Abstract
The utilization of immunosuppressive agents presents patients with autoimmune nephrosis at a high risk of infection. The present trial was to investigate the efficacy and safety of Broncho-Vaxom on preventing infection in immunosuppressive patients with autoimmune nephrosis.
Methods: 40 patients with autoimmune nephrosis were randomly divided into two groups. The control group (20 cases) routinely received corticosteroid and (or) immunosuppressive therapy, while the treatment group (20 cases) received a capsule containing 7 mg Broncho-Vaxom daily for the first 10 d of each month for 3 consecutive months on the basis of conventional corticosteroid and (or) immunosuppressive therapy. The condition of infection and blood lymphocyte were assessed.
Results: 4 patients in the treatment group and 5 patients in the control group were lost during the follow-up period. 25% of patients in the treatment group and 40% of patients in the control group suffered infection. There was no difference in the incidence of infection between the two groups (p > 0.05), while Broncho-Vaxom treated patients suffered a shorter infection period and of which fewer patients need to receive antibiotics therapy (p < 0.05). After the treatment with Broncho-Vaxom, the total number of blood T lymphocyte, proportion of CD4+ T lymphocyte, CD4+/CD8+ reduced less and the serum IgG rose more obviously (p < 0.05), but the blood lymphocyte, B lymphocyte, CD8+ T lymphocyte, IgA and IgM have no differences between the two groups (p > 0.05).
Conclusion: Broncho-Vaxom might be a good choice for preventing the respiratory infection in nephrosis, especially in the patients under the therapy of immunosuppressive agents.
Keywords: Glomerulonephritis, Immunostimulating Bacterial Lysate, Nephrotic Syndrome, Respiratory infection, T lymphocyte subsets, glomerulonephritis
Introduction
Infection is one of the most common complications of autoimmune nephrosis, which has been reported in up to 20% of adult patients with nephrotic syndrome.1 Currently, corticosteroid and the other immunosuppressant are the major therapy of autoimmune nephrosis. With the non-specific immunosuppressive effect of these agents, infection is one of the most common and serious complication. Infection not only can induce autoimmune nephrosis, but also can make a recurrent of kidney disease. It is estimated that 50 to 70% of relapses of nephrotic syndrome among children in developing countries follow infections chiefly of the upper respiratory tract.2 Serious bacterial infection also has long been recognized as a major, potentially life-threatening complication of autoimmune nephrosis. Prior to the advent of antibiotics, sepsis was responsible for the death of approximately one-third of patients.3 With the widespread use of antibiotics, patients died from infection decrease, but still have an important proportion. The International Study Group of Kidney Disease in Children indicated that, of the 10 deaths among the nearly 400 children with minimal change disease followed for 5 to 10 y, six occurred after infection, resulting in a cumulative infection-related mortality incidence of 1.5%.4 Therefore, how to prevent infection effectively has become an important link of the treatment for autoimmune nephrosis.
At present, multiple different prophylactic interventions are used and/or recommended for reducing the risk of infection in nephrosis, such as chemoprophylaxis with antibiotics, pneumococcal vaccines, immunoglobulin replacements and immunity regulator therapies. But the effects of these treatments are controversial.5
Broncho-Vaxom is one of the immunostimulants, an extract from 8 bacteria frequently responsible for respiratory tract infection (Hemophilus influenzae, Klebsiella pneumoniae and ozaenae, Staphylococcus aureus, Streptococcus pneumoniae, pyogenes and viridans, Moraxella catarrhalis), which has been widely used to prevent respiratory tract infection,6-10 improve COPD condition and reduce the rate and duration of wheezing attack.11-13 But there was no related research had reported that Broncho-Vaxom could also enhance the immunity of autoimmune nephrosis in an immunosuppressive situation. This clinic trail was due to observe the efficacy and safety of Broncho-Vaxom in preventing the respiratory infection and regulating the immunity of autoimmune nephrosis.
