Abstract
Common wheat is a hexaploid species with most of the genes present as triplicate homoeologs. Expression divergences of homoeologs are frequently observed in wheat as well as in other polyploid plants. However, little is known about functional variances among homologous genes arising from polyploidy. Expansins play diverse roles in plant developmental processes related to the action of cell wall loosening. Expression of the three TaEXPA1 homoeologs varied dynamically at different stages and organs, and epigenetic modifications contribute to the expression divergence of three TaEXPA1 homoeologs during wheat development. Nevertheless, their functions remain to be clarified. We found that over expression of TaEXPA1-A, -B and -D produced similar morphological changes in transgenic Arabidopsis plants, including increased germination and growth rate during seedling and adult stages, indicating that the proteins encoded by these three wheat TaEXPA1 homoeologs have similar (or conserved) functions in Arabidopsis. Collectively, our present study provided an example of a set of homoeologous genes expression divergence in different developmental stages and organs in hexaploid wheat but functional retention in transgenic Arabidopsis plants.
Introduction
Polyploidization plays an important role in plant evolution. It has been demonstrated that approximately 70% of flowering plants experienced polyploidization events during their evolution [1], [2]. Polyploidy, along with genomic segmental duplications, could benefit plants by increasing overall gene expression levels and cell sizes, and providing sources for novel variants and genome “buffering” of deleterious mutations [3]. When two or more different genomes are combined into a single cell, they must respond to the consequences of genome duplication [2], [4], [5], [6]. There are three possible evolutionary fates for homoeologous genes in polyploids: retention of original or similar function, functional diversification, and gene silencing [2].
Hexaploid bread wheat (T. aestivum, 2n = 6x = 42, genome constitution AABBDD) is an allopolyploid that was formed through hybridization and successive chromosome doubling of three ancestral diploid species (2n = 14), T. urartu (AA), Aegilops speltoides (SS ≈ BB), and Ae. squarrosa (DD) [7], [8]. Allopolyploidization leads to the generation of duplicated homoeologous genes (homoeologs). Therefore, most genes in hexaploid wheat are present as triplicate homoeologs. However, the presence of three homoeologs in wheat does not necessarily imply that three independent mRNAs are transcribed, and transcriptional divergence between homoeologous genes is widely documented in hexaploid wheat [9], [10], [11], [12], [13], [14]. Organ-specific regulation of homoeologous gene expression is also frequently observed [9]. Although there are numerous studies analyzing the genomic structures and transcriptional divergences of the homoeologous genes in hexaploid wheat, little is known about functional variances among homologous genes arising from polyploidy in wheat. Such information, however, is fundamentally important for a better understanding of the complex mechanisms involved in variant biological pathways in the hexaploid wheat and for the benefit of the genetic engineering of plants with polyploid backgrounds. For example, systematic study revealed that homoeologous Bx genes (TaBx1–TaBx5) were highly conserved in the coding sequences and in the exon/intron structures, but were transcribed differentially in which the homoeologs from the B genome generally contributed the most to Bx biosynthesis in hexaploid wheat [15]. Recently, Abdollahi et al (2012) reported that WAP2AQ fully recovered their flower organs similar to the wild-type, WAP2D showed less recovery, and WAP2Aq rescued the least mutant flower phenotype [16].
Expansins are a family of closely related nonenzymatic proteins that induce cell wall extension and stress relaxation at acidic pH condition in plants [17]. Based on phylogenetic analysis and shared intron patterns, expansins can be mainly divided into three discrete subfamilies, α-, β and γ-expansins [18], [19], [20]. They are similar to each other in size and have distant but significant sequence similarity throughout the length of the protein backbone. A number of sequence features are common to the three expansin families, including a signal peptide in the N-terminal region, a cellulose-binding domain in the C-terminal region, and a central catalytic domain that contains a histidine-phenylalanin-aspartate (HFD) motif and several cysteine residues thought to function in catalytic activity [21]. It has been shown that expansins play diverse roles in plant developmental processes related to the action of cell wall loosening [22], [23], [24], [25], [26].
In previous study, we identified three TaEXPA1 homoeologous genes (TaEXPA1-A, TaEXPA1-B and TaEXPA1-D) from hexaploid wheat. Chromosome mapping using aneuploid lines revealed that the TaEXPA1-A, TaEXPA1-B and TaEXPA1-D genes located on chromosomes, 1A, 1B, and 1D, respectively. The orthologs of TaEXPA1 also were found in the three ancestral diploid species. We demonstrated that epigenetic modifications contribute to the expression divergence of three TaEXPA1 homoeologs during wheat development [27]. Due to its highly conserved sequence but transcriptional divergences in different organs and tissues, it is of great interest to reveal functional variances among three TaEXPA1 homologous genes in wheat. In present study, we found that over expression of TaEXPA1-A, -B and -D produced similar morphological changes in transgenic Arabidopsis plants, including increased germination and growth rate during seedling and adult stages, indicating that the proteins encoded by these three wheat TaEXPA1 homoeologs have similar (or conserved) functions in Arabidopsis.
