Abstract
One of the most effective molecular diversity techniques is phage display. This technology is based on a direct linkage between phage phenotype and its encapsulated genotype, which leads to presentation of molecule libraries on the phage surface. Phage display is utilized in studying protein-ligand interactions, receptor binding sites and in improving or modifying the affinity of proteins for their binding partners. Generating monoclonal antibodies and improving their affinity, cloning antibodies from unstable hybridoma cells and identifying epitopes, mimotopes and functional or accessible sites from antigens are also important advantages of this technology. Techniques originating from phage display have been applied to transfusion medicine, neurological disorders, mapping vascular addresses and tissue homing of peptides. Phages have been applicable to immunization therapies, which may lead to development of new tools used for treating autoimmune and cancer diseases. This review describes the phage display technology and presents the recent advancements in therapeutic applications of phage display.
Keywords: antibody libraries, immune-diagnostics, phage display, tumor targeting, autoimmune diseases, neurological disorders, transfusion medicine
Introduction
Phage display was created by G. Smith in 19851 as a method for presenting polypeptides on the surface of lysogenic filamentous bacteriophages. Since then, this method has become one of the most effective ways for producing large amounts of peptides, proteins and antibodies. This technology is based on the fact that phage phenotype and genotype are physically linked. Indeed, the gene encoding the displayed molecule is packed within the same virion as a single-strained DNA (ssDNA) and the displayed peptides or proteins are expressed in fusion with phage coat protein.2 This genotype-phenotype linkage ensures that identical phage particles will be obtained from the same Escherichia coli clone. The phage display technique allows the creation of libraries which contain up to 1010 different variants and could be used for affinity screening of combinatorial peptide libraries to study protein-ligand interactions and to characterize these ligands,3 receptor and antibody-binding sites,4 define epitopes for monoclonal antibodies, select enzyme substrates and screen cloned antibody repertoires.5
This review focuses on selected applications of phage display in health and medical biotechnology but it also highlights the basis of the phage display technique, methods for the construction of displayed molecules and types of antibody libraries.
Phage display technology
Phage display systems
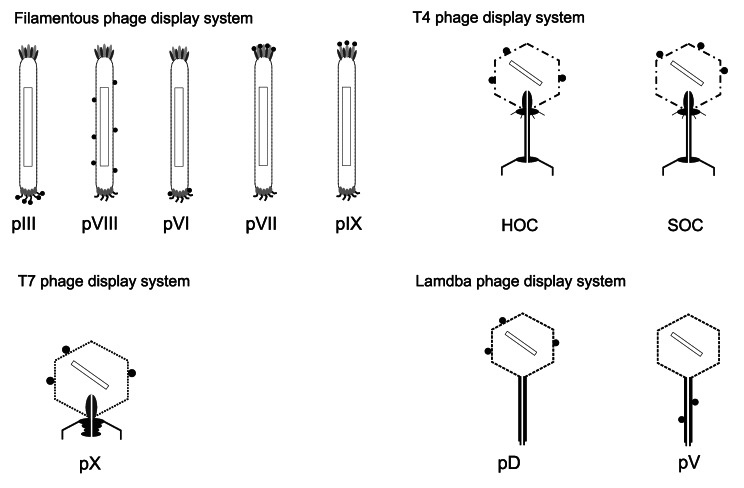
E . coli filamentous bacteriophages (f1, fd, M13) are commonly used for phage display. Most antibodies and peptides are displayed at phage proteins pIII6 and pVIII.7 The major coat protein (pVIII) is a product of gene 8 expression and occurs in nearly 3000 copies, therefore it is used to enhance detection signal when phage displayed antibody associates with antigen. Morover modifications of pVIII are made to increase the efficiency of display onto pVIII.7 In comparison, minor coat protein (pIII) consists of 406 amino acid residues and occurs at the phage tip in 3 to 5 copies. The vast majority of peptides and folded proteins are displayed as fusions with pIII protein, whereas pVIII, for preserving its functionality, could be coupled only with short (6–7 residues) not containing cysteine peptides.8 The loss of coat protein functionality was the major limitation of the phage display technology, however this problem was overcome by hybrid phages and coat protein modifications.7 These virions consist of the complete wild type genome and a copy of fusion gene which might occur as an insert in phage genome9 or as phagemid10 a vector that contains the origins of replication for phage and its host, gene 3 with appropriate cloning sites and an antibiotic-resistance gene. Moreover, the phagemid encoding polypeptide-pIII fusion requires hybrid with helper phage for packing into M13 particle. The helper phage contains a slightly defective origin of replication (such as M13KO7 or VCSM13) and supplies all the structural proteins required for generating a complete virion. Thus, both wild pIII protein and polypeptide-pIII fusion protein will be present on the phage surface. The ratio of polypeptide-pIII fusion protein to wild type pIII may range between 1 to 9 and 1 to 1000 depending on the type of phagemid, growth conditions, the nature of the polypeptide fused to pIII and proteolytic cleavage of antibody-pIII fusions.11 This ratio ensures that the fusion protein, as a minor component of the phage coat, does not affect phage viability. However, it should be noted that when hyperphage is used, achieving this ratio is unnecessary. Hyperphage has wild-type pIII phenotype, but due to lack of functional pIII gene, the fusion of pIII and antibody is the only source of pIII for phage assembly. Therefore, it allows to increase the number of presented scFv by more than two orders of magnitude and also 10-fold increases the binding of phage to antigen comparing to M13KO7 helper phage. The predominance of this phage is its utility in stoichiometric situations, when single phage could hardly locate the desired antigen.12
Moreover, hybrid phage system enables displaying large proteins with all five M13 coat proteins as N-terminal fusions with pIII, pVIII,13 pVII and pIX14,15 and also as C-terminal fusions with pVI, pIII, and pVIII.10,16,17
Due to the naturally occurring translational stop codon in the 3′-region of reverse transcribed mRNAs in M13 display system, expression of cDNA libraries could be difficult. For expression in M13 phage display system, cDNA cannot contain in-frame stop codons. Moreover cDNA has to be in the same reading frame as the pIII protein and the secretory leader sequence. There are several possibilities to overcome this problem, for instance cDNA fragmentation prior incorporation to plasmid but it could also lead to obtain a large number of clones with non-functional inserts. The usage of T7 is an alternative for M13 display.18 T7 phage display system has been widely used,19,20 due to its extreme robustness and stability in conditions that inactivate other phages. It found application in displaying small peptides (less than 50 residues) in high copy number, larger peptides or proteins in low or mid-copy number and displaying inserts with stop codon on the C-terminal of pX capsid protein. These advantages of using T7 over M13 display techniques is connected with the fact that the capsid is not involved in the phage to host adsorption and also with the possibility to obviate the need of secretion of displayed peptides through the periplasm and the cell membrane, however this approach restricts the possibility of posttranslational modification of polypeptides in eukaryotic systems.18
The phage T4 HOC/SOC bipartite display system21 could be applied to cDNA expression. It displays larger proteins in high copy number and inserts with stop codon on the C-terminal of SOC (small outer capsid) protein that occurs in 810 copies or N-terminal of HOC (highly antigenic outer capsid) protein that occurs in 155 copies.
Phage lambda is capable of displaying complicated, high molecular mass proteins as fusions with N- or C-terminal of pD head protein that occurs in 405 copies or C-terminal of pV tail protein that occurs in 6 copies.22,23 Moreover, in this system translocation through the Escherichia coli membrane is not required. Therefore, in comparison to filamentous phage system, lambda display gives the higher immune response in spite of displaying a wide variety of proteins in multiple copies. Representations of different phage types used for the display technique are shown in Figure 1.
Figure 1. Schematic presentation of phage display systems. The black circle represents molecule displayed on various phage proteins (listed below the scheme). The rectangle inside the phage represents nucleic acid.
Display of antibody fragments
The structure of most antibodies consists of two heavy chains (variable (VH), diversity (D), joining (JH), and constant (CH) region) and a pair of light chains (variable (VL), joining (JL) and constant (CL) region) linked by noncovalent bonds and disulfide bridges. However, in the early 90s an unusual type of antibodies without any light chain was discovered in the serum of Camelidae.24 The antigen is bound by these antibodies with a specific VHH fragment which could recognize unique conformational epitopes due to the significant contribution of its long complementary-determining region 3 (CDR3).
Antibody fragments have been expressed in E. coli periplasm or cytoplasm and to ensure the correct activity and function of antibody fragments the in vivo refolding of molecules obtained by cytoplasmic expression is required. Recently, a method for the soluble expression of recombinant proteins in the cytoplasm of the Origami DE3 Escherichia coli strain has been described.25 In this bacterial strain the disulfide bond-dependent folding of heterologous proteins is improved via disruption of the trxB and gor genes fused to the N-terminal of pET 32b plasmid sequences that encode two reductase enzymes, which allows the formation of disulfide bonds in the E. coli cytoplasm. Interestingly, the functional scFv recognizing MCF-7 which was expressed in bacterial cytoplasm shows significantly better binding characteristics and affinities than the one expressed in the periplasm.
