Abstract
Objective: Vaccination provides the most effective protection against maternal, fetal and neonatal complications of influenza infection. This study aimed to determine the uptake rate of influenza vaccination including 2009 pandemic H1N1 influenza and seasonal influenza vaccination and the reasons for acceptance or rejection among pregnant women.
Result: Mean age of the 416 pregnant women enrolled in this study was 27.06 ± 5.27 y. Only 25 (6%) of 397 women had history of vaccination. Of 383 (92.06%) pregnant women who had rejected vaccination, 116 (30.28%) declared that they lacked information about influenza vaccination and 44 (11.48%) felt that they did not need vaccination. Concerns about the safety of influenza vaccination were reported by only 2 women (0.52%). Of the 25 (6%) pregnant women who were vaccinated against influenza, 15 (60%) accepted because of advice they received from persons other than physicians, 5 (20%) believed that influenza vaccination is necessary for everyone, and 3 (12%) accepted because of a history of frequent influenza virus infections in previous years.
Method: This questionnaire based study was conducted at obstetrics and maternity hospitals affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. Pregnant women were interviewed individually and privately. SPSS was used for data analysis.
Conclusion: Most of the unvaccinated and vaccinated pregnant women lacked sufficient knowledge about influenza. Education of pregnant women about influenza vaccination and encouragement from physicians may have a remarkable effect on turning poor compliance into high flu vaccination uptake among pregnant women.
Keywords: pregnant women, 2009 Pandemic H1N1 influenza, A(H1N1) pdm09 influenza, seasonal influenza, vaccination, physician
Introduction
On 11 June 2009, in response to the global spread of a new strain of H1N1 influenza, the World Health Organization (WHO) declared the outbreak to be an influenza pandemic, the first since 1968.1 The novel “H1N1 swine flu” was first identified in California in late April 2009.1 By August 2010, more than 214 countries and overseas territories or communities had reported laboratory-confirmed A(H1N1) pdm09 influenza, which had resulted in more than 18 449 deaths.1,2 On 10 August 2010 WHO announced that the H1N1 Influenza virus had moved into the post-pandemic period, while noting that localized outbreaks of various magnitudes are likely to continue.3 A look at the influenza virus pandemics in the 20th century (H1N1 in 1918, H2N2 in 1957 and H3N2 in 1968)4 and epidemics of seasonal influenza shows4,5 that pregnant women have been more vulnerable to death due to influenza and its complications than the general population. During the recent 2009 H1N1 pandemic it was shown that pregnant women and their newborns were among the groups at risk for higher rates of hospitalization, intensive care unit (ICU) admission and morbidity or mortality compared with the general population.5-9 This risk was 13-fold greater (9.6 to 18.3) for pregnant women at 20 or more weeks of gestation.10 Diminished lung capacity and altered immunity place pregnant women (especially in the third trimester) at increased risk of severe and deadly complications from variant H1N1.10-12 The complications of influenza during pregnancy include death, sepsis, pneumonia, adult respiratory distress syndrome, intrauterine fetal death, spontaneous abortion, and preterm labor or delivery.6,13,14
Vaccination is the most effective method of protection against influenza, and increasing vaccine coverage is the only way to eliminate uncertainties regarding possible future waves of 2009 pandemic influenza A(H1N1).15 The Centers for Disease Control (CDC) Advisory Committee on Immunization Practices and the American College of Obstetricians and Gynecologists has recommended that pregnant women and especially women who are or will become pregnant during the influenza season should routinely receive the seasonal influenza vaccine, regardless of their pregnancy trimester.15,16 Despite the attenuated response of pregnant women against 2009 H1N1 virus infection, their responses to 2009 H1N1 and other seasonal influenza vaccines have been reported to be well preserved.9 The safety14,17 and efficacy14,18,19 of inactivated influenza vaccination for pregnant women and their newborns have been reported in several studies. The cost effectiveness of inactivated trivalent seasonal influenza vaccine, especially when the timing and the trimester of pregnancy are considered, has been also demonstrated.20,21 In Iran, with a population of 75 million, including 23 million women of reproductive age (15–49 y), antenatal care and pregnant women immunization are integrated into primary health services and provided through public primary health care facilities and hospitals. According to expanded program on immunization (EPI) in Iran, pregnant women immunization limited only to diphtheria-tetanus (Td) toxoids vaccination in 6th month of pregnancy provided history of lacking or prior incomplete Td vaccination or lasting more than 10 y of previously complete Td immunization. However, it is recommended by ministry of health of Iran that pregnant women that their pregnancy coincides with cold seasons, should be vaccinated against influenza. To the best of our knowledge, there is no any official report about influenza vaccination status of pregnant women in Iran. Therefore we conducted this study to determine the uptake rate of combined 2009 pandemic H1N1 influenza + seasonal influenza vaccination and the reasons (such as physicians’ recommendations) or barriers to acceptance among pregnant women.
