Abstract
A higher incidence of stomach cancer in ABO blood type A individuals than in those with blood type O has been known for a long time. We studied this association in relation to Helicobacter pylori (Hp) of different cagA status.
For this study we used baseline gastric histopathology data and DNAs from frozen gastric biopsies of 2077 subjects enrolled in a chemoprevention trial for gastric precancerous lesions in Venezuela. We analyzed 6 single nucleotide polymorphisms in the ABO gene and we assessed the presence of the Hp cagA gene. Odds ratios for risk of advanced precancerous gastric lesions were calculated using individuals with normal gastric epithelium or non-atrophic gastritis as a reference.
Among individuals carrying a cagA negative Hp infection or no Hp infection, those with blood type A had a lower risk of intestinal metaplasia and dysplasia than those with blood type O (OR=0.60; 95% CI 0.38-0.94). In carriers of cagA positive Hp strains, individuals with blood type A had a higher risk of intestinal metaplasia or dysplasia than those with blood type O (OR=1.42, 95% CI 1.09-1.86) and a higher risk if compared with subjects carrying cagA− strain and non-A blood group (OR=3.82, 95%CI=2.80-5.20). The interaction between Hp cagA status and blood type was statistically significant (P=0.0006).
We showed that SNPs in the ABO gene, predictive of ABO blood groups, are associated with risk of advanced precancerous gastric lesions in individuals infected with Hp, but the assessment of the risk is strictly dependent on cagA status.
Keywords: Helicobacter pylori, ABO blood groups, risk of preneoplastic gastric lesions
Introduction
Helicobacter pylori (Hp) is one of the most common chronic bacterial infections in humans and it has been acknowledged to be a causative factor for gastric adenocarcinoma. To colonize mucosal surfaces and invade the epithelium, microbes, including Hp, commonly interact with glycan structures of the host glycocalyx. In particular, the adherence of Hp to the human gastric epithelial lining can be mediated by the blood-group antigen-binding adhesin (BabA) that targets human fucosylated blood group antigens H type I (type O substance) and Lewis b (Leb) 1, 2. Secure attachment is crucial for bacteria to transfer their virulence molecules, such as the CagA protein, to host cells. The cagA gene resides within the cytotoxin-associated gene pathogenicity island (cagPAI) of the Hp genome, and is responsible for most of the Hp-associated malignant phenotypes: it triggers IL-8 secretion priming an inflammatory response, promotes cell proliferation, scattering and migration through phosphorylation-dependent and independent mechanisms 3, 4.
A higher proportion of ABO blood type A in gastric cancer patients than in control individuals was noticed as early as in the 1950s 5.
The ABO gene encodes enzymes known as glycosyltransferases which transfer specific sugar residues to a precursor substance (the H antigen) to produce the A and B antigens. Glycosylation is one of the most prevalent modifications mediated by complex enzymatic machinery, whereby glycans (sugars) are covalently attached to specific amino acid sites of proteins. Glycans have important biological functions in protein maturation and turnover, cell adhesion and trafficking, and receptor binding and activation 6.
There are three major alleles at the ABO locus on chromosome 9q34: A, B and O, defined by single base deletions and substitutions (SNP) occurring in exons 6 and 7. The A allele encodes α1→3 N-acetylgalactosaminyltransferase, which adds N-acetylgalactosamine (GalNAc) to the H antigen to form the A antigen. The B allele encodes α1→3 galactosyltransferase which transfers galactose to the H antigen to construct the B antigen 7. The O allele does not produce an active enzyme 7. Four SNPs at nucleotides (nt) 526, 703, 796 and 803 resulting in amino acid substitutions (Arg176Gly, Gly235Ser, Leu266Met and Gly268Ala) explain all the differences in the activity and the nucleotide-sugar donor specificity of the A and B transferases. In addition, a base substitution (rs1053878) at nt 467, resulting in an amino acid substitution (Pro156Leu), distinguishes the A1 from A2 subtypes. A2 is present in approximately 20% of subjects with A blood group among Caucasians and shows an intermediate phenotype, between the “full” enzymatic activity defined by the A1 allele and the nonfunctioning enzyme defined by the O allele 8.
