Abstract
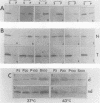
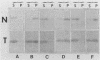
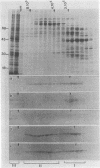
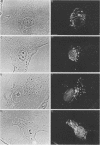
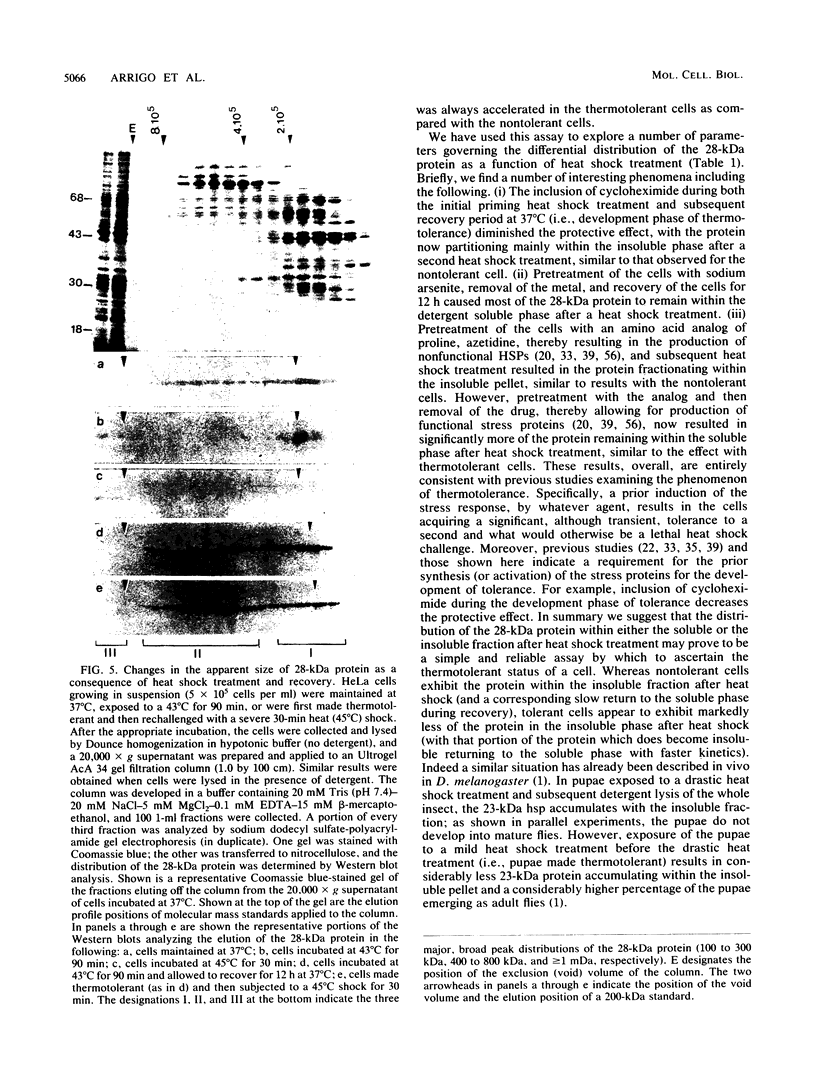
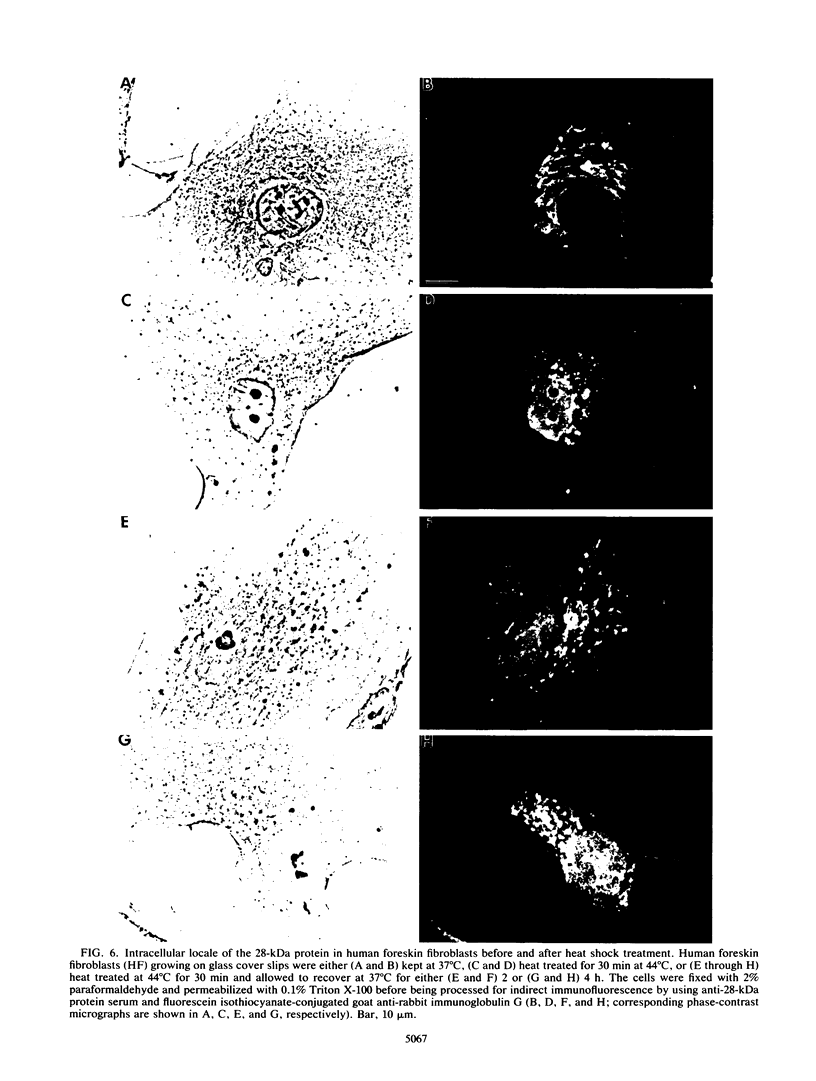
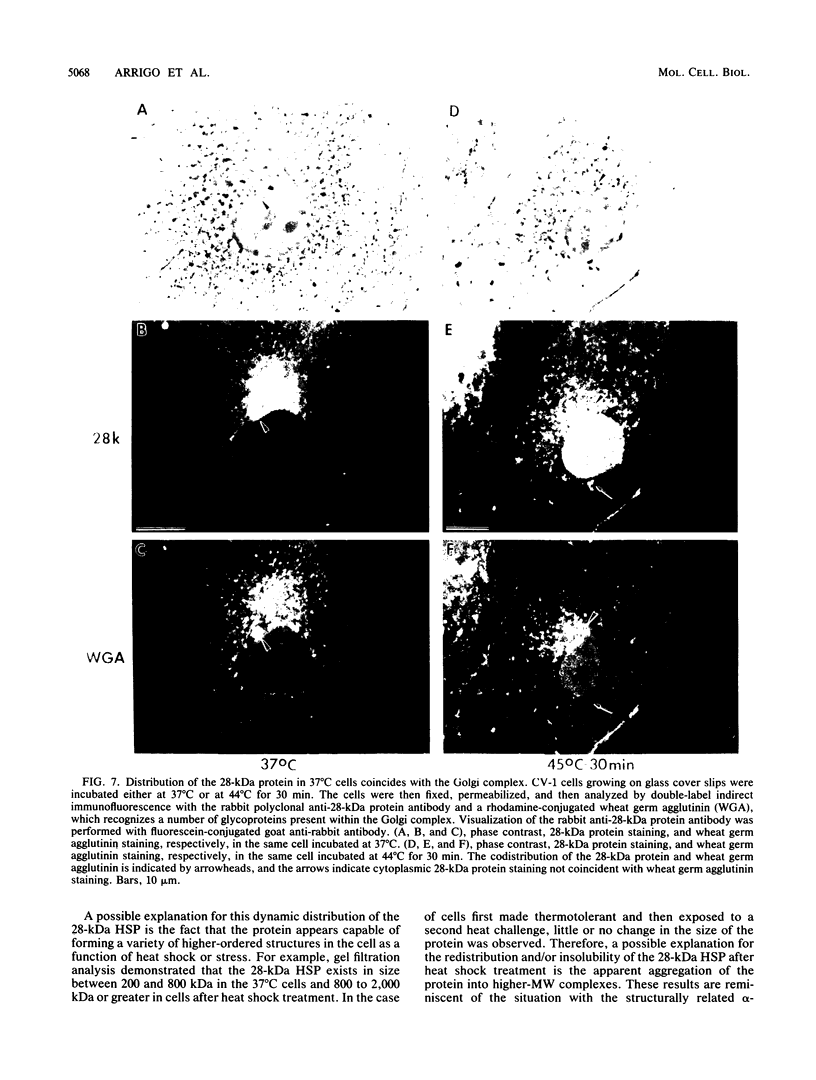
Mammalian cells grown at 37 degrees C contain a single low-molecular-weight heat shock (or stress) protein with an apparent mass of 28 kilodaltons (kDa) whose synthesis increases in cells after exposure to elevated temperatures or other forms of physiologic stress. Herein we present data demonstrating that heat shock protein 28 exists in a number of dynamic states depending upon the physiologic state of the cell. Biochemical fractionation of 37 degrees C cells in the absence of nonionic detergent revealed that the 28-kDa protein partitioned approximately equally between the soluble and insoluble fractions. The addition of detergent in the fractionation procedure resulted in all of the protein distributed within the soluble phase. In contrast, in cells first heat shocked and then fractionated in the presence of detergent, most of the 28-kDa protein was found within the insoluble fraction. These biochemical results appeared entirely consistent with indirect immunofluorescence experiments, demonstrating that the 28-kDa protein resided within the perinuclear region of 37 degrees C cells in close proximity to the Golgi complex. After heat shock treatment, the 28-kDa protein relocalized within the nucleus and resisted detergent extraction. The extent of 28-kDa protein redistribution into the nucleus and its detergent insolubility increased as a function of the severity of the heat shock treatment. With time of recovery from the heat treatment there occurred a gradual return of the 28-kDa protein into the detergent-soluble phase. Concomitant with these changes in 28-kDa protein solubility was a corresponding change in the apparent size of the protein as determined by