Results
Four hundred 16 pregnant women participated in this study. Their mean of age was 27.06 ± 5.27 y (median 26, range 16 to 42). Of 397 pregnant women whose gestational age was determined, 178 (44.83%) were in the third trimester, 180 (45.34%) in the 2nd and 39 (9.82%) in the 1st trimester of their pregnancy. Seventeen (4.08%) were illiterate, 33 (7.93%) had graduated from university and 366 (87.98%) had attended primary school or high school. Two hundred nine women (69.47%) resided in urban areas and 118 (28.36%) in rural areas. Only 22 (5.28%) were employed outside the home (Table 1).
Table 1. Univariate analysis of factors associated with influenza vaccination in pregnant women referred to obstetrics and maternity hospitals affiliated with Shiraz University of Medical Sciences, 2010−2011*.
| Items | Total (% all participants) n = 416 (100%) |
Vaccinated group (% all participants) n = 25 (6%) |
Unvaccinated group (% all participants) n = 383 (92.06%) |
Statistics | P value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) Mean Median Minimum Maximum |
27.06 ± 5.27 26 16 42 |
27.04 ± 5.96 26 17 39 |
27.06 ± 5.23 26 15 42 |
MWU‡ |
0.97 |
| Body mass index before this pregnancy |
24.28 ± 4.25 |
25.68 ± 5.19 |
24.19 ± 4.18 |
MWU |
0.14 |
| Residence Urban Rural |
289 (69.47%) 118 (28.36%) |
19 (4.56%) 6 (1.44%) |
270 (64.90%) 112 (26.92%) |
χ2† = 0.32 |
0.57 |
| Occupation Employed Unemployed |
22 (5.28%) 386 (92.78%) |
2 (0.48%) 23 (5.52%) |
20 (4.8%) 363 (87.25%) |
FE* |
0.63 |
| Educational level University High school Up to middle school Primary school Illiterate |
33 (7.93) 176 (42.30) 190 (45.67) 17 (4.08) |
2 (0.48%) 12 (2.88%) 11 (2.64%) 0 (0%) |
31 (7.45%) 164 (39.42%) 179 (43.02%) 9 (2.16%) |
χ2 = 0.27 |
0.87 |
| Number of persons in household Median |
3.43 ± 1.85 3 |
3.92 ± 3.62 3 |
3.40 ± 1.67 3 |
MWU |
0.79 |
| Number of children Median |
66 ± 0.86 0 |
0.80 ± 0.91 1 |
0.65 ± 0.86 1 |
MWU |
0.39 |
| Obstetrics characteristics | |||||
| Gestational age First trimester (≤ 14 weeks) Second trimester (14+1-28 weeks) Third trimester (≥ 28+1 weeks) |
39 (9.82%) 180 (45.34%) 178 (42.78%) |
2 (0.48%) 11 (2.64%) 10 (2.40%) |
37 (8.89%) 169 (40.62%) 168 (40.38%) |
χ2 = 0.075 |
0.96 |
| Gravidity 1st 2nd 3rd or more |
175 (42.06) 133 (31.97) 100 (24.03) |
10 (2.4%) 6 (1.44%) 9 (2.16%) |
165 (39.66%) 127 (30.52%) 91 (21.87%) |
χ2 = 7.81 |
0.25 |
| Years since previous pregnancy Median |
3.45 ± 3.75 2 |
4.53 ± 3.91 3.5 |
3.38 ± 3.74 2 |
MWU |
0.18 |
| Body mass index in 1st trimesters (kg/m2) |
27.70 ± 4.68 |
28.72 ± 5.63 |
27.64 ± 4.61 |
MWU |
0.25 |
| Number of fetuses in this pregnancy Singleton Multiple gestation |
372 (89.42%) 12 (2.88%) |
22 (5.28%) 3 (0.72%) |
374 (89.90%) 9 (2.16%) |
FE* |
0.03 |
| History of stillbirth Yes No |
91 (21.9) 317 (76.20%) |
7 (1.68%) 18 (4.32%) |
84 (20.19%) 299 (71.87%) |
χ2 = 0.49 |
0.48 |
| Influenza related | |||||
| Had influenza in the past 2 y Yes No |
69 (16.58%) 338 (81.25%) |
7 (1.68%) 17 (4.08%) |
62 (14.90%) 321 (77.16%) |
χ2 = 2.70 | 1.00 |
Eight (1.92%) of studied pregnant women with unclear Influenza vaccination history did not include in comparisons. MWU‡, Man- Whitney U test; χ2†, Chi-square test; FE*, Fischer’s exact test.
