Abstract
Incidence of meningococcal diseases is high in children, and effective vaccines are needed for this age group. In this phase II, open, controlled study, 309 children aged 2–10 y from Finland were randomized (3:1) into two parallel groups to receive one dose of meningococcal ACWY-tetanus toxoid conjugate vaccine (ACWY-TT group; n = 231) or a licensed meningococcal ACWY polysaccharide vaccine (Men-PS group; n = 78). Serum bactericidal activity using rabbit complement (rSBA) was evaluated up to three years post-vaccination. Exploratory comparisons suggested that rSBA vaccine response rates and geometric mean titers (GMTs) for each serogroup at one month post-vaccination and rSBA GMTs for serogroups A, W-135 and Y up to three years post-vaccination were higher in the ACWY-TT compared with Men-PS group, but did not detect any difference between groups in terms of rSBA-MenC GMTs at three years post-vaccination; this is explained by the higher proportion of children from the Men-PS group who were excluded because they were re-vaccinated with a monovalent meningococcal serogroup C vaccine due to loss of protective antibody levels against this serogroup. Although there was a higher incidence of local reactogenicity in the ACWY-TT group, general and unsolicited symptoms reporting rates were comparable in both groups. This study showed that MenACWY-TT was immunogenic with a clinically acceptable safety profile in children aged 2–10 y. MenACWY-TT induced higher functional antibody titers for all serogroups, which persisted longer for serogroups A, W-135 and Y, than the MenACWY polysaccharide vaccine. This study has been registered at www.clinicaltrials.gov NCT00427908.
Keywords: tetravalent meningococcal vaccine, conjugate vaccine, polysaccharide vaccine, bactericidal activity, child, safety, immunogenicity, persistence
Introduction
Neisseria meningitidis is responsible for invasive bacterial infections associated with high levels of mortality, especially in children and adolescents.1,2 Although the current level of meningococcal disease is low in industrialized countries,3 the number of confirmed meningococcal disease cases reported to the European Centre for Disease Prevention and Control in 2009 was 7.37 per 100,000 children under five years of age4 and the case fatality ratio of meningococcal disease was estimated to be 8% in Europe in 2004.5
N. meningitidis is classified into serogroups based on differences in the capsular polysaccharides, and invasive meningococcal diseases are mostly caused by five serogroups (A, B, C, W-135 and Y).1,2 In the European Union (EU), serogroup B was responsible for 71%, serogroup C for 13%, and serogroups Y for 4% of reported cases of invasive meningococcal disease in 2009.6 The incidence of serogroup C has declined in Europe since the introduction of conjugate vaccines against this serogroup in 1999,2 and an increase of meningococcal disease due to serogroup Y has recently been observed in Scandinavian countries and in the United Kingdom.7-10 Of note, there may be substantial regional variation in the relative distribution of each serogroup, and new serogroups may appear in some countries as a result of strain importation and evolution.2,11
Vaccination remains the best strategy to prevent meningococcal disease, and broadly effective vaccines are needed.11 Plain capsular polysaccharide vaccines providing protection against meningococcal serogroups A, C, W-135 and Y have been widely used in Europe over the last few decades. However, plain polysaccharide vaccines have limitations: they have lower immunogenicity among young children, they usually do not elicit long-term protection, they afford no herd immunity and no immune memory and they induce immunological hyporesponsiveness and a T-cell independent immune response.12,13
To overcome these limitations, capsular polysaccharides were covalently coupled to carrier proteins in meningococcal conjugate vaccines.12-16 The first meningococcal conjugate vaccines were monovalent vaccines against serogroup C using mutant diphtheria toxoid (CRM197) or tetanus toxoid (TT) as carrier protein.17 These vaccines were introduced in vaccination programs in Europe and were highly successful in reducing the incidence of meningococcal disease due to serogroup C, including in the youngest age groups.12,14,16-21 Subsequently, two tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccines using diphtheria toxoid (DT) or CRM197 as carrier protein were licensed for use in various countries,22-25 and a monovalent meningococcal serogroup A conjugate vaccine using TT as carrier protein was designed specifically for Africa.26-29 In addition, a new tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine using TT as carrier protein [Nimenrix™ (GlaxoSmithKline Vaccines); MenACWY-TT] has been recently approved by the European Medicines Agency for the active immunization of subjects older than 12 mo of age. This vaccine has been shown to be immunogenic with a clinically acceptable safety profile in toddlers, children, adolescents and young adults.30-36
This study assessed the immunogenicity, antibody persistence, reactogenicity and safety of one dose of the EU-licensed MenACWY-TT vaccine compared with one dose of a licensed monovalent meningococcal serogroup C conjugate vaccine in toddlers, and with one dose of a licensed tetravalent meningococcal serogroups A, C, W-135 and Y plain polysaccharide vaccine (Men-PS) in children aged 2–10 y. This manuscript will discuss the results obtained in children while those obtained in toddlers are presented in a separate publication.
