Abstract
A tetravalent DNA vaccine formulated with Vaxfectin adjuvant was shown to elicit high levels of neutralizing antibody against all four dengue virus serotypes (Porter et al.,16), warranting further testing in humans. In preparation for a phase 1 clinical testing, the vaccine and the adjuvant were manufactured under current good manufacturing practice guidelines. The formulated vaccine and the adjuvant were tested for safety and/or immunogenicity in New Zealand white rabbits using a repeat dose toxicology study. The formulated vaccine and the adjuvant were found to be well tolerated by the animals. Animals injected with formulated vaccine produced strong neutralizing antibody response to all four dengue serotypes.
Keywords: dengue, DNA vaccine, vaxfectin, immunogenicity, rabbits
Dengue viruses belong to the family Flaviviridae. Four antigenically distinct serotypes of dengue virus have similar clinical presentation, epidemiology, and distribution, especially in the tropical and subtropical regions of the world, where nearly 2.5 billion people are at risk of infection.1 Infection with any of the four dengue virus serotypes can cause diseases ranging from mild febrile illness and classic dengue fever to the severe and potentially fatal forms of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).2 Natural infection with any of the dengue virus serotypes provides only long-term homotypic immunity, and available epidemiologic data suggest an increased risk for DHF/DSS during secondary infections with a heterologous serotype.3,4 Global expansion of dengue virus infections in recent decades has made the development of vaccines for dengue viruses a public health priority. Traditional vaccine approaches such as live attenuated viruses,5,6 inactivated viruses,7 and subunit vaccines,8 as well as novel approaches such as cloned, engineered viruses9 and chimeric viruses using yellow fever virus backbone10 are being pursued. Several have advanced to clinical evaluation; however, a licensed vaccine is not yet available.
To avoid the potential for increased risk of DHF/DSS due to postulated immune enhancement,11 a dengue virus vaccine should elicit immunity simultaneously to all four serotypes. We have developed plasmid DNA vaccines directed against each of the four dengue serotypes that express respective pre-membrane and envelope antigens. These have been tested in small animal and non-human primate models.12-14 A nonadjuvanted, monovalent dengue-1 DNA vaccine was recently tested in a phase 1 proof-of-concept clinical trial in which modest antibody responses were demonstrated in a subset of vaccinated individuals.15 In order to enhance antibody responses, we recently tested in rhesus macaques a tetravalent dengue DNA vaccine (a mixture of 4 plasmids encoding the 4 dengue antigens) formulated with Vaxfectin® adjuvant. Significantly higher, and longer lasting, neutralizing antibodies to all four serotypes were demonstrated compared with vaccine without the adjuvant,16 warranting further investigation in a phase 1 clinical trial. Here, we report the safety and immunogenicity results of a Vaxfectin®-adjuvanted tetravalent dengue DNA vaccine (TVDV) in New Zealand white rabbits. This study was conducted according to Good Laboratory Practices (GLP) in support of an Investigational New Drug application (IND).
Plasmids expressing pre-membrane and envelope proteins of dengue virus type-1, -2 and -3 have been described previously.12-14 Minor changes were introduced in the sequences just upstream of the initiating methionine codon in order to remove certain redundant sequences. A dengue-4 vaccine construct similar to the other plasmids was prepared. All four DNA plasmid constructs were manufactured by Vical Inc. according to current Good Manufacturing Practice (cGMP) guidelines. The plasmids were mixed (1:1:1:1, wt/wt) to produce TVDV. Vaxfectin®,17 a cationic lipid-based adjuvant also manufactured by Vical according to cGMP guidelines, was combined with TVDV to produce an adjuvanted vaccine containing both DNA and lipid at final concentrations of 1 mg/mL (TVDVVax).