Result
Demographic Characteristics
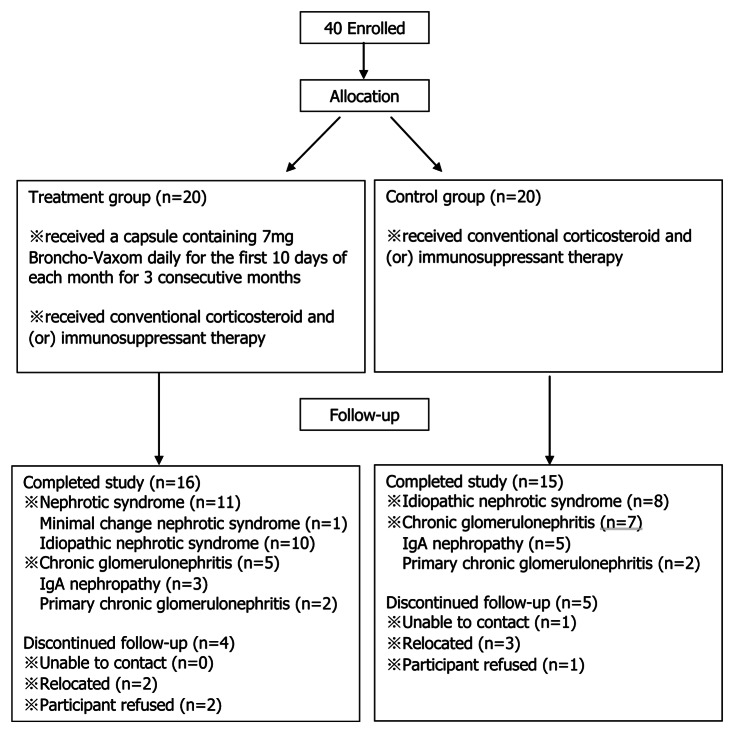
There were nine cases in the two groups of patients lost during the period of follow-up, 4 patients in the treatment group and 5 patients in the control group. All of them dropped out because of unable to contact, relocated or participant refused. (Fig. 1) Both the treatment group (16 cases) and the control group (15 cases) had similar demographic characteristics at the beginning of the trial (p > 0.05). (Table 1)
Figure 1. Allocation and follow-up of the study.
Table1 . Patient characteristic at enrollment.
| Characteristics | The treatment group(n = 16) | The control group(n = 15) |
|---|---|---|
| Age, yr(means ± SD) |
22.94 ± 8.20 |
27.27 ± 12.95 |
| Sex, No. (%) |
|
|
| Male |
11(69%) |
9(60%) |
| Female |
5(31%) |
6(40%) |
| Disease, No. (%) |
|
|
| Nephrotic syndrome |
11(69%) |
8(53%) |
| Chronic glomerulonephritis |
5(31%) |
7(47%) |
| Weight, kg |
62.25 ± 13.37 |
61.9 ± 10.42 |
| Duration, median (min, max) |
105d (6d,6yr) |
90d (5d,4yr) |
| Serum total protein, g/l |
49.83 ± 9.85 |
50.91 ± 10.63 |
| Serum albumin, g/l |
23.60 ± 10.71 |
25.49 ± 9.65 |
| Blood urea nitrogen, mmol/l |
5.63 ± 2.60 |
6.59 ± 3.75 |
| Serum creatinine, umol/l | 85.50 ± 33.18 | 84.47 ± 21.60 |
All p > 0.05 (Mann–Whitney U test or Chi Square test).
The adoption of corticosteroid and immunosuppressive therapy
The kind of corticosteroid used in the two groups was prednisone. The dosage of prednisone was not obviously different between the two groups (p > 0.05). The numbers of patients accepted immunosuppressive therapy also had no obvious difference in the three months between the two groups, 5 0f 15 (33%) patients in the control group and 6 of 16 (37.5%) patients in the treatment group (p > 0.05). (Fig. 2) In the treatment group, five of the patients accepted the therapy of Tripterygium wilfordii 60mg per day, one of the patients accepted the therapy of leflunomide 20mg per day. In the control group, three of patients were treated with Tripterygium wilfordii 60mg per day, two were treated with leflunomide 20mg per day. We might safely draw the conclusion that the use of prednisone and immunosuppressant were same in the two groups. Thus they would not influence the results of the experiment.