Eighty six (20.67%) of pregnant women had history of at least one chronic disease, including 7 (28%) of vaccinated and 79 (20.62%) of non-vaccinated groups. Thirty two (7.69%) of pregnant women had Diabetes mellitus and 28 (6.73%) had neurological diseases.
Twenty one (5%) were hypertensive, excluding 18(4.32%) that had other cardiovascular diseases. Twelve (2.88%), 7 (1.68%), 3 (0.72%) and 2 (0.48%) had hypothyroidism, Asthma, hyperthyroidism and HIV/AIDS respectively. Five (1.2%) were on chronic corticosteroid treatment.
Three hundreds 80 three (92.06%) of pregnant women had not been vaccinated against flu, including pandemic H1N1 influenza and/or seasonal influenza, since 2 y before conducting this study. And 25 (6%) had history of flu vaccination. In 8 (1.92%), status of influenza vaccination could not be defined. In 19 (4.56%) of the 416 pregnant women initially included, both gestational age and history of influenza vaccination were unknown. Two hundred two (48.55%) of the women were able to describe at least two influenza symptoms. Thirty seven (8.89%) reported at least one complication from influenza during pregnancy, and 146 (35.09%) identified at least one difference between influenza and the common cold. Their total mean score for knowledge on influenza-related items and methods of prevention was 2.05 ± 1.63 (out of 10). Mean score for preventive measures against influenza was 1.11 ± 1.01 (out of 7). Less than 30% (1.44–28.12%) of the women were able to correctly explain how to prevent influenza (Table 2). Of 383 (92.06%) pregnant women who had refused influenza vaccination, 116 (30.28%) declared that the main reason was lack of information and 44 (11.48%) stated that they did not need vaccination. Thirty seven (9.66%) reported that they did not get influenza and 30 (7.83%) stated that influenza vaccination did not matter to them. Concerns about the side effects of the influenza vaccine for pregnant women, the fetus or the newborn were reported by 2 women (0.52%). One woman (0.26%) expressed uncertainties about the efficacy of flu vaccination. More than one reason for declining vaccination was mentioned by 147 (38.38%) women, but none of them said that lack of access was the cause of non-vaccination (Table 3).
Table 2. Knowledge of influenza and methods to prevent it, reported by pregnant women seen in obstetrics and maternity hospitals affiliated with Shiraz University of Medical Sciences, 2010−2011.
| Groups Knowledge of |
Total participants (n = 416) |
Vaccinated group (n = 25) |
Nonvaccinated group (n = 383) |
Statistics | P value | Odds ratio | 95% Confidence interval Odds ratio |
|---|---|---|---|---|---|---|---|
| Influenza symptoms (at least 2) |
202 (48.55%) |
11 (45.8%) |
191 (49.9%) |
χ2† = 0.14 |
0.70 |
1.17 |
0.51–2.69 |
| Difference between influenza and the common cold (at least one) |
37 (8.89%) |
11 (45.8%) |
135 (35.2%) |
χ2 = 1.10 |
0.29 |
0.64 |
0.28–1.47 |
| Influenza complications during pregnancy (at least one) |
146 (35.09%) |
1 (4.2%) |
36 (9.4%) |
FE* |
0.71 |
2.38 |
0.31–18.19 |
| Influenza vaccination |
64 (15.38%) |
3 (12.5%) |
61 (15.9%) |
FE* |
1.00 |
1.32 |
0.38–4.58 |
| Proper hand-washing |
74 (17.78%) |
6 (25%) |
68 (17.8%) |
χ2 = 0.79 |
0.37 |
0.64 |
0.24–1.69 |
| Keeping 1–2 min away from patients with flu |
117 (28.12%) |
5 (20.8%) |
112 (29.2%) |
χ2 = 0.78 |
0.37 |
1.57 |
0.57–4.30 |
| Using a tissue when coughing or sneezing |