Three groups of 16 New Zealand white rabbits (8 males and 8 females) were vaccinated with phosphate buffered saline (PBS control, Group 1,), TVDVVax at 2 mg (Group 2, 0.5 mg DNA of each of the 4 vaccine constructs) or Vaxfectin® at 4 mg (Group 3). All treatments were delivered as bilateral 1 mL injections to the vastus lateralis. The TVDVVax dose was selected based on the proposed clinical protocol. The Vaxfectin® alone group was included to assess the potential contribution of Vaxfectin® adjuvant to any responses observed in the TVDVVax group; as the adjuvant was supplied at a 2 mg/mL concentration, delivering bilateral 1 mL injections yielded a 4 mg total body dose. Animals were vaccinated on Days 1, 30, 60 and 90. Euthanasia time-points were scheduled for Day 92 (acute toxicity, 48 h following final dose) and 120 (recovery toxicity, 30 d following final dose). Parameters evaluated included clinical observations, dermal irritation, body weight and temperature, food consumption, clinical pathology, ophthalmology, gross necropsy findings, absolute and relative organ weights, and histopathology findings. Serum samples were also prepared from blood collected on Day 60 (post 2nd dose) and Day 120 (post 4th dose) and used for measurement of neutralizing antibody titers by plaque reduction neutralization test (PRNT).18
One Group 3 female was found dead on Day 62. This animal had mistakenly received 6 mg of Vaxfectin® instead of 4 mg on Day 30. Because no unusual clinical, gross or microscopic observations were seen, the death could not be attributed to test article administration and the cause of death was undetermined. All other animals survived until scheduled euthanasia. Cage side observations and dermal irritation scoring revealed the presence of varying degrees of erythema and edema in all groups, including PBS controls. Erythema and edema were increased in Group 2 and 3 males when compared with controls, but were minor in extent (scoring very slight to slight) and duration (resolving in 1–5 d), and did not appear different between the test article groups 2 and 3. Body weights and body weight changes during the study were mostly unremarkable, and there were no statistically significant test article-related changes. Ophthalmic examinations were normal across groups.
There were no test article-related changes in organ weights at necropsy. Microscopic examination did reveal pyogranulomatous and/or granulomatous inflammatory lesions at both injection site skin and muscle in both TVDVVax- and Vaxfectin®-treated rabbits. The incidence of muscle lesions in male and female animals and skin lesions in females was higher with TVDVVax than with Vaxfectin® on Day 92 (acute toxicology time point). Recovery was apparent by Day 120.
Clinical chemistry/pathology panels revealed several test article-related changes that were likely secondary to the injection site inflammation, generally occurring on Day 3 and 92 (48 h post-dosing) but resolving by later time-points. These included elevated levels of total white blood cells, segmented neutrophils, and lymphocytes in Group 2 males, and elevated segmented neutrophils in Groups 2 and 3 females (Table 1). On study day 3, somewhat decreased levels of hemoglobin and hematocrit were noted in group 2 males and females. Liver function tests indicated elevated levels of aspartate aminotransferase (AST) in group 2 animals on study day 92 (Table 2). These responses were transient and had resolved by study day 120 (not shown). Forty-eight hours after the first administration of test articles (study day 3), animals in both groups 2 and 3 had decreased protohrombin times and elevated activated partial thromboplastin times (Table 3). Both test article treated groups also showed elevated fibrinogen levels on days 3 and 92. C-reactive protein (CRP), which was included as an inflammatory marker endpoint, was also transiently increased in both test article groups at Days 3 and 92 (Table 2). This marker (CRP) rises but quickly resolves, providing a sensitive measure of inflammatory response. Transient increases in CRP in this study offer additional support for concluding that the clinical pathology changes observed were likely secondary to injection site reactogenicity/inflammation related to intramuscular injection of TVDVVax or Vaxfectin®.
Table 1. Key Hematology Indicators.