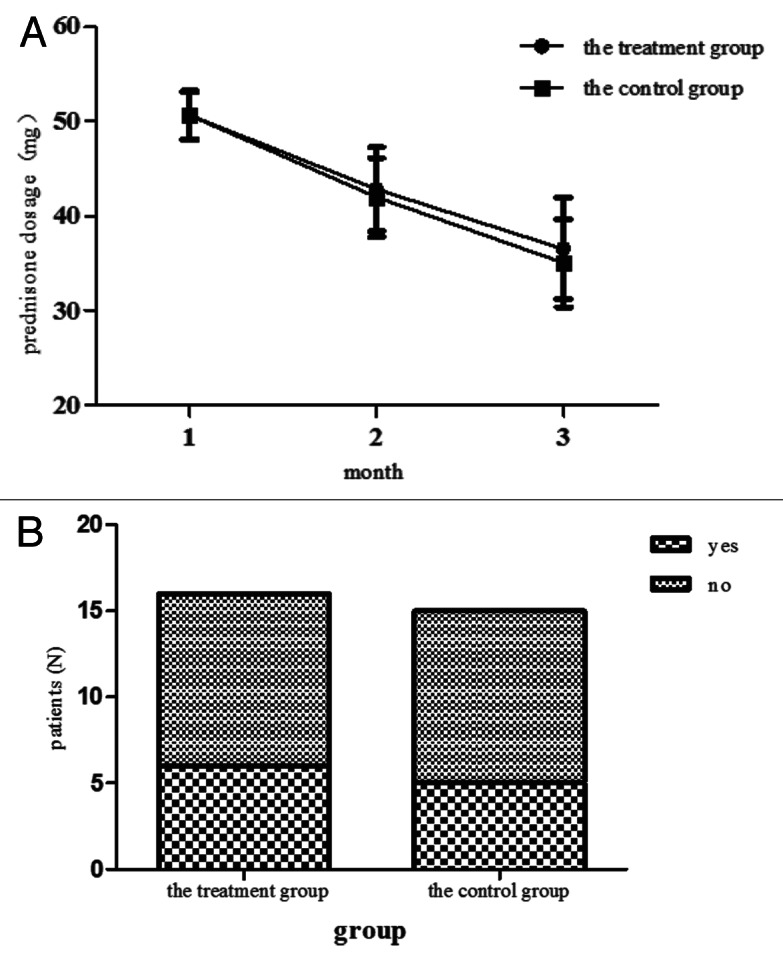
Figure 2. The dosage of the predisone for every month and the numbers of patients accepted immunouppressive therapy in three months in each group. (A) The dosage of predisone was collected per month. Data shows the mean ± sd of all patients in each group. All the p > 0.05 by the Mann-Whitney U test. (B) The numbers of patients accepted immunosuppressnt were recorded in 3 mo. Data shows the totle numbers of patients accepted and did not accept immunosuppressant.The p > 0.05 by the Chi-Square test.
Lymphocyte number and Lymphocyte subsets proportions
We compared the totally number of blood lymphocyte and the proportions of lymphocyte subsets before and after treatment in each groups separately. The proportion of T lymphocyte in the control group decreased obviously after the treatment (p < 0.01), while it had no significant change in the treatment group (p > 0.05). The percentage of CD4+ T lymphocyte and CD4+/CD8+ in the two groups decreased obviously after the treatment, the proportion of CD8+ T lymphocyte rose obviously after treatment (p < 0.05). The total number of lymphocyte and the proportion of B lymphocyte had no obvious change after treatment in each group (p > 0.05). (Fig. 3)
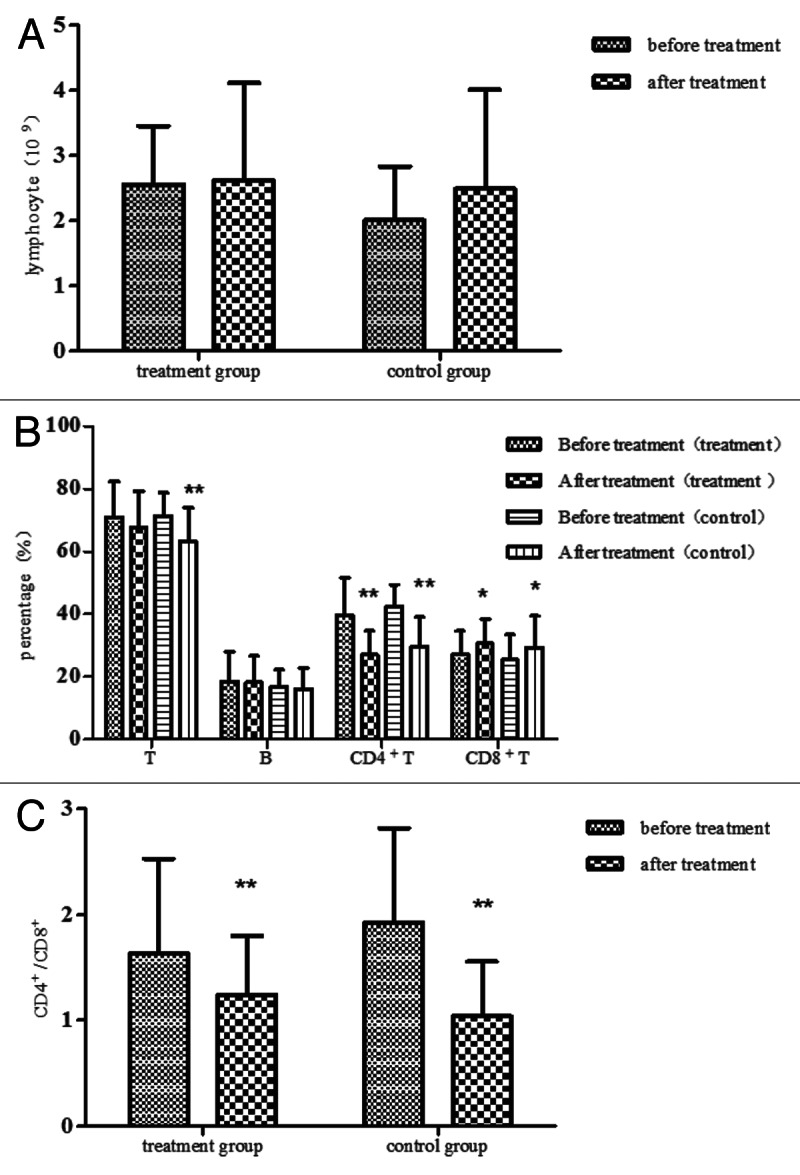