Although the association between ABO blood groups and risk of gastric cancer is well established, very little is known about the possible relation between ABO blood groups and preneoplastic gastric lesions, in particular advanced ones such as intestinal metaplasia and dysplasia. Here, we conducted a study to assess the impact of ABO genotype on the risk of advanced precancerous lesions in a Venezuelan population in relation with the infection with different strains of Hp. In particular we tested the relevance of the presence of the cagA gene which is known to increase the risk of more severe gastric lesions 9.
Materials and Method
Study population
The randomized trial that provided the infrastructure for this study has been described previously 10. Briefly, eligible subjects were participants in the gastric cancer control program of Tachira State, Venezuela, between 35 and 69 years of age. After they gave written informed consent, all subjects underwent gastroscopic examination with collection of gastric biopsies, blood, and urine specimens, and they were administered a questionnaire on sociodemographic and lifestyle variables by a trained interviewer. During the study recruitment period from July 1991 to February 1995, there were 4349 eligible subjects, of whom 2272 were invited to participate in the trial. Of these, 72 refused to participate. All participants signed an informed written consent. The study was approved by the ethical review boards of the institutions responsible for subject recruitment in each of the recruitment centres.
Ethical clearance for the study was obtained from the International Agency for Research on Cancer (IARC) Ethical Committee in Lyon, France, and the Cancer Control Center in San Cristobal, Venezuela.
The presence of the cagA gene in gastric biopsies from the study subjects was previously assessed by reverse hybridization using a line probe assay or a DNA enzyme immunoassay at Delft Diagnostic Laboratory as described 11. Basic characteristics of this study population are presented in Table 1.
Table 1.
Characteristics of study population.
| Characteristics | No.a | (%) | |
|---|---|---|---|
| Gender | Male | 971 | 47.1% |
| Female | 1091 | 52.9% | |
| Age | ≤39 | 479 | 23.2% |
| 40-49 | 753 | 36.5% | |
| 50-59 | 532 | 25.8% | |
| ≥60 | 298 | 14.5% | |
| Years of schooling | 0-5 | 688 | 33.4% |
| 6-8 | 701 | 34.0% | |
| 9+ | 672 | 32.6% | |
| Years of refrigerator use | 0-9 | 276 | 13.4% |
| 10-19 | 348 | 16.9% | |
| 20-29 | 615 | 29.8% | |
| 30+ | 823 | 39.9% | |
| Cigarette smoking | Never | 1459 | 70.8% |
| Ever | 603 | 29.2% | |
| Family history of gastric cancer | No | 1767 | 85.7% |
| Yes | 294 | 14.3% | |
| Hp status | No Hp | 181 | 8.8% |
| cagA− Hp | 567 | 27.5% | |
| cagA+ Hp | 1314 | 63.7% | |
| Histological diagnosis | Normal | 10 | 0.5% |
| Superficial gastritis | 78 | 3.8% | |
| Chronic gastritis | 979 | 47.5% | |
| Atrophic gastritis | 323 | 15.7% | |
| Intestinal metaplasia | 560 | 27.2% | |
| Dysplasia | 112 | 5.4% | |
| Blood groups | O | 1202 | 58.3% |
| A | 690 | 33.5% | |
| B | 141 | 6.8% | |
| AB | 29 | 1.4% |
2200 subjects accepted to participate in the study. For 138 subjects the DNA from biopsies was not available anymore or the quality was insufficient for genotyping, leaving thus a total of 2062 subjects who were included in statistical analyses.
Genotyping
Total DNA was extracted from gastric biopsy specimens after digestion with Proteinase K. Briefly, biopsies were incubated in 250 μL of a solution of 10 mM Tris – HCl (pH 8.0), 5 mM EDTA, 0.1% sodium dodecyl sulfate, and 0.1 mg/mL Proteinase K for at least 2 hours at 55°C. Proteinase K was inactivated by incubation at 95°C for 10 minutes.
In this study we examined 6 single nucleotide polymorphisms (SNPs) on the ABO gene: rs505922 (tagging rs8176719 12), rs1053878, rs8176720, rs8176741, rs8176746 (tagging rs7853989, rs8176743 and rs8176749), and rs8176747. They account for all the variability in the functional polymorphisms and predict the ABO blood groups, as shown in Table 2.
Table 2.