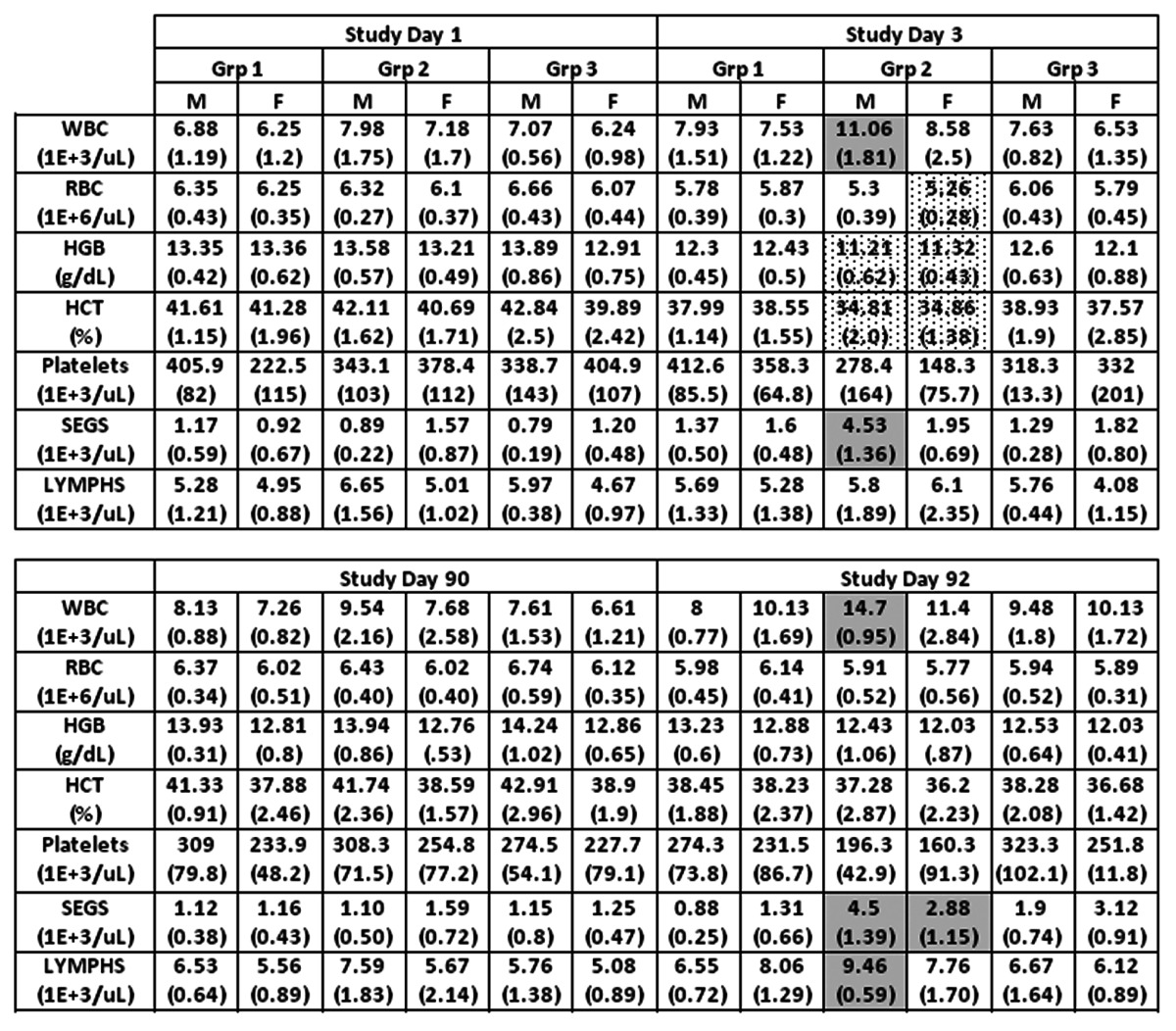
Groups 1, 2 and 3 are treated with PBS, TVDV and Vaxfectin respectively. M and F refer to male and female animals. Shown are group averages with standard deviation in parentheses for white and red blood cells (WBC, RBC), hemoglobin (HGB), hematocrit (HCT), platelets, segmented neutrophils (SEGS) and lymphocytes (LYMPHS). Group 2 an3 values that are significantly higher or lower compared to group 1 (p<0.05) are shown in shaded or dotted boxes respectively.
Table 2. Clinical chemistry.