Figure 3. The serum lymphocyte and lymphocyte subsets in the two groups. (A) The serum lymphocyte was detected before the treatment and 3 mo after treatment both in the treatment group and the control group. Compared with before treatment, all p > 0.05 by the Student's T test. (B) The serum lymphocyte subsets, such as the serum T lymphocyte, B lymphocyte, CD4+ T lymphocyte and CD8+ T lymphocyte were detected by flow cytometry before and after treatment. Compared with before treatment, *p < 0.05, ** p < 0.01 by the Student's T test or the Wilcoxon test. (C) The serum CD4+/CD8+ in the two groups, compared with before treatment, **p < 0.01 by the Student's T test or the Wilcoxon test.
The variations of CD4+ T lymphocytes, CD8+ T lymphocytes and CD4+ / CD8+
We compared the ratios of the value after the treatment to it before the treatment about the CD4+ T lymphocyte, CD8+ T lymphocyte and CD4+/CD8+ between the two groups. Compared with before treatment, the CD4+ T lymphocyte declined about 31% in the control group, 13% in the treatment group. The CD4+ T lymphocyte reduced more obviously in the control group (p < 0.01). The CD4+/CD8+ declined about 45% in the control group, 21% in the treatment group. The CD4+/CD8+ reduced more obviously in the control group (p < 0.01). The ratios of the CD8+ T lymphocyte have no significant differences in the two groups (p > 0.05). (Fig. 4)
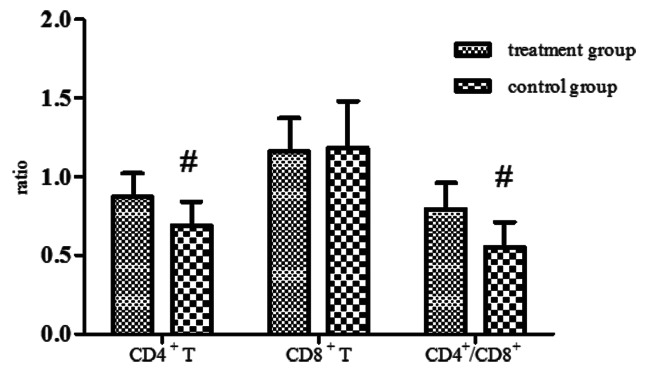
Figure 4. The ratios of the value after the treatment to it before the treatment about the CD4+ T lymphocyte, CD8+ T lymphocyte and CD4+/CD8+. Compared with the control group, #p < 0.01 by the Student's T test or the Mann-Whitney U test.