ABO gene SNP selection.
| Blood groups |
|||||||
|---|---|---|---|---|---|---|---|
| cDNAa | aab | SNP | Tagc | O | A1 | A2 | B |
|
|
|||||||
| aa | aa | aa | |||||
| 261 | 87 | rs8176719 | rs5059221 | del (frame shift) | |||
| 293 | 99 | rs8176720 | gly | gly | gly | ||
| 467 | 156 | rs1053878 | pro | leu | pro | ||
| 526 | 176 | rs7853989 | rs8176746 | arg | arg | gly | |
| 657 | 219 | rs8176741 | his | his | his | ||
| 703 | 235 | rs8176743 | rs8176746 | gly | gly | ser | |
| 796 | 266 | rs8176746 | leu | leu | met | ||
| 803 | 268 | rs8176747 | gly | gly | ala | ||
| 930 | 268 | rs8176749 | rs8176746 | leu | leu | leu | |
Position (nucleotide number) within the ABO cDNA
Position (aminoacid/codon number) within the ABO protein
SNP that can be used as surrogate because of complete linkage disequilibrium(r2=1 in HapMap CEU subjects)
Genotyping was performed at the German Cancer Research Center (Heidelberg, Germany) using an allele-specific PCR-based KASPar SNP genotyping system (KBiosciences, Hoddesdon, UK). Thermocycling was performed according to the manufacturer’s instructions. Detection was performed using an ABI PRISM 7900 HT sequence detection system with SDS 2.4 software (Applied Biosystems, Foster City, CA, USA).
Haplotype blocks were constructed from genotyping data using Phase software 13 and SNP tool (http://www.dkfz.de/de/molgen_epidemiology/tools/SNPtool.html)14.
In addition, we typed two SNPs in the cagA gene in position 154 (cagA154_GA) and 858 (cagA858_CT), by allele-specific PCR-based KASPar SNP genotyping system. The presence of the two polymorphic sites has been assessed by sequencing in a small subset of the same population 15. The results obtained with the KASPar assays were compared with the sequencing results with 100% concordance. A sample was defined as cagA positive when it showed a signal in at least two out of three PCRs (i.e. the reverse hybridization/DNA enzyme immunoassay and the two SNP assays).
Statistical analysis
After excluding 138 subjects whose DNA samples were unavailable or failed in ABO genotyping assays, 2062 subjects were left for statistical analysis.
The response variable in this study was histological diagnosis, which was divided into 6 groups: dysplasia, intestinal metaplasia (IM), atrophic gastritis, chronic gastritis, superficial gastritis and normal epithelium. The last three groups were combined to create the control group in this study because the combined frequency of normal epithelium and superficial gastritis in this population was less than 5%. Multinomial logistic regression analysis was employed, using the SAS CATMOD procedure, to estimate odds ratios (ORs) and 95% confidence intervals (CIs) associated with ABO SNPs for atrophic gastritis, IM and dysplasia, in comparison with controls. All ORs were adjusted for basic demographic variables (sex, age and educational levels), and other environmental risk factors reported previously (family history of gastric cancer, cigarette smoking, quintile levels of fruit and starchy vegetable intakes, and duration of refrigerator use) 16. In addition we calculated the ORs for the association between cagA+ HP infection and ABO genotypes in the control group by unconditional logistic regression model including the same covariates.
Results
Basic characteristics of the population included in this study are presented in Table 1. Genotype success was >95%. Blinded duplicate samples (16.7%) included for quality control showed >99% genotype concordance. The genotype frequencies for all SNPs in controls were in accordance with Hardy–Weinberg equilibrium and any deviation from the expected was not statistically significant (data not shown).
We reconstructed the ABO blood groups of the study subjects by using their genotypes at the 6 SNPs we genotyped, as shown in Table 2. The concordance between blood groups assessed by use of genotyping data and serology-obtained blood group data collected at baseline was 96% (data not shown).
We assessed the risk for gastric precancerous lesions, in comparisons with normal epithelium and non-atrophic gastritis, according to blood types and cagA status (Table 3).
Table 3.
