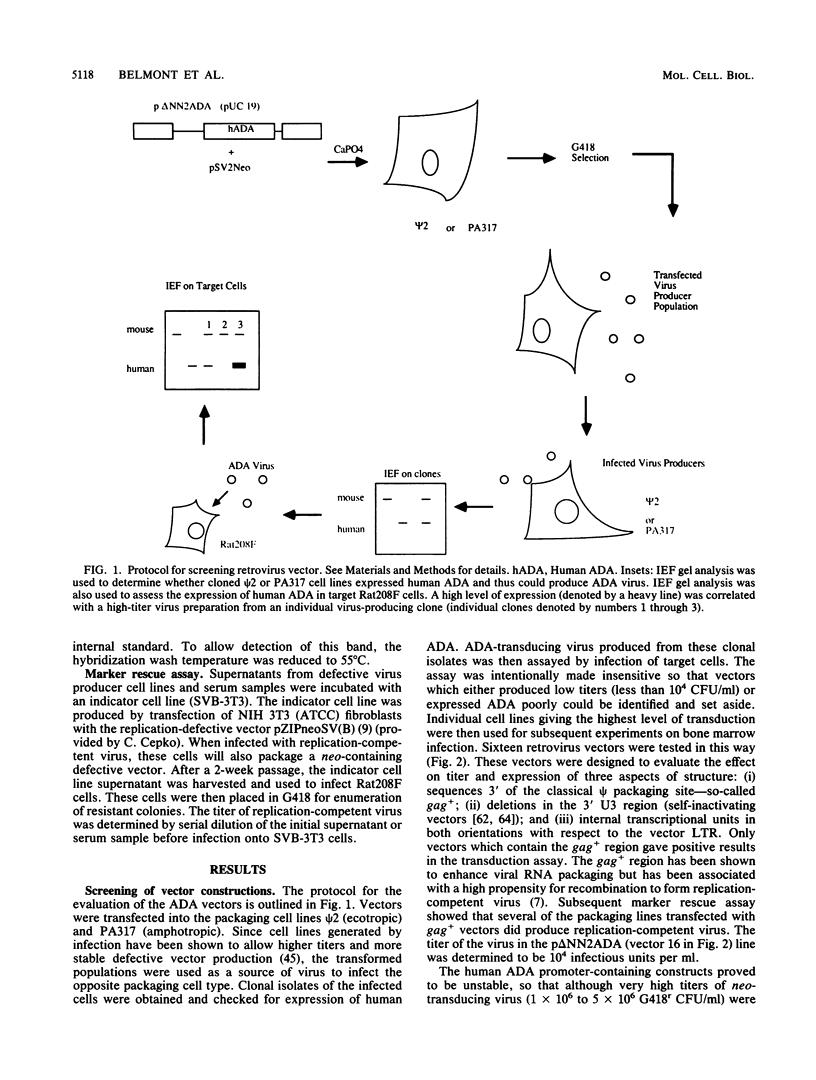
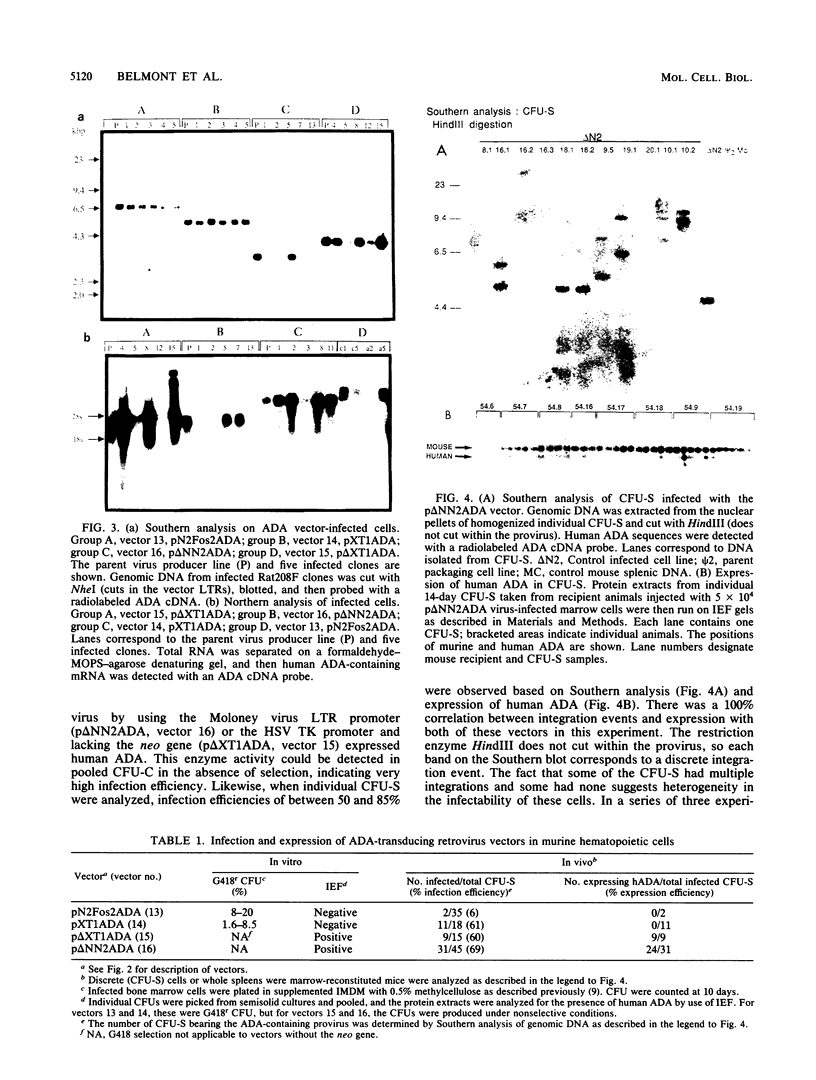
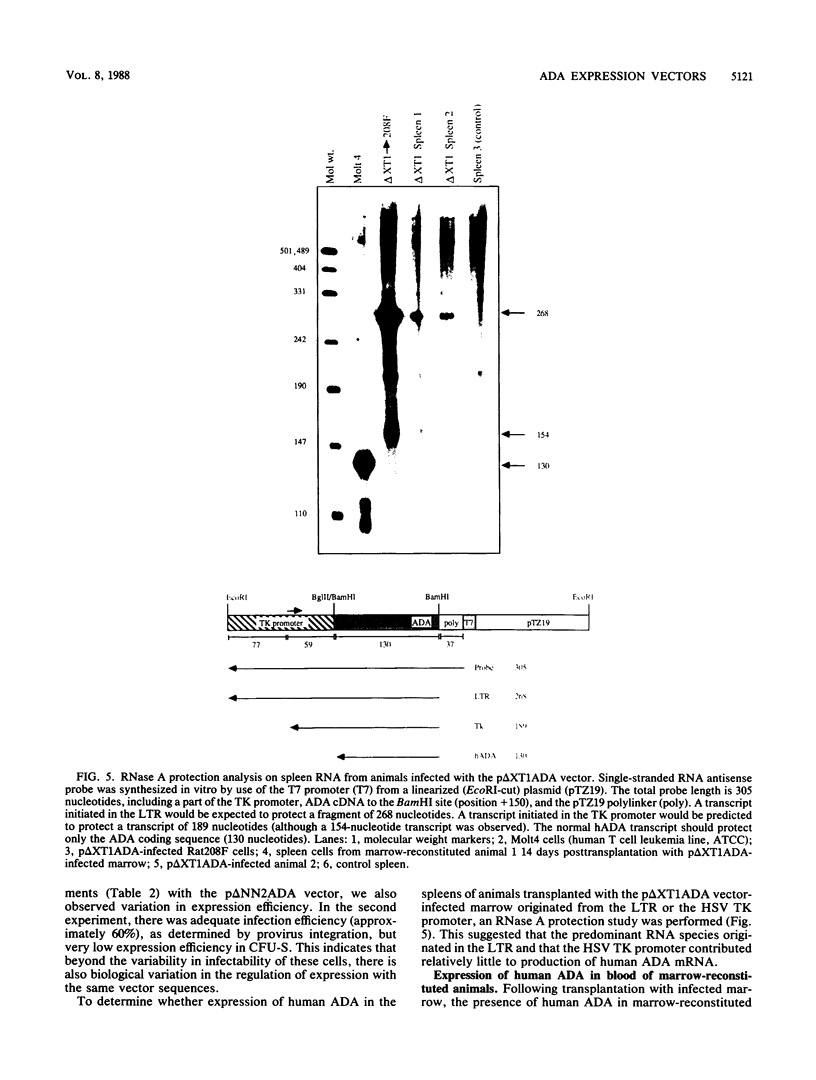
Abstract
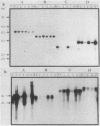
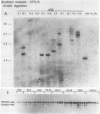
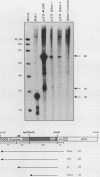
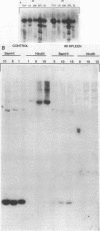
Multiple replication-defective retrovirus vectors were tested for their ability to transfer and express human adenosine deaminase in vitro and in vivo in a mouse bone marrow transplantation model. High-titer virus production was obtained from vectors by using both a retrovirus long terminal repeat promoter and internal transcriptional units with human c-fos and herpes virus thymidine kinase promoters. After infection of primary murine bone marrow with one of these vectors, human adenosine deaminase was detected in 60 to 85% of spleen colony-forming units and in the blood of 14 of 14 syngeneic marrow transplant recipients. This system offers the opportunity to assess methods for increasing efficiency of gene transfer, for regulation of expression of foreign genes in hematopoietic progenitors, and for long-term measurement of the stability of expression in these cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeson A. L., Wiginton D. A., States J. C., Perme C. M., Dusing M. R., Hutton J. J. Mutations in the human adenosine deaminase gene that affect protein structure and RNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5947–5951. doi: 10.1073/pnas.84.16.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont J. W., Henkel-Tigges J., Chang S. M., Wager-Smith K., Kellems R. E., Dick J. E., Magli M. C., Phillips R. A., Bernstein A., Caskey C. T. Expression of human adenosine deaminase in murine haematopoietic progenitor cells following retroviral transfer. Nature. 1986 Jul 24;322(6077):385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter C. A., Wagner E. F. A universal retroviral vector for efficient constitutive expression of exogenous genes. Nucleic Acids Res. 1987 Sep 11;15(17):7194–7194. doi: 10.1093/nar/15.17.