Immunoglobulin
The serum IgA and IgM reduced significantly after the treatment in each group (p < 0.05). The serum IgG in the treatment group rose obviously after treatment (p < 0.05), but it had no significant change in the control group (p > 0.05). (Fig. 5)
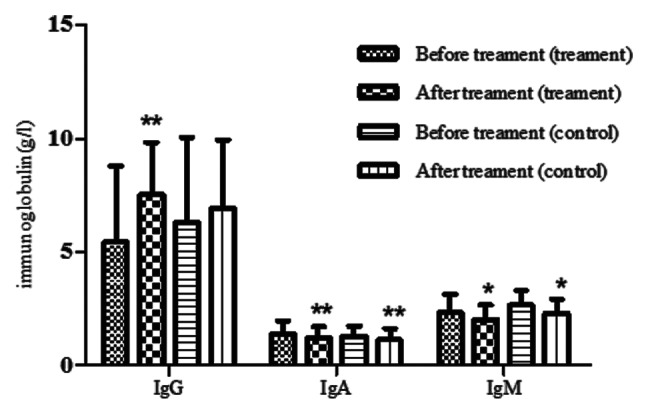
Figure 5. The serum immunoglobulins in the two groups. The serum IgG, IgA and IgM were detected before and after treatment in the two groups. Compared with before treatment, *p < 0.05, **p < 0.01 by the Student's T test.
Respiratory infection and antibiotic use
In the 3 mo, the numbers of patients suffer from respiratory tract infection had no difference in the two groups, 6 0f 15 (40%) in the control group and 4 0f 16 (25%) in the treatment group (p = 0.46), a patient in the control group suffered two infections in the 3 mo. However the total duration of the respiratory tract infection in the treatment group was shorter than the control group, 18 d in the treatment group and 38 d in the control group (p < 0.01). There was no patient accepting antibiotics in the treatment group, but 4 patients in the control group. The patients accepted antibiotics in the treatment group were also fewer than the control group (p < 0.05). (Table 2) During the 3 mo, there was no patient replasing in the treatment group, but 1 patient in the control group.
Table 2. Respiratory infection and the use of antibiotics in the two groups.
| The treatment group | The control group | |
|---|---|---|
| Respiratory tract infection, No. (%) |
4(25%) |
6(40%) |
| Total duration of the respiratory tract infection, Day |
18 |
37** |
| Antibiotic threapy, No. (%) | 0(0) | 4(27%)* |
p < 0.05, ** p < 0.01 (T-test or Chi Square test).
Original disease condition
The percentage of patients in complete remission, partial remission, or no response was not different between the two groups after treatment (p > 0.05). (Table 3)
Table 3. The outcome of nephrosis in the two groups.
| The treatment group | The control group | |
|---|---|---|
| Complete remmision, No. (%) |
8(50.00%) |
7(46.67%) |
| Partial remission, No. (%) |
5(31.25%) |
5(33.33%) |
| No response, No. (%) | 3(18.75%) | 3(20%) |
p > 0.05 (Chi Square test)
Discussion
Infection is a major complication of autoimmune nephrosis, especially under immunosuppressive circumstance. Infection not only can induce autoimmune nephrosis, but also makes a recurrent of kidney disease. Severe infection can even lead to death. Infection is a major reason for death in the patients of autoimmune nephrosis. Preventing infection effectively can reduce the recurrence and lead to lower mortality. Currently, prophylactic interventions used to prevent infection include avoidance of nephrosis, chemoprophylaxis with antibiotics, pneumococcal vaccines, immunoglobulin replacements and immunity regulator therapies (such as the thymus preparations, traditional Chinese medicines, etc.). Some scholars had made a META analysis in 2004 confirmed that there was no effective way to prevent infection in patients with nephrotic syndrome.5 As an immunomodulator, Broncho-Vaxom has been widely used to prevent respiratory tract infection in patients with recurrent respiratory tract infections.6-10 A META analysis published in 2010 said that Broncho-Vaxom could reduce the numbers of infectious patients approximately 26.2% in the people suffer recurrent respiratory tract infection.8 Razi, CH et al. also found that Broncho-Vaxom could significantly reduce asthma attacks in patients with recurrent respiratory tract infections by reducing the number of respiratory infections.13 However in patients with autoimmune nephrosis, particularly in utilization of corticosteroid or other immunosuppressant therapy, the effectiveness of Broncho-Vaxom was debatable.