Results
Study participants
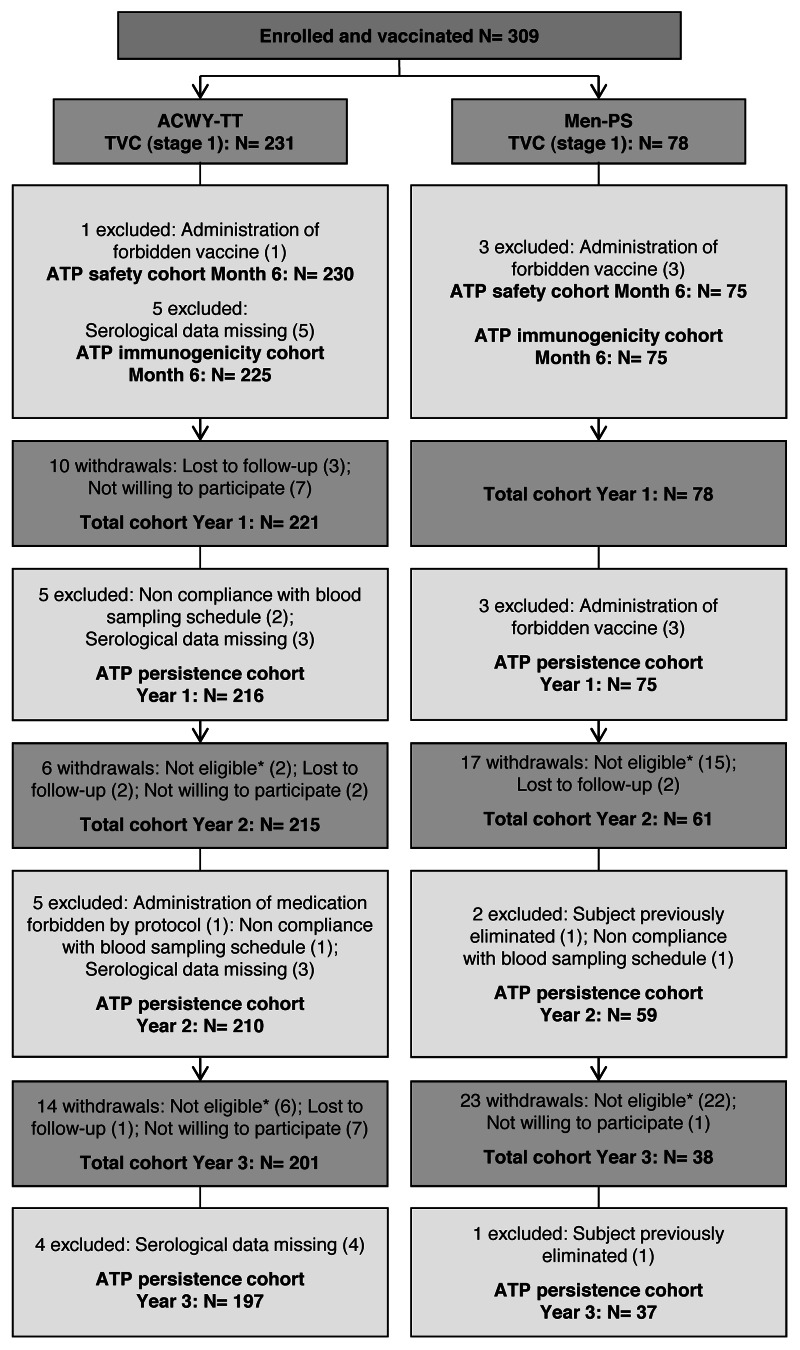
In this study, 309 Finnish children aged 2–10 y were enrolled and randomized (3:1) to receive either the MenACWY-TT vaccine (ACWY-TT group) or the MenACWY polysaccharide vaccine (Men-PS group) (Fig. 1). Of these, 306 and 300 children completed the primary stage at one month post-vaccination and the six month safety follow-up of the vaccination phase, respectively. One child in the ACWY-TT group was withdrawn from the primary stage of the study due to a non-serious adverse event (AE; grade 3 swelling for which medical attention was sought). In the persistence follow-up stage, 299 children were enrolled at one year, 276 children at two years and 239 children at three years post-vaccination. In Figure 1, 45 children were not included in the persistence stage at two or three years post-vaccination because they were not eligible; 4/8 ineligible children in the ACWY-TT and 37/37 ineligible children in the Men-PS group were withdrawn because they had been re-vaccinated with a monovalent meningococcal serogroup C conjugate vaccine as they did not retain rSBA titers ≥ 1:8 for serogroup C. The two treatment groups were comparable in terms of demographic characteristics (Table 1).
Figure 1. Participants’ progression through the study. ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; ATP, according to protocol; TVC, total vaccinated cohort; N, number of children; *Reasons for non-eligibility: suboptimal responder re-vaccinated with a monovalent meningococcal serogroup C vaccine (n = 41); suboptimal responder who did not come to the booster vaccination visit (n = 2); consent withdrawal (n = 1); and acute leukemia (n = 1)
Table 1. Demographic characteristics of enrolled and vaccinated children (total vaccinated cohort).
| Characteristic | ACWY-TT group | Men-PS group | |
|---|---|---|---|
| N |
|
231 |
78 |
| Age (months) |
Mean (SD) |
71.9 (31.19) |
71.6 (30.22) |
| |
Range |
24–131 |
24–125 |
| Gender |
Female n (%) |
115 (49.8) |
37 (47.4) |
| |
Male n (%) |
116 (50.2) |
41 (52.6) |
| Race |
White - Caucasian / European heritage n (%) |
225 (97.4) |
76 (97.4) |
| |
African heritage / African American n (%) |
1 (0.4) |
0 (0.0) |
| |
Asian - East Asian heritage n (%) |
1 (0.4) |
0 (0.0) |
| |
White - Arabic / North African heritage n (%) |
1 (0.4) |
0 (0.0) |
| Other n (%) | 3 (1.3) | 2 (2.6) | |
ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; N, number of children; n, number of children with the specified characteristic; %, percentage (n/N x 100) of children with the specified characteristic; SD, standard deviation.
Immunogenicity
One month after vaccination, the vaccine response rates, measured with a serum bactericidal activity assay using rabbit complement (rSBA), were at least 94.3% in the ACWY-TT group and 81.2% in the Men-PS group. Since the lower limit (LL) of the asymptotic standardized 95% confidence interval (CI) for the difference between the ACWY-TT and the Men-PS groups in terms of vaccine response rates was above -15% for each antigen, the primary objective to demonstrate the non-inferiority of MenACWY-TT vs. the MenACWY polysaccharide vaccine was met (Table 2).