In contrast to that, the periplasmic expression provides conditions for VH and VL pairing, similar to those in endoplasmic reticulum of the lymphocyte, which allows to generate the fully functional molecules . It was shown that co-expression of two periplasmic chaperones, Skp and FkpA, had strong effects on the productivity of soluble scFv. However, cytoplasmic chaperones, including GroELS, DnaKJE, TF, and SecB, did not enhance the secretory production.26
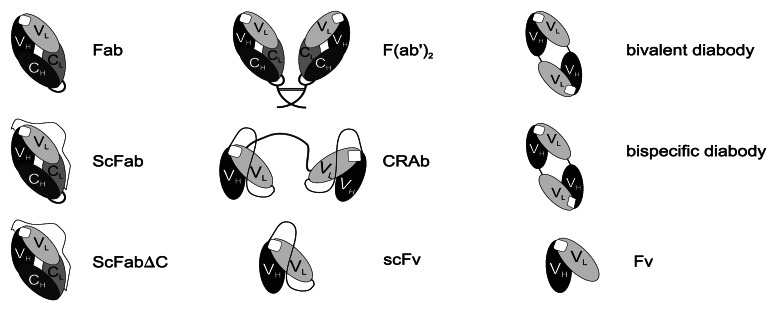
Indeed, many different antibody fragments are used in the phage display technology, including Fab (antigen-binding fragment), Fv (fragment variable), scFv (single chain fragment variable) and its modifications,27,28 antibodies with one V-gene domain,29 bivalent or bispecific diabodies30 and other oligomers.31 In Fab, VH-CH and VL-CL segments are linked by disulfide bonds and radiolabeled Fabs found application in tumor imaging.32 Fv molecule consists of the VL and VH regions only as well as its modification– the scFv, presently the most commonly used antibody fragment. Generally, the (Gly4Ser)3 linker is used to stabilize VL-VH connection and to ensure the proper antigen-binding site formation in scFv.33,34 These fragments have been expressed on phage surface without loss of antibody affinity. Furthermore, high binding affinity has been achieved by fusion of several different scFv segments. An example could be the CRAbs construct that consists of two scFv fragments specific to the same antigen but to adjacent epitopes.35 These fragments are connected by short linker (up to 10 amino acids), thereby dimerization of molecules and diabodies forming is possible.36 Representations of different antibody fragments are shown in Figure 2.
Figure 2. Schematic presentation of antibody fragments. Fab (the antigen-binding fragment), scFab (the single chain antigen-binding fragment), scFabΔC (the scFab variant without cysteins), scFv (the single chain fragment variable), Fv (the fragment variable), VHdAb (the antibody with one variable heavy chain domain), CRAb (the construct specific to adjacent epitopes on the antigen).
Library technology
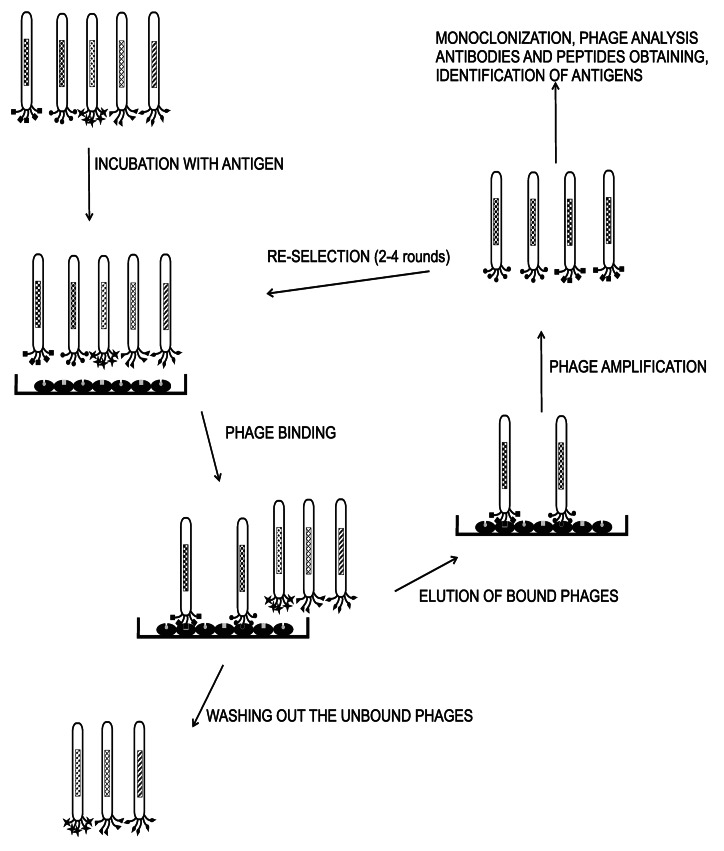
The phage display technology has provided the ability to create antibody libraries that contain a great number of phage particles, from which each one encodes and displays different molecules (106‒1011 different ligands in a population of > 1012 phage molecules). Therefore biopanning—the procedure of specific binders selection—is essential for enriching the desired molecule level. The biopanning method is based on repeated cycles of incubation, washing, amplification and re-selection of bound phage. The target molecule is immobilized on solid support as microtiter plate wells,37 PVDF membrane,38 column matrix or immunotubes,39 magnetic beads40 and even on whole cells.41 The several rounds of selection cycle are necessary to achieve the desired binding activity of obtained monoclonal phage antibodies. For determining this activity several tests are used, for example enzyme-linked immunosorbent assay (ELISA),42 fluorometric microvolume assay technology (FMAT)43 or chromophore-assisted laser inactivation (CALI).44,45 The type of solid support, time of binding and washing as well as antigen concentration have affected the level of selection. A proper design of the biopanning procedure enables the selection of antibodies to unique epitopes.46 Representations of the biopanning procedureis shown in Figure 3.
Figure 3. Schematic presentation of biopanning. Phage display library is incubated with target molecule immobilized on solid support. Specific library phage is bound to molecule and unbound phages are washed out. The specific phages are eluted and amplified in bacteria. After several rounds, amplified phages could be analyzed and amplified to obtain diagnostic and therapeutic agents.
Immune libraries have been used to produce high quality and high affinity antigen-specific antibodies providing good analytical tools. In these libraries, source of V-genes is the IgG mRNA of immunized animals or human B-cells. Human immunization allows to obtain phage display libraries from the peripheral blood or spleen lymphocytes and provides phage-displayed antibodies to red cell antigens D and B,47 platelet antigen HPA-Ia,48 glycoprotein IIb/IIIa,49 native T-cell receptor TCR-Vα50 and specific major histocompatibility complex (MHC)/peptide construct.51 Furthermore, humanized immune repertoires are obtained using preparation of RNA from spleen material taken from hyperimmunised animals such as chickens,52 rabbits,53 sheep,54 cows, nonhuman primates.55 Moreover for this purpose the human antibody-producing XenoMouse strain,56 has been engineered. These transgenic mice contain the majority of the human antibody gene repertoire on megabase-sized fragments from the human heavy and kappa light chain loci and its immunization leads to production in B-cell the human-like antibodies. The advantage of these libraries is that generated antibodies will have undergone affinity maturation by the immune system, which allows to achieve high-affinity antibodies even when the library is relatively small (107 clones).57,58 However, the immunization of animals is a time consuming process and each antigen requires the construction of new antibody library. Moreover, there are some limitations in the creation of human antibodies and prediction of the immune response is not always possible. An obstacle may be also lack of immune response to self or toxic antigens.,However, recent studies have shown that the immune library may contain a significant number of unimmunized clones and (that) a sufficiently large immune library can be utilized similarly to a naive library.59
In naive libraries, V-genes derived from the IgM mRNA of non-immunized human donors B-cells are isolated from diverse lymphoid sources such as peripheral blood lymphocytes, spleen cells, tonsils, bone marrow or from animal sources.59 Lymphocytes from over 40 non-immunized donors were used to construct the library that contains 1.4 × 1010 clones.60 Naive libraries offer the possibility to produce human monoclonal antibodies against red cell antigens E, D, Kbp, H and B.47 Furthermore, small-sized human single-pot libraries, containing 3 × 107 antibodies clones, were used to obtain antibodies to haptens, tumor necrosis factor α (TNFα), mucin, CD4, bovine serum albumin or lysozyme61 with efficient but lower in comparison to immune library antibodies affinity and reactivity. Predominance of the single-pot library is the ability to produce a single library for all antigens in a short time (2 to 6 times faster than obtaining antibodies from immune library)62. A further advantage of these libraries is the possibility of direct isolation of high affinity antibodies when very large repertoires are used and isolation of human antibodies to self, unimmunogenic or toxic antigens.63 However, the use of naive libraries could provide some difficulties in obtaining high affinity antibodies when small sized library is used. Also quality, size and content of the library depend on V-gene repertoire expression, which level is not constant. Potentially limited diversity of the IgM repertoire, the unknown history of the B-cell donor and tendency to achieve increased cross-reactivity are the main disadvantages of naive libraries.64
In synthetic libraries, B cells are not involved in antibodies construction. Antibody genes, in this library, are obtained by the polymerase chain reaction (PCR) to randomize the hypervariable regions of a generic set of human germline encoded variable region genes.65,66 These libraries were used for isolating a group of E and D antibodies.67
Libraries in which the complementary determining regions (CDRs) contain changes in the most diverse loop in composition and length of all CDRs, the VH-CDR3 region, are often called semi-synthetic libraries. VH-CDR3 is the most central to the antigen-binding site and it is randomized by PCR or oligonucleotide directed mutagenesis.65 In some cases synthetic repertoires were constructed by differentiation of three CDR loops in one V-gene segment68 or by randomization of light and heavy chain CDR3s.69 The advantage of these libraries is the overall diversity, local variability of repertoires, the ability to control and define the contents.70 Predominance of this library is composition which is not constrained by in vivo tolerance mechanisms.71 Moreover, the synthetic libraries provide a tool which can be useful when antigen deficiency occurs or antigen is toxic or unimmunogenicity occurs and immunization is not possible
Application
The search for new biotechnological components which could serve as pharmaceuticals or novel diagnostic agents is one of the major challenges of modern science. Phage display provides a valuable technique for obtaining large amounts of specific proteins, enzymes and peptides in a relatively short time. These molecules are used, inter alia, in many areas of modern medicine. Some of them will be discussed here.