37 (8.89%) |
4 (16.7%) |
33 (8.6%) |
FE* |
0.25 |
0.47 |
0.15–1.46 |
| Using mask when indicated |
73 (17.54%) |
8 (33.3%) |
65 (17%) |
χ2 = 4.10 |
0.04 |
0.40 |
0.16–0.99 |
| Enough rest when getting flu |
6 (1.44%) |
0 (0%) |
6 (1.6%) |
FE* |
1.00 |
0.94 |
0.91–0.96 |
| Avoid kissing, hugging or shaking hands with a person who has flu |
81 (19.47%) |
4 (16.7%) |
77 (20.1%) |
FE* |
0.79 |
1.25 |
0.41–3.78 |
| Total score out of 10 (mean ± SD) | 2.05 ± 1.63 | 2.20 ± 1.95 | 2.04 ± 1.61 | MWU‡ | 0.80 | - | - |
Eight (1.92%) of studied pregnant women with unclear Influenza vaccination history did not include in comparisons. χ2†, Chi-square test; FE*, Fischer’s exact test; MWU‡, Man- Whitney U test
Table 3. Reasons for declining or accepting the influenza vaccination reported by pregnant women referred to obstetrics and maternity hospitals affiliated with Shiraz University of Medical Sciences, Shiraz, Iran, 2010–2011.
| Reason | Frequency (%) | |
|---|---|---|
| Nonvaccinated (n = 383) |
“I don’t know anything about influenza vaccination.” |
116 (30.28) |
| |
“I think that I don’t need influenza vaccination” |
44 (11.48) |
| |
“I do not get influenza” |
37 (9.66) |
| |
“Influenza vaccination doesn’t matter to me” |
30 (7.83) |
| |
“Only some special groups other than pregnant women need to be vaccinated against influenza” |
3 (0.77) |
| |
“I’m afraid of the side effects of the vaccine for myself, my fetus and my newborn” |
2 (0.52) |
| |
“I’m afraid of injections” |
2 (0.52) |
| |
“I don’t believe the vaccine is effective” |
1 (0.26) |
| |
More than one reason |
147 (38.38) |
| |
No reason given |
1 (0.26) |
| Vaccinated (n = 25) |
Recommendation of someone other than a physician |
15 (60) |
| |
“Influenza vaccination is a must and necessary for everyone” |
5 (20) |
| |
“I get influenza several times each year” |
3 (12) |
| |
“Influenza vaccination is an effective method to prevent flu in pregnant women” |
1 (4) |
| More than one reason | 1 (4) |
Eight (1.92%) of studied pregnant women with unclear Influenza vaccination history did not include in comparisons
Of the 25 (6%) pregnant women who had been vaccinated against influenza, 15 (60%) reported being vaccinated on the advice of someone other than a physician. None of the women reported being vaccinated as a result of advice from a physician. Five (20%) believed that influenza vaccination was necessary for everyone. Three (12%) reported getting influenza several times each year, and 1 (4%) believed that vaccination was an effective method of prevention (Table 3).
Non Parametric tests showed that among demographic, obstetrics and influenza related characteristics and also history of chronic diseases, influenza vaccination in pregnant women only correlated significantly with the number of fetuses in the current pregnancy (single vs. multiple gestation). (p = 0.01, Table 1). However, forward logistic regression analysis including factors with a P value up to 0.30 (number of fetuses in the current pregnancy, BMI before pregnancy and BMI in the 1st trimester of pregnancy, current gravidity, years since the previous pregnancy, history of hyperthyroidism, knowledge of the difference between influenza and the common cold, and using a mask or tissue during coughing or sneezing, Tables 1 and 2) showed that none of these factors was an independent predictor of influenza vaccination in pregnant women.