Table 2. rSBA vaccine response rates at one month after administration of MenACWY-TT or MenACWY polysaccharide vaccine (ATP immunogenicity cohort).
| Antibody | ACWY-TT group |
Men-PS group |
Difference in vaccine response rate (ACWY-TT minus Men-PS) |
||||
|---|---|---|---|---|---|---|---|
| N | % [95% CI] | N | % [95% CI] | % | 95% CI |
||
| LL | UL | ||||||
| rSBA-MenA |
185 |
98.4* [95.3 – 99.7] |
62 |
91.9 [82.2 – 97.3] |
6.44 |
1.15 |
16.04 |
| rSBA-MenC |
212 |
94.3* [90.3 – 97.0] |
69 |
81.2 [69.9 – 89.6] |
13.18 |
4.79 |
24.32 |
| rSBA-MenW-135 |
199 |
100* [98.2 – 100] |
68 |
95.6 [87.6 – 99.1] |
4.41 |
1.51 |
12.21 |
| rSBA-MenY | 219 | 99.1* [96.7 – 99.9] | 70 | 82.9 [72.0 – 90.8] | 16.23 | 8.99 | 26.78 |
ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; N, number of children with an rSBA result both pre- and post-vaccination; %, percentage of children with an rSBA vaccine response; 95% CI, 95% confidence interval; LL, lower limit; UL, upper limit; Vaccine response, for initially seronegative children (pre-vaccination rSBA titer < 1:8), post-vaccination rSBA titer ≥ 1:32; for initially seropositive children (pre-vaccination rSBA titer ≥ 1:8), post-vaccination rSBA titer ≥ 4-fold the pre-vaccination rSBA titer; *Indicates higher rSBA vaccine response in the MenACWY-TT group than in the Men-PS group (exploratory analysis).
At one month post-vaccination, all children in both groups had rSBA titers ≥ 1:8 and at least 99.6% and 94.6% of them had rSBA titers ≥ 1:128 for each individual serogroup in the ACWY-TT and Men-PS groups, respectively (Table 3). For the four serogroups, exploratory analyses suggested that vaccine response rates and post-vaccination rSBA geometric mean titers (GMTs) adjusted for baseline titers were higher among MenACWY-TT recipients than among children who received the MenACWY polysaccharide vaccine. Moreover, exploratory analyses suggested that a higher percentage of children had rSBA titers ≥ 1:128 for serogroup C in the ACWY-TT group compared with the Men-PS group.
Table 3. Percentage of children with rSBA titers equal to or above cut-off values, and rSBA GMTs before and one month after vaccination (ATP immunogenicity cohort), one year after vaccination (ATP persistence cohort Year 1), two years after vaccination (ATP persistence cohort Year 2) and three years after vaccination (ATP persistence cohort Year 3). ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine; GMT, geometric mean antibody titer calculated on all children; N, number of children with available results; %, percentage of children with titer within the specified range; 95% CI, 95% confidence interval; Pre, pre-vaccination; Month 1, Year 1, Year 2, Year 3, one month, one year, two years and three years after vaccination. *Indicates higher value in the ACWY-TT group compared with the Men-PS group (exploratory analyses). Differences between groups in terms of GMTs are based on values adjusted for pre-vaccination measurements.
| Antibody | Group | Timing | N | % ≥ 1:8 (95% CI) | % ≥ 1:128 (95% CI) | GMT (95% CI) |
|---|---|---|---|---|---|---|
| rSBA- MenA |
ACWY-TT |
Pre |
185 |
67.0 (59.7 – 73.7) |
51.9 (44.4 – 59.3) |
57.9 (43.3 – 77.5) |
| Month 1 |
225 |
100 (98.4 – 100) |
99.6 (97.5 – 100) |
7300.9* (6586.0 – 8093.4) |
||
| Year 1 |
216 |
99.5* (97.4 – 100) |
99.5* (97.4 – 100) |
2448.1* (2149.6 – 2788.1) |
||
| Year 2 |
208 |
100* (98.2 – 100) |
99.0* (96.6 – 99.9) |
1333.4* (1181.9 – 1504.2) |
||
| Year 3 |
192 |
100* (98.1 – 100) |
99.0* (96.3 – 99.9) |
1184.2* (1054.2 – 1330.3) |
||
| Men-PS |
Pre |
62 |
64.5 (51.3 – 76.3) |
51.6 (38.6 – 64.5) |
58.2 (33.8 – 100.1) |
|
| Month 1 |
75 |
100 (95.2 – 100) |
100 (95.2 – 100) |
2033.4 (1667.1 – 2480.2) |
||
| Year 1 |
71 |
90.1 (80.7 – 95.9) |
80.3 (69.1 – 88.8) |
358.5 (230.2 – 558.4) |
||
| Year 2 |
56 |
91.1 (80.4 – 97.0) |
75.0 (61.6 – 85.6) |
202.5 (135.3 – 303.0) |
||
| Year 3 |
34 |
91.2 (76.3 – 98.1) |
79.4 (62.1 – 91.3) |
218.8 (128.9 – 371.5) |
||
| rSBA- MenC |
ACWY-TT |
Pre |
212 |
62.7 (55.8 – 69.3) |
27.8 (21.9 – 34.4) |
33.5 (26.0 – 43.1) |
| Month 1 |
225 |
100 (98.4 – 100) |
99.6* (97.5 – 100) |
2435.3* (2105.8 – 2816.3) |
||
| Year 1 |
215 |
99.5* (97.4 – 100) |
89.3* (84.4 – 93.1) |
489.5* (419.5 – 571.1) |
||
| Year 2 |
210 |
98.6* (95.9 – 99.7) |
75.7* (69.3 – 81.4) |
256.0* (213.9 – 306.2) |
||
| Year 3 |
192 |
98.4* (95.5 – 99.7) |
72.9 (66.0 – 79.1) |
244.3 (200.8 – 297.3) |
||
| Men-PS |
Pre |
70 |
51.4 (39.2 – 63.6) |
27.1 (17.2 – 39.1) |
24.1 (15.2 – 38.2) |
|
| Month 1 |
74 |
100 (95.1 – 100) |
94.6 (86.7 – 98.5) |
750.2 (555.2 – 1013.7) |
||
| Year 1 |
65 |
80.0 (68.2 – 88.9) |