Phage display in transfusion medicine
The significance of phage display in hematological applications is growing. To date, new antibody reagents for cell subpopulation discrimination, targeted therapeutics and reagents for in vivo imaging have been developed. Among the first obtained antibodies against red blood antigens used for hemagglutination assays were anti-ABO, anti-Rh and anti-Kell antibodies.72 A large amount of anti-Rh(D) antibodies is required for blood group typing and for the preparation of Rh(D)-immune globulin. These antibodies could be produced only in humans and the availability of alloimmunized donors is dwindling, thus the phage display technique seems to be the best method for obtaining large amounts of antibodies in a short time. Moreover, antibodies expressed as Fab on phage surface allow to generate highly sensitive (6 to 15 times more than IgG) self-replication typing reagents. It is noteworthy that the phage display technology had led to design an anti-Rh(D) and anti-HPA-Ia bispecific diabody that might be useful in the diagnosis and treatment of neonatal alloimmune thrombocytopenia. With this approach, the development of a sensitive, inexpensive, accurate and automative hemagglutination assay for HPA-Ia was possible.48 Furthermore, phage display allows to obtain antibody reagents against fetal red blood cells.73 Besides, in white blood cells (WBC) fraction, antibodies against dendritic cells74 , hairy cells75 , paraproteins,76 B and T cells77were obtained from scFv naive human library. Moreover, generating antibodies against a variety of cluster of differentiation (CD) antigens,78,79 AITP, GPIa, and GPIIIa platelets antigens,80 11-dehydro-thromboxane B2 (11D-TX)81 and clotting factors82 has been reported.
Interestingly, phage display library from individuals with chronic immune thrombocytopenic purpura (AITP) was applied for autoantibodies cloning. Platelet GPIIb/IIIa specific H44L4 antibody, originating from this library could be used to prevent cardiac ischemic complications in patients undergoing percutaneous coronary intervention since it was shown to inhibit ADP-induced platelet aggregation and to stay unbound (with) to vitronectin receptor αvβ3.49
Diagnostic and therapeutic agents for autoimmune diseases
Phage display gives the possibility to clone and characterize human immune libraries, thereby the study of autoimmune diseases has been facilitated and more information about diseases pathophysiology has been acquired. To date, many autoimmune disorders have been investigated by phage display. One of the most commonly reported diseases is the previously mentioned AITP, a hematologic disorder caused by anti-platelet autoantibodies. Screening of random heptapeptide phage-displayed library allowed to obtain a panel of affinity-selected phage clones specific to autoantibodies from AITP individuals.83 Moreover, phage display provides tools for studying antigen-autoantibody reactions in thrombotic thrombocytopenic purpura (TTP),84 acute anterior uveitis (AAU)85 and other autoimmune ocular inflammatory disorders.86 Phage display proved to be an important technique in investigating the Wegener’s granulomatosis,87 autoimmune thyroid disease88 and autoimmune diabetes89 as well as two blistering skin diseases, pemphigus vulgaris (PV)90 and pemphigus foliaceus (PF).91 Primary biliary cirrhosis (PBC) and autoimmune cholangitis (AIC) are serologic expressions of biliary ductular cells affecting autoimmune liver disease. Phage clones that react with anti–PDC-E2 (E2 subunit of the pyruvate dehydrogenase complex) were generated by biopanning and used to establish that PBC and AIC have a similar autoimmune targeting.92 Recently, it has been reported that sera from Crohn’s disease patients contain more anti-galectin-3 IgG autoantibodies than sera from healthy individuals and ulcerative colitis, primary biliary cirrhosis or autoimmune hepatitis patients. Phage display was applied to generate galectin-3 mimotopes that might be useful in regulating the immune responses in Crohn’s disease patients.93 It is noteworthy that characterization of an antibody (scFv) phage library from a patient with celiac disease has led to isolation of different scFv against the toxic antigen (A-gliadin) and dietary antigens (α/βlactoglobulin).94
Phage display could be useful in designing potential diagnostic and therapeutic agents for autoimmune disease. In myasthenia gravis (MG) antibodies against nicotinic acetylcholine receptors (AChR) induce loss of functional receptors at the neuromuscular junction. It was reported that anti-huAChR Fab was able to protect against AChR loss by antigenic modulation induced by MG serum antibodies.95 The PMTLPENYFSERPYH peptide was used to generate cyclic molecule capable of preventing the antigenic modulation of AChR by the anti-main immunogenic region antibody by in vivo inhibiting its binding to AChR.96
Several biotechnology companies use the phage display technology to produce human antibody therapeutics.97 Rheumatoid arthritis (RA) is considered to be a common, chronic, idiopathic autoimmune disease. Standard treatment for RA typically consists of traditional disease modifying anti-rheumatic drugs (DMARDs), corticosteroids, non-steroidal anti-inflammatory drugs or analgesics. It is often ineffective and allows further progression of the disease. However, the phage display technology has enabled the development of a new drug based on anti-TNFα antibodies.98 Multiple sclerosis (MS) is an inflammatory autoimmune-mediated disease of the nervous system and the second most common neurological disability affecting young and middle-aged adults. It is noteworthy that in June 2010 drug for MS based on anti-CD52 antibody was granted fast-track designation by the US Food and Drug Administration (FDA).99,100 Antibodies for autoimmune diseases generated by phage display are increasingly introduced to the market.
Phage display in neurological disorders
Intracellular antibody fragments (intrabodies) are considered potential therapeutics for neurological disorders due to their ability to selective recognition of abnormal intracellular proteins. However, there are several problems that have to be considered when intrabodies are used. This molecules could specifically bind the target antigen outside the cell but it could fail in the intracellular environment. Reducing conditions of the intracellular environment has influence on the stability of newly-synthesized intrabodies by affecting disulfide bond formation and antibody folding. It was shown that the intrinsic stability of the intrabody, rather than its affinity for the antigen, dictates its intracellular efficacy.101 This leads to the formation of non-functional, low solubility antibodies with reduced half-life. Another problem is internalization of DNA. Transfected recombinant DNA could be applied into cell in in vivo studies by the use of viral-based vector, lipofection102 or electroporation.103 However, these methods are not efficient enough and could significantly influence cell viability. It was reported that this problem could be overcome by fusing protein transduction domains (PTD) to antibodies.104
It was reported that humanized-camel phage display library was utilized in elaborating novel immunotherapeutic strategy for botulism by using a cell penetrating, humanized-single domain antibody that inhibits the botulinum neurotoxin.105
Furthermore, phage display libraries were used for selection of human anti-prion scFv and Fab antibodies, which inhibit in vivo the conformational change of normal prion protein (PrPC) in PrPSc. Accumulation of abnormal prion protein (PrPSc) is responsible for Creutzfeld–Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS), kuru disease and familial fatal insomnia.106
Other studies have reported the applicability of intrabodies in the treatment of Huntington’s and Parkinson diseases. Development of anti-α-synuclein scFv resulted in progress in research on synucleinopathies, including Parkinson disease.107,108 Phage display system allowed, inter alia, the investigation of the proteins that bind to 2-methylnorharman, a structural and functional analog of the parkinsonian-inducing toxin, MPP+.109 Furthermore, the human scFv specific for the 17 N-terminal residues of huntingtin, adjacent to the polyglutamine in HD exon 1, were selected by phage display library. It was shown that this intrabodies could prevent in situ expanded-repeat exon 1 analog aggregation in cellular models of this disease.110 In addition, a peptide motif that inhibits polyglutamine aggregation in vitro has been identified.111
Recently, the advantages of intrabodies in the inhibition study of β-amyloid formation have been described.112 Interestingly, EFRH epitope, corresponding to amino acids 3–6 within the human β-amyloid peptide, acts as a regulatory site in the β-amyloid fibrils formation and disaggregation process. The method of production of effective anti-aggregating β-amyloid antibodies, which could be used in vaccinations against Alzheimer disease, has been elaborated on the basis of phage display methodology.113,114
Many neurological disorders are characterized by the expression of different peptide motifs connected with vascular remodeling. This approach might be used for directed enzyme therapy in the central nervous system defects.115 Antibodies were also considered therapeutic and neuroprotective targeting agents to the brain vasculature and the brain parenchyma.116,117
Tissue homing and anti-angiogenic strategies
The vascular endothelium from different tissues or organs expresses unique receptors for trafficking. This organ’s specific molecular diversity has been characterized by in vivo panning with phage display libraries, allowing isolation of peptides homing to brain, kidney, lung, skin, adrenal gland, intestine, pancreas, retina, prostate, breast tissue and uterus.118,119 Indeed, targeting stem cells might prove to be an excellent tool for studying the embryonic stem cell, controlling proliferation and differentiation of neural stem cells and development of stem cell-based regenerative medicine.120 Phage display system for selection of skeletal stem cells could be useful in acceleration of bone and cartilage repair process.121