Associations between ABO blood types determined by 6 ABO SNPs and risk of gastric precancerous lesions, in comparison with normal epithelium or non-atrophic gastritis
| Normal/non- atrophic gastritis |
Atrophic gastritis | Intestinal metaplasia | Dysplasia | Intestinal metaplasia + Dysplasia |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hp cagA status |
Blood genotypes |
No. | No | ORa | (95% CI) | No. | ORa | (95% CI) | No. | ORa | (95% CI) | No. | ORa | (95% CI) |
| Negative | O | 285 | 59 | 1 | 76 | 1 | 16 | 1 | 92 | 1 | ||||
| B/AB | 44 | 16 | 1.69 | (0.88-3.26) | 14 | 1.18 | (0.60-2.33) | 1 | - | 15 | 1.05 | (0.54-2.02) | ||
| A | 172 | 30 | 0.83 | (0.51-1.36) | 33 | 0.68 | (0.43-1.08) | 2 | - | 35 | 0.60 | (0.38-0.94) | ||
| AO | 155 | 27 | 0.83 | (0.50-1.38) | 31 | 0.70 | (0.43-1.12) | 1 | - | 32 | 0.60 | (0.38-0.95) | ||
| AA | 17 | 3 | 0.89 | (0.24-3.20) | 2 | 0.48 | (0.11-2.17) | 1 | - | 3 | 0.63 | (0.18-2.26) | ||
| A1b | 132 | 24 | 0.84 | (0.49-1.42) | 25 | 0.65 | (0.39-1.07) | 1 | - | 26 | 0.56 | (0.34-0.91) | ||
| A2c | 40 | 6 | 0.81 | (0.32-2.04) | 8 | 0.80 | (0.33-1.82) | 1 | - | 9 | 0.78 | (0.36-1.71) | ||
|
| ||||||||||||||
| Positive | O | 348 | 127 | 1 | 244 | 1 | 47 | 1 | 291 | 1 | ||||
| B/AB | 46 | 19 | 1.12 | (0.63-2.00) | 26 | 0.88 | (0.52-1.48) | 4 | 0.69 | (0.23-2.04) | 30 | 0.85 | (0.51-1.41) | |
| A | 172 | 72 | 1.14 | (0.80-1.61) | 167 | 1.36 | (1.03-1.79) | 42 | 1.78 | (1.11-2.85) | 209 | 1.42 | (1.09-1.86) | |
| AO | 156 | 63 | 1.11 | (0.77-1.59) | 152 | 1.38 | (1.04-1.84) | 38 | 1.78 | (1.09-2.89) | 190 | 1.44 | (1.10-1.90) | |
| AA | 16 | 9 | 1.38 | (0.59-3.26) | 15 | 1.16 | (0.55-2.45) | 4 | 1.77 | (0.55-5.76) | 19 | 1.25 | (0.62-2.54) | |
| A1b | 122 | 46 | 1.02 | (0.69-1.53) | 118 | 1.34 | (0.98-1.83) | 32 | 1.88 | (1.13-3.15) | 150 | 1.42 | (1.06-1.92) | |
| A2c | 50 | 26 | 1.40 | (0.83-2.36) | 49 | 1.40 | (0.90-2.17) | 10 | 1.50 | (0.69-3.23) | 59 | 1.42 | (0.93-2.17) | |
| Pinteractiond | 0.189 | 0.006 | 0.0006 | |||||||||||
Odd ratios were adjusted for age, gender, family history of gastric cancer, smoking status, length of refrigerator use, educational level, fruit and starchy vegetable intakes. Analyses adjusted only for age and gender showed essentially the same results (data not shown). Values in bold are statistically significant (p<0.05). Statistical analysis was not performed for the dysplasia group among cagA negative subjects because of the very small numbers.
A1 includes homozygotes A1/A1 and heterozygotes A1/O
A2 includes heterozygotes A2/A1, heterozygotes A2/O and homozygotes A2/A2
P-value of interaction between Hp cagA status and blood type A
In individuals carrying cagA negative strains or not infected with Hp (748 cases) we found no associations between blood types and risk of atrophic gastritis (105 cases). Individuals with A blood type showed a lower risk of intestinal metaplasia (123 cases) and dysplasia (19 cases) with an OR of 0.60 (95% CI 0.38-0.94) than individuals with blood type O. The association is shown in heterozygous AO subjects with an OR=0.60 (95% CI 0.38-0.95) but not in the homozygotes, due to the small number (3 cases).