Materials and Methods
Plant Materials
The hexaploid wheat genotype Nongda 3338 (Triticum aestivum) was used in this study. Wheat seeds were germinated on moist filter papers and transplanted into pots to grow in the greenhouse under a 12-hr photoperiod provided by cool white fluorescent and incandescent light (intensity≥3000 lx). The plants were watered with a 1/10-strength Hoagland’s solution when necessary. The Columbia (Col-0) ecotype of Arabidopsis thaliana was used as the wild type. All genotype plants were grown in a growth room with a 16-h light/8-h-dark cycle at 22 to 24°C for general growth and seed harvesting.
Phylogenetic Analysis of α-expansin Genes
All deduced amino acid sequences were aligned using Clustal method with the MEGA4.0 program [28]. Distance analyses used the program ProtDist of the Phylip 3.5c package with a PAM250 substitution matrix. Phylogenetic trees were then calculated from the matrices by the neighbor-joining algorithm. Bootstrap analyses consisted of 1,000 to 5,000 replicates using the same protocol. The dendrogram was generated on the basis of the alignment of the deduced amino-acid sequences encoded by 26 α-expansin genes of Arabidopsis, 33 α-expansin genes of rice (http://www.personal.psu.edu/fsl/ExpCentral/), and 3 TaEXPA1 homoeologs of wheat. The GenBank accession numbers of selected expansins can be found in the Table S1.
RNA Extraction
Wheat Nongda 3338 were planted in pots in silt-loam/coarse soil mix and grown in green house. In two-leaf stage, the first leaf in the bottom, the second fully expanded leaf in the middle and the third unexpanded leaf were collected, and designated as mature, fully expanded and juvenile leaves, respectively. Total RNA extracted from different leaves for the gene expression analysis, using Trizol RNA isolation protocol (Life Technologies, USA). First-strand cDNA synthesis was performed using 2 µg of DNase-digested total RNA with oligo (dT) primer according to the protocol for RT-PCR first-strand synthesis (Promega, WI, USA).
Quantitative PCR Analysis
Real-time qPCR experiments using SYBR Green PCR master mix (Applied Biosystems) on 96-well optical reaction plates with optical adhesive covers (ABI PRISM) were designed, performed, and analyzed using the StepOnePlus Real-Time PCR system with its accompanying StepOne Software (version 2.2; Applied Biosystems). Gene-specific primers for quantitative PCR were designed based on nucleotide polymorphisms in the cDNA sequences of three TaEXPA1 homoeologous genes, Table S2). The specificity of the primers was confirmed using plasmids containing genomic DNA sequences of TaEXPA1 homoeologs. The PCR efficiencies of each primer pair were determined from standard curve experiments. Any amplification with detected primer dimers from melt-curve analysis was excluded from the data analysis [29]. In comparative cycle threshold experiments for quantitative analysis of gene expression levels, β-actin was amplified as an endogenous control.
Binary Constructs and Arabidopsis Transformation
To examine heterologous expression of TaEXPA1 homoeologous genes, genomic DNA fragments of the genes were cloned downstream of the cauliflower mosaic virus 35S promoter in pB2GW7 vectors. Binary vectors were introduced into Agrobacterium tumefaciens GV3101. Six-week-old Arabidopsis plants were transformed via Agrobacterium-mediated transformation by the floral-dip method [30]. Transgenic plants were selected on Murashige and Skoog medium containing herbicides. The herbicides-resistant seedlings were transferred to soil. T3 generation transgenic plants were used for further experiments.
Germination Assays
Seed lots to be compared were harvested on the same day from individual plants grown in identical environmental conditions. Seeds of each genotype were sterilized and cold treated for 3 d in the dark, then sown in triplicate (55–65 seeds from one individual plant a quarter of Petri dish) on MS medium. The plates incubated in a climate-controlled room (22°C, 16-h light/8-h-dark cycle). Germination was scored after 16 h imbibitions, we used radicle tip break through the seed coat as the seed germination standard. The number of geminating seeds was counted and converted into germination rate per 8 hour intervals for both transgenic lines and wild type plants. The average germination percentages ± SE of triplicates were calculated.
Image Processing and Measurements
For leaf size measurement and analysis of Col-0 wild-type and transgenic plants, cotyledons and sixth leaves were harvested and measured digitally on photographs taken using a digital camera (Nikon D90) with a standard 15-cm ruler for scaling on a black background. Acquired photographs were processed using Photoshop (Adobe version CS5) with manual tracing of leaf outlines to generate leaf silhouettes. Based on these measurements, leaf area was calculated.
Fresh Weight and Height Measurement
All genotype plants were grown in a growth chamber with a 16-h light/8-h-dark cycle at 22°C. Plants were grown for 3 weeks after sowing and were measured. The actual times were 22, 25 and 29 days after sowing (DAS). The fresh rosette was cut with a pair of scissors just above ground and for weight determination with electronic balance and calculation of the amount of green biomass. Plant height was measured from the base of the plant (aerial parts) to the tip using a cotton thread. The actual times were 29, 32 and 35 DAS. Each line 15 plants were selected for fresh weight and height analysis.
Results
Structure and Phylogenetic Analysis of TaEXPA1 Genes
The alignment of genomic and their corresponding cDNA sequences of three TaEXPA1 homeologous genes showed a significant conservation of the intron/exon organization within the open reading frame (ORF). The structures of three TaEXPA1 homeologous genes were also confirmed by the identification of potential exon/intron junction sequences, which were consistent with the universal rule GT.AG. The ORF sequences of three TaEXPA1 genes showed single nucleotide substitutions only, whereas the introns had more frequent base substitutions and insertions/deletions (Figure S1).