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. M., Wager-Smith K., Tsao T. Y., Henkel-Tigges J., Vaishnav S., Caskey C. T. Construction of a defective retrovirus containing the human hypoxanthine phosphoribosyltransferase cDNA and its expression in cultured cells and mouse bone marrow. Mol Cell Biol. 1987 Feb;7(2):854–863. doi: 10.1128/mcb.7.2.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Meijlink F., Verma I. M. Identification of a transcriptional enhancer element upstream from the proto-oncogene fos. Science. 1985 Dec 6;230(4730):1174–1177. doi: 10.1126/science.3865371. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Caskey C. T. Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science. 1987 Apr 17;236(4799):303–305. doi: 10.1126/science.3563511. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Covarrubias L., Hawley T., Mintz B. Handicapped retroviral vectors efficiently transduce foreign genes into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2406–2410. doi: 10.1073/pnas.84.8.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Buckley R. H., Greenberg M. L., Melton A. L., Schiff R., Hatem C., Kurtzberg J., Markert M. L., Kobayashi R. H., Kobayashi A. L. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987 Mar 5;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Krodich N. M. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1978 Nov 17;202(4369):757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner-Maniscalco V., Kuritsky L., Rosen F. S. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981 Dec;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Honig J., Martiniuk F., D'Eustachio P., Zamfirescu C., Desnick R., Hirschhorn K., Hirschhorn L. R., Hirschhorn R. Confirmation of the regional localization of the genes for human acid alpha-glucosidase (GAA) and adenosine deaminase (ADA) by somatic cell hybridization. Ann Hum Genet. 