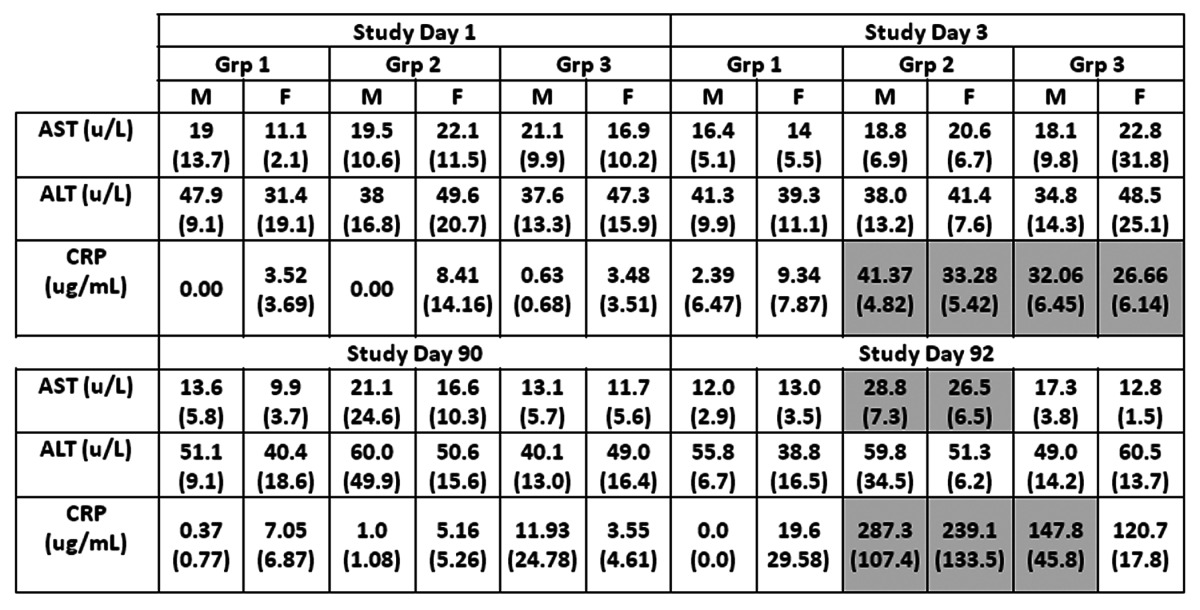
Groups 1, 2 and 3 are treated with PBS, TVDV and Vaxfectin respectively. M and F refer to male and female animals. Shown are group averages with standard deviation in parentheses for alanine and aspartate amino- transferases (ALT and AST), and C-reactive protein (CRP). Group 2 an3 values that are significantly higher or lower compared to group 1 (p<0.05) are shown in shaded or dotted respectively.
Table 3. Coagulation tests.
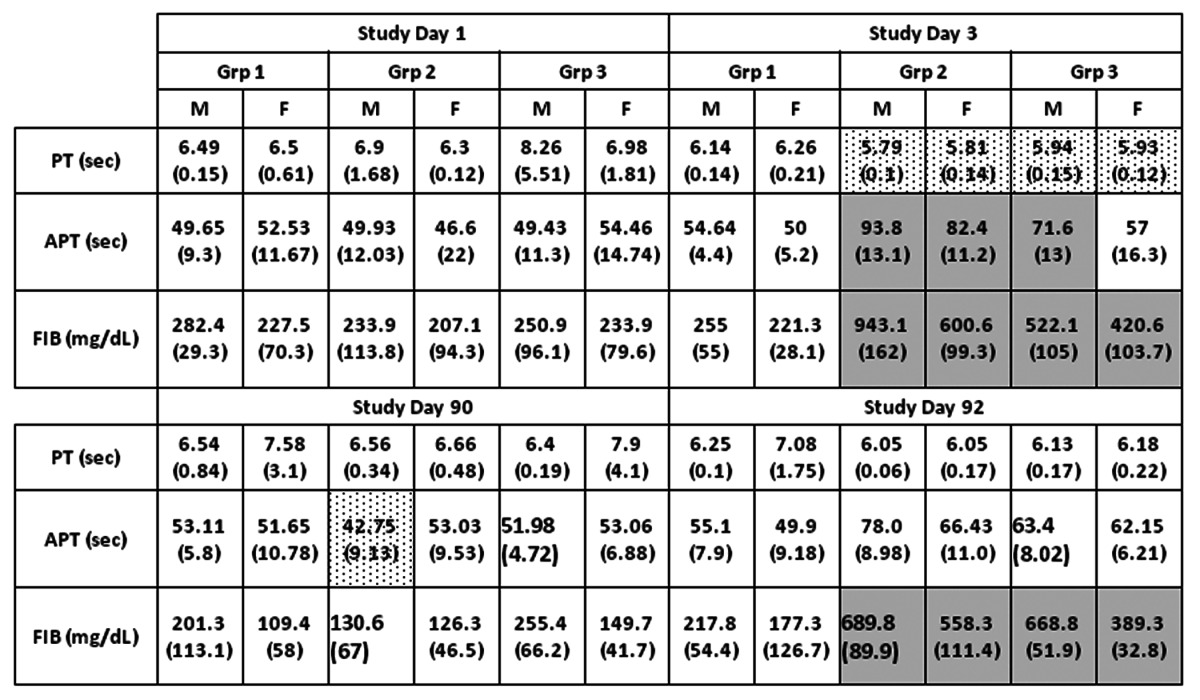
Groups 1, 2 and 3 are treated with PBS, TVDV and Vaxfectin respectively. M and F refer to male and female animals. Shown are group averages with standard deviation in parentheses for prothrombin time (PT), activated prothrombin time (APT) and fibrinogen protein. Group 2 an3 values that are significantly higher or lower compared to group 1 (p<0.05) are shown in shaded or dotted boxes respectively.
Dengue virus neutralizing antibodies were determined for Days 60 and 120 sera of all TVDVVax vaccinated animals and two each of PBS and Vaxfectin® vaccinated animals. No neutralizing antibodies against any of the dengue serotypes were detected in animals vaccinated