Abstract
The pond snail Lymnaea stagnalis learns taste aversion and consolidates it into long-term memory (LTM). This is referred to as conditioned taste aversion (CTA). The superfusion of molluscan insulin-related peptides (MIPs) over the isolated snail brain causes a long-term enhancement of synaptic input between the cerebral giant cell and the B1 buccal motor neuron. This enhancement is hypothesized to underlie CTA. The synaptic enhancement caused by the superfusion of MIPs can be blocked by the application of human insulin receptor antibody, which recognizes the extracellular domain of human insulin receptor and acts as an antagonist even for MIP receptors. An injection of the human insulin receptor antibody into the abdominal cavity of trained snails blocks the consolidation process leading to LTM, even though the snails acquire taste aversion. Here, we examined whether or not taste-aversion training changes the mRNA expression level of MIP receptor in the snail brain and found that it does not. This result, taken together with previous findings, suggest that the MIPs’ effect on synaptic function in the snail brain is attributable to a change in the MIP concentration, and not to a change in the mRNA expression level of MIP receptor, which is thought to reflect the number of MIP receptors.
Keywords: conditioned taste aversion, insulin, insulin receptor, long-term memory, Lymnaea, molluscan insulin-related peptide
One of the remarkable associative learning abilities of the pond snail Lymnaea stagnalis is that it can establish taste aversion and consolidate it into long-term memory (LTM).1 This is referred to as conditioned taste aversion (CTA), which persists for more than a month.2,3 Our DNA microarray experiments showed that gene expression of some molluscan insulin-related peptides (MIPs) was upregulated in snails exhibiting CTA-LTM.4 Our recent electrophysiological approaches showed that the application of MIPs that had been partially purified from the central nervous system (CNS) evoked a long-term enhancement of synaptic transmission between the cerebral giant cell (a key interneuron for CTA) and the B1 motor neuron (a buccal motor neuron).5 This change in synaptic efficacy is thought to underlie the CTA-LTM consolidation process.6-8 When the human insulin receptor antibody is injected into the snail, it blocks the long-term synaptic enhancement caused by the MIPs. That is, it acts as an antagonist to MIP receptors. The human insulin receptor antibody recognizes the extracellular domain of human insulin receptor. Further, while the human insulin antibody blocks the establishment of LTM, it does not block the acquisition of taste aversion.5 Here, we ask whether or not the mRNA expression level of MIP receptor, which is thought to reflect the number of MIP receptors, in the Lymnaea CNS changes during the learning process.
To examine whether or not taste-aversion training changes the mRNA expression level of MIP receptor, we applied a previously described training procedure to three groups of snails: (1) snails trained for taste aversion; (2) a backward-conditioned control group; and (3) a naive control group.5,9 The conditioned stimulus (CS) was 10 mM sucrose, and the unconditioned stimulus (US) was 10 mM KCl. Application of the CS to the lips increases the feeding response in snails, whereas application of the US inhibits feeding behavior and evokes a withdrawal response. In the taste-aversion-training procedure, the CS is paired with the US 10 times. After these repeated contingent presentations of the CS and US, the CS no longer elicits the feeding response. In all three groups, we first performed a pretest with the CS. Posttests to the CS were performed 10 min after training. The number of feeding responses (rasping movements of the buccal mass) was counted in distilled water in a 1 min observation period, following a 15 sec application of the CS to the lips of the snail.
The results obtained from the behavioral experiments were as follows. Taste-aversion-trained snails (mean ± SEM): Pretest = 13.1 ± 0.8 (biting/min, n = 10 snails), Posttest = 0.2 ± 0.1 (biting/min, n = 10 snails). Backward-conditioned control snails: Pretest = 14.1 ± 0.9 (biting/min, n = 10 snails), Posttest = 12.4 ± 1.2 (biting/min, n = 10 snails). Naive control snails: Pretest = 13.2 ± 0.6 (biting/min, n = 10 snails), Posttest = 12.0 ± 0.7 (biting/min, n = 10 snails). As can be observed from these data, the feeding response (i.e., the number of bites) was significantly lower in the taste-aversion-trained snails than in the control snails (p < 0.01 by one-way ANOVA and post hoc Scheffé test).