The in vivo selection method in which peptides that home to specific vascular beds are selected after intravenous administration of a phage-display random peptide library have been described.122 This approach has led to tissue-specific targeting of angiogenesis-related targeting of tumor blood vessels or peptides. Moreover, it has been used to guide the delivery of proapoptotic peptides, cytotoxic drugs, metalloprotease inhibitors and cytokines to obtain more efficient and less toxic therapeutics.122 Interestingly, peptides binding to the extracellular domain of LOX-1 receptor, which is upregulated in dysfunctional endothelial cells associated with hypertension and atherogenesis, have been identified.123 Other studies have reported that RGD-motif-containing peptide homing to angiogenic vasculature was linked to a proapoptotic peptide and successfully used in treatment collagen-induced arthritis in mice.124
Furthermore, in vivo phage display was applied to development of potential therapeutic peptide for the anti-obesity therapy. It was reported that targeting the proapoptotic CKGGRAKDC peptide to prohibitin in the adipose vasculature caused ablation of white fat with no apparent side effects125
Recently, phage displayed molecules have been identified as factors that may affect the angiogenesis. For instance, peptides that block the interaction between MMP and avb3 integrin126 and scFv that target receptor-bound vascular endothelial growth factor (VEGF)127 have been described. Both molecules exhibit potent anti-angiogenic and anti-tumor activity in vivo. Tissue-specific targeting can also be used for imaging techniques in cancer patients.128
Molecular imaging and tumor targeting
Phage displayed peptides could be successfully used as tumor targeting agents.,However, many of these anti-tumor agents show no activity in vivo. The scFv (MFE-23) molecule specific for CEA was the first phage displayed recombinant antibody used for tumor targeting.129
Obtaining molecular imaging agents that will be able to visualize pathogenic processes in vivo has been one of the main objects of interest in recent years.130-132 Although labeled antibodies (radiolabeled, biotin/streptavidin labeled) have been used in tumor targeting and imaging for decades, their use can cause adverse effects and lower the natural immunity.133 Nevertheless, phage displayed peptides seem to be better molecular imaging agents due to their small size, rapid blood clearance, lack of immunogenicity, tissue penetration and increased diffusion.134 By the use of the phage display technology numerous peptides for tumor targeting were isolated using human B-cell lymphoma,135 cervical,136 colon,137 gastric,138 breast,139 lung,140 glioblastoma,141 hepatic,142 prostate,143 neuroblastoma144 and thyroid145 carcinoma cell cultures. However, as it has been mentioned, about 80% of these peptides have not been reported to function in vivo, for example, peptides recognizing MDM2/p53,146 IL-11 receptor,147 prostate specific antigen (PSA)148 heat shock protein 90149 and growth factors.150
To date, much fewer phage display peptides that could be considered as molecular imaging and tumor targeting agents in vivo have been reported.151,152 One of them, the prostate carcinoma binding IAGLATPGWSHWLAL peptide151 has been used in vivo. The CPIEDRPMC (RPMrel) peptide was reported as binding agent to five colon cancer cell lines: HT29, CaCo-2, RKO, SW480, and DLD-1. It is encouraging that fluorescein-conjugated RPMrel peptide stained, ex vivo, tissue sections from colon adenocarcinoma and leaves normal colon, lungs, lung sarcoma, liver, liver sarcoma and stomach unstained. RPMrel conjugated to a mitochondrial toxin induced the death of HT29.130 Interestingly, peptide sequence the CLSYYPSYC phosphatidylserine-recognizing moiety was proposed as in vivo imaging molecule for apoptosis and the single dose of an anti-cancer drug indicated peptide homing to the tumor.153
Besides, phage displayed peptides have been used to inhibit tumor growth140,154,155 and most attention is focused on identifying progressive disease markers and therapeutic agents. The NPNWGPR heptapeptide labeled with 188-rhenium has been reported as human tumor melanin-binding molecule. In vivo administration of this peptide results in inhibition of tumor growth. The tumor uptake of this molecule was similar to other peptides but the level of kidney uptake was lower by about 85% at 3h and 24h after injection. Furthermore, this radiolabeled heptapeptide appears to have advantages for goal directed therapy due to its ability to bind only extracellular melanin, which increases the safety of the therapy.156 Peptide K237-(HTMYYHHYQHHL) isolated from phage-displayed library binds to kinase domain receptor. It was reported to inhibit the growth of solid tumors implanted beneath breasts by 70% and reduce the metastases to lungs by 53%.150 The LyP-1 peptide is another anti-tumor agent that inhibits tumor growth and has a proapoptotic/cytotoxic effect. This CGNKRTRGC sequence binds to tumor lymphatics. After injection it accumulates in breast cancer xenografts localizing preferentially in hypoxic areas. The treated tumors contain foci of apoptotic cells and a reduced number of lymphatic vessels.157 Indeed, phage display provides a tool for designing novel nanoparticle-based diagnostics and therapeutics. The nanoparticle system that provides effective accumulation of the particles in tumors has been described.158 It is based on the CERKA peptide which binds to PyMT tumors, MDA-MB-435 human breast cancer xenografts and it might be useful in effective tumor imaging, tumor homing and partially inhibiting of tumor growth by blood vessel occlusion. The inhibition of tumor growth may be enhanced by additional drug-carrying function of peptide-nanoparticle complex that could accumulate in tumor vessels and release the drug.
Screening of phage display libraries for binding to anti-carbohydrate antibodies has resulted in the generation of peptides that imitate carbohydrate ligands. These peptides might be applied to inhibition of carbohydrate-dependent tumor metastasis and design of anti-cancer vaccines.159
The phage display libraries could be used to isolate human monoclonal antibodies (mAbs) directed toward various antigens, including markers of tumor. Several phage-derived antibodies are being investigated in clinical trials. Recently, a first generation of chimeric rabbit/human Fab and IgG1 that bind receptor tyrosine kinase ROR1 has been described as target for monoclonal antibodies based therapy for chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL).160 Furthermore, the in vitro immunization method to induce antigen-specific immune responses in human peripheral blood mononuclear cells (PBMCs) by using multiple antigen peptide (MAP) instead of monovalent peptide has been reported. This method has resulted in obtaining four independent human monoclonal antibodies specific for tumor necrosis factor-α (TNF-α).161 It is noteworthy that the phage display technique allowed to obtain human antibody against psoriasin, the calcium-binding protein that is upregulated in many types of cancer and often associated with reduced patients survival. Obtained antibodies could be utilized in diagnostic and therapy of breast cancer, oral squamous carcinomas or bladder cancer.162 Also anti-human Cyr61 monoclonal antibodies named 093G9 have shown a promising therapeutic characteristic in breast cancer.163 In other studies antibody phage technology was used to generate good-quality F16 and P12 human recombinant antibodies, which are specific to the alternatively spliced domains A1 and D of the large isoform of tenascin-C. This glycoprotein is strongly overexpressed in adult tissue undergoing tissue remodeling.164 (131)I-labeled antibodies specific to A1 and D domain of tenascin-C have been successfully used for the treatment of glioma165 and of lymphoma.166
The generation of 3 human monoclonal antibodies (F8, B7 and D5), which recognize the same epitope of the extra-domain A (EDA) has been recently described. These antibodies specifically recognize tumor cells and were considered as versatile tumor targeting agents. The antibodies displayed a dissociation constant to the antigen in the low nanomolar range and exhibited an impressive preferential localization at the tumor site(as assessed by quantitative biodistribution studies following intravenous administration). The anti-EDA antibodies was suggest to be useful for the development of selective and potent anti-cancer biopharmaceuticals167. The novel library utilized in isolating human mAbs for various antigens, including the alternatively-spliced EDA domain of fibronectin—a marker of tumor angiogenesis has been recently described. 2H7 mAb, derived from this library, binds to a novel epitope on EDA, which does not overlap with the one recognized by the clinical-stage F8 antibody. These antibodies were used to construct chelating recombinant antibodies (CRAbs). Both anti-EDA CRAb (F8–10aa-2H7) and CRAb (F8–18aa-2H7) antibodies were able to accumulate selectively at the tumor site what may be useful in the development of improved anti-cancer biopharmaceuticals.
Conclusions
The phage display technology is a valuable tool in biomedical applications which offers rapid, efficient and relatively inexpensive methods for investigating protein-protein interactions, receptor binding sites, identifying epitopes, mimotopes, functional and accessible sites from antigens. Due to the high flexibility of this method, it has become the object of interest of many researchers allowing the application of techniques based on phage display to explore the mechanisms of disease, improve diagnostic methods and design potential therapeutic agents and vaccines. The application of the phage display technique in vaccine development and delivery will be discussed in the second part of this paper. Phage display and its application is the subject of many patents and the first therapeutic products obtained through this technology are available on the market. It is a reasonable expectation that the role of phage display in medicine, diagnostic and healthcare will continue to increase.