The immunity of autoimmune nephrosis, especially who accepted corticosteroid and (or) immunosuppressant therapy, was inhibited. Numerous studies document abnormalities of immunity in patients with nephrosis. IgG concentrations, for example, are signiðcantly reduced during exacerbation and may persist following remission.14 Nephrotic patients may also show disturbances of complement, particularly factor B of the alternative pathway, which provides protection against encapsulated organisms such as pneumococcus.15 Disturbances of neutrophil phagocytosis and T lymphocyte function in vitro have been documented during exacerbations of the nephrotic syndrome.3 Kemper et al. found the alterations of T cell subpopulations in nephrotic syndrome, such as an increased number of cytotoxic/suppressor T cells (CD8+) or reduction of CD4+ helper cells.16 Currently, corticosteroid and the other immunosuppressant are the major therapy of autoimmune nephrosis. They can further suppress cellular and humoral immunity. Corticosteroid could promote the apoptosis of activated T lymphocyte, inhibited T lymphocyte proliferation, and also affected the redistribution of T lymphocyte. In addition, corticosteroid also affected the function of B lymphocyte, led to the reduction of immunoglobulin synthesis.17 Kemper et al. found that CD4+ T lymphocyte in steroid-treated patients with nephrotic syndrome decreased more significantly than untreated patients not only in relapse, but also in remission in 2005.16 It could be observed from this experiment that compared with before treatment with corticosteroid and (or) immunosuppressant, the numbers of the blood T lymphocyte, CD4+ T lymphocyte, CD4+/CD8+, serum IgA and IgM reduced obviously after the treatment, the blood CD8+ T lymphocyte rose remarkably. The immunity of patients in these two groups was suppressed. All of these induce a high susceptibility to a wide range of infections.
Broncho-Vaxom is one of the immunostimulants, an extract from 8 bacteria frequently responsible for respiratory tract infection (Hemophilus influenzae, Klebsiella pneumoniae and ozaenae, Staphylococcus aureus, Streptococcus pneumoniae, pyogenes and viridans, Moraxella catarrhalis). It can stimulate the specificity and nonspecific immunity of the respiratory system. Broncho-Vaxom was proved to affect acquired immune response regulated by lymphocytes and synthesis of immunoglobulins.18 It had been mentioned that in newborn animals Broncho-Vaxom encouraged preferential development of the Th1-type immunity characterized by amplified IFN-γ and decreased IL-4 production.19 In human studies, an increased content of serum IgG, IgA and IgM levels were observed upon Broncho-Vaxom treatment.20 Maestroni et al. confirmed that Broncho-Vaxom could improve the secretory IgA.18,20-22 The secretory IgA exists in the secretion of mucosa, is the most important protective activity against respiratory tract infection, providing help in ongoing and future contact with the same antigen. As well as antigen-specific defensive mechanisms, Broncho-Vaxom also evoked a non-specific response by influencing macrophages, neutrophils activity and proinflammatory cytokines production.23 It could be observed from this experiment that the level of the blood T lymphocyte and serum IgG improved more obviously, the CD4+ T lymphocyte as well as CD4+/CD8+ reduced less after treated with Broncho-Vaxom. After treating with Broncho-Vaxom, the cellular and humoral immunity of patients were enhanced obviously. Autoimmune nephrosis accepted the therapy of Broncho-Vaxom showed stronger immunity. Result of the experiment revealed that Broncho-Vaxom mainly affected the T lymphocyte, especially the CD4+ T lymphocyte and serum IgG, but had no significant influence on other lymphocytes and immunoglobulins, such as the B lymphocyte, CD8+ T lymphocyte, IgA and IgM. These changes may be related to that Broncho-Vaxom selectively applied Th1 function.19 TH1 cells mainly regulate the cellular immunity, so that the B lymphocytes, IgA and IgM were not significantly affected. But the elevation of the serum IgG might be related to IFN-γ which could be secreted by TH1 cells. IFN-γ could promote secretion of immunoglobulin IgG for B lymphocytes.
This experiment showed that, 25% of patients in the treatment group and 40% of patients in the control group suffered infections. The incidence of infections in the treatment group was 15% less than the control group. But there was no statistically significant difference between the two groups, which may be due to the number of patients enrolled into the study was scarce. So that it still need some large clinical researches to further prove the result. In addition, it could be observed from this experiment that the total duration of infection was significantly shorter than the control group and the numbers of patients accepted antibiotics also reduced significantly. Treatment with Broncho-Vaxom to some extent relieved the severity of infection and was helpful for the control of infection. In addition, despite of less immunosuppression by using Broncho-Vaxom, it did not influence the control of the underlying nephrosis. The percentage of patients in complete remission, partial remission, or no response was not different between the two groups after treatment. And we evaluated the condition of nephrosis by the result of qualitation analysis of albuminuria and quantitative analysis of serum albumin. However, we did not have a quantitative tracking of renal function. So further research is still needed to evaluated the effect of Broncho-Vaxom for controlling nephrosis.