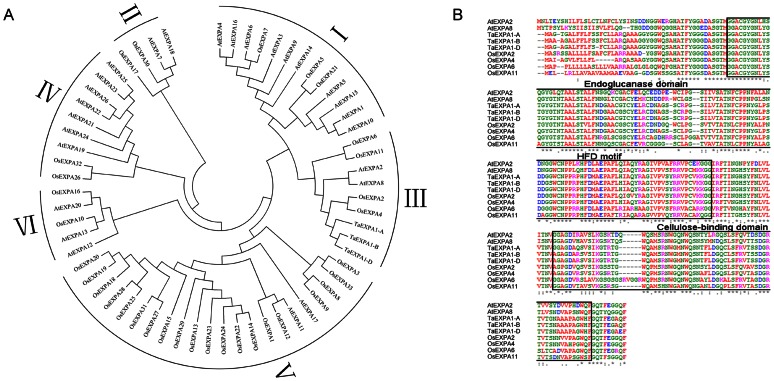
Based our previous results, the TaEXPA1 belongs to the α-expansin class [31]. To further investigate the relationship between three TaEXPA1 homeologous genes and α-expansins of Arabidopsis and rice, we performed a neighbor-joining phylogenetic analysis using MEGA 4.0. These proteins were classified into six different classes according to the phylogenetic analysis, among which TaEXPA1 belongs to the class III (Figure 1A). Thus, these plant expansins proteins of class III were aligned using ClustalW, and the results showed that our identified wheat EXPA1 sequences contain highly conserved cellulose-binding domain, and a central catalytic domain that contains a histidine-phenylalanine-aspartate (HFD) motif and several cysteine residues thought to function in catalytic activity regions, which are thought to be critical for inducing cell wall extension and stress relaxation (Figure 1B).
Figure 1. Sequence analysis of three TaEXPA1 homoeologous genes.
(A) Phylogenetic relationship of three TaEXPA1 homoeologs with Arabidopsis and rice α-expansins. The accession numbers of selected expansins are listed as Table S1. (B) Comparison of nine α-expansins amino acids in class III. Identical amino acids are indicated by asterisks, and black boxes indicate residues conserved among the seven proteins.
It should be noted that, there were two and four expansin protein members that can be clustered into class III in Arabidopsis and rice, respectively (Figure 1A). Therefore, we speculate that there was at least another member that was not cloned in wheat.
Expression Analysis of Three TaEXPA1 Homoeologous Genes
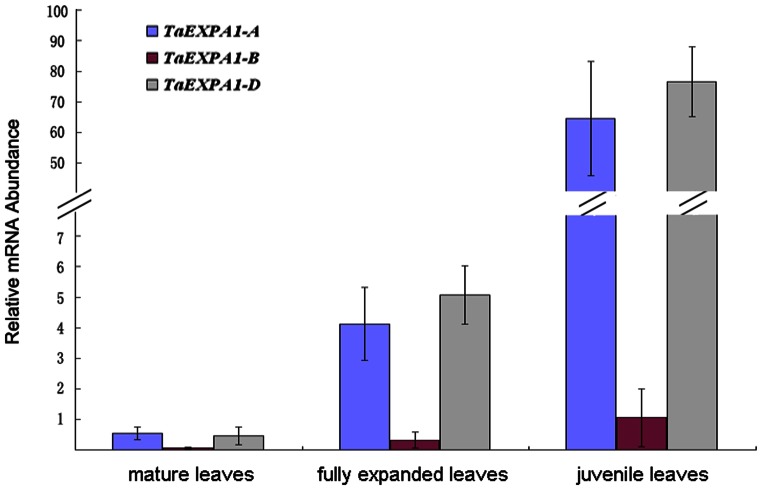
Previously, we examined the expression profiles of TaEXPA1 homoeologs in different organs at different developmental stages. The results indicated that all three TaEXPA1 homoeologous genes were expressed in dry seed embryos, shoots of 1d after germination seedlings, jointing stage stems, heading stage flag leaves and heading stage immature ears. However, expression levels of the three TaEXPA1 homoeologs were significantly divergent in different tissues and different development stages. For example, all of the three TaEXPA1 homoeologs were silenced in seedling roots. In the leaves of the same stage, TaEXPA1-A and TaEXPA1-D were expressed, but TaEXPA1-B was silenced [27]. Due to its important role in regulating cell wall extension during growth, here we examined the expression of three TaEXPA1 homoeologs in different leaves of the two-leaf stage (Figure 2). Interestingly, expression divergence was observed between the three TaEXPA1 homoeologs in seedling leaves of different development stages. Most notably, TaEXPA1-A and TaEXPA1-D homoeologs were with higher expression in the third juvenile leaves than the other expanded leaves, whereas the mRNA of TaEXPA1-B was below detection level (Figure 2).
Figure 2. Expression patterns of EXPA1 homoeologous genes by gene-specific PCR.
Total RNA was isolated from different leaves of wheat genotype nongda 3338 in the two-leaf stage; β-actin was used as an endogenous control.