Acknowledgments
Financial support for this work by the European Union within the European Social Fund is gratefully acknowledged.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21703
References
- 1.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan G, Gershoni JM. A general insert label for peptide display on chimeric filamentous bacteriophages. Anal Biochem. 2012;420:68–72. doi: 10.1016/j.ab.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann S, Funke SA, Wiesehan K, Moedder S, Glück JM, Feuerstein S, et al. Competitively selected protein ligands pay their increase in specificity by a decrease in affinity. Mol Biosyst. 2010;6:126–33. doi: 10.1039/b910945e. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Li H, Wang X, Qi H, Miao X, Zhang T, et al. Phage-derived fully human antibody scFv fragment directed against human vascular endothelial growth factor receptor 2 blocked its interaction with VEGF. Biotechnol Prog. 2012;28:981–9. doi: 10.1002/btpr.1559. [DOI] [PubMed] [Google Scholar]
- 5.Schüller C, Wiebe JC, Pegel A, Kramer K, Skerra A, Hock B. A system for repertoire cloning and phage display of murine and leporid antibody fragments. J AOAC Int. 2010;93:66–79. [PubMed] [Google Scholar]
- 6.Cho W, Fowler JD, Furst EM. Targeted binding of the M13 bacteriophage to thiamethoxam organic crystals. Langmuir. 2012;28:6013–20. doi: 10.1021/la300522g. [DOI] [PubMed] [Google Scholar]
- 7.Hess GT, Cragnolini JJ, Popp MW, Allen MA, Dougan SK, Spooner E, et al. M13 bacteriophage display framework that allows sortase-mediated modification of surface-accessible phage proteins. Bioconjug Chem. 2012;23:1478–87. doi: 10.1021/bc300130z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagerlund A, Myrset AH, Kulseth MA. Construction and characterization of a 9-mer phage display pVIII-library with regulated peptide density. Appl Microbiol Biotechnol. 2008;80:925–36. doi: 10.1007/s00253-008-1630-z. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Arumuganathan K, Gill KS, Song Y. Flow sorting and microcloning of maize chromosome 1. Hereditas. 2004;141:55–60. doi: 10.1111/j.1601-5223.2004.01847.x. [DOI] [PubMed] [Google Scholar]
- 10.Garbe D, Thiel IV, Mootz HD. Protein trans-splicing on an M13 bacteriophage: towards directed evolution of a semisynthetic split intein by phage display. J Pept Sci. 2010;16:575–81. doi: 10.1002/psc.1243. [DOI] [PubMed] [Google Scholar]
- 11.Oh MY, Joo HY, Hur BU, Jeong YH, Cha SH. Enhancing phage display of antibody fragments using gIII-amber suppression. Gene. 2007;386:81–9. doi: 10.1016/j.gene.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Rondot S, Koch J, Breitling F, Dübel S. A helper phage to improve single-chain antibody presentation in phage display. Nat Biotechnol. 2001;19:75–8. doi: 10.1038/83567. [DOI] [PubMed] [Google Scholar]
- 13.Sidhu SS, Weiss GA, Wells JA. High copy display of large proteins on phage for functional selections. J Mol Biol. 2000;296:487–95. doi: 10.1006/jmbi.1999.3465. [DOI] [PubMed] [Google Scholar]
- 14.Løset GA, Bogen B, Sandlie I. Expanding the versatility of phage display I: efficient display of peptide-tags on protein VII of the filamentous phage. PLoS One. 2011;6:e14702. doi: 10.1371/journal.pone.0014702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Løset GA, Roos N, Bogen B, Sandlie I. Expanding the versatility of phage display II: improved affinity selection of folded domains on protein VII and IX of the filamentous phage. PLoS One. 2011;6:e17433. doi: 10.1371/journal.pone.0017433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuh G, Pisabarro MT, Li Y, Quan C, Lasky LA, Sidhu SS. Analysis of PDZ domain-ligand interactions using carboxyl-terminal phage display. J Biol Chem. 2000;275:21486–91. doi: 10.1074/jbc.275.28.21486. [DOI] [PubMed] [Google Scholar]
- 17.Speck J, Arndt KM, Müller KM. Efficient phage display of intracellularly folded proteins mediated by the TAT pathway. Protein Eng Des Sel. 2011;24:473–84. doi: 10.1093/protein/gzr001. [DOI] [PubMed] [Google Scholar]
- 18.Krumpe LR, Atkinson AJ, Smythers GW, Kandel A, Schumacher KM, McMahon JB, et al. T7 lytic phage-displayed peptide libraries exhibit less sequence bias than M13 filamentous phage-displayed peptide libraries. Proteomics. 2006;6:4210–22. doi: 10.1002/pmic.200500606. [DOI] [PubMed] [Google Scholar]
- 19.Kang HT, Bang WK, Yu YG. Identification and characterization of a novel angiostatin-binding protein by the display cloning method. J Biochem Mol Biol. 2004;37:159–66. doi: 10.5483/BMBRep.2004.37.2.159. [DOI] [PubMed] [Google Scholar]
- 20.Ishi K, Sugawara F. A facile method to screen inhibitors of protein-protein interactions including MDM2-p53 displayed on T7 phage. Biochem Pharmacol. 2008;75:1743–50. doi: 10.1016/j.bcp.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Tu C, Yu X, Zhang M, Zhang N, Zhao M, et al. Bacteriophage T4 nanoparticle capsid surface SOC and HOC bipartite display with enhanced classical swine fever virus immunogenicity: a powerful immunological approach. J Virol Methods. 2007;139:50–60. doi: 10.1016/j.jviromet.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Cicchini C, Ansuini H, Amicone L, Alonzi T, Nicosia A, Cortese R, et al. Searching for DNA-protein interactions by lambda phage display. J Mol Biol. 2002;322:697–706. doi: 10.1016/S0022-2836(02)00851-3. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Wang Y, Liu Z, Wang Z. Phage display and its application in vaccine design. Ann Microbiol. 2010;60:13–9. doi: 10.1007/s13213-009-0014-7. [DOI] [Google Scholar]
- 24.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–8. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 25.Mahgoub IO. Expression and Characterization of a Functional Single-Chain Variable Fragment (scFv) Protein Recognizing MCF7 Breast Cancer Cells in E. coli Cytoplasm. Biochem Genet. 2012;50:625–41. doi: 10.1007/s10528-012-9506-4. [DOI] [PubMed] [Google Scholar]
- 26.Sonoda H, Kumada Y, Katsuda T, Yamaji H. Effects of cytoplasmic and periplasmic chaperones on secretory production of single-chain Fv antibody in Escherichia coli. J Biosci Bioeng. 2011;111:465–70. doi: 10.1016/j.jbiosc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson F, Trilling M, Perez F, Ohlin M. A dimerized single-chain variable fragment system for the assessment of neutralizing activity of phage display-selected antibody fragments specific for cytomegalovirus. J Immunol Methods. 2012;376:69–78. doi: 10.1016/j.jim.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Wen K, Nölke G, Schillberg S, Wang Z, Zhang S, Wu C, et al. Improved fluoroquinolone detection in ELISA through engineering of a broad-specific single-chain variable fragment binding simultaneously to 20 fluoroquinolones. Anal Bioanal Chem. 2012;403:2771–83. doi: 10.1007/s00216-012-6062-z. [DOI] [PubMed] [Google Scholar]
- 29.Hughes DL, Stafford P, Hamaia SW, Harmer J, Schoolmeester A, Deckmyn H, et al. Platelet integrin alpha2 I-domain specific antibodies produced via domain specific DNA vaccination combined with variable gene phage display. Thromb Haemost. 2005;94:1318–26. [PubMed] [Google Scholar]
- 30.Shimazaki K, Lepin EJ, Wei B, Nagy AK, Coulam CP, Mareninov S, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367–77. doi: 10.1158/1078-0432.CCR-08-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki T, Onodera K, Kamijo S. Identification of novel short peptide inhibitors of soluble 37/48 kDa oligomers of amyloid β42. Biosci Biotechnol Biochem. 2011;75:1496–501. doi: 10.1271/bbb.110198. [DOI] [PubMed] [Google Scholar]
- 32.Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, Bouwman WH, van der Graaf WT, Oyen WJ, et al. Optimization of IGF-1R SPECT/CT imaging using (111)In-labeled F(ab')(2) and Fab fragments of the monoclonal antibody R1507. Mol Pharm. 2012 doi: 10.1021/mp300232n. In press. [DOI] [PubMed] [Google Scholar]
- 33.Markiv A, Beatson R, Burchell J, Durvasula RV, Kang AS. Expression of recombinant multi-coloured fluorescent antibodies in gor -/trxB- E. coli cytoplasm. BMC Biotechnol. 2011;11:117. doi: 10.1186/1472-6750-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang YM, Ning BT, Cao J, Shen HQ, Qian BQ. Construction and expression of single-chain antibody derived from a new clone of monoclonal antibody against human CD14 in CHO cells. Immunopharmacol Immunotoxicol. 2007;29:375–86. doi: 10.1080/08923970701619927. [DOI] [PubMed] [Google Scholar]
- 35.Neri D, Momo M, Prospero T, Winter G. High-affinity antigen binding by chelating recombinant antibodies (CRAbs) J Mol Biol. 1995;246:367–73. doi: 10.1006/jmbi.1994.0091. [DOI] [PubMed] [Google Scholar]
- 36.Wright MJ, Deonarain MP. Phage display of chelating recombinant antibody libraries. Mol Immunol. 2007;44:2860–9. doi: 10.1016/j.molimm.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Lomonosova AV, Laman AG, Fursova KK, Shepelyakovskaya AO, Vertiev YV, Brovko FA, et al. Generation of scFv phages specific to Staphylococcus enterotoxin C1 by panning on related antigens. MAbs. 2011;3:513–6. doi: 10.4161/mabs.3.6.18089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, Huang L, Sihlbom C, Burlingame A, Marks JD. Towards proteome-wide production of monoclonal antibody by phage display. J Mol Biol. 2002;315:1063–73. doi: 10.1006/jmbi.2001.5276. [DOI] [PubMed] [Google Scholar]