In carriers of cagA positive Hp strains (1314 subjects) we detected a higher risk of IM (437 cases) and dysplasia (93 cases) in individuals with blood type A compared with blood type O, with an OR of 1.42 (95%CI 1.09-1.86). Due to the larger numbers in the the cagA positive stratum, it was possible to estimate separate odds ratios for IM (OR=1.36, 95% CI 1.03-1.79), and dysplasia (OR=1.78, 95% CI 1.11-2.85).
We observed a statistically significant interaction between Hp cagA status and blood type, with a P=0.0006 for the combined group of subjects with IM or dysplasia (Table 3).
SNPs in the ABO gene were not associated with risk of cagA positive Hp infection in the subjects without advanced precancerous lesions (normal epithelium to non-atrophic gastritis; data not shown).
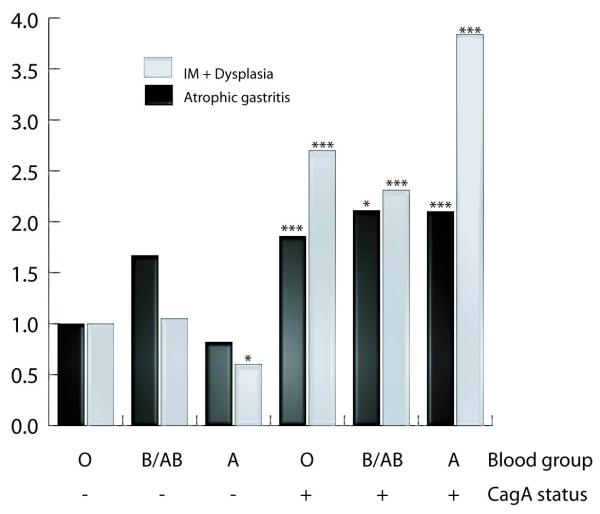
Furthermore, we assessed the risk for advanced gastric precancerous lesions, in comparison with normal epithelium and non-atrophic gastritis, combining blood types and cagA status (Figure 1). For this analysis we used subjects with normal epithelium or non-atrophic gastritis carrying cagA negative strains as reference group. This analysis confirmed a decreased risk of IM or dysplasia in subjects with blood group A and carriers of cagA negative strain (OR=0.60, 95%CI 0.38-0.93). Infection with cagA positive strains showed an increased risk of atrophic gastritis and IM or dysplasia in all subjects, but in particular among subjects with blood group A (OR=2.10; 95%CI 1.41-3.13 for atrophic gastritis and OR=3.84; 95%CI 2.78-5.31 for IM or dysplasia).
Figure 1.
Risk of gastric lesions in subjects infected with different strains of Helicobacter pylori according to cagA status and blood group. Subjects with normal epithelium or non-atrophic gastritis carrying cagA negative strains were considered the reference group (* p<0.05; ***p<0.001)
We also tested if there was an association between individual SNPs and risk of preoneoplastic lesions (Table 4). We found an association with SNP rs505922, which discriminates the O phenotype from A or B: the T allele of the SNP is associated with a increased risk of dysplasia (OR=1.57; 95% CI 1.00-2.48) in carriers of cagA positive strains.
Table 4.
Associations between individual ABO SNPs and risk of gastric precancerous lesions, in comparison with normal epithelium or non-atrophic gastritis.