Overexpression of Three TaEXPA1 Homoeologous Genes Displayed Increased Germination Rate in Arabidopsis
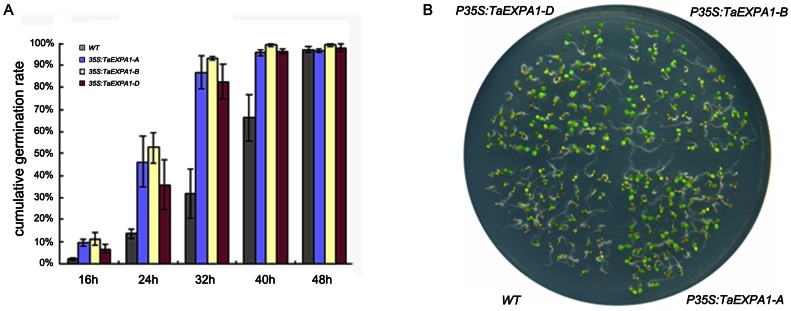
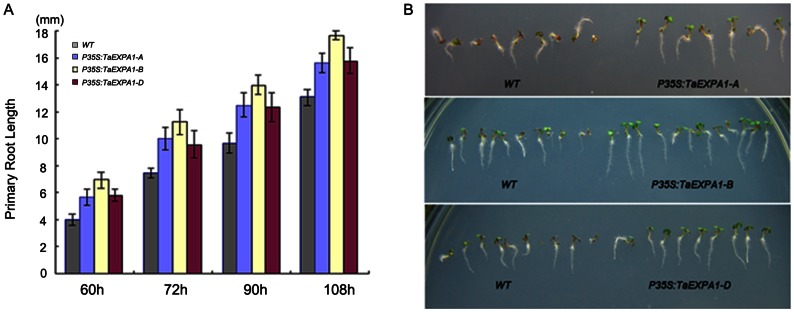
In order to gain further insight into the functional divergence of three TaEXPA1 homoeologs genes in wheat, ectopic expression of TaEXPA1-A, TaEXPA1-B and TaEXPA1-D were performed using an Arabidopsis transgenic system. For every transgenic line, three independent T3 homozygous overexpression lines were used for further characterization. RT-PCR analysis indicated that three TaEXPA1 homoeologs were expressed in all the transgenic Arabidopsis plants, respectively, but was absent in the wild-type (Figure S2). Overt phenotype was observed in transgenic Arabidopsis plants compared with control wild-type plants, and the transgenic plants with three TaEXPA1 homoeologs genes constructs exhibited similar phenotypes. Firstly, we measured the germination rate between transgenic plants and wild-type plants. The number of geminating seeds was counted and converted into germination rate at 8 hour intervals for both transgenic lines and wild-type plants. The germination rate for transgenic lines were more than 40% in the first 24 hours after sowing, but only less than 20% for wild-type during the same time period. The cumulative germination rate of transgenic plants was more than 80%, where in contrast with only 30% for wild-type after 32 hours after sowing (Figure 3A). The phenotype differences were also observed in other independent transgenic lines, these data indicated that the transgenic lines germinated faster than the wild-type (Figure 3A, 3B). We also measured the primary root length of both transgenic and wild-type plants at 60, 72, 90 and 108 hours after sowing. At all four time points, primary root lengths of transgenic plants were longer than that of wild-type plants. This indicated that primary roots of transgenic plants grew faster than the wild-type (Figure 4).
Figure 3. Transgenic Arabidopsis showed faster germination rates.
(A) Germination was scored after 16 h imbibitions. The average germination percentages ± SE of triplicates were calculated. (B) Arabidopsis seeds germinated on MS medium after 90 h.
Figure 4. Transgenic Arabidopsis showed longer primary roots.
(A) The primary root length of the transgenic lines and the wild-type plants were measured after 60 h at four time points. (B) Transgenic plants and wild-type sowed in MS medium after 72 h.