- 39.Garet E, Cabado AG, Vieites JM, González-Fernández A. Rapid isolation of single-chain antibodies by phage display technology directed against one of the most potent marine toxins: Palytoxin. Toxicon. 2010;55:1519–26. doi: 10.1016/j.toxicon.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Chang C, Takayanagi A, Yoshida T, Shimizu N. Screening of scFv-displaying phages recognizing distinct extracellular domains of EGF receptor by target-guided proximity labeling method. J Immunol Methods. 2011;372:127–36. doi: 10.1016/j.jim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Noronha EJ, Wang X, Ferrone S. Isolation of human tumor-associated cell surface antigen-binding scFvs. Methods Mol Biol. 2002;178:227–33. doi: 10.1385/1-59259-240-6:227. [DOI] [PubMed] [Google Scholar]
- 42.Chakravarthy B, Ménard M, Brown L, Atkinson T, Whitfield J. Identification of protein kinase C inhibitory activity associated with a polypeptide isolated from a phage display system with homology to PCM-1, the pericentriolar material-1 protein. Biochem Biophys Res Commun. 2012;424:147–51. doi: 10.1016/j.bbrc.2012.06.093. [DOI] [PubMed] [Google Scholar]
- 43.Hallborn J, Carlsson R. Automated screening procedure for high-throughput generation of antibody fragments. Biotechniques. 2002;(Suppl):30–7. [PubMed] [Google Scholar]
- 44.Rubenwolf S, Niewöhner J, Meyer E, Petit-Frère C, Rudert F, Hoffmann PR, et al. Functional proteomics using chromophore-assisted laser inactivation. Proteomics. 2002;2:241–6. doi: 10.1002/1615-9861(200203)2:3<241::AID-PROT241>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Konthur Z. High-throughput applications of phage display in proteomic analyses. Targets. 2003;2:261–70. doi: 10.1016/S1477-3627(03)02383-3. [DOI] [Google Scholar]
- 46.Almagro JC, Raghunathan G, Beil E, Janecki DJ, Chen Q, Dinh T, et al. Characterization of a high-affinity human antibody with a disulfide bridge in the third complementarity-determining region of the heavy chain. J Mol Recognit. 2012;25:125–35. doi: 10.1002/jmr.1168. [DOI] [PubMed] [Google Scholar]
- 47.Hughes-Jones NC, Gorick BD, Bye JM, Finnern R, Scott ML, Voak D, et al. Characterization of human blood group scFv antibodies derived from a V gene phage-display library. Br J Haematol. 1994;88:180–6. doi: 10.1111/j.1365-2141.1994.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 48.Watkins NA, Armour KL, Smethurst PA, Metcalfe P, Scott ML, Hughes DL, et al. Rapid phenotyping of HPA-1a using either diabody-based hemagglutination or recombinant IgG1-based assays. Transfusion. 1999;39:781–9. doi: 10.1046/j.1537-2995.1999.39070781.x. [DOI] [PubMed] [Google Scholar]
- 49.Siegel D, Loftu JC, McMillan R. Characterization of an ITP patient-derived anti-alpha(2b)beta(3) monoclonal antibody that inhibits platelet aggregation. Blood. 2003;102:87A-A. [Google Scholar]
- 50.Dahan R, Tabul M, Chou YK, Meza-Romero R, Andrew S, Ferro AJ, et al. TCR-like antibodies distinguish conformational and functional differences in two- versus four-domain auto reactive MHC class II-peptide complexes. Eur J Immunol. 2011;41:1465–79. doi: 10.1002/eji.201041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dogan I, Dorgham K, Chang HC, Parizot C, Lemaître F, Ferradini L, et al. Phage-displayed libraries of peptide/major histocompatibility complexes. Eur J Immunol. 2004;34:598–607. doi: 10.1002/eji.200324721. [DOI] [PubMed] [Google Scholar]
- 52.Finlay WJ, Bloom L, Cunningham O. Optimized generation of high-affinity, high-specificity single-chain Fv antibodies from multiantigen immunized chickens. Methods Mol Biol. 2011;681:383–401. doi: 10.1007/978-1-60761-913-0_21. [DOI] [PubMed] [Google Scholar]
- 53.Hofer T, Tangkeangsirisin W, Kennedy MG, Mage RG, Raiker SJ, Venkatesh K, et al. Chimeric rabbit/human Fab and IgG specific for members of the Nogo-66 receptor family selected for species cross-reactivity with an improved phage display vector. J Immunol Methods. 2007;318:75–87. doi: 10.1016/j.jim.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charlton K, Harris WJ, Porter AJ. The isolation of super-sensitive anti-hapten antibodies from combinatorial antibody libraries derived from sheep. Biosens Bioelectron. 2001;16:639–46. doi: 10.1016/S0956-5663(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 55.Chahboun S, Hust M, Liu Y, Pelat T, Miethe S, Helmsing S, et al. Isolation of a nanomolar scFv inhibiting the endopeptidase activity of botulinum toxin A, by single-round panning of an immune phage-displayed library of macaque origin. BMC Biotechnol. 2011;11:113. doi: 10.1186/1472-6750-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59:1236–43. [PubMed] [Google Scholar]
- 57.Tabares-da Rosa S, Rossotti M, Carleiza C, Carrión F, Pritsch O, Ahn KC, et al. Competitive selection from single domain antibody libraries allows isolation of high-affinity antihapten antibodies that are not favored in the llama immune response. Anal Chem. 2011;83:7213–20. doi: 10.1021/ac201824z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu YY, Li ZG, Yang YW, Deng W, Duan W, Miao Q, et al. Isolation of single chain variable fragments against six esters of pyrethrins by subtractive phage display. Biosci Biotechnol Biochem. 2009;73:1541–9. doi: 10.1271/bbb.90043. [DOI] [PubMed] [Google Scholar]
- 59.Moon SA, Ki MK, Lee S, Hong ML, Kim M, Kim S, et al. Antibodies against non-immunizing antigens derived from a large immune scFv library. Mol Cells. 2011;31:509–13. doi: 10.1007/s10059-011-2268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–14. doi: 10.1038/nbt0396-309. [DOI] [PubMed] [Google Scholar]
- 61.Griffiths AD, Malmqvist M, Marks JD, Bye JM, Embleton MJ, McCafferty J, et al. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993;12:725–34. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babel I, Barderas R, Peláez-García A, Casal JI. Antibodies on demand: a fast method for the production of human scFvs with minimal amounts of antigen. BMC Biotechnol. 2011;11:61. doi: 10.1186/1472-6750-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoyanova V, Aleksandrov R, Lukarska M, Duhalov D, Atanasov V, Petrova S. Recognition of Vipera ammodytes meridionalis neurotoxin vipoxin and its components using phage-displayed scFv and polyclonal antivenom sera. Toxicon. 2012;60:802–9. doi: 10.1016/j.toxicon.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Azzazy HM, Highsmith WE., Jr. Phage display technology: clinical applications and recent innovations. Clin Biochem. 2002;35:425–45. doi: 10.1016/S0009-9120(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 65.Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 66.Krebs B, Rauchenberger R, Reiffert S, Rothe C, Tesar M, Thomassen E, et al. High-throughput generation and engineering of recombinant human antibodies. J Immunol Methods. 2001;254:67–84. doi: 10.1016/S0022-1759(01)00398-2. [DOI] [PubMed] [Google Scholar]
- 67.Hughes-Jones NC, Bye JM, Gorick BD, Marks JD, Ouwehand WH. Synthesis of Rh Fv phage-antibodies using VH and VL germline genes. Br J Haematol. 1999;105:811–6. doi: 10.1046/j.1365-2141.1999.01412.x. [DOI] [PubMed] [Google Scholar]
- 68.van den Beucken T, van Neer N, Sablon E, Desmet J, Celis L, Hoogenboom HR, et al. Building novel binding ligands to B7.1 and B7.2 based on human antibody single variable light chain domains. J Mol Biol. 2001;310:591–601. doi: 10.1006/jmbi.2001.4703. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Zhu Z, Feng Y, Dimitrov DS. A large human domain antibody library combining heavy and light chain CDR3 diversity. Mol Immunol. 2010;47:912–21. doi: 10.1016/j.molimm.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markovic-Plese S, Hemmer B, Zhao Y, Simon R, Pinilla C, Martin R. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: implications for the initiation of autoimmune response in multiple sclerosis. J Neuroimmunol. 2005;169:31–8. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Chan CE, Chan AH, Lim AP, Hanson BJ. Comparison of the efficiency of antibody selection from semi-synthetic scFv and non-immune Fab phage display libraries against protein targets for rapid development of diagnostic immunoassays. J Immunol Methods. 2011;373:79–88. doi: 10.1016/j.jim.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marks JD, Ouwehand WH, Bye JM, Finnern R, Gorick BD, Voak D, et al. Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology (N Y) 1993;11:1145–9. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 73.Huie MA, Cheung MC, Muench MO, Becerril B, Kan YW, Marks JD. Antibodies to human fetal erythroid cells from a nonimmune phage antibody library. Proc Natl Acad Sci U S A. 2001;98:2682–7. doi: 10.1073/pnas.051631798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitting J, Killian D, Junghanss C, Willenbrock S, Murua Escobar H, Lange S, et al. Generation of recombinant antibody fragments that target canine dendritic cells by phage display technology. Vet Comp Oncol. 2011;9:183–95. doi: 10.1111/j.1476-5829.2010.00246.x. [DOI] [PubMed] [Google Scholar]
- 75.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Nuallain B, Allen A, Ataman D, Weiss DT, Solomon A, Wall JS. Phage display and peptide mapping of an immunoglobulin light chain fibril-related conformational epitope. Biochemistry. 2007;46:13049–58. doi: 10.1021/bi701255m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maeda M, Ito Y, Hatanaka T, Hashiguchi S, Torikai M, Nakashima T, et al. Regulation of T cell response by blocking the ICOS signal with the B7RP-1-specific small antibody fragment isolated from human antibody phage library. MAbs. 2009;1:453–61. doi: 10.4161/mabs.1.5.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barth S, Huhn M, Matthey B, Tawadros S, Schnell R, Schinköthe T, et al. Ki-4(scFv)-ETA’, a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood. 2000;95:3909–14. [PubMed] [Google Scholar]
- 79.Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–103. [PubMed] [Google Scholar]