58.5 (45.6 – 70.6) |
113.5 (67.3 – 191.5) |
||
| Year 2 |
59 |
66.1 (52.6 – 77.9) |
45.8 (32.7 – 59.2) |
59.9 (33.0 – 108.7) |
||
| Year 3 |
37 |
83.8 (68.0 – 93.8) |
67.6 (50.2 – 82.0) |
163.5 (83.8 – 319.2) |
||
| rSBA- MenW-135 |
ACWY-TT |
Pre |
199 |
60.3 (53.1 – 67.2) |
45.2 (38.2 – 52.4) |
43.1 (32.4 – 57.4) |
| Month 1 |
225 |
100 (98.4 – 100) |
100 (98.4 – 100) |
11777.0* (10666.2 – 13003.5) |
||
| Year 1 |
216 |
100 (98.3 – 100) |
99.1* (96.7 – 99.9) |
2983.3* (2628.2 – 3386.3) |
||
| Year 2 |
210 |
99.5* (97.4 – 100) |
99.0* (96.6 – 99.9) |
1298.0* (1135.5 – 1483.7) |
||
| Year 3 |
196 |
100* (98.1 – 100) |
98.0* (94.9 – 99.4) |
1737.1* (1503.8 – 2006.7) |
||
| Men-PS |
Pre |
68 |
57.4 (44.8 – 69.3) |
35.3 (24.1 – 47.8) |
40.1 (23.9 – 67.3) |
|
| Month 1 |
75 |
100 (95.2 – 100) |
100 (95.2 – 100) |
2186.3 (1723.1 – 2773.9) |
||
| Year 1 |
75 |
100 (95.2 – 100) |
93.3 (85.1 – 97.8) |
463.0 (367.4 – 583.5) |
||
| Year 2 |
54 |
85.2 (72.9 – 93.4) |
68.5 (54.4 – 80.5) |
144.0 (90.1 – 230.2) |
||
| Year 3 |
35 |
82.9 (66.4 – 93.4) |
60.0 (42.1 – 76.1) |
112.9 (59.9 – 212.6) |
||
| rSBA- MenY |
ACWY-TT |
Pre |
219 |
67.1 (60.5 – 73.3) |
44.7 (38.0 – 51.6) |
57.3 (43.7 – 75.2) |
| Month 1 |
225 |
100 (98.4 – 100) |
99.6 (97.5 – 100) |
6641.4* (6044.3 – 7297.4) |
||
| Year 1 |
216 |
100* (98.3 – 100) |
99.5* (97.4 – 100) |
2172.1* (1939.6 – 2432.5) |
||
| Year 2 |
210 |
100* (98.3 – 100) |
99.5* (97.4 – 100) |
1530.2* (1339.2 – 1748.4) |
||
| Year 3 |
195 |
100* (98.1 – 100) |
100* (98.1 – 100) |
1551.6* (1381.2 – 1743.1) |
||
| Men-PS | Pre |
70 |
60.0 (47.6 – 71.5) |
40.0 (28.5 – 52.4) |
45.5 (26.8 – 77.0) |
|
| Month 1 |
75 |
100 (95.2 – 100) |
97.3 (90.7 – 99.7) |
1409.9 (1085.9 – 1830.5) |
||
| Year 1 |
71 |
90.1 (80.7 – 95.9) |
78.9 (67.6 – 87.7) |
332.4 (213.5 – 517.7) |
||
| Year 2 |
55 |
74.5 (61.0 – 85.3) |
54.5 (40.6 – 68.0) |
96.9 (54.1 – 173.6) |
||
| Year 3 | 37 | 81.1 (64.8 – 92.0) | 51.4 (34.4 – 68.1) | 103.8 (54.3 – 198.3) |
The rSBA GMTs decreased in both groups between one month and one year post-vaccination and between one and two years post-vaccination, with a smaller decrease observed in the ACWY-TT group than in the Men-PS group for all serogroups (Table 3). At three years after vaccination, at least 98.4% of children in the ACWY-TT group retained rSBA antibody titers ≥ 1:8 for each serogroup while in the Men-PS group, the percentage of children retaining rSBA antibody titers ≥ 1:8 had fallen to 81.1–91.2%. At one year, two years and three years after vaccination, exploratory analyses suggested that the percentages of children retaining rSBA antibody titers ≥ 1:8 and ≥ 1:128 and post-vaccination rSBA GMTs adjusted for pre-vaccination titers were higher in the ACWY-TT group than in the Men-PS group for all serogroups, apart from serogroup W-135 for which 100% of the children in both groups retained rSBA titers ≥ 1:8 at one year post-vaccination, and serogroup C for which no difference was observed for adjusted rSBA GMTs or percentages of children with rSBA titers ≥ 1:128 at three years post-vaccination (Table 3). Of note, a selection bias was introduced in the persistence stage since a higher proportion of children in the Men-PS [37 children (47.4%)] compared with the ACWY-TT group [4 children (1.7%)] were excluded from the analyses because they had been re-vaccinated with a monovalent meningococcal serogroup C conjugate vaccine due to loss of protective antibody levels against this serogroup.
Exploratory analyses at one month post-vaccination suggested that the percentage of children with anti-TT concentrations ≥ 1.0 IU/mL was higher in the ACWY-TT group [100%; 95% CI (89.1–100)] than in the Men-PS group [71.4%; 95% CI (29.0–96.3)] (data not shown).
Safety
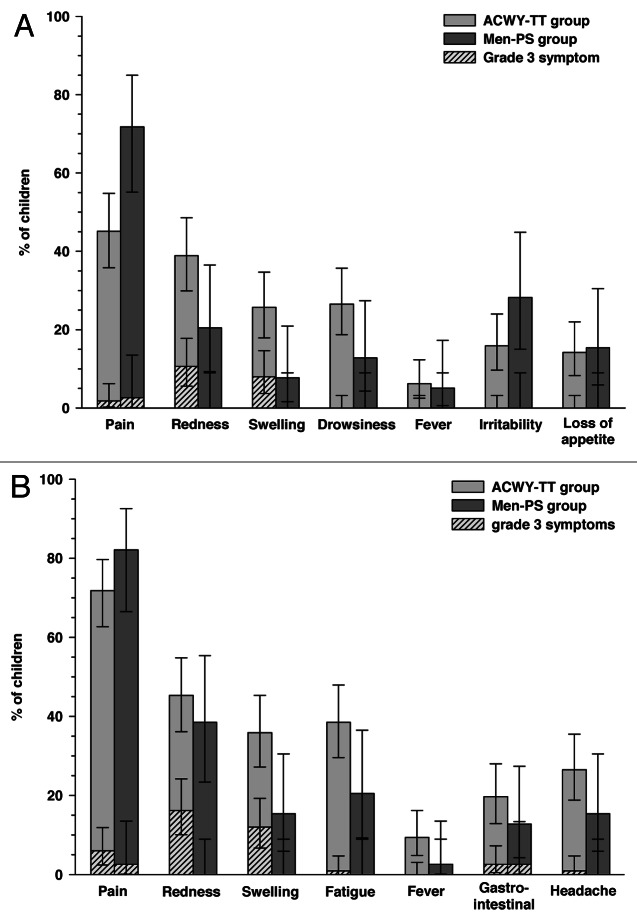
Within four days following vaccination, pain was the most frequent solicited local symptom in children aged 2–5 y, which was reported in 45.1% [51/113] of children in the ACWY-TT group and 71.8% [28/39] of children in the Men-PS group (Fig. 2A). In this age stratum, drowsiness was the most common solicited general symptom in the ACWY-TT group [26.5% (30/113) of children] and irritability in the Men-PS group [28.2% (11/39) of children]. In children aged 6–10 y, pain was also the most frequently reported solicited local symptom in both groups [71.8% (84/117) of children in the ACWY-TT group and 82.1% (32/39) of children in the Men-PS group] and fatigue was the most common solicited general symptom reported within four days following vaccination [38.5% (45/117) and 20.5% (8/39) of children in the ACWY-TT and Men-PS groups, respectively] (Fig. 2B).