1984 Jan;48(Pt 1):49–56. doi: 10.1111/j.1469-1809.1984.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Huszar D., Balling R., Kothary R., Magli M. C., Hozumi N., Rossant J., Bernstein A. Insertion of a bacterial gene into the mouse germ line using an infectious retrovirus vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8587–8591. doi: 10.1073/pnas.82.24.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Rosner M. R. Characterization of murine-specific leukemia virus receptor from L cells. J Virol. 1986 Jun;58(3):900–908. doi: 10.1128/jvi.58.3.900-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A., Keller G., Phillips R. A., Bernstein A. Retrovirus transfer of a bacterial gene into mouse haematopoietic progenitor cells. Nature. 1983 Oct 6;305(5934):556–558. doi: 10.1038/305556a0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C. F., Hardy C., Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984 Sep;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- Kwok W. W., Schuening F., Stead R. B., Miller A. D. Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4552–4555. doi: 10.1073/pnas.83.12.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli M. C., Dick J. E., Huszar D., Bernstein A., Phillips R. A. Modulation of gene expression in multiple hematopoietic cell lineages following retroviral vector gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(3):789–793. doi: 10.1073/pnas.84.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Wiginton D. A., States J. C., Ward F. E., Bigner S. H., Buckley R. H., Kaufman R. E., Hutton J. J. Identification of a deletion in the adenosine deaminase gene in a child with severe combined immunodeficiency. J Immunol. 1987 May 15;138(10):3203–3206. [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Suh E. J., Hershfield M. S. Regional localization of the human genes for S-adenosylhomocysteine hydrolase (cen----q131) and adenosine deaminase (q131----qter) on chromosome 20. Hum Genet. 1984;66(4):292–295. doi: 10.1007/BF00287630. [DOI] [PubMed] [Google Scholar]