| ABO SNPs | Comparison | Normal/n on atrophic gastritis |
Atrophic gastritis | Intestinal metaplasia | Dysplasia | Intestinal metaplasia + Dysplasia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No* | No* | ORa | (95% CI) | No* | ORa | (95% CI) | No* | ORa | (95% CI) | No* | ORa | (95%CI) | ||
| CagA negative | ||||||||||||||
| rs505922 | (CT+CC)/TT | 216/283 | 46/58 | 1.02 | (0.66-1.58) | 46/72 | 0.80 | (0.53-1.22) | 3/16 | - | 49/88 | 0.70 | (0.47-1.05) | |
| rs8176720 | (AG+AA)/GG | 361/133 | 75/29 | 1.01 | (0.62-1.64) | 88/33 | 1.01 | (0.64-1.60) | 12/7 | - | 100/40 | 0.95 | (0.62-1.46) | |
| rs8176746 | (AC+AA)/CC | 46/453 | 16/88 | 1.72 | (0.92-3.24) | 14/108 | 1.28 | (0.67-2.46) | 1/18 | - | 15/126 | 1.17 | (0.62-2.21) | |
| rs8176747 | (CG+CC)/GG | 46/455 | 16/89 | 1.71 | (0.91-3.21) | 14/109 | 1.28 | (0.66-2.46) | 1/18 | - | 15/127 | 1.16 | (0.61-2.21) | |
| rs1053878 | (TC+TT)/CC | 52/440 | 9/92 | 0.95 | (0.44-2.03) | 9/109 | 0.71 | (0.35-1.58) | 2/17 | - | 11/126 | 0.82 | (0.41-1.65) | |
| rs8176741 | (TC+TT)/CC | 45/448 | 16/88 | 1.72 | (0.91-3.23) | 14/105 | 1.32 | (0.68-2.54) | 1/18 | - | 15/123 | 1.19 | (0.63-2.27) | |
|
| ||||||||||||||
| Caga positive | ||||||||||||||
| rs505922 | (CT+CC)/TT | 216/345 | 89/124 | 1.14 | (0.82-1.57) | 189/24 3 |
1.25 | (0.96-1.62) | 46/47 | 1.57 |
(1.00-
2.48) |
235/290 | 1.30 | (1.01-1.67) |
| rs8176720 | (AG+AA)/GG | 430/133 | 163/54 | 0.92 | (0.63-1.32) | 329/10 4 |
0.98 | (0.72-1.34) | 73/20 | 1.16 | (0.67-2.02) | 402/124 | 1.01 | (0.75-1.36) |
| rs8176746 | (AC+AA)/CC | 48/518 | 19/198 | 1.02 | (0.58-1.79) | 26/411 | 0.74 | (0.44-1.24) | 4/89 | 0.52 | (0.18-1.50) | 30/500 | 0.70 | (0.43-1.15) |
| rs8176747 | (CG+CC)/GG | 48/518 | 19/199 | 1.01 | (0.58-1.78) | 26/411 | 0.74 | (0.44-1.24) | 4/89 | 0.51 | (0.18-1.50) | 30/500 | 0.70 | (0.43-1.15) |
| rs1053878 | (TC+TT)/CC | 69/493 | 33/183 | 1.29 | (0.82-2.04) | 55/381 | 1.02 | (0.69-1.51) | 10/83 | 0.85 | (0.41-1.75) | 65/464 | 1.00 | (0.68-1.45) |
| rs8176741 | (TC+TT)/CC | 48/502 | 17/186 | 0.94 | (0.52-1.69) | 28/391 | 0.82 | (0.50-1.36) | 4/84 | 0.55 | (0.18-1.60) | 32/475 | 0.79 | (0.48-1.26) |
Odd ratios were adjusted for age, gender, family history of gastric cancer, smoking status, length of refrigerator use, educational level, fruit and starchy vegetable intakes. Analyses adjusted only for age and gender showed essentially the same results (data not shown). Values in bold are statistically significant (p<0.05). SNPs were analyzed according to a dominant model. Statistical analysis was not performed for the dysplasia group among cagA negative subjects because of the very small numbers.
None of the other SNPs showed any statistically significant association with the risk of preneoplastic lesions, either in cagA positive carriers or in cagA negative subjects.
Discussion
ABO genotype has been investigated as a risk factor for a number of different cancer sites. A recent genome-wide association study (GWAS) has revealed associations between variants in the ABO locus, predicting blood groups, and susceptibility to pancreatic cancer 17. The association has been confirmed in other recent studies 8, 12, 18. Studies of other cancer sites that have tested ABO blood groups by genotyping have shown mixed results: they have not confirmed old epidemiological evidence for an association with breast cancer risk and survival 19, 20, nor with risk for colorectal cancer 21, while the B blood group was positively associated with ovarian cancer incidence 22.
The results of the present study suggest that the A allele exerts its biological effects in gastric carcinogenesis in the presence of the bacterial cagA gene. This may account for inconsistent associations between blood type A and gastric cancer observed in earlier studies that did not take into account the prevalence of Hp infection in the study population.