Figure 2. Incidence (with 95% CI) of solicited local and general symptoms occurring within 4 d after the first vaccination in children aged 2–5 y (A) or 6–10 y (B) (total vaccinated cohort). ACWY-TT group, group of children who received one dose of MenACWY-TT; Men-PS group, group of children who received one dose of the MenACWY polysaccharide vaccine. Error bars represent 95% confidence intervals
During the 31-d post-vaccination follow-up period, unsolicited symptoms were reported in 35.9% (83/231) and 30.8% (24/78) of children for any unsolicited symptom and 6.5% (15/231) and 5.1% (4/78) of children for grade 3 unsolicited symptoms in the ACWY-TT and Men-PS groups, respectively. The most frequently reported unsolicited AEs in the ACWY-TT group were pyrexia and otitis media [both reported in 5.2% (12/231) of children] and in the Men-PS group, upper respiratory tract infection [reported in 5.1% (4/78) of children]. One grade 3 unsolicited symptom considered as related to vaccination (hematoma) was reported in a child from the ACWY-TT group.
During the six-month safety follow-up period of the primary stage, serious adverse events (SAEs) were reported in two children in the ACWY-TT group (laryngitis and hyperglycemia and pneumonia) and one child in the Men-PS group (testicular torsion). No SAEs were considered causally related to vaccination up to three years post-vaccination. A new onset of chronic illness (NOCI) was reported in six children [two children (0.9%) in the ACWY-TT group and four children (5.1%) in the Men-PS group] during the six-month safety follow-up period of the primary stage. The NOCIs were all allergic in nature, and none of the reported NOCIs were considered causally related to vaccination. No deaths were reported throughout the study.
Discussion
This study was designed to compare the immunogenicity, antibody persistence and safety profile of the EU-licensed MenACWY-TT vaccine and a licensed tetravalent meningococcal plain polysaccharide vaccine in children 2–10 y of age. The primary objective was reached as the non-inferiority of the MenACWY-TT vaccine vs. the MenACWY polysaccharide vaccine in terms of rSBA vaccine response to the four serogroups was demonstrated.
At one month post-vaccination, exploratory analyses detected higher rSBA vaccine response rates and GMTs for all serogroups in the children who received MenACWY-TT as compared with those who received the MenACWY polysaccharide vaccine. These results are consistent with other studies, in which MenACWY-TT was found to induce higher functional antibody titers than plain polysaccharide vaccines in children32,33 or in adolescents and young adults.31,34 This observation is also in line with previous studies comparing the post-vaccination functional antibody titers induced by tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccines, using DT or CRM197 as carrier protein, and plain polysaccharide vaccines in children 2–10 y of age24,25 or in adolescents and adults.22,23,37
Persistence of rSBA antibodies up to three years after vaccination was observed for both vaccines in at least 81.1% of children. Greater antibody persistence was observed in the children who received the MenACWY-TT vaccine than in those who received the MenACWY polysaccharide vaccine. This was expected as plain polysaccharide vaccines induce T-cell independent immune responses, which elicit shorter-term protection.15,24 As expected, the observed rSBA GMTs for the four serogroups decreased between one month and one year post-vaccination, and a further decline was observed between one year and two years post-vaccination. In contrast, no significant decline in terms of rSBA GMTs was observed between two and three years post-vaccination in both treatment groups.
For serogroup C, exploratory analyses at three years post-vaccination did not detect any difference between the two groups in terms of rSBA GMTs. This is largely explained by the higher proportion of children vaccinated with the MenACWY polysaccharide vaccine [37 children (47.4%)] compared with MenACWY-TT [4 children (1.7%)] who were excluded from the persistence analyses because they had been re-vaccinated with a monovalent meningococcal serogroup C conjugate vaccine due to loss antibody against serogroup C. This introduced a selection bias in the persistence stage of the study since children who did not maintain rSBA titers ≥ 1:8 were excluded, leading to an overestimation of the persistence as the study progressed.
A significant proportion of children in this study had rSBA antibodies against meningococcal serogroups A, C, W-135 and Y prior to vaccination, and the observed pre-vaccination seropositivity rates were comparable between the two treatment groups. As observed in a previous study, the percentage of seropositive children increased with age, suggesting a rising probability of acquiring functional antibodies against N. meningitidis through natural immunity mechanisms in older children.38 Therefore, the vaccine response rate was an important primary endpoint as it assessed the ability of children to respond to the vaccine regardless of their serostatus at pre-vaccination. Here, significantly higher rSBA vaccine response rates for all four serogroups were observed in children who received the MenACWY-TT vaccine than in children who received the MenACWY polysaccharide vaccine.
As expected, the anti-TT antibody concentrations increased between pre- and post-vaccination in the children who received the MenACWY-TT vaccine. An increase in anti-TT antibody concentrations was also observed after vaccination with MenC-TT and MenA-TT monovalent conjugate vaccines.29,39 However, functional anti-TT antibodies have not been assessed after administration of either vaccine.