- 80.Chu XX, Hou M, Peng J, Zhu YY, Ji XB, Wang L, et al. Effects of IgG and its F(ab’)2 fragments of some patients with idiopathic thrombocytopenic purpura on platelet aggregation. Eur J Haematol. 2006;76:153–9. doi: 10.1111/j.1600-0609.2005.00557.x. [DOI] [PubMed] [Google Scholar]
- 81.Tsuruta LR, Tomioka Y, Hishinuma T, Kato Y, Itoh K, Suzuki T, et al. Characterization of 11-dehydro-thromboxane B2 recombinant antibody obtained by phage display technology. Prostaglandins Leukot Essent Fatty Acids. 2003;68:273–84. doi: 10.1016/S0952-3278(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 82.Suggett S, Kirchhofer D, Hass P, Lipari T, Moran P, Nagel M, et al. Use of phage display for the generation of human antibodies that neutralize factor IXa function. Blood Coagul Fibrinolysis. 2000;11:27–42. [PubMed] [Google Scholar]
- 83.Gevorkian G, Manoutcharian K, Almagro JC, Govezensky T, Dominguez V. Identification of autoimmune thrombocytopenic purpura-related epitopes using phage-display peptide library. Clin Immunol Immunopathol. 1998;86:305–9. doi: 10.1006/clin.1997.4502. [DOI] [PubMed] [Google Scholar]
- 84.Luken BM, Kaijen PH, Turenhout EA, Kremer Hovinga JA, van Mourik JA, Fijnheer R, et al. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:2355–64. doi: 10.1111/j.1538-7836.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y, Caberoy NB, Alvarado G, Davis JL, Feuer WJ, Li W. Identification of Hnrph3 as an autoantigen for acute anterior uveitis. Clin Immunol. 2011;138:60–6. doi: 10.1016/j.clim.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Davis JL, Li W. Identification of tribbles homolog 2 as an autoantigen in autoimmune uveitis by phage display. Mol Immunol. 2005;42:1275–81. doi: 10.1016/j.molimm.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 87.Finnern R, Pedrollo E, Fisch I, Wieslander J, Marks JD, Lockwood CM, et al. Human autoimmune anti-proteinase 3 scFv from a phage display library. Clin Exp Immunol. 1997;107:269–81. doi: 10.1111/j.1365-2249.1997.254-ce1127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Latrofa F, Pichurin P, Guo J, Rapoport B, McLachlan SM. Thyroglobulin-thyroperoxidase autoantibodies are polyreactive, not bispecific: analysis using human monoclonal autoantibodies. J Clin Endocrinol Metab. 2003;88:371–8. doi: 10.1210/jc.2002-021073. [DOI] [PubMed] [Google Scholar]
- 89.Farilla L, Tiberti C, Luzzago A, Yu L, Eisenbarth GS, Cortese R, et al. Application of phage display peptide library to autoimmune diabetes: identification of IA-2/ICA512bdc dominant autoantigenic epitopes. Eur J Immunol. 2002;32:1420–7. doi: 10.1002/1521-4141(200205)32:5<1420::AID-IMMU1420>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 90.Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–99. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–48. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ikuno N, Scealy M, Davies JM, Whittingham SF, Omagari K, Mackay IR, et al. A comparative study of antibody expressions in primary biliary cirrhosis and autoimmune cholangitis using phage display. Hepatology. 2001;34:478–86. doi: 10.1053/jhep.2001.27013. [DOI] [PubMed] [Google Scholar]
- 93.Jensen-Jarolim E, Neumann C, Oberhuber G, Gscheidlinger R, Neuchrist C, Reinisch W, et al. Anti-Galectin-3 IgG autoantibodies in patients with Crohn’s disease characterized by means of phage display peptide libraries. J Clin Immunol. 2001;21:348–56. doi: 10.1023/A:1012240719801. [DOI] [PubMed] [Google Scholar]
- 94.Sblattero D, Not T, Marzari R, Bradbury A. Phage Antibody Library From Celiac Disease Patient. J Pediatr Gastroenterol Nutr. 1999;28:568. doi: 10.1097/00005176-199905000-00117. [DOI] [Google Scholar]
- 95.Graus YF, de Baets MH, van Breda Vriesman PJ, Burton DR. Anti-acetylcholine receptor Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. Immunol Lett. 1997;57:59–62. doi: 10.1016/S0165-2478(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 96.Venkatesh N, Im SH, Balass M, Fuchs S, Katchalski-Katzir E. Prevention of passively transferred experimental autoimmune myasthenia gravis by a phage library-derived cyclic peptide. Proc Natl Acad Sci U S A. 2000;97:761–6. doi: 10.1073/pnas.97.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osbourn JK, Jermutus L, Duncan A. Current methods for the generation of human antibodies for the treatment of autoimmune diseases. Drug Discov Today. 2003;8:845–51. doi: 10.1016/S1359-6446(03)02803-4. [DOI] [PubMed] [Google Scholar]
- 98.Rau R. Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials. Ann Rheum Dis. 2002;61(Suppl 2):ii70–3. doi: 10.1136/ard.61.suppl_2.ii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klotz L, Meuth SG, Wiendl H. Immune mechanisms of new therapeutic strategies in multiple sclerosis-A focus on alemtuzumab. Clin Immunol. 2012;142:25–30. doi: 10.1016/j.clim.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 101.Rajpal A, Turi TG. Intracellular stability of anti-caspase-3 intrabodies determines efficacy in retargeting the antigen. J Biol Chem. 2001;276:33139–46. doi: 10.1074/jbc.M101332200. [DOI] [PubMed] [Google Scholar]
- 102.Tsai MK, Lin RH, Hsu BR, Lee PH. Lipofection of pcDNA3-CTLA4-Ig into B cells promotes allogeneic hyporesponsiveness. Transplant Proc. 2003;35:548–9. doi: 10.1016/S0041-1345(02)03768-5. [DOI] [PubMed] [Google Scholar]
- 103.Jazi MH, Dabaghian M, Tebianian M, Gharagozlou MJ, Ebrahimi SM. In vivo electroporation enhances immunogenicity and protection against influenza A virus challenge of an M2e-HSP70c DNA vaccine. Virus Res. 2012;167:219–25. doi: 10.1016/j.virusres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Langedijk JP, Olijhoek T, Schut D, Autar R, Meloen RH. New transport peptides broaden the horizon of applications for peptidic pharmaceuticals. Mol Divers. 2004;8:101–11. doi: 10.1023/B:MODI.0000025653.26130.ce. [DOI] [PubMed] [Google Scholar]
- 105.Thanongsaksrikul J, Chaicumpa W. Botulinum neurotoxins and botulism: a novel therapeutic approach. Toxins (Basel) 2011;3:469–88. doi: 10.3390/toxins3050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skrlj N, Serbec VC, Dolinar M. Single-chain Fv antibody fragments retain binding properties of the monoclonal antibody raised against peptide P1 of the human prion protein. Appl Biochem Biotechnol. 2010;160:1808–21. doi: 10.1007/s12010-009-8699-4. [DOI] [PubMed] [Google Scholar]
- 107.Emadi S, Liu R, Yuan B, Schulz P, McAllister C, Lyubchenko Y, et al. Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004;43:2871–8. doi: 10.1021/bi036281f. [DOI] [PubMed] [Google Scholar]
- 108.Zhou C, Emadi S, Sierks MR, Messer A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed alpha-synuclein. Mol Ther. 2004;10:1023–31. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 109.Gearhart DA, Toole PF, Warren Beach J. Identification of brain proteins that interact with 2-methylnorharman. An analog of the parkinsonian-inducing toxin, MPP+ Neurosci Res. 2002;44:255–65. doi: 10.1016/S0168-0102(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 110.Lecerf JM, Shirley TL, Zhu Q, Kazantsev A, Amersdorfer P, Housman DE, et al. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington’s disease. Proc Natl Acad Sci U S A. 2001;98:4764–9. doi: 10.1073/pnas.071058398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kenan DJ, Strittmatter WJ, Burke JR. Phage display screening for peptides that inhibit polyglutamine aggregation. Methods Enzymol. 2006;413:253–73. doi: 10.1016/S0076-6879(06)13014-1. [DOI] [PubMed] [Google Scholar]
- 112.Solomon B, Frenkel D. Generation and brain delivery of anti-aggregating antibodies against beta-amyloid plaques using phage display technology. J Neural Transm Suppl. 2002;(62):321–5. doi: 10.1007/978-3-7091-6139-5_30. [DOI] [PubMed] [Google Scholar]
- 113.Solomon B. Active immunization against Alzheimer’s beta-amyloid peptide using phage display technology. Vaccine. 2007;25:3053–6. doi: 10.1016/j.vaccine.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 114.Frenkel D, Katz O, Solomon B. Immunization against Alzheimer’s beta -amyloid plaques via EFRH phage administration. Proc Natl Acad Sci U S A. 2000;97:11455–9. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–8. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia H, Anderson B, Mao Q, Davidson BL. Recombinant human adenovirus: targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J Virol. 2000;74:11359–66. doi: 10.1128/JVI.74.23.11359-11366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther. 2000;292:1048–52. [PubMed] [Google Scholar]
- 118.Essler M, Ruoslahti E. Molecular specialization of breast vasculature: a breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci U S A. 2002;99:2252–7. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci U S A. 2002;99:1527–31. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu S, Xu X, Zhao W, Wu W, Yuan H, Shen H, et al. Targeting of embryonic stem cells by peptide-conjugated quantum dots. PLoS One. 2010;5:e12075. doi: 10.1371/journal.pone.0012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gothard D, Tare RS, Mitchell PD, Dawson JI, Oreffo RO. In search of the skeletal stem cell: isolation and separation strategies at the macro/micro scale for skeletal regeneration. Lab Chip. 2011;11:1206–20. doi: 10.1039/c0lc00575d. [DOI] [PubMed] [Google Scholar]
- 122.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–7. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 123.White SJ, Nicklin SA, Sawamura T, Baker AH. Identification of peptides that target the endothelial cell-specific LOX-1 receptor. Hypertension. 2001;37:449–55. doi: 10.1161/01.HYP.37.2.449. [DOI] [PubMed] [Google Scholar]