Discussion
Evidence from previous pandemics and the most recent 2009 H1N1 influenza pandemic and from seasonal influenza epidemics shows that pregnant women and their children are at increased risk for influenza-related complications.4-14 In USA, six percent of hospitalization and six percent of mortality due to 2009 H1N1 pandemic in adults who had underlying conditions were related to pregnancy.1 There is no any official report about total number of admitted pregnant women or their proportion of total hospital admissions due to influenza in Iran. According to a cohort study that compared pregnant women with non-pregnant women of childbearing age, pregnant or postpartum women infected with the 2009 H1N1 influenza virus were at increased risk of admission to intensive care (relative risk 7.4, 95% confidence interval 5.5–10.0).10 The safety and efficacy of influenza vaccination have been demonstrated in several studies, and antibody transfer from the immune mother to her neonate adds to the benefits of maternal influenza immunization.15-19 Influenza vaccination is not included in EPI in Iran and only after experiencing 2009 H1N1 pandemic, more attention was driven toward it, especially for high risk groups such as pregnant women. We studied the rate of influenza vaccination among pregnant women, and their reasons for accepting or rejecting vaccination. Our findings show that only 6% of the pregnant women in our study were vaccinated against influenza, a poor uptake rate that is somewhat lower than the 6.9% rate reported in Hungary,22 and much lower than of 16% - 81% rates in other countries such as the USA.14,16,23-30 This differences may be explained in part by different strategies used in different countries to promote flu vaccination in pregnant women. In some countries only specific groups are targeted. In Iran, for example, only pregnant women with a history of chronic disease have been included in the national vaccination program. Other countries have taken a universal approach to encourage flu vaccination for all pregnant women.22,31,32 On a global level, however, influenza vaccination uptake among pregnant women has remained low.32
This study shows that only one out of every five pregnant women had an acceptable level of knowledge about influenza in general and ways to prevent it. And one third of the women in the nonvaccinated group reported that they had no information about the flu vaccine or vaccination. These results are consistent with the findings of another survey that found pregnant women to have insufficient knowledge of influenza.25
Our survey shows that concerns regarding the side effects of the vaccine for the pregnant women herself or her fetus or newborn (< 1%) and the belief that the vaccine is not effective (< 1%) were minor barriers to vaccination, in contrast to the results of many other studies that considered these two factors as major barriers.16,25,26,28,30 In the group of vaccinated women, which made up a small proportion of our total sample, recommendations from people other than physicians played a large role, a finding that suggests that physicians in our setting do not recommend vaccination or encourage pregnant women to be vaccinated. This finding is in agreement with other studies that reported that health care providers and especially physicians rarely recommend flu vaccination to pregnant women.22,28 One earlier study even found that pregnant women had been advised by their general practitioners against vaccination during pregnancy.30 On the other hand, some studies noted physicians’ key role in encouraging pregnant women to get vaccinated against flu.24,25 The results of this study are consistent with other studies that concluded that education is essential for both pregnant women16,23-28,30,33,34 and physicians22,30 regarding influenza, its possible complications during pregnancy and the importance of vaccination.
We found that none of the factors included in the logistic regression analysis was an independent predictor of the likelihood of influenza vaccination in pregnant women. This contrasts with other studies that found age, race, care provider and educational level to have a moderate predictive value (area under curve = −0.86) for flu vaccination,26 and reported that low socioeconomic level or immigrant status were associated with a low rate of vaccination uptake.27 Another survey found lower vaccination rates in women with a lower educational level or younger than 35 y.24
Our study had some limitations. We included only pregnant women who were referred to university-affiliated hospitals; women referred to private hospitals were not included. Although nearly all obstetricians affiliated with Shiraz University of Medical Sciences also work in private hospitals and clinics, there seem to be no grounds to believe that their policies regarding influenza vaccination would differ for women seen at public vs. private clinics. Future studies should be designed to ask obstetricians about their knowledge and attitudes toward influenza vaccination in pregnant women. Another limitation was that the only source of information regarding chronic disease history was self-reporting by the pregnant women we surveyed.
Conclusion
A multidisciplinary approach seems advisable in efforts to improve influenza vaccination uptake among pregnant women. Effective interventions such as education for pregnant women, their physicians and other caregivers are essential to promote and enhance flu vaccination coverage among pregnant women.
Patients and Methods
This cross-sectional questionnaire-based study was conducted from November 2010 to January 2011 at Hafez and Zeinabieh hospitals (the main reference obstetrics and maternity centers in our area), both of which are affiliated with Shiraz University of Medical Sciences in Shiraz, the capital city of Fars province, southern Iran.
A convenience sampling method was used. The sample size necessary for this study was 384 pregnant women, calculated with the formula z2pq/d2, assuming that p (vaccination rate) = 50%, for a confidence level of 95% and an error rate of 5%. The only exclusion criterion was the woman’s refusal to participate in this study. Initially, three trained, experienced midwives explained the aims of the study to each woman and filled out the related informed consent forms and questionnaires. Each woman was interviewed privately in a separate room at the obstetrics clinics of each hospital.