with PBS or Vaxfectin® alone (not shown). Geometric mean 50% neutralization titers for each of the 4 dengue serotypes are shown in Figure 1. After two doses of TVDVVax, all animals had seroconverted to all 4 serotypes except one male and one female animal in which antibody to dengue-4 could not be detected. The 50% neutralization titers ranged between 128–716 (dengue-1), 156–672 (dengue-2), 128–716 (dengue-3) and 38–140 (dengue-4). By Day 120, however, 100% of the animals had significant levels of neutralizing antibody to all 4 serotypes. The range of antibody titers had increased to 724–1349 (dengue-1), 1287–2454 (dengue-2), 848–1632 (dengue-3) and 194–290 (dengue-4). From Day 60 (n = 16) to Day 120 (n = 8), the geometric mean titers had increased from 261 to 1032 (dengue-1), 253 to 1729 (dengue-2), 222 to 1107 (dengue-3) and 41 to 241 (dengue-4).
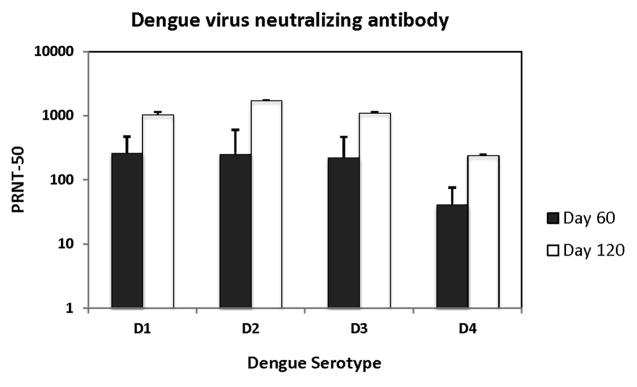
Figure 1. Virus neutralizing antibody in rabbits vaccinated with TVDVVax. Geometric mean fifty percent virus neutralization titers (PRNT-50) for each of the 4 dengue serotypes (D1-D4) using sera after 2 doses of vaccine (Day 60) and 4 doses of vaccine (Day 120) are shown.