In the present study, the MenACWY-TT vaccine induced a higher rate of injection site redness and swelling than the MenACWY polysaccharide vaccine, and this is likely due to the increased protein content in the conjugate vaccine. This was previously observed with the same vaccines in children,32 adolescents and young adults31,34 as well as with the two other licensed tetravalent meningococcal conjugate vaccines when compared with a plain polysaccharide vaccine.22-25 However, this observation is in contrast with results of another study, in which MenACWY-TT was compared with the MenACWY polysaccharide vaccine in children aged 2–10 y, where rates of injection site reactions were more comparable.33 In the present study, the incidence of pain at the injection site was lower in the children aged 2–5 y who received MenACWY-TT compared with the MenACWY polysaccharide vaccine. This observation is consistent with results of the previous study comparing the same vaccines in children aged 2–10 y.33
A limitation of this study was its open design because the study vaccines differed both in appearance and route of administration (intramuscular for MenACWY-TT and subcutaneous for the MenACWY polysaccharide vaccine). The open design would not have influenced the immunogenicity results, but had the potential to bias the safety, although this bias would most likely be in favor of the control MenACWY polysaccharide vaccine vs. the MenACWY-TT vaccine. The multiple exploratory comparisons for both safety and immunogenicity endpoints could also have limited this study, which was only powered to address immunological non-inferiority of MenACWY-TT vs. the MenACWY polysaccharide vaccine in terms of rSBA vaccine response rates. Another limitation in the persistence stage of this study was the high number of children who were excluded because they failed to retain protective levels of rSBA antibodies against serogroup C and were therefore re-vaccinated with a monovalent meningococcal serogroup C conjugate vaccine. Re-vaccination was done to ensure that children remained protected against meningococcal serogroup C disease, but it means that observed titers at future persistence timepoints may be overestimated due to the differential drop-out of seronegative children in both groups.
In conclusion, this study showed that the MenACWY-TT vaccine induced an immune response against meningococcal serogroups A, C, W-135 and Y in children aged 2–10 y, which persisted up to three years post-vaccination. Exploratory analyses suggested higher rSBA vaccine response rates and GMTs for each serogroup at one month post-vaccination and higher rSBA GMTs for serogroups A, W-135 and Y up to three years post-vaccination in children vaccinated with MenACWY-TT compared with the MenACWY polysaccharide vaccine. Additional antibody persistence data will be generated at subsequent timepoints to see if the higher functional antibody titers further translate into a longer duration of protection. The MenACWY-TT vaccine had a clinically acceptable safety profile, although it was followed by more local injection site reactions than the MenACWY polysaccharide vaccine. This study supports the administration of the MenACWY-TT vaccine in children aged 2–10 y to provide long-term protection against meningococcal diseases.
Methods
Study design
This phase II, open, randomized, controlled study was conducted in 11 centers in Finland between February 2007 and May 2010. The study was designed to evaluate the immunological non-inferiority, antibody persistence and safety profile of MenACWY-TT vs. a licensed tetravalent plain polysaccharide vaccine in children aged 2–10 y and a licensed monovalent meningococcal serogroup C conjugate vaccine in toddlers. The study was conducted in two stages: a six-month vaccination and safety follow-up primary stage, and a five-year persistence stage. In this manuscript, we present the results obtained in children 2–10 y of age up to three years after vaccination.
The children were stratified according to age (2–5 y and 6–10 y) to assess different profiles of solicited symptoms in the two age strata. Within each age stratum, children were randomized (3:1) into two parallel treatment groups: children in the ACWY-TT group received one dose of MenACWY-TT and children in the Men-PS group received one dose of the MenACWY polysaccharide vaccine. The randomization list was generated at GlaxoSmithKline, Rixensart, Belgium, using a standard SAS® program and was used to number the vaccines. Treatment allocation at the investigator site was performed using a central internet randomization system. The randomization algorithm used a minimization procedure (block size of 4) accounting for center in order to ensure a balanced distribution of the population in each group. The study was open in design because the study vaccines differed both in appearance and route of administration (intramuscular for MenACWY-TT and subcutaneous for the MenACWY polysaccharide vaccine).
The study was conducted in accordance with the Good Clinical Practice Guidelines and the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by the Ethics Committee of Pirkanmaa Hospital District. Written informed consent was obtained from the parents/guardians of all the children prior to study entry. In addition, written assent was obtained from the children who were able to give assent to decisions about their participation in the study. This study has been registered at www.clinicaltrials.gov NCT00427908. A summary of the protocol is available at http://www.gsk-clinicalstudyregister.com (GlaxoSmithKline study ID 108658, 108660, 108661 and 108663).
Study participants
Study participants were healthy children aged 2–10 y who previously completed routine childhood vaccinations. Children were not eligible to participate if they had used any investigational product other than the study vaccine within 30 d preceding the study or had planned use during the study period; if they were immunosuppressed from any cause; if they were planned to receive a vaccine not foreseen by the study protocol within one month post-vaccination; if they had previously received a meningococcal conjugate vaccine at any time, a meningococcal polysaccharide vaccine within the last five years, or a vaccine containing TT within the last month; if they had history of meningococcal disease, neurologic disorders or seizures, allergic disease or reactions likely to be exacerbated by any component of the vaccine; if they had a major congenital defect or a serious chronic illness; if they had acute disease at the time of enrolment; or if they had received immunoglobulins or blood products within three months preceding the study or had planned administration during the study period. During the three-year persistence of the study, toddlers who did not retain rSBA titers ≥ 1:8 for meningococcal serogroup C and were re-vaccinated with a monovalent meningococcal serogroup C conjugate vaccine were excluded from the analyses performed at subsequent timepoints.
Vaccines
One dose of MenACWY-TT contained 5μg of each of the meningococcal serogroups A, C, W-135 and Y polysaccharides, conjugated to TT (44µg). The lyophilized vaccine was reconstituted with saline (0.5mL) and administered intramuscularly into the non-dominant deltoid.
One dose of the MenACWY polysaccharide vaccine (Mencevax™ACWY, GlaxoSmithKline, Rixensart, Belgium) contained 50μg of each of the meningococcal serogroups A, C, W-135 and Y polysaccharides. The lyophilized vaccine was reconstituted with saline (0.5mL) and administered subcutaneously into the non-dominant upper arm.