Glycoconjugates, such as the ABO antigen, are important mediators of intercellular adhesion and membrane signaling, which are both critical to the progression and spread of malignant cells 23. Altered expression of ABO blood group antigen has been described in colorectal adenocarcinomas, lung carcinoma and urinary bladder cancer 24. Moreover, as cell surface molecules they are also recognized by the host immune response and may influence immunosurveillance for malignant cells 25.
Studies have found that ABO antigens including H antigen can be present on epidermal growth factor receptor (EGFR), integrins, cadherins, and CD-44 (a cell-surface glycoprotein), which are involved in cell proliferation, cell-cell interaction, cell adhesion and motility, as well as angiogenicity 26, 27.
In addition to gastric cancer, ABO blood types and secretor phenotypes have been associated with various kinds of infection including norovirus, cholera and malaria 28.
Furthermore, recent GWASs suggest that SNPs of the ABO gene are associated with several serum markers of inflammation and cell adhesion: TNF-α, soluble intercellular adhesion molecule-1 (ICAM-1), soluble E-selectin, and soluble P-selectin 29-32. These findings support the possibility that ABO blood group alleles might correlate with systemic inflammatory state and immune cell recruitment, and thereby influence the risk of several cancers 8.
In the gastric epithelium, the ABO blood group antigens and their related carbohydrate structures, such as the Lewis b antigens, are one of the major functional receptors for Hp 8. The observed association between ABO blood groups and risk of Hp-induced gastric cancer can thus be explained by differential binding of the bacterium to the blood group antigens. In particular, on the bacterial side, the binding is mediated by the outer-membrane protein BabA, encoded by the gene baba2 8. baba2-positive Hp strains are associated with an increased risk of gastric adenocarcinoma 1. The binding between BabA and Lewis b antigen is important not only for Hp to adhere to the stomach surface but also to anchor the bacterial secretion system (T4SS) to the host cell surface so that bacterial factors, including the CagA protein, can be effectively injected into the host cell cytosol. This interaction plays an important role in potentiating T4SS-mediated secretion, resulting in inflammation and intestinal metaplasia 33, although we cannot address how specifically blood group type A affects HP attachment to gastric epithelial cells. The presence of babA2 is correlated with the presence of cagA and vacA s1; strains positive for the three genes carry the highest risk of gastric cancer 1.
Some South American (Amerindian) strains use exclusively blood group O antigen for attachment to gastric epithelial cells 34 and Amerindian strains are known to carry distinct genetic structures from Western and Eastern strains. While attenuated virulence of CagA protein from those strains has been reported35, sequence variations in the other regions of the genome 36 may account for reduced risk of advanced precursor lesion with type A compared with type O in CagA-negative patients.
The association between ABO blood groups and Hp infection is still unclear because of discordant results. In particular, nine studies have tested the association between ABO blood group and Hp infection in healthy subjects; two of them have found a statistically significant association between the infection and A blood type (in Bangladeshi young children 37 and Estonian blood donors 38). In other studies no association was detected, either in children 39, 40 or adults 41-44. Other studies that focused on gastric preneoplastic or gastric cancer cases (two of them in the Japanese population 18, 45) showed an increased risk for atrophic gastritis in individuals with blood group A. Nevertheless other studies in different populations on gastritis or gastric cancer patients did not confirm the association 46-48.
We realize that our study has some limitations. First, although the overall study is rather large, the sample sizes for some gastric histologies are rather small. Furthermore another possible limitation with the cross-sectional study design is difficulty in inferring causal relationship for the observed associations because temporal relations between exposures and outcomes are not clear. Yet, the cross-sectional analysis has an advantage in accumulating histological changes developing over several decades as Hp is generally acquired in the childhood in high-risk populations 49. Finally, our study was limited to a Venezuelan population and the results cannot necessarily be extrapolated to other populations.
In summary, our cross sectional study in a Venezuelan population showed that SNPs at the ABO gene, predictive of ABO blood groups, were associated with the risk of more severe gastric preneoplastic lesion depending on cagA status. In particular, in carriers of cagA positive Hp strains, we detected a significantly increased risk of IM and dysplasia associated with blood group A. On the contrary, among individuals carrying cagA negative strains or not infected with Hp, presence of the A blood