By evaluating the security of Broncho-Vaxom, we did not observe any adverse reactions, such as malaise and/or fatigue, headache, nausea, vomiting, chills, arthralgia etc. during the follow-up. But we still need broader data such as a complete blood count, blood urea nitrogen, serum creatinine, ALT, AST, to further assess the safety of using Broncho-Vaxom in nephrosis. In summary, Broncho-Vaxom could enhance the antimicrobial immunity in immunosuppressive patients with autoimmune nephrosis by increasing the blood T lymphocyte, especially the CD4+ T lymphocyte and the level of IgG. Meanwhile, it could shorten the average duration of infection and reduce the use of antibiotics. Therefore, Broncho-Vaxom might be a good choice for preventing the respiratory infection in nephrosis, especially in the patients under the therapy of immunosuppressive agents. But in this study, the number of patients enrolled was scarce and all of them were observed only for three months. So it still need a larger sample size and longer follow-up period to further prove the prevention of infection in immunosuppressive patients with autoimmune nephrosis with Broncho-Vaxom.
Materials and Methods
Patients
Forty patients from our hospital fulfilled the case definition and diagnostic criteria of nephrotic syndrome or chronic glomerulonephritis were enrolled into the study. Patients with systemic autoimmune disease, primary immunodeficiency disease and major surgical procedure within 3 mo before the commencement of the study, recent immunosuppressant or immunostimulants therapy were excluded. The patients not caused by immune factors were excluded either. The patients were randomly enrolled into the control and treatment groups. The control group (20 cases) routinely received corticosteroid and (or) immunosuppressant therapy, while the treatment group (20 cases) received a capsule containing 7mg Broncho-Vaxom daily for the first 10 d of each month for 3 consecutive months on the basis of conventional corticosteroid and (or) immunosuppressant therapy. (Fig. 1) Hospital’s ethics committee approval and written informed consent directly from participants were obtained before initiation of the study.
Follow-up
All the patients were followed up for 3 mo, recorded the condition of respiratory tract infection, the use of antibiotics and the outcome of primary disease. The dosage of corticosteroid and immunosuppressant were also collected.
Blood test and urinalysis
Blood lymphocyte, lymphocyte subsets (T lymphocyte, B lymphocyte, CD4+T lymphocyte, CD8+T lymphocyte, CD4+/CD8+), Serum immunoglobulins (IgG, IgA, IgM) were detected before and after the treatment. Lymphocyte was detected by Blood cell analysis instrument. Lymphocyte subsets were detected by flow cytometry. Immunoglobulins were detected by chemiluminescence immunoassay. The urinalysis, serum albumin and serum total protein were tested monthly.
The criteria for the outcome of nephrosis
Complete remission: Symptom and sign disappear completely; urinary protein and urinary red blood cell are negative.
Partial remission: Amelioration of symptom and sign, reduction of urinary protein or urinary red blood cell, but not turning negative.
No response: Symptom and sign have no improvement, urinary protein, urinary red blood cell do not reduce obviously.
Statistical analysis
The data according with mormal distribution were analyzed by the Student’s T test, while the data not according with mormal distribution were analyzed by the nonparametric Mann-Whitney U test or Wilcoxon test using spss13.0. The data were expressed as the means ± SD. Frequencies were analyzed by the Chi Square test. p < 0.05 was considered to be statistically signiðcant different.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21874
References
- 1.Hull R, Goldsmith D. Identifying and managing nephrotic syndrome in adults. Practitioner. 2008;252:30–, 32-4, 37. [PubMed] [Google Scholar]
- 2.Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Arch Dis Child. 2008;93:226–8. doi: 10.1136/adc.2007.116079. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth JA, Gracey DM, Pussell BA. Adult nephrotic syndrome: non-specific strategies for treatment. Nephrology (Carlton) 2008;13:45–50. doi: 10.1111/j.1440-1797.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 4.Report of the International Study of Kidney Disease in Children Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years’ observation. Pediatrics. 1984;73:497–501. [PubMed] [Google Scholar]