Overexpression of Three TaEXPA1 Homoeologous Genes Lines Showed Larger Cotyledons and Rosette Leaves in Arabidopsis
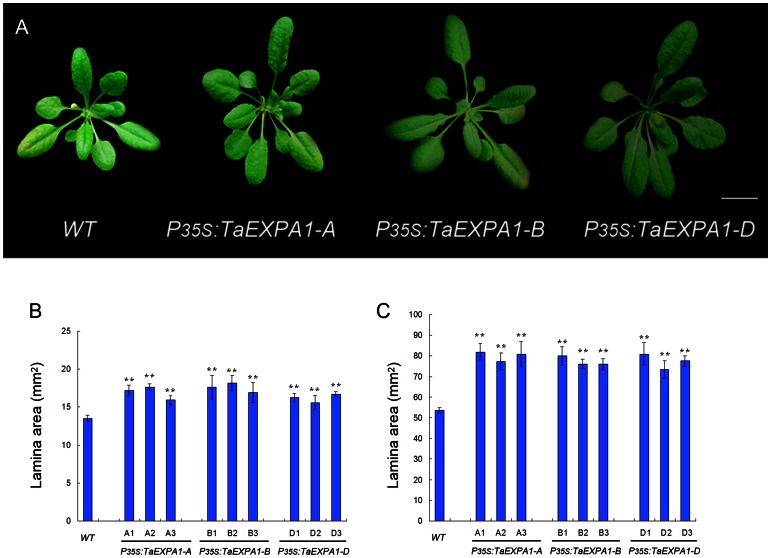
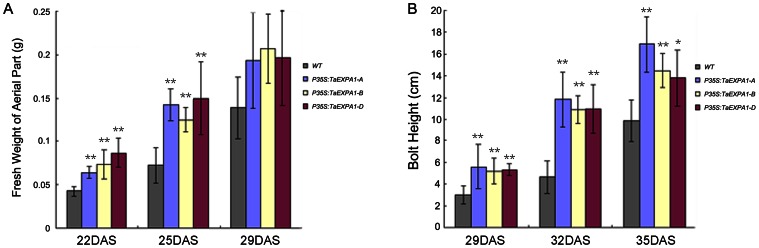
Due to the divergent expression pattern of three TaEXPA1 homoeologs genes in leaves, we focused on leaf growth phenotype between transgenic plants and wild-type plants. In comparison with control wild-type plants, transgenic lines of three TaEXPA1 homoeologous genes displayed larger cotyledons (Figure 5A, B). Previous study showed that decreased expansin gene expression leads to a more marked repression of the leaf 6 growth during the later stage of leaf development [32]. To investigate the individual leaf basis of the changes after the overexpression of three TaEXPA1 homoeologs, we analyzed the lamina area of leaf 6 at maturity (30 DAS) of the transgenic Arabidopsis plants with that of wild-type plants. These results showed that leaf lamina areas were all significantly increased in the overexpression of three TaEXPA1 homoeologous genes plants (Figure 5A, C). Organ size is determined by final cell size and cell numbers. Previous studies indicated that repression of expansin gene expression leads to decreased cell number in both lamina and petiole [32]. To quantify this trait, we measured abaxial epidermal cell size of fully expanded sixth leaf (30 DAS) for both transgenic and wild-type plants using an inverted microscope. Cytological observation showed that epidermal cell size of transgenic and wild-type plants did not differ significantly (Figure S3), while the transgenic plants rosette leaves was significantly larger than that of wild-type (Figure 5A, C), implying that larger transgenic plants are mainly caused by increased cell numbers. Biomass is usually used as an index of plant growth rate. We then measured the fresh weight of aerial tissues in transgenic seedlings and wild-type plants at 22 days after sowing. The three P35S:TaEXPA1 homoeolog transformants appeared heavier of fresh weight than non-transgenic seedlings at 22 and 25 DAS (Figure 6A). In addition, Arabidopsis plants transformed with P35S:TaEXPA1 exhibited a taller phenotype than the wild-type plants (Figure 6B).
Figure 5. Transgenic Arabidopsis plants leads to an increase in rosette growth.
(A) Transgenic Arabidopsis showed larger cotyledons and rosette leaves, Bar = 1cm. (B) Data show a comparison of lamina size between cotyledons from overexpression transgenic Arabidopsis plants and wild-type plants and measured at 30 DAS. Values are means±SE (n = 10). (C) Data show a comparison of lamina size between sixth leaves from overexpression transgenic Arabidopsis plants and wild-type plants and measured at 30 DAS. Values are means±SE (n = 10). T-test compared with the wild type control: *P<0.05, **P<0.01.
Figure 6. Transgenic Arabidopsis grow faster than non-transgenic seedlings.
(A) Change of fresh weight of aerial tissues with time (DAS) between transgenic seedlings and wild-type plants. Values are means±SE (n = 15). T-test compared with the wild type control: *P<0.05, **P<0.01. (B) Change of bolt height with time (DAS) between transgenic seedlings and wild-type plants. Values are means±SE (n = 15). T-test compared with the wild type control: *P<0.05, **P<0.01.
Discussion
Sequence Conservation, Expression Divergence and Functional Retention of Three TaEXPA1 Homoeologous Genes
Previous classifications of the expansin gene family divided proteins into two subfamilies, α and β, based on substrate specificity and sequence conservation [21]. Further studies revealed a total of 38 expansin or expansin-like sequences within the scope of whole genome in Arabidopsis, these expansins fall into three discrete subfamilies (α-, β and γ) based on overall sequence similarity and shared structural features [20]. In previous work, we identified three TaEXPA1 homoeologous genes TaEXPA1-A, TaEXPA1-B and TaEXPA1-D from hexaploid wheat [27]. Here, the relationship between three TaEXPA1 homeologous genes and α-expansins of Arabidopsis and rice was further investigated by neighbor-joining phylogenetic analysis. These α-expansins were classified into six different classes according to the phylogenetic tree, among which three TaEXPA1 homoeologs belong to the class III with AtEXPA2, AtEXPA8 and OsEXPA2, OsEXPA4, OsEXPA6, OsEXPA11 (Figure 1A), suggested functional similar between them. Based on an analysis with the ClustalW program, it was found that three TaEXPA1 homoeologs expansin proteins had a cleavable signal peptide at the N-terminus. Two domains characteristic of expansin proteins, a cysteine-rich region and a tryptophan-rich C-terminal portion, were also observed in all three TaEXPA1 homoeologs, implied they may have conserved function.