The questionnaire consisted of an introductory explanation of the aims of the study and the identity of the researchers, and items regarding the participants’ demographic and obstetric characteristics. A history of influenza or vaccination against the disease during the previous two years and any history of chronic disease were also recorded. These histories were based on the women’s self reports. Information about pregnancy-related items was obtained from each woman’s records at the obstetrics clinics. Knowledge of pregnant women about influenza in general was measured through questions about at least two symptoms of influenza, at least one difference between influenza and common cold symptoms and at least one influenza induced complication during pregnancy. We also asked about knowledge of pregnant women toward prevention of influenza. These questions were about need to influenza vaccination in pregnancy and applying precautions (proper hand washing, keeping 1–2 m distance, avoiding kissing, hugging or shaking hands, using tissue or mask when coughing or sneezing, having enough rest) when they become exposed to or in contact with flu patients or when getting influenza. Reasons for non-compliance toward influenza vaccination in un-vaccinated pregnant women and reasons for flu vaccination adherence in vaccinated group, were also queried. The scores were based on three options: Yes, No and I do not know.
The content and face validity of the questionnaire were ensured by expert opinion, and its reliability as calculated with the Kuder-Richardson 20 formula was 0.65. The data were analyzed with Non-Parametric tests (Chi-square, Fischer’s Exact and Mann-whitney U) and Forward logistic regression test as appropriate using SPSS version 11.5 software (SPSS), and graphs were produced with Microsoft Office Excel 2007. The accuracy of data entry was ensured by randomly selecting and checking completed questionnaires against their corresponding data in the SPSS software.
Univariate analysis was used initially to detect differences between women based on demographic characteristics, influenza-related items, disease history and history of influenza vaccination; P values < 0.05 were considered significant. Factors that yielded a P value < 0.3 were then used in forward logistic regression analysis to detect relationships between these variables as independent factors and influenza vaccination as the dependent factor. Pregnant women with a gestational age (documented by their last menstrual period or pelvic sonography) of up to 14 weeks, 14+1 to 28 weeks, or more than 28+1 weeks were categorized as being in their first, second, or third trimester of pregnancy, respectively.35 The body mass index (BMI) of all participants before their current pregnancy and for women in their 1st trimester was calculated as weight (kg) divided by the square of height per meter (m2).36
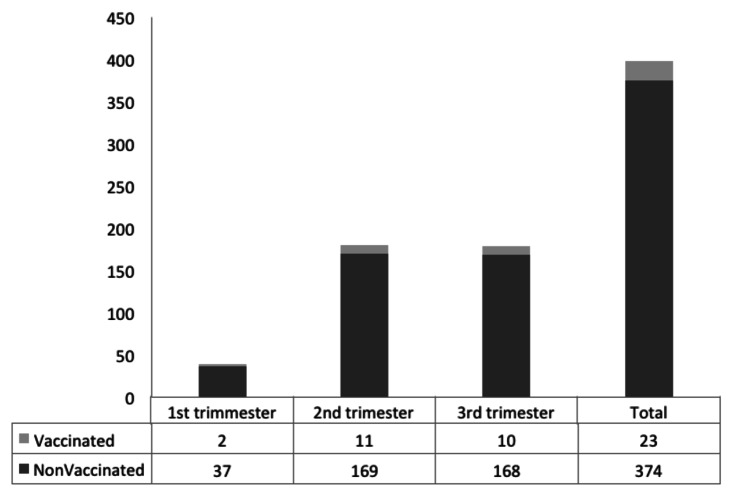
The Ethics Committee of the Health Policy Research Center affiliated to Shiraz University of Medical Sciences approved this study based on the protocol described below. (Author, please cite Fig. 1)
Figure 1. Numbers of pregnant women in each trimester of pregnancy who accepted or declined combined vaccination against influenza vaccination. Shiraz, southern Iran, 2010−2011 (In 19 of the 416 pregnant women initially included, gestational age and history of influenza vaccination were unknown).