- 5.Wu HM, Tang JL, Sha ZH, Cao L, Li YP. Interventions for preventing infection in nephrotic syndrome. Cochrane Database Syst Rev. 2004:CD003964. doi: 10.1002/14651858.CD003964.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Debbas N, Derenne JP. Preventive effects of an immunostimulating product on recurrent infections of chronic bronchitis in the elderly. Lung. 1990;168(Suppl):737–40. doi: 10.1007/BF02718202. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez-Tarango MD, Berber A. Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest. 2001;119:1742–8. doi: 10.1378/chest.119.6.1742. [DOI] [PubMed] [Google Scholar]
- 8.Schaad UB. OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr. 2010;6:5–12. doi: 10.1007/s12519-010-0001-x. [DOI] [PubMed] [Google Scholar]
- 9.Schaad UB, Mütterlein R, Goffin H, BV-Child Study Group Immunostimulation with OM-85 in children with recurrent infections of the upper respiratory tract: a double-blind, placebo-controlled multicenter study. Chest. 2002;122:2042–9. doi: 10.1378/chest.122.6.2042. [DOI] [PubMed] [Google Scholar]
- 10.Del-Río-Navarro BE, Luis Sienra-Monge JJ, Berber A, Torres-Alcántara S, Avila-Castañón L, Gómez-Barreto D. Use of OM-85 BV in children suffering from recurrent respiratory tract infections and subnormal IgG subclass levels. Allergol Immunopathol (Madr) 2003;31:7–13. doi: 10.1157/13042838. [DOI] [PubMed] [Google Scholar]
- 11.Solèr M, Mütterlein R, Cozma G, Swiss-German OM-85 Study Group Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration. 2007;74:26–32. doi: 10.1159/000093933. [DOI] [PubMed] [Google Scholar]
- 12.Steurer-Stey C, Bachmann LM, Steurer J, Tramèr MR. Oral purified bacterial extracts in chronic bronchitis and COPD: systematic review. Chest. 2004;126:1645–55. doi: 10.1378/chest.126.5.1645. [DOI] [PubMed] [Google Scholar]
- 13.Razi CH, Harmancı K, Abacı A, Özdemir O, Hızlı S, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. 2010;126:763–9. doi: 10.1016/j.jaci.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Kemper MJ, Altrogge H, Ganschow R, Müller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002;17:413–7. doi: 10.1007/s00467-001-0817-7. [DOI] [PubMed] [Google Scholar]
- 15.McLean RH, Forsgren A, Björkstén B, Kim Y, Quie PG, Michael AF. Decreased serum factor B concentration associated with decreased opsonization of Escherichia coli in the idiopathic nephrotic syndrome. Pediatr Res. 1977;11:910–6. doi: 10.1203/00006450-197708000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kemper MJ, Zepf K, Klaassen I, Link A, Muller-Wiefel DE. Changes of lymphocyte populations in pediatric steroid-sensitive nephrotic syndrome are more pronounced in remission than in relapse. Am J Nephrol. 2005;25:132–7. doi: 10.1159/000085357. [DOI] [PubMed] [Google Scholar]
- 17.Cupps TR, Fauci AS. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–55. doi: 10.1111/j.1600-065X.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 18.Maestroni GJ, Losa GA. Clinical and immunobiological effects of an orally administered bacterial extract. Int J Immunopharmacol. 1984;6:111–7. doi: 10.1016/0192-0561(84)90005-5. [DOI] [PubMed] [Google Scholar]
- 19.Bowman LM, Holt PG. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun. 2001;69:3719–27. doi: 10.1128/IAI.69.6.3719-3727.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puigdollers JM, Serna GR, Hernandez del Rey I, Barruffet MT, Torroella JJ. Immunoglobulin production in man stimulated by an orally administered bacterial lysate. Respiration. 1980;40:142–9. doi: 10.1159/000194264. [DOI] [PubMed] [Google Scholar]
- 21.Lusuardi M, Capelli A, Carli S, Spada EL, Spinazzi A, Donner CF. Local airways immune modifications induced by oral bacterial extracts in chronic bronchitis. Chest. 1993;103:1783–91. doi: 10.1378/chest.103.6.1783. [DOI] [PubMed] [Google Scholar]
- 22.Emmerich B, Emslander HP, Pachmann K, Hallek M, Milatovic D, Busch R. Local immunity in patients with chronic bronchitis and the effects of a bacterial extract, Broncho-Vaxom, on T lymphocytes, macrophages, gamma-interferon and secretory immunoglobulin A in bronchoalveolar lavage fluid and other variables. Respiration. 1990;57:90–9. doi: 10.1159/000195827. [DOI] [PubMed] [Google Scholar]
- 23.Rozy A, Chorostowska-Wynimko J. Bacterial immunostimulants--mechanism of action and clinical application in respiratory diseases. Pneumonol Alergol Pol. 2008;76:353–9. [PubMed] [Google Scholar]