Up to date, many wheat expansin genes were identified and their expression were analyzed. For example, 18 expansin genes were isolated from wheat and nine of them (TaEXPA1–TaEXPA9) represent α-expansins, while TaEXPB1–TaEXPB5 and TaEXPB7–TaEXPB10 belong to the β-expansin class [31]. Jin et al (2006) identified two wheat expansin genes that are expressed during male gametophyte development [33]. Gao et al (2008) evaluated the characteristics of wheat expansins protein TaEXPB23, and found the growth of wheat coleoptiles is intimately correlated with its expression [34]. In our previous report, it was found that all the three TaEXPA1 homoeologs were silenced in seedling roots, but TaEXPA1-A and -D were expressed in seedling leaves [27]. In present study, expression pattern of these three TaEXPA1 homoeologs was further investigated in seedling leaves of different development stages. Interestingly, TaEXPA1-A and TaEXPA1-D homoeologs appeared to be highly expressed in the young leaves, as compare to fully expanded leaves. In Arabidopsis, it was reported that expansin genes play important role during leaf development. Collectively, it can be concluded that the highly expression of TaEXPA1 in the young leaves may impact cell proliferation or expansin, then affect leaf development in turn.
Plenty of studies have shown that polyploidization is often accompanied by changes in genomic structure and gene expression, including loss of function, gene silencing and homoeologous gene expression diversification [2], [9], [11], [12], [35], [36]. For example, comparison of three WLHS1 homoeologous gene sequences in wheat indicated that WLHS1-A has a 3479 base pair insertion, leading to the loss of gene function [10]. The expression of duplicated genes resulting from polyploidization can be partitioned between the duplicates so that only one copy is expressed and functions only in some organs, while the other copy is expressed only in other organs, indicative of subfunctionalization [37], [38]. Evolution of the Q/q loci in polyploid wheat resulted in the hyperfunctionalization of 5AQ, pseudogenization of 5Bq, and subfunctionalization of 5Dq [39]. In present study, overexpression of TaEXPA1-A, -B and -D produced similar morphological changes in transgenic Arabidopsis plants, including increased germination and growth rate during the seedling and adult stages (Figure 3–6). These results indicated that three TaEXPA1 homoeologs all functioned in leaf growth of transgenic Arabidopsis plants, although the TaEXPA1-B silenced in seedling leaves of wheat. Therefore, our present study provided an example of a set of homoeologous genes expression divergence in different developmental stages and organs in hexaploid wheat but functional retention in transgenic Arabidopsis plants. It should to be noted that, in our present study, only seed germination and seedling related traits (root and leaf) were analyzed between transgenic lines and wild-type, further analysis of other processes, such as pollination, floral opening, meristem dynamics, fruit softening, as well as in adaptive responses to submergence, GA and wounding, will provide more valuable information for the function retention of three TaEXPA1 homoeologs.
Overexpression of TaEXPA1 Genes in Arabidopsis Leads to Increased Growth Rate
Expansin genes have been isolated from a variety of plant species and are a large multigene family [40], [41], [42]. It has been shown that expansins play important roles in plant development, including seed germination [23], root hair emergence [24], [43], leaf growth [25], [26], stem elongation [44], pollination [45], floral opening [46], meristem dynamics [47], fruit softening [22], as well as in adaptive responses to submergence, GA and wounding [48], and other developmental processes where cell wall loosening occurs [21]. Different members of expansin are expressed in various parts of plants at different developmental stages. For example, LeEXP4 was shown to be expressed specifically in the endosperm cap tissue enclosing the radicle tip [49]. However, LeEXP8 and LeEXP10 were expressed in the embryo. The tissue localization and expression patterns of both LeEXP8 and LeEXP10 suggested that they played roles in the initial elongation stage of the radicle and seedling growth [23]. The GmEXP1 was expressed mainly in the regions undergoing cell division and elongation in the primary and secondary roots, suggesting a role in root elongation in soybean [50]. The complexity of expansin gene expression is reflected in the similarly diverse expression patterns found in wheat, in which three TaEXPA1 homoeologs were differentially expressed in different tissues and developmental stages [27]. The distinct spatial and temporal expression patterns of three TaEXPA1 homoeologs suggested that TaEXPA1 may play multiple roles in wheat development. In present study, we found that germination rate and primary root length of transgenic plants were faster than control plants, suggesting that TaEXPA1 gene plays an important biological role in control of seed germination and initial elongation of the radicle. A previous study demonstrated that AtEXP10 is expressed predominantly in young growing petioles and leaf blades, overexpression of AtEXP10 produced larger rosettes and matured earlier as compared to the control plants, and leaf size was substantially reduced in antisense lines with suppressed AtEXP10 expression [51]. A quantitative growth analysis by down-regulating the expression of four expansin genes (EXPA1, −3, −5, and −10) demonstrated that expansins are required for leaf growth [32]. Overexpression of three TaEXPA1 homoeologs in Arabidopsis increased leaf size during the seedling stage, combined higher mRNA abundance of TaEXPA1-A and TaEXPA1-D gene in tender leaves than mature leaves, suggesting that TaEXPA1-A and -D genes may play an important biological role in leaf growth in wheat. However, transgenic study using heterologous Arabidopsis system only provides the function of protein from transgene in Arabidopsis plants. Therefore, the relationship of TaEXPA1 genes to leaf growth in wheat is still an area to be further elucidated.
Supporting Information
Alignment of genomic and their corresponding cDNA sequences of three TaEXPA1 homoeologous genes. Exons and introns are showed in shaded and gray texts, respectively; ATG and TAG boxes are the start and stop codon, respectively.