These results demonstrate that repeated intramuscular injections of 2 mg TVDVVax or 4 mg Vaxfectin® were well tolerated by rabbits. The observations and findings made during this toxicology study were representative of the type of changes expected following the intramuscular injection of an adjuvanted vaccine such as TVDVVax, and are characteristic of the successful induction of an acquired immune response. Similar qualitative changes were seen following delivery of Vaxfectin® (at double the dose present in TVDVVax) but with some indications of a lower degree of severity (e.g., as with the Day 92 inflammatory lesions). The Vaxfectin®-related findings are in agreement with a previous report19 and once again are not unexpected for an adjuvant whose proposed mechanism of action is the induction/modulation of immune responses.17 In addition, all animals elicited strong neutralizing antibodies to all 4 dengue serotypes. The antibody responses were much higher than previously reported for monovalent vaccine constructs in mice and monkeys,12-14,18 or for Vaxfectin®-adjuvanted TVDV in monkeys.16 This could be due to the differences in animal species, or different doses used in different animal species. It will be interesting to see how this adjuvanted DNA vaccine will perform in humans. A phase 1 clinical trial of this vaccine was initiated in early 2012.
Acknowledgments
This work was supported by funds from the Navy Bureau of Medicine and Surgery. Animal work was performed through a contract at the AAALAC accredited facilities of Bioreliance Corporation, in compliance with the Animal Welfare Act. The authors, except Thomas Luke and John Doukas are military service members or employees of the US Government. This work was prepared as part of official duties. Title 17 U.S.C. s105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. s101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Navy, Department of Defense, nor the US Government.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21806
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–9. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 4.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–51. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 5.Sun W, Cunningham D, Wasserman SS, Perry J, Putnak JR, Eckels KH, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naïve adults. Hum Vaccin. 2009;5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 6.Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69(Suppl):48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 7.Robert Putnak J, Coller BA, Voss G, Vaughn DW, Clements D, Peters I, et al. An evaluation of denguetype-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23:4442–52. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine. 2011;29:7267–75. doi: 10.1016/j.vaccine.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durbin AP, Whitehead SS. Next-generation dengue vaccines: novel strategies currently under development. Viruses. 2011;3:1800–14. doi: 10.3390/v3101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29:7229–41. doi: 10.1016/j.vaccine.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 11.Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS Res Hum Retroviruses. 1990;6:993–8. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 12.Raviprakash K, Porter KR, Kochel TJ, Ewing D, Simmons M, Phillips I, et al. Dengue virus type 1 DNA vaccine induces protective immune responses in rhesus macaques. J Gen Virol. 2000;81:1659–67. doi: 10.1099/0022-1317-81-7-1659. [DOI] [PubMed] [Google Scholar]
- 13.Raviprakash K, Marques E, Ewing D, Lu Y, Phillips I, Porter KR, et al. Synergistic neutralizing antibody response to a dengue virus type 2 DNA vaccine by incorporation of lysosome-associated membrane protein sequences and use of plasmid expressing GM-CSF. Virology. 2001;290:74–82. doi: 10.1006/viro.2001.1136. [DOI] [PubMed] [Google Scholar]
- 14.Blair PJ, Kochel TJ, Raviprakash K, Guevara C, Salazar M, Wu SJ, et al. Evaluation of immunity and protective efficacy of a dengue-3 pre-membrane and envelope DNA vaccine in Aotus nancymae monkeys. Vaccine. 2006;24:1427–32. doi: 10.1016/j.vaccine.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine. 2011;29:960–8. doi: 10.1016/j.vaccine.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine. 2012;30:336–41. doi: 10.1016/j.vaccine.2011.10.085. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010;7:1433–46. doi: 10.1517/17425247.2010.538047. [DOI] [PubMed] [Google Scholar]
- 18.Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18:2426–34. doi: 10.1016/S0264-410X(99)00570-8. [DOI] [PubMed] [Google Scholar]
- 19.Doukas J, Morrow J, Bellinger D, Hilgert T, Martin T, Jones D, et al. Nonclinical biodistribution, integration, and toxicology evaluations of an H5N1 pandemic influenza plasmid DNA vaccine formulated with Vaxfectin®. Vaccine. 2011;29:5443–52. doi: 10.1016/j.vaccine.2011.05.060. [DOI] [PubMed] [Google Scholar]


