. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169:481–9. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez JA, Burchfield JG, Blair DH, Mele K, Ng Y, Vallotton P, et al. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20:3918–29. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–35. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]