To determine the mRNA expression level of MIP receptor by taste-aversion training, we dissected the buccal and cerebral ganglia 90 min after the end of training. One buccal ganglion and one cerebral ganglion were dissected from each of the 10 snails in each of the three groups. The total RNA samples of single buccal and cerebral ganglia were purified using the RNAqueous-Micro Kit (Ambion, Life Technologies). Reverse transcription (RT) was performed using 10 μl of each total RNA preparations, SuperScript II Reverse Transcriptase (Invitrogen, Life Technologies) and RNaseOUT (Invitrogen, Life Technologies) following the product manuals. After 1/5 dilution with distilled water, RT samples were mixed with SYBR Green Realtime PCR Master Mix (Toyobo) and a primer set selectively amplifying the MIP receptor or actin of Lymnaea. The nucleotide sequences of the primer sets of MIP receptor and actin were as follows. The forward primer for the MIP receptor: 5′-AAT GGC TGG AGA AAT AGC AGA TG-3′; the reverse primer for the MIP receptor: 5′-TGT CAT ACC AAA GTC TCC AAT TTT AAC-3′; the forward primer for actin: 5′-TCC CTT GAG AAG AGC TAC GAG C-3′; and the reverse primer for actin: 5′-GAG TTG TAG GTG GTT TCG TGG-3′. The reaction was performed at 95°C for 1 min followed by 50 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 30 sec each using the StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies). Each preparation of the MIP receptor and actin was applied to a 96-well plate in triplicate. Relative mRNA levels of the MIP receptor were calculated by the comparative CT (ΔΔCT) method (Applied Biosystems, Life Technologies)10 using the mRNA of actin as a reference. That is, the mRNA expression level of MIP receptor was normalized to that of actin. All the behavioral and quantitative real-time PCR experiments were performed blindly.
The ΔΔCT method for quantitative real-time PCR showed that the mRNA expression levels of MIP receptor were not changed (p > 0.05 by one-way ANOVA) in the three groups (taste-aversion training, backward-conditioned control training and naive control training; Fig. 1). Because the training did not change the mRNA expression level of MIP receptor, which is thought to reflect the number of MIP receptors, and because the gene expression of some MIPs was upregulated in snails exhibiting CTA-LTM as we showed previously,4 our results supported the hypothesis that CTA-LTM is the result of the release of more MIPs from the MIP-containing neurons (the light green cells).11-18 Because we do not possess an anti-MIP antibody, we cannot at this time determine the concentration of MIPs in the CNS following the taste-aversion training.
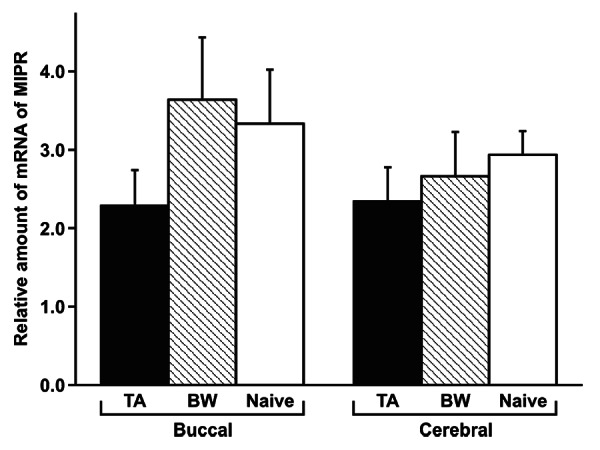
Figure 1. Comparison of MIP receptor mRNA levels in the snail brain among three different training procedures. At 90 min after training, the buccal and cerebral ganglia were dissected. The expression level was normalized to that of actin according to the comparative CT (ΔΔCT) method for quantitative real-time PCR. The data showed that training did not change the expression level (p > 0.05) at the single-ganglion level. The data are expressed as means ± SEM. Ten ganglia were obtained from each of 10 different snails. MIPR, MIP receptor; TA, taste-aversion-trained snails; BW, backward-conditioned control snails; Naive, naive control snails.
In conclusion, we found that taste-aversion training did not change the mRNA expression level of MIP receptor, which is thought to reflect the number of MIP receptors, in the snail brain. Our results thus supported the hypothesis that taste-aversion training results in the release of more MIPs from MIP-containing neurons. Finally, it appears that the increase in MIP concentrations, which brings about changes in synaptic efficacy between the cerebral giant cell and the B1 motor neuron,5 results from changes occurring on the postsynaptic B1 motor neuron.19 These data add weight to Glanzman’s findings that significant changes in synaptic efficacy in molluscan preparations are the result of postsynaptic changes and are not due solely to presynaptic changes.20
Acknowledgments
This work was supported by KAKENHI from JSPS (No. 23780051 to D.H. and No. 24657055 to E.I.) and by a grant from CIHR (No. MOP 64339 to K.L.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23955
References
- 1.Ito E, Kobayashi S, Kojima S, Sadamoto H, Hatakeyama D. Associative learning in the pond snail, Lymnaea stagnalis. Zoolog Sci. 1999;16:711–23. doi: 10.2108/zsj.16.711. [DOI] [Google Scholar]