Study objectives
The primary objective of this study was to evaluate the non-inferiority of the MenACWY-TT vaccine vs. the licensed MenACWY polysaccharide vaccine in terms of vaccine response rates to serogroups A, C, W-135 and Y in children aged 2–10 y. The vaccine response was defined as a post-vaccination rSBA titer ≥ 1:32 for initially seronegative children (rSBA titer < 1:8 at pre-vaccination) and as at least a 4-fold increase in rSBA titer from pre- to post-vaccination for initially seropositive children (rSBA titer ≥ 1:8 at pre-vaccination). Non-inferiority of MenACWY-TT compared with the MenACWY polysaccharide vaccine was demonstrated if the LL of the asymptotic standardized 95% CI for the difference between the ACWY-TT group and the Men-PS group in terms of percentage of children having a bactericidal vaccine response to serogroups A, C, W-135 and Y at one month after vaccination was ≥ -15%.
The secondary objectives included the comparison of the immunogenicity, antibody persistence and safety profile between MenACWY-TT and the MenACWY polysaccharide vaccine.
Immunogenicity assessment
Blood samples were collected from all the children before and one month, one year, two years and three years after vaccination. Functional antibody titers for meningococcal serogroups A, C, W-135, and Y were measured using a rSBA assay.40 The presumed correlate of protection against meningococcal disease due to serogroup C was a rSBA titer ≥ 1:841 and this threshold had historically been extended to the other serogroups.42 Moreover, the percentages of children with rSBA titers ≥ 1:128, which is the more conservative threshold for protection, were also evaluated.
Blood samples collected before and one month after vaccination were also analyzed to determine anti-TT antibody concentrations by enzyme-linked immunosorbent assay (ELISA) with a cut-off of 0.1IU/mL.43 All immunological assays were performed at GlaxoSmithKline laboratories.
Safety and reactogenicity assessment
The primary safety evaluation was performed separately on the 2–5 y and 6–10 y age strata, because the nature of the solicited general symptoms and the severity grading of the solicited local symptoms differed. In the 2–5 y age strata, solicited local symptoms (pain, redness and swelling) and general symptoms [drowsiness, irritability, loss of appetite and fever (rectal temperature ≥ 38.0°C)] were recorded using diary cards completed by the parent/legally-authorized representative up to four days after vaccination. Redness and swelling were of grade 3 intensity if their diameter was > 30mm, pain if the child cried when the limb was moved or if the limb was spontaneously painful, loss of appetite if the child did not eat at all, fever if rectal temperature was > 40.0°C, and all other symptoms if they prevented normal activity.
In the 6–10 y age stratum, solicited local symptoms (pain, redness and swelling) and general symptoms (fatigue, gastro-intestinal, headache and fever) were also recorded using diary cards completed by the parent/legally-authorized representative up to four days after vaccination. Redness and swelling were of grade 3 intensity if their diameter was > 50 mm, fever if rectal temperature was > 40°C, and all other symptoms if they prevented normal activity.
The occurrence of SAEs and NOCIs was recorded up to six months post-vaccination. SAEs were defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in disability or incapacity, or was an important medical event. NOCIs were identified by the investigator on the case report form, and confirmed by the GlaxoSmithKline Vaccines’ physician responsible for the study at the time of data review. All solicited local (injection site) reactions were considered causally related to vaccination. Causality of all other AEs was assessed by the investigator. SAEs considered causally related to vaccination by the investigator were reported throughout the study.
Statistical analyses
With a sample size of 272 children aged 2–10 y evaluable for immunogenicity at one month post-vaccination (204 children in the ACWY-TT group and 68 children in the Men-PS group), the study had 76.5% power to meet the primary non-inferiority objective of MenACWY-TT vs. the MenACWY polysaccharide vaccine in terms of vaccine response to serogroups A, C, W-135 and Y.
The total vaccinated cohort, on which the safety analyses were performed, included all vaccinated children. The according to protocol (ATP) immunogenicity cohort, on which the primary immunogenicity analyses were performed, included all vaccinated children who had complied with protocol-defined procedures and had results available for at least one immunogenicity endpoint at one month after vaccination. The ATP persistence cohorts Year 1, Year 2 or Year 3 on which the antibody persistence analyses were performed included all vaccinated children who had complied with protocol-defined procedures, had not received a previous dose of meningococcal vaccine other than the study vaccine during the study, and had results available for at least one immunogenicity endpoint at one year, two years or three years post-vaccination, respectively.
GMTs were calculated by taking the anti-log of the mean of the log10 titer transformations. For each treatment group and for each antibody assessed, rSBA GMTs were tabulated with their asymptotic 95% CIs, and the percentages of toddlers with rSBA titers ≥ 1:8 and ≥ 1:128, or anti-TT concentrations ≥ 0.1 IU/mL and ≥ 1.0 IU/mL were calculated with their exact 95% CIs.
As specified in the protocol, potential differences between the ACWY-TT and the Men-PS groups were highlighted in exploratory analyses if the standardized asymptotic 95% CI for the group difference in the percentage of toddlers with titers above the specified cut-offs did not include the value 0, or if the 95% CIs of the GMT ratio between the two groups did not include the value 1. The GMT ratios were calculated using an ANCOVA model on the log10 transformation of the titers or concentrations using the pre-vaccination log10 transformation of the titers or concentrations, the age strata and the vaccine group as covariates. The results of the exploratory group comparisons should be interpreted with caution considering that there was no adjustment for multiplicity of endpoints.
The incidence and intensity of each solicited and unsolicited local and general symptom was calculated with exact 95% CI per age strata for each group. SAEs and NOCIs were described in detail.
The statistical analyses were performed using the SAS® software version 9.1 (SAS Institute Inc.) and Proc StatXact 7.0.
Mencevax ACWY and Nimenrix are trademarks of the GlaxoSmithKline Group of Companies.