- Petersen M. B., Tranebjaerg L., Tommerup N., Nygaard P., Edwards H. New assignment of the adenosine deaminase gene locus to chromosome 20q13 X 11 by study of a patient with interstitial deletion 20q. J Med Genet. 1987 Feb;24(2):93–96. doi: 10.1136/jmg.24.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Introduction of genes into preimplantation mouse embryos by use of a defective recombinant retrovirus. Proc Natl Acad Sci U S A. 1986 Jan;83(2):366–368. doi: 10.1073/pnas.83.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Cone R. D., Mulligan R. C., Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986 Dec 12;234(4782):1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Vanek M., Wagner E. F. Expression of foreign genes from retroviral vectors in mouse teratocarcinoma chimaeras. EMBO J. 1985 Dec 30;4(13B):3701–3709. doi: 10.1002/j.1460-2075.1985.tb04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Cone R., Mulligan R. C., Jaenisch R. Introduction of a selectable gene into different animal tissue by a retrovirus recombinant vector. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7151–7155. doi: 10.1073/pnas.81.22.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K., Moores J. C., Jolly D. J., Wolff J. A., Respess J. G., Friedmann T. Gene expression from transcriptionally disabled retroviral vectors. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5197–5201. doi: 10.1073/pnas.84.15.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C. Y., Ingolia D. E., Bobonis C., Dunbar B. S., Riser M. E., Siciliano M. J., Kellems R. E. Selective overproduction of adenosine deaminase in cultured mouse cells. J Biol Chem. 1983 Jul 10;258(13):8338–8345. [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]