Acknowledgments
This research was financially supported by the Health Policy Research Center (HPRC), Shiraz University of Medical Sciences. We thank the pregnant women and their partners for their consent to participate in this study, and the obstetrics clinic staff members at Hafez and Zeinabieh Hospitals in Shiraz for providing a comfortable environment for the interviews that was respectful of participants’ privacy. We also thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Glossary
Abbreviations:
- WHO
World Health Organization
- ICU
intensive care unit
- CDC
Centers for Disease Control and Prevention
- SPSS
Statistical Package for Social Sciences
- BMI
body mass index
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22008
References
- 1.Centers for Disease Control and Prevention (CDC). 2009 H1N1: overview of a pandemic, April 2009-August 2010. [cited January 10 2012]. Available at: http://www.cdc.gov/h1n1flu/yearinreview/yir5.htm
- 2.World Health Organization (WHO). Global Alert Response (GAR). Weekly Update. Pandemic (H1N1) 2009 - update 112 [cited January 10 2012]. Available from: http://www.who.int/csr/don/2010_08_06/en/
- 3.World Health Organization (WHO). Pandemic (H1N1) 2009. Global Alert and Response (GAR). [cited January 10 2012]. Available at: http://www.who.int/csr/disease/swineflu/en/
- 4.Peiris JS, Tu WW, Yen HL. A novel H1N1 virus causes the first pandemic of the 21st century. Eur J Immunol. 2009;39:2946–54. doi: 10.1002/eji.200939911. [DOI] [PubMed] [Google Scholar]
- 5.Creanga AA, Kamimoto L, Newsome K, D’Mello T, Jamieson DJ, Zotti ME, et al. Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. Am J Obstet Gynecol. 2011;204(Suppl 1):S38–45. doi: 10.1016/j.ajog.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Honarvar B, Asadi N, Ghaffarpasand F, Moghadami M, Kasraeian M. Pregnancy outcomes among patients infected with pandemic H1N1 influenza virus in Shiraz, Iran. Int J Gynaecol Obstet. 2010;111:86–7. doi: 10.1016/j.ijgo.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Michaan N, Amzallag S, Laskov I, Cohen Y, Fried M, Lessing JB, et al. Maternal and neonatal outcome of pregnant women infected with H1N1 influenza virus (swine flu) J Matern Fetal Neonatal Med. 2012;25:130–2. doi: 10.3109/14767058.2011.562569. [DOI] [PubMed] [Google Scholar]
- 8.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic H1N1 Influenza in Pregnancy Working Group Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan S, Gordon DL, Honda-Okubo Y, Petrovsky N, Phillips P, Huddleston S, et al. Serological responses following influenza A H1N1 2009 infection in adults. J Infect. 2011;62:388–93. doi: 10.1016/j.jinf.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10.ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oluyomi-Obi T, Avery L, Schneider C, Kumar A, Lapinsky S, Menticoglou S, et al. Perinatal and maternal outcomes in critically ill obstetrics patients with pandemic H1N1 Influenza A. J Obstet Gynaecol Can. 2010;32:443–7, 448-52. doi: 10.1016/S1701-2163(16)34497-8. [DOI] [PubMed] [Google Scholar]
- 12.Moghadami M, Honarvar B, Sabaeian B, Zamiri N, Pourshahid O, Rismanchi M, et al. H1N1 influenza infection complicated with diabetic ketoacidosis. Arch Iran Med. 2012;15:55–8. [PubMed] [Google Scholar]
- 13.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–8. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Tamma PD, Steinhoff MC, Omer SB. Influenza infection and vaccination in pregnant women. Expert Rev Respir Med. 2010;4:321–8. doi: 10.1586/ers.10.26. [DOI] [PubMed] [Google Scholar]
- 15.Chlibek R, Anca I, André F, Bakir M, Ivaskeviciene I, Mangarov A, et al. Central European Vaccination Advisory Group (CEVAG) guidance statement on recommendations for 2009 pandemic influenza A(H1N1) vaccination. Vaccine. 2010;28:3758–66. doi: 10.1016/j.vaccine.2010.03.072. [DOI] [PubMed] [Google Scholar]
- 16.Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal influenza vaccine coverage among pregnant women: pregnancy risk assessment monitoring system. J Womens Health (Larchmt) 2011;20:649–51. doi: 10.1089/jwh.2011.2794. [DOI] [PubMed] [Google Scholar]