(TIF)
Identification of TaEXPA1-A , TaEXPA1-B and TaEXPA1-D gene expression in homozygous T3 transgenic Arabidopsis by RT-PCR. 1 represented wide-type Arabidopsis; 2–4 represented overexpression TaEXPA1-A transgenic line of OEA-2-12, OEA-8-10, and OEA-11-12, respectively; 5–7 represented TaEXPA1-B transgenic line of OEB-4-6, OEB-9-9, and OEB-17-5, respectively; 8–10 represented TaEXPA1-D transgenic line of OED-5-3, OED-7-5, and OED-12-7, respectively.
(TIF)
Cytological observation showed that the epidermal cell size of transgenic plants and wild-type did not differ significantly, while the transgenic plants rosette leaves were significantly greater than the wild-type, implying that transgenic plants were larger mainly due to an increased number of cells. Bar = 100 µm.
(TIF)
The GenBank accession numbers of selected expansions.
(DOC)
Gene-specific primer pairs used in PCR.
(DOC)
Funding Statement
This work was supported by the Major Program of the National Natural Science Foundation of China (31290210, 31290212) and National Science Foundation for Distinguished Young Scholars (30925023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Masterson J (1994) Stomatal size in fossil plants, evidence for polyploidy in majority of angiosperms. Science 264: 421–423. [DOI] [PubMed] [Google Scholar]
- 2. Wendel JF (2000) Genome evolution in polyploids. Plant Mol Biol 42: 225–249. [PubMed] [Google Scholar]
- 3. Udall JA, Wendel JF (2006) Polyploidy and crop improvement. Crop Science 46: S3–14. [Google Scholar]
- 4. Osborn TC, Pires JC, Birchler JA, Auger D, Chen ZJ, et al. (2003) Understanding mechanisms of novel gene expression in polyploids. Trends in Genetics, 19 (3): 141–147. [DOI] [PubMed] [Google Scholar]
- 5. Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr. Opin. Plant Bio 8: 135–141. [DOI] [PubMed] [Google Scholar]
- 6. Liu ZL, Adams KL (2007) Expression partitioning between genes duplicated by polyploidy under abiotic stress and during organ development. Current Biology 17: 1669–1674. [DOI] [PubMed] [Google Scholar]
- 7. Huang SX, Sirikhachornkit A, Su XJ, Faris J, Gill B, et al. (2002) Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc. Natl. Acad. Sci. USA 99: 8133–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldman M, Levy AA (2005) Allopolyploidy -A shaping force in the evolution of wheat genomes. Cytogenet Genome Res 109: 250–258. [DOI] [PubMed] [Google Scholar]
- 9. Bottley A, Xia GM, Koebner RM (2006) Homoeologous gene silencing in hexaploid wheat. Plant J 47: 897–906. [DOI] [PubMed] [Google Scholar]
- 10. Shitsukawa N, Tahira C, Kassai K, Hirabayashi C, Shimizu T, et al. (2007) Genetic and epigenetic alteration among three homoeologous genes of a class E MADS box gene in hexaploid wheat. Plant Cell 19: 1723–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hovav R, Udall JA, Chaudhary B, Rapp R, Flagel, (2008) Partitioned expression of duplicated genes during development and evolution of a single cell in a polyploid plant. Proc Natl Acad Sci USA 105: 6191–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaudhary B, Flagel L, Stupar RM, Udall JA, Verma N, et al. (2009) Reciprocal silencing, transcriptional bias and functional divergence of homoeologs in polyploid cotton (gossypium). Genetics 182: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu ZR, Yu Y, Wang R, Yao YY, Peng HR, et al. (2011) Expression divergence of TaMBD2 homoeologous genes encoding methyl CpG-binding domain proteins in wheat (Triticum aestivum L.). Gene 471(1–2): 13–18. [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Truksa M, Snyder C, EI-Mezawy A, Shah S, et al. (2011) Three homologous genes encoding sn-Glycerol-3-Phosphate Acyltransferase 4 exhibit different expression patterns and functional divergence in Brassica napus . Plant Physiol 155: 851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nomura T, Ishihara A, Yanagita RC, Endo TR, Iwamura H (2005) Three genomes differentially contribute to the biosynthesis of benzoxazinones in hexaploid wheat. Proc Natl Acad Sci USA 102(45): 16490–16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdollahi P, Kamiya Y, Kawaura K, Ogihara Y (2012) Overexpression of Q/q-related homoeoalleles of hexaploid wheat reveals distinct recovery of flower transformation in the apetala2 mutant of Arabidopsis. Plant Biotechnology. 29: 245–252. [Google Scholar]
- 17. McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce wall extension. Plant Cell 4: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shcherban TY, Shi J, Durachko DM, Guiltinan MJ, McQueen-Mason SJ, et al. (1995) Molecular cloning and sequence analysis of expansins–a highly conserved multigene family of proteins that mediate cell wall extension in plants. Proc Natl Acad Sci USA 92: 9245–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee Y, Choi D, Kende H (2001) Expansins: ever-expanding numbers and functions. Curr Opin Plant Biol 4: 527–532. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, et al. (2002) Plant Expansins Are a Complex Multigene Family with an Ancient Evolutionary Origin. Plant Physiol 128: 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- 22. Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, et al. (1999) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11: 2203–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen F, Dahal P, Bradford KJ (2001) Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol 127: 928–936. [PMC free article] [PubMed] [Google Scholar]