- 124.Gerlag DM, Borges E, Tak PP, Ellerby HM, Bredesen DE, Pasqualini R, et al. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Res. 2001;3:357–61. doi: 10.1186/ar327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 126.Boger DL, Goldberg J, Silletti S, Kessler T, Cheresh DA. Identification of a novel class of small-molecule antiangiogenic agents through the screening of combinatorial libraries which function by inhibiting the binding and localization of proteinase MMP2 to integrin alpha(V)beta(3) J Am Chem Soc. 2001;123:1280–8. doi: 10.1021/ja003579+. [DOI] [PubMed] [Google Scholar]
- 127.Cooke SP, Boxer GM, Lawrence L, Pedley RB, Spencer DI, Begent RH, et al. A strategy for antitumor vascular therapy by targeting the vascular endothelial growth factor: receptor complex. Cancer Res. 2001;61:3653–9. [PubMed] [Google Scholar]
- 128.Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL, et al. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–36. [PubMed] [Google Scholar]
- 129.Chester KA, Begent RH, Robson L, Keep P, Pedley RB, Boden JA, et al. Phage libraries for generation of clinically useful antibodies. Lancet. 1994;343:455–6. doi: 10.1016/S0140-6736(94)92695-6. [DOI] [PubMed] [Google Scholar]
- 130.Kelly KA, Jones DA. Isolation of a colon tumor specific binding peptide using phage display selection. Neoplasia. 2003;5:437–44. doi: 10.1016/s1476-5586(03)80046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer. 2008;123:1979–90. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edwards WB, Xu B, Akers W, Cheney PP, Liang K, Rogers BE, et al. Agonist-antagonist dilemma in molecular imaging: evaluation of a monomolecular multimodal imaging agent for the somatostatin receptor. Bioconjug Chem. 2008;19:192–200. doi: 10.1021/bc700291m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Adachi S, Yoshimura T, Matsuoka T, Okada K, Yasuda T, Kamei K. [Appearance of skin and nail toxicity in patients with breast cancer who underwent trastuzumab-containing chemotherapy] Gan To Kagaku Ryoho. 2011;38:1453–6. [PubMed] [Google Scholar]
- 134.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 135.McGuire MJ, Samli KN, Chang YC, Brown KC. Novel ligands for cancer diagnosis: selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp Hematol. 2006;34:443–52. doi: 10.1016/j.exphem.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 136.Robinson P, Stuber D, Deryckère F, Tedbury P, Lagrange M, Orfanoudakis G. Identification using phage display of peptides promoting targeting and internalization into HPV-transformed cell lines. J Mol Recognit. 2005;18:175–82. doi: 10.1002/jmr.723. [DOI] [PubMed] [Google Scholar]
- 137.Rasmussen UB, Schreiber V, Schultz H, Mischler F, Schughart K. Tumor cell-targeting by phage-displayed peptides. Cancer Gene Ther. 2002;9:606–12. doi: 10.1038/sj.cgt.7700476. [DOI] [PubMed] [Google Scholar]
- 138.Liang S, Lin T, Ding J, Pan Y, Dang D, Guo C, et al. Screening and identification of vascular-endothelial-cell-specific binding peptide in gastric cancer. J Mol Med (Berl) 2006;84:764–73. doi: 10.1007/s00109-006-0064-2. [DOI] [PubMed] [Google Scholar]
- 139.Askoxylakis V, Zitzmann S, Mier W, Graham K, Krämer S, von Wegner F, et al. Preclinical evaluation of the breast cancer cell-binding peptide, p160. Clin Cancer Res. 2005;11:6705–12. doi: 10.1158/1078-0432.CCR-05-0432. [DOI] [PubMed] [Google Scholar]
- 140.Chang DK, Lin CT, Wu CH, Wu HC. A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS One. 2009;4:e4171. doi: 10.1371/journal.pone.0004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu C, Lo SL, Boulaire J, Hong ML, Beh HM, Leung DS, et al. A peptide-based carrier for intracellular delivery of proteins into malignant glial cells in vitro. J Control Release. 2008;130:140–5. doi: 10.1016/j.jconrel.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 142.Du B, Qian M, Zhou Z, Wang P, Wang L, Zhang X, et al. In vitro panning of a targeting peptide to hepatocarcinoma from a phage display peptide library. Biochem Biophys Res Commun. 2006;342:956–62. doi: 10.1016/j.bbrc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 143.Zitzmann S, Mier W, Schad A, Kinscherf R, Askoxylakis V, Krämer S, et al. A new prostate carcinoma binding peptide (DUP-1) for tumor imaging and therapy. Clin Cancer Res. 2005;11:139–46. [PubMed] [Google Scholar]
- 144.Askoxylakis V, Mier W, Zitzmann S, Ehemann V, Zhang J, Krämer S, et al. Characterization and development of a peptide (p160) with affinity for neuroblastoma cells. J Nucl Med. 2006;47:981–8. [PubMed] [Google Scholar]
- 145.Zitzmann S, Krämer S, Mier W, Hebling U, Altmann A, Rother A, et al. Identification and evaluation of a new tumor cell-binding peptide, FROP-1. J Nucl Med. 2007;48:965–72. doi: 10.2967/jnumed.106.036699. [DOI] [PubMed] [Google Scholar]
- 146.Pazgier M, Liu M, Zou G, Yuan W, Li C, Li C, et al. Structural basis for high-affinity peptide inhibition of p53 interactions with MDM2 and MDMX. Proc Natl Acad Sci U S A. 2009;106:4665–70. doi: 10.1073/pnas.0900947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zurita AJ, Troncoso P, Cardó-Vila M, Logothetis CJ, Pasqualini R, Arap W. Combinatorial screenings in patients: the interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Res. 2004;64:435–9. doi: 10.1158/0008-5472.CAN-03-2675. [DOI] [PubMed] [Google Scholar]
- 148.Pakkala M, Jylhäsalmi A, Wu P, Leinonen J, Stenman UH, Santa H, et al. Conformational and biochemical analysis of the cyclic peptides which modulate serine protease activity. J Pept Sci. 2004;10:439–47. doi: 10.1002/psc.557. [DOI] [PubMed] [Google Scholar]
- 149.Kim Y, Lillo AM, Steiniger SC, Liu Y, Ballatore C, Anichini A, et al. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–44. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 150.Hetian L, Ping A, Shumei S, Xiaoying L, Luowen H, Jian W, et al. A novel peptide isolated from a phage display library inhibits tumor growth and metastasis by blocking the binding of vascular endothelial growth factor to its kinase domain receptor. J Biol Chem. 2002;277:43137–42. doi: 10.1074/jbc.M203103200. [DOI] [PubMed] [Google Scholar]
- 151.Newton JR, Kelly KA, Mahmood U, Weissleder R, Deutscher SL. In vivo selection of phage for the optical imaging of PC-3 human prostate carcinoma in mice. Neoplasia. 2006;8:772–80. doi: 10.1593/neo.06331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Landon LA, Peletskaya EN, Glinsky VV, Karasseva N, Quinn TP, Deutscher SL. Combinatorial evolution of high-affinity peptides that bind to the Thomsen-Friedenreich carcinoma antigen. J Protein Chem. 2003;22:193–204. doi: 10.1023/A:1023483232397. [DOI] [PubMed] [Google Scholar]
- 153.Thapa N, Kim S, So IS, Lee BH, Kwon IC, Choi K, et al. Discovery of a phosphatidylserine-recognizing peptide and its utility in molecular imaging of tumour apoptosis. J Cell Mol Med. 2008;12(5A):1649–60. doi: 10.1111/j.1582-4934.2008.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zang L, Shi L, Guo J, Pan Q, Wu W, Pan X, et al. Screening and identification of a peptide specifically targeted to NCI-H1299 from a phage display peptide library. Cancer Lett. 2009;281:64–70. doi: 10.1016/j.canlet.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 155.Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974–9. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 156.Howell RC, Revskaya E, Pazo V, Nosanchuk JD, Casadevall A, Dadachova E. Phage display library derived peptides that bind to human tumor melanin as potential vehicles for targeted radionuclide therapy of metastatic melanoma. Bioconjug Chem. 2007;18:1739–48. doi: 10.1021/bc060330u. [DOI] [PubMed] [Google Scholar]
- 157.Laakkonen P, Akerman ME, Biliran H, Yang M, Ferrer F, Karpanen T, et al. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc Natl Acad Sci U S A. 2004;101:9381–6. doi: 10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007;104:932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fukuda MN, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, Ruoslahti E, et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Res. 2000;60:450–6. [PubMed] [Google Scholar]
- 160.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS One. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fujiki T, Tsuji A, Matsumoto SE, Yamashita M, Teruya K, Shirahata S, et al. Generation of a human anti-tumor necrosis factor-α monoclonal antibody by in vitro immunization with a multiple antigen peptide. Biosci Biotechnol Biochem. 2010;74:1836–40. doi: 10.1271/bbb.100243. [DOI] [PubMed] [Google Scholar]
- 162.Cyranka-Czaja A, Wulhfard S, Neri D, Otlewski J. Selection and characterization of human antibody fragments specific for psoriasin - a cancer associated protein. Biochem Biophys Res Commun. 2012;419:250–5. doi: 10.1016/j.bbrc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 163.Lin J, Huo R, Wang L, Zhou Z, Sun Y, Shen B, et al. A novel anti-Cyr61 antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2012;61:677–87. doi: 10.1007/s00262-011-1135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12:3200–8. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 165.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, et al. Phase II trial of murine (131)I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol. 2002;20:1389–97. doi: 10.1200/JCO.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 166.Rizzieri DA, Akabani G, Zalutsky MR, Coleman RE, Metzler SD, Bowsher JE, et al. Phase 1 trial study of 131I-labeled chimeric 81C6 monoclonal antibody for the treatment of patients with non-Hodgkin lymphoma. Blood. 2004;104:642–8. doi: 10.1182/blood-2003-12-4264. [DOI] [PubMed] [Google Scholar]
- 167.Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122:2405–13. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]