- 17.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201:547–52. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Ohfuji S, Fukushima W, Deguchi M, Kawabata K, Yoshida H, Hatayama H, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203:1301–8. doi: 10.1093/infdis/jir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puleston RL, Bugg G, Hoschler K, Konje J, Thornton J, Stephenson I, et al. Observational study to investigate vertically acquired passive immunity in babies of mothers vaccinated against H1N1v during pregnancy. Health Technol Assess. 2010;14:1–82. doi: 10.3310/hta14550-01. [DOI] [PubMed] [Google Scholar]
- 20.Jit M, Cromer D, Baguelin M, Stowe J, Andrews N, Miller E. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine. 2010;29:115–22. doi: 10.1016/j.vaccine.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 21.Roberts S, Hollier LM, Sheffield J, Laibl V, Wendel GD., Jr. Cost-effectiveness of universal influenza vaccination in a pregnant population. Obstet Gynecol. 2006;107:1323–9. doi: 10.1097/01.AOG.0000210225.45986.99. [DOI] [PubMed] [Google Scholar]
- 22.Czeizel E. [Influenza vaccination of pregnant women and the experiences related to the pandemic influenza A-virus H1N1 infection in Hungary, 2009] Lege Artis Med. 2011;21:89–95. [PubMed] [Google Scholar]
- 23.Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011;204(Suppl 1):S112–5. doi: 10.1016/j.ajog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Steelfisher GK, Blendon RJ, Bekheit MM, Mitchell EW, Williams J, Lubell K, et al. Novel pandemic A (H1N1) influenza vaccination among pregnant women: motivators and barriers. Am J Obstet Gynecol. 2011;204(Suppl 1):S116–23. doi: 10.1016/j.ajog.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Fisher BM, Scott J, Hart J, Winn VD, Gibbs RS, Lynch AM. Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am J Obstet Gynecol. 2011;204(Suppl 1):S107–11. doi: 10.1016/j.ajog.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Fridman D, Steinberg E, Azhar E, Weedon J, Wilson TE, Minkoff H. Predictors of H1N1 vaccination in pregnancy. Am J Obstet Gynecol. 2011;204(Suppl 1):S124–7. doi: 10.1016/j.ajog.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Freund R, Le Ray C, Charlier C, Avenell C, Truster V, Tréluyer JM, et al. Inserm COFLUPREG Study Group Determinants of non-vaccination against pandemic 2009 H1N1 influenza in pregnant women: a prospective cohort study. PLoS ONE. 2011;6:e20900. doi: 10.1371/journal.pone.0020900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharbanda EO, Vargas CY, Castaño PM, Lara M, Andres R, Stockwell MS. Exploring pregnant women’s views on influenza vaccination and educational text messages. Prev Med. 2011;52:75–7. doi: 10.1016/j.ypmed.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Bone A, Guthmann JP, Nicolau J, Lévy-Bruhl D. Population and risk group uptake of H1N1 influenza vaccine in mainland France 2009-2010: results of a national vaccination campaign. Vaccine. 2010;28:8157–61. doi: 10.1016/j.vaccine.2010.09.096. [DOI] [PubMed] [Google Scholar]
- 30.White SW, Petersen RW, Quinlivan JA. Pandemic (H1N1) 2009 influenza vaccine uptake in pregnant women entering the 2010 influenza season in Western Australia. Med J Aust. 2010;193:405–7. doi: 10.5694/j.1326-5377.2010.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 31.Luteijn JM, Dolk H, Marnoch GJ. Differences in pandemic influenza vaccination policies for pregnant women in Europe. BMC Public Health. 2011;11:819. doi: 10.1186/1471-2458-11-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz JR, Englund JA, Neuzil KM. Influenza vaccine for pregnant women in resource-constrained countries: a review of the evidence to inform policy decisions. Vaccine. 2011;29:4439–52. doi: 10.1016/j.vaccine.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen SA, Kissin DM, Yeung LF, MacFarlane K, Chu SY, Turcios-Ruiz RM, et al. Pandemic Influenza and Pregnancy Working Group Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol. 2011;204(Suppl 1):S13–20. doi: 10.1016/j.ajog.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe KK, Eckert LO. Achieving high coverage of H1N1 influenza vaccine in an ethnically diverse obstetric population: success of a multifaceted approach. Infect Dis Obstet Gynecol. 2011;2011:746214. doi: 10.1155/2011/746214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham FG, Lenovo KJ, Bloom SL, Haut JC, Rouse DJ, Spong CY. Prenatal care. In: Williams Obstetrics, 23rd Ed. New York: MC Graw-Hill, 2010:195. [Google Scholar]
- 36.Cunningham FG, Lenovo KJ, Bloom SL, Haut JC, Rouse DJ, Spong CY. Prenatal care. In: Williams Obstetrics, 23rd Ed. New York: MC Graw-Hill, 2010:201. [Google Scholar]