- 2.Kojima S, Yamanaka M, Fujito Y, Ito E. Differential neuroethological effects of aversive and appetitive reinforcing stimuli on associative learning in Lymnaea stagnalis. Zoolog Sci. 1996;13:803–12. doi: 10.2108/zsj.13.803. [DOI] [Google Scholar]
- 3.Kita S, Hashiba R, Ueki S, Kimoto Y, Abe Y, Gotoda Y, et al. Does conditioned taste aversion learning in the pond snail Lymnaea stagnalis produce conditioned fear? Biol Bull. 2011;220:71–81. doi: 10.1086/BBLv220n1p71. [DOI] [PubMed] [Google Scholar]
- 4.Azami S, Wagatsuma A, Sadamoto H, Hatakeyama D, Usami T, Fujie M, et al. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea. J Neurosci Res. 2006;84:1610–20. doi: 10.1002/jnr.21045. [DOI] [PubMed] [Google Scholar]
- 5.Murakami J, Okada R, Sadamoto H, Kobayashi S, Mita K, Sakamoto Y, et al. Involvement of insulin-like peptide in long-term synaptic plasticity and long-term memory of the pond snail Lymnaea stagnalis. J Neurosci. 2013;33:371–83. doi: 10.1523/JNEUROSCI.0679-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima S, Nanakamura H, Nagayama S, Fujito Y, Ito E. Enhancement of an inhibitory input to the feeding central pattern generator inLymnaea stagnalis during conditioned taste-aversion learning. Neurosci Lett. 1997;230:179–82. doi: 10.1016/S0304-3940(97)00507-7. [DOI] [PubMed] [Google Scholar]
- 7.Kawai R, Kobayashi S, Fujito Y, Ito E. Multiple subtypes of serotonin receptors in the feeding circuit of a pond snail. Zoolog Sci. 2011;28:517–25. doi: 10.2108/zsj.28.517. [DOI] [PubMed] [Google Scholar]
- 8.Ito E, Otsuka E, Hama N, Aonuma H, Okada R, Hatakeyama D, et al. Memory trace in feeding neural circuitry underlying conditioned taste aversion in Lymnaea. PLoS ONE. 2012;7:e43151. doi: 10.1371/journal.pone.0043151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugai R, Shiga H, Azami S, Watanabe T, Sadamoto H, Fujito Y, et al. Taste discrimination in conditioned taste aversion of the pond snail Lymnaea stagnalis. J Exp Biol. 2006;209:826–33. doi: 10.1242/jeb.02069. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Geraerts WPM. Neurohormonal control of growth and carbohydrate metabolism by the light green cells in Lymnaea stagnalis. Gen Comp Endocrinol. 1992;86:433–44. doi: 10.1016/0016-6480(92)90068-U. [DOI] [PubMed] [Google Scholar]
- 12.Li KW, Geraerts WP, Ebberink RH, Joosse J. Purification and sequencing of molluscan insulin-related peptide I (MIP I) from the neuroendocrine light green cells of Lymnaea stagnalis. Mol Cell Endocrinol. 1992;85:141–50. doi: 10.1016/0303-7207(92)90133-Q. a. [DOI] [PubMed] [Google Scholar]
- 13.Li KW, Geraerts WP, Joosse J. Purification and sequencing of molluscan insulin-related peptide II from the neuroendocrine light green cells in Lymnaea stagnalis. Endocrinology. 1992;130:3427–32. doi: 10.1210/en.130.6.3427. b. [DOI] [PubMed] [Google Scholar]
- 14.Meester I, Ramkema MD, van Minnen J, Boer HH. Differential expression of four genes encoding molluscan insulin-related peptides in the central nervous system of the pond snail Lymnaea stagnalis. Cell Tissue Res. 1992;269:183–8. doi: 10.1007/BF00384739. [DOI] [PubMed] [Google Scholar]
- 15.Smit AB, Vreugdenhil E, Ebberink RH, Geraerts WP, Klootwijk J, Joosse J. Growth-controlling molluscan neurons produce the precursor of an insulin-related peptide. Nature. 1988;331:535–8. doi: 10.1038/331535a0. [DOI] [PubMed] [Google Scholar]
- 16.Smit AB, Geraerts PM, Meester I, van Heerikhuizen H, Joosse J. Characterization of a cDNA clone encoding molluscan insulin-related peptide II of Lymnaea stagnalis. Eur J Biochem. 1991;199:699–703. doi: 10.1111/j.1432-1033.1991.tb16173.x. [DOI] [PubMed] [Google Scholar]
- 17.Smit AB, Thijsen SF, Geraerts WP, Meester I, van Heerikhuizen H, Joosse J. Characterization of a cDNA clone encoding molluscan insulin-related peptide V of Lymnaea stagnalis. Brain Res Mol Brain Res. 1992;14:7–12. doi: 10.1016/0169-328X(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 18.Smit AB, van Marle A, van Elk R, Bogerd J, van Heerikhuizen H, Geraerts WP. Evolutionary conservation of the insulin gene structure in invertebrates: cloning of the gene encoding molluscan insulin-related peptide III from Lymnaea stagnalis. J Mol Endocrinol. 1993;11:103–13. doi: 10.1677/jme.0.0110103. [DOI] [PubMed] [Google Scholar]
- 19.Murakami J, Okada R, Fujito Y, Sakakibara M, Lukowiak K, Ito E. Paired pulse ratio analysis of insulin-induced synaptic plasticity in the snail brain. J Exp Biol. 2013 doi: 10.1242/jeb.083469. In press. [DOI] [PubMed] [Google Scholar]
- 20.Glanzman DL. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr Biol. 2010;20:R31–6. doi: 10.1016/j.cub.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