gel filtration. While at 37 degrees C cells the protein exhibited a mass of 200 to 800 kDa; after heat shock the protein assumed sizes of 2 MDa or greater. Using immunoelectron microscopy, we show an accumulation of these aggregates of 28-kDa protein within the nucleus. Finally, we show that the heat-dependent redistribution of the 28-kDa protein from the cytoplasm into the nucleus was greatly diminished when the cells were first rendered thermotolerant, and we suggest that this simple assay (i.e., 28-kDa protein detergent solubility) may prove useful in evaluating the thermotolerant status of a cell or tissue.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Ahmad-Zadeh C. Immunofluorescence localization of a small heat shock protein (hsp 23) in salivary gland cells of Drosophila melanogaster. Mol Gen Genet. 1981;184(1):73–79. doi: 10.1007/BF00271198. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P. Cellular localization of HSP23 during Drosophila development and following subsequent heat shock. Dev Biol. 1987 Jul;122(1):39–48. doi: 10.1016/0012-1606(87)90330-7. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Pauli D. Characterization of HSP27 and three immunologically related polypeptides during Drosophila development. Exp Cell Res. 1988 Mar;175(1):169–183. doi: 10.1016/0014-4827(88)90264-9. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Tanaka K., Goldberg A. L., Welch W. J. Identity of the 19S 'prosome' particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988 Jan 14;331(6152):192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Augusteyn R. C., Koretz J. F. A possible structure for alpha-crystallin. FEBS Lett. 1987 Sep 28;222(1):1–5. doi: 10.1016/0014-5793(87)80180-1. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Heuser J., Levy M. A., Schlesinger M. J. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol. 1988 Apr;106(4):1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier N. C., Schlesinger M. J. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol. 1986 Oct;103(4):1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Intracellular localization of heat shock proteins in maize. Plant Physiol. 1987 Aug;84(4):1197–1203. doi: 10.1104/pp.84.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Jacobsen K. Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature sensitive growth. Cell. 1984 Oct;38(3):841–849. doi: 10.1016/0092-8674(84)90279-4. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Gerner E. W., Schneider M. J. Induced thermal resistance in HeLa cells. Nature. 1975 Aug 7;256(5517):500–502. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- Henle K. J., Leeper D. B. Modification of the heat response and thermotolerance by cycloheximide, hydroxyurea, and lucanthone in CHO cells. Radiat Res. 1982 May;90(2):339–347. [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Shuman J., Sette M., Przybyla A. Nuclear localization and phosphorylation of three 25-kilodalton rat stress proteins. Mol Cell Biol. 1984 Mar;4(3):468–474. doi: 10.1128/mcb.4.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Ledger P. W., Uchida N., Tanzer M. L. Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol. 1980 Dec;87(3 Pt 1):663–671. doi: 10.1083/jcb.87.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht B. G., Biessmann H., Palter K. B., Bonner J. J. Small heat shock proteins of Drosophila associate with the cytoskeleton. Proc Natl Acad Sci U S A. 1986 Jan;83(1):90–94. doi: 10.1073/pnas.83.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983 May;115(2):116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Li G. C., Laszlo A. Amino acid analogs while inducing heat shock proteins sensitize CHO cells to thermal damage. J Cell Physiol. 1985 Jan;122(1):91–97. doi: 10.1002/jcp.1041220114. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Welch W. J. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988 Apr;106(4):1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983 Sep;3(9):1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Dunia I., Dodemont H. J., Benedetti E. L., Bloemendal H. Lenticular intermediate-sized filaments: biosynthesis and interaction with plasma membrane. Proc Natl Acad Sci U S A. 1982 May;79(10):3208–3212. doi: 10.1073/pnas.79.10.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R. H., Candido E. P. Locus encoding a family of small heat shock genes in Caenorhabditis elegans: two genes duplicated to form a 3.8-kilobase inverted repeat. Mol Cell Biol. 1985 Jun;5(6):1268–1278. doi: 10.1128/mcb.5.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Siezen R. J., Bindels J. G., Hoenders H. J. The quaternary structure of bovine alpha-crystallin. Size and charge microheterogeneity: more than 1000 different hybrids? Eur J Biochem. 1978 Nov 15;91(2):387–396. doi: 10.1111/j.1432-1033.1978.tb12691.x. [DOI] [PubMed] [Google Scholar]
- Sinibaldi R. M., Morris P. W. Putative function of Drosophila melanogaster heat shock proteins in the nucleoskeleton. J Biol Chem. 1981 Nov 10;256(21):10735–10738. [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Lectin-binding sites as markers of Golgi subcompartments: proximal-to-distal maturation of oligosaccharides. J Cell Biol. 1983 Oct;97(4):1243–1248. doi: 10.1083/jcb.97.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R., Blose S. H. The mammalian stress response and the cytoskeleton: alterations in intermediate filaments. Ann N Y Acad Sci. 1985;455:57–67. doi: 10.1111/j.1749-6632.1985.tb50403.x. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-dalton mammalian stress proteins. J Biol Chem. 1985 Mar 10;260(5):3058–3062. [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Nyffenegger T., De Robertis E. M. Nucleocytoplasmic distribution of snRNPs and stockpiled snRNA-binding proteins during oogenesis and early development in Xenopus laevis. Cell. 1983 Feb;32(2):425–434. doi: 10.1016/0092-8674(83)90462-2. [DOI] [PubMed] [Google Scholar]