Abstract
When cytotoxic T-lymphocytes (CTLs) kill infected or cancerous cells they secrete cytolytic proteins (perforin and granzymes) into the target cell. These “death factors” are pre-stored in cytolytic granules within the CTL until an increase in the intracellular Ca2+ drives granule exocytosis. However, not all sources of Ca2+ stimulate exocytosis: we have recently demonstrated that it is the cytolytic granules themselves that are the source of the Ca2+ that most efficiently drives their own exocytosis; release of Ca2+ from the granules is only activated by the Ca2+-mobilizing messenger NAADP (nicotinic acid adenine dinucleotide phosphate) that acts upon target two-pore channels (TPCs) present on the granules. That NAADP is a unique stimulus of exocytosis may be of fundamental importance not only to immunology but to cell biology in general.
Keywords: NAADP, calcium, exocytosis, T-cell, TPC
The role of acidic Ca2+ stores in immune cells is, at present, poorly understood but our recent findings indicate that they may be crucially important for fighting infection. One powerful mechanism that cytotoxic T lymphocytes (CTLs) use to kill virus-infected or tumorigenic target cells is the exocytosis of cytolytic proteins (e.g., granzymes and perforin) at the immunological synapse formed at the contact interface between the CTL and its target cell.1,2 These “death factors” are pre-stored in specialized secretory lysosomes (termed cytolytic granules) within the CTL until an increase in intracellular Ca2+ concentration drives granule exocytosis.3
A trigger for CTL stimulation is the activation of its T-cell receptor (TCR) by antigen-MHC complexes on the target cell resulting in a biphasic elevation of Ca2+ i.e., an initial release of Ca2+ from intracellular stores followed by Ca2+ entry across the plasma membrane. Because intracellular Ca2+ release in T-cells can make a relatively small contribution to the global Ca2+ signal, Ca2+ influx has understandably garnered more attention and the store-operated Ca2+ influx pathway [Stim/Orai, that is regulated by the Ca2+-filling state of the endoplasmic reticulum, (ER)] has been implicated in supporting granule exocytosis and target-cell killing.
Nonetheless, Ca2+ release from intracellular stores may assume a greater importance than merely “tuning” Ca2+ influx. A theme in Ca2+ signaling is that not all sources or patterns of intracellular Ca2+ are equivalent and Ca2+ channels can differentially couple to cellular processes.1,4 In CTLs, the exocytosis of cytolytic factors is clearly Ca2+-dependent; however, although store-operated Ca2+ influx across the plasma membrane is a necessary component for the exocytosis of cytolytic proteins, it is not a sufficient stimulus per se because it requires the additional activation of protein kinases.5,6 We therefore hypothesized that a different Ca2+ channel family couples more directly to exocytosis.
Although the ER is the largest and the best-characterized Ca2+ store, acidic organelles (e.g., endo-lysosomes) are emerging as important Ca2+ stores but ones using a different second messenger pathway to the familiar Ins(1,4,5)P3: acidic Ca2+ stores are preferentially mobilized by the Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate (NAADP) that activates two-pore channels (TPCs).7-10 We tested whether this pathway is important for granule exocytosis and cell killing in CTLs.11
Using pharmacological and genetic approaches, we first showed that this acidic Ca2+ store/NAADP/TPC pathway was present and contributed to TCR-stimulated Ca2+ signals.11 More remarkably, NAADP-induced Ca2+ release could drive exocytosis of cytolytic granules whereas the Ins(1,4,5)P3/Orai system or ionomycin (Ca2+ ionophore) were ineffective i.e., there was a selectivity for the acidic Ca2+ store pathway and NAADP.11 A further twist in the tale was that the targets for NAADP, TPCs, were on the cytolytic granules themselves, suggesting that the granules served a dual function: they contributed to as well as responded to Ca2+ signals to efficiently drive their own exocytosis.11 We hypothesize that privileged local Ca2+ nanodomains around TPCs on the acidic Ca2+ stores are sensed by the neighboring exocytotic machinery and this locally high Ca2+ is what distinguishes NAADP from the other stimuli.
In summary, TCR activation recruits NAADP to activate target TPC channels resident on the exocytotic granules themselves, and TPCs consequently translocate toward the immunological synapse. Therefore, these granules store and release the Ca2+ for their own exocytosis and deliver Ca2+ in an “autocrine” fashion via TPCs, presumably acting in local perigranular Ca2+ nanodomains (Fig. 1). That NAADP is a unique stimulus of exocytosis may be of fundamental importance not only to immune cell function but may impact on stimulus-secretion coupling in wider cellular contexts.
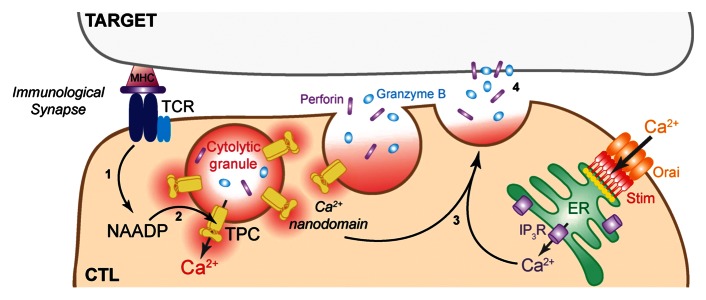
Figure 1. Activation of the T-cell receptor (TCR) by contact with the target cell results in Ca2+ signals in the CTL: (1) the second messenger, NAADP, is synthesized; (2) NAADP activates target TPCs (two-pore channels) on the acidic cytolytic granules themselves; (3) TPCs generate local Ca2+ domains around the granules that act in concert with the ER/Ca2+ influx pathway to evoke exocytosis (4).
Acknowledgments
Figure 1 is reprinted from the graphical abstract from Current Biology 22, 2331–2337, Davis L. C., et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing, Copyright (2012), with permission from Elsevier.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24175
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Pores-Fernando AT, Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev. 2009;231:160–73. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 3.Page LJ, Darmon AJ, Uellner R, Griffiths GM. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401:146–56. doi: 10.1016/S0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- 4.Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grybko MJ, Pores-Fernando AT, Wurth GA, Zweifach A. Protein kinase C activity is required for cytotoxic T cell lytic granule exocytosis, but the theta isoform does not play a preferential role. J Leukoc Biol. 2007;81:509–19. doi: 10.1189/jlb.0206109. [DOI] [PubMed] [Google Scholar]
- 6.Ma JS, Haydar TF, Radoja S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J Immunol. 2008;181:4716–22. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- 7.Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439:349–74. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 8.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–9. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, et al. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–9. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis LC, Morgan AJ, Chen JL, Snead CM, Bloor-Young D, Shenderov E, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–7. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