- 24. Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis . Plant Cell 14: 3237–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reidy B, McQueen-Mason S, Nosberger J, Fleming A (2001) Differential expression of α- and β-expansin genes in the elongating leaf of Festuca pratensis . Plant Mol Biol 46: 491–504. [DOI] [PubMed] [Google Scholar]
- 27. Hu ZR, Han ZF, Song N, Chai LL, Yao YY, et al. (2013) Epigenetic modification contributes to the expression divergence of three TaEXPA1 homoeologs in hexaploid wheat (Triticum aestivum L.). New Phytol 197: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 28. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 29. Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis . Plant Physiol 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 31. Lin Z, Ni ZF, Zhang Y, Yao YY, Wu HY, et al. (2005) Isolation and characterization of 18 genes encoding α- and β-expansins in wheat (Triticum aestivum L.). Mol. Gen. Genomics 274: 548–556. [DOI] [PubMed] [Google Scholar]
- 32. Goh HH, Sloan J, Dorca-Fornell C, Fleming A (2012) Inducible repression of multiple expansin genes leads to growth suppression during leaf development. Plant Physiol 159(4): 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin Y, Tashpulatov AS, Katholnigg H, Heberle-Bors E, Touraev A (2006) Isolation and characterisation of two wheat β-expansin genes expressed during male gametophyte development. Protoplasma 228: 13–19. [DOI] [PubMed] [Google Scholar]
- 34. Gao Q, Zhao M, Li F, Guo Q, Xing S, et al. (2008) Expansins and coleoptile elongation in wheat. Protoplasma 233: 73–81. [DOI] [PubMed] [Google Scholar]
- 35. Kashkush K, Feldman M, Levy A (2002) Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160: 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC (2007) Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 19: 3403–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lynch M, Force A (2000) The probability of duplicate gene preservation by subfunctionalization. Genetics 154: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams KL, Cronn R, Percifield R, Wendel JF (2003) Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proc Natl Acad Sci USA 10: 4369–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang ZC, Belcram H, Gornicki P, Charles M, Just J, et al. (2011) Duplication and partitioning in evolution and function of homoeologous Q loci governing domestication characters in polyploid wheat. Proc Natl Acad Sci USA 108(46): 18737–18742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cosgrove DJ (1998) Cell wall loosening by expansins. Plant Physiol 118: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu YJ, Meeley RB, Cosgrove DJ (2001) Analysis and expression of the α-expansin and β-expansin gene families in maize. Plant Physiol 126: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Y, Jones L, McQueen-Mason SJ (2003) Expansins and cell growth. Curr Opin Plant Biol 6: 603–610. [DOI] [PubMed] [Google Scholar]
- 43. Zhang NG, Hasenstein KH (2000) Distribution of expansins in graviresponding maize roots. Plant Cell Physiol 41: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 44. Cho HT, Kende H (1997) Expansins and internodal growth of deepwater rice. Plant Physiol 113: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cosgrove DJ, Bedinger P, Durachko DM (1997) Group I allergens of grass pollen as cell wall-loosening agents. Proc Natl Acad Sci USA 94: 6559–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gookin TE, Hunter DA, Reid MS (2003) Temporal analysis of alpha and beta-expansin expression during floral opening and senescence. Plant Sci 164: 769–781. [Google Scholar]
- 47. Fleming AJ, McQueen-Mason SJ, Mandel T, Kuhlemeier C (1997) Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415–1418. [Google Scholar]
- 48. Lee Y, Kende H (2001) Expression of β-expansins is correlated with internodal elongation in deepwater rice. Plant Physiol 127: 645–654. [PMC free article] [PubMed] [Google Scholar]
- 49. Chen F, Bradford KJ (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee DK, Ahn JH, Song SK, Choi YD, Lee JS (2003) Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol 131: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cho HT, Cosgrove DJ (2000) Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana . Proc Natl Acad Sci USA 97: 9783–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of genomic and their corresponding cDNA sequences of three TaEXPA1 homoeologous genes. Exons and introns are showed in shaded and gray texts, respectively; ATG and TAG boxes are the start and stop codon, respectively.
(TIF)
Identification of TaEXPA1-A , TaEXPA1-B and TaEXPA1-D gene expression in homozygous T3 transgenic Arabidopsis by RT-PCR. 1 represented wide-type Arabidopsis; 2–4 represented overexpression TaEXPA1-A transgenic line of OEA-2-12, OEA-8-10, and OEA-11-12, respectively; 5–7 represented TaEXPA1-B transgenic line of OEB-4-6, OEB-9-9, and OEB-17-5, respectively; 8–10 represented TaEXPA1-D transgenic line of OED-5-3, OED-7-5, and OED-12-7, respectively.
(TIF)
Cytological observation showed that the epidermal cell size of transgenic plants and wild-type did not differ significantly, while the transgenic plants rosette leaves were significantly greater than the wild-type, implying that transgenic plants were larger mainly due to an increased number of cells. Bar = 100 µm.
(TIF)
The GenBank accession numbers of selected expansions.
(DOC)
Gene-specific primer pairs used in PCR.
(DOC)