Acknowledgments
The authors are indebted to the study participants and their parents, clinicians, nurses and laboratory technicians at the study site as well as to clinical investigators for their contribution to this study. We would also like to thank the following employees of GlaxoSmithKline Vaccines for their valuable contributions: P. Vink for his input into the study design and protocol development; S. Ledant, M. Pulkinen and T. Puumalainen for their assistance in coordination of the study; L. Moerman and M. Paste for assistance in preparation of, or contribution to, study reports; L. Fissette for performing the statistical analysis; and P. Lestrate and K. Maleux for conducting laboratory assays. Finally we thank C. Verbelen (XPE Pharma and Science) who provided medical writing services, and V. Durbecq, J. Gray and N. Van Driessche (XPE Pharma and Science on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Sources of support
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Glossary
Abbreviations:
- (S)AE
(serious) adverse event
- ATP
according to protocol
- CI
confidence interval
- CRM197
mutant diphtheria toxoid
- DT
diphtheria toxoid
- ELISA
enzyme-linked immunosorbent assay
- EU
European Union
- GMT
geometric mean titer
- LL
lower limit
- MenACWY-TT
tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine
- MenPS
meningococcal tetravalent polysaccharide vaccine
- NOCI
new onset of chronic illness
- rSBA
serum bactericidal assay using baby rabbit complement
- SD
standard deviation
- TT
tetanus toxoid
- UL
upper limit
Disclosure of Potential Conflicts of Interest
T.V. received consulting fees as well as support for meetings, travel or accommodation expenses from GlaxoSmithKline group of companies in the past 3 y. M.V.W., V.B., D.B. and J.M. are employees of GlaxoSmithKline group of companies. M.V.W., D.B. and J.M. declare stock ownership in GlaxoSmithKline group of companies. D.B. is also inventor of certain GlaxoSmithKline group of companies patents. A.F. has no conflict to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22165
References
- 1.Harrison LH, Pass MA, Mendelsohn AB, Egri M, Rosenstein NE, Bustamante A, et al. Invasive meningococcal disease in adolescents and young adults. JAMA. 2001;286:694–9. doi: 10.1001/jama.286.6.694. [DOI] [PubMed] [Google Scholar]
- 2.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 3.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285–98. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control. Annual epidemiological report. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. Stockholm ECDC 2011; 155-57. [Google Scholar]
- 5.Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. 2007;31:27–36. doi: 10.1111/j.1574-6976.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC). Surveillance of invasive disease in Europe 2008/09. Available at: http://ecdc.europa.eu/en/publications/Publications/1107_SUR_IBD_2008-09.pdf [Accessed 10/08/2012].
- 7.Kriz P, Wieffer H, Holl K, Rosenlund M, Budhia S, Vyse A. Changing epidemiology of meningococcal disease in Europe from the mid-20th to the early 21st Century. Expert Rev Vaccines. 2011;10:1477–86. doi: 10.1586/erv.11.117. [DOI] [PubMed] [Google Scholar]
- 8.Thulin HS, Toros B, Fredlund H, Olcen P, Molling P. Genetic characterisation of the emerging invasive Neisseria meningitidis serogroup Y in Sweden, 2000 to 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 9.Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala’Aldeen DA, Bayliss CD. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49:506–12. doi: 10.1128/JCM.01322-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The European Meningococcal Disease Society (EMGM). 11th EMGM meeting, May 18-20, 2011, Ljubljana, Slovenia. Emergence of serogroup Y: Poster abstracts P035-P40. [Google Scholar]
- 11.Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(Suppl):S274–9. [PubMed] [Google Scholar]
- 12.Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev. 2006;19:142–64. doi: 10.1128/CMR.19.1.142-164.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bröker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Med Infect Dis. 2010;8:47–50. doi: 10.1016/j.tmaid.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichichero ME. Meningococcal conjugate vaccines. Expert Opin Biol Ther. 2005;5:1475–89. doi: 10.1517/14712598.5.11.1475. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow R, Miller E. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev Vaccines. 2006;5:851–7. doi: 10.1586/14760584.5.6.851. [DOI] [PubMed] [Google Scholar]
- 18.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–7. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 20.de Voer RM, Mollema L, Schepp RM, de Greeff SC, van Gageldonk PG, de Melker HE, et al. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS ONE. 2010;5:e12144. doi: 10.1371/journal.pone.0012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larrauri A, Cano R, García M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097–100. doi: 10.1016/j.vaccine.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 22.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, et al. V59P13 Study Group Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49:e1–10. doi: 10.1086/599117. [DOI] [PubMed] [Google Scholar]
- 24.Pichichero M, Casey J, Blatter M, Rothstein E, Ryall R, Bybel M, et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis J. 2005;24:57–62. doi: 10.1097/01.inf.0000148928.10057.86. [DOI] [PubMed] [Google Scholar]
- 25.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2-10 years of age. Vaccine. 2010;28:657–63. doi: 10.1016/j.vaccine.2009.10.104. [DOI] [PubMed] [Google Scholar]
- 26.Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine. 2009;27(Suppl 2):B13–9. doi: 10.1016/j.vaccine.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Kuo WC, Beri S, Kapre S, Joshi JS, Bouveret N, et al. Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine. 2009;27:726–32. doi: 10.1016/j.vaccine.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 28.Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 29.Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Préziosi M-P, et al. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine. 2007;25(Suppl 1):A101–7. doi: 10.1016/j.vaccine.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 30.Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J. 2011;30:e41–8. doi: 10.1097/INF.0b013e3182054ab9. [DOI] [PubMed] [Google Scholar]
- 31.Bermal N, Huang L-M, Dubey AP, Jain H, Bavdekar A, Lin T-Y, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7:239–47. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 32.Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine. 2010;28:744–53. doi: 10.1016/j.vaccine.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 33.Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J. 2011;30:e56–62. doi: 10.1097/INF.0b013e31820e6e02. [DOI] [PubMed] [Google Scholar]
- 34.Östergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine. 2009;27:161–8. doi: 10.1016/j.vaccine.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 35.Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine. 2011;29:4264–73. doi: 10.1016/j.vaccine.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine. 2011;29:4274–84. doi: 10.1016/j.vaccine.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC) Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 38.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richmond P, Goldblatt D, Fusco PC, Fusco JD, Heron I, Clark S, et al. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine. 1999;18:641–6. doi: 10.1016/S0264-410X(99)00276-5. [DOI] [PubMed] [Google Scholar]
- 40.Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al. The Multilaboratory Study Group Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–67. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine. 2005;23:2222–7. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) Inadvertent misadministration of meningococcal conjugate vaccine--United States, June-August 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1016–7. [PubMed] [Google Scholar]
- 43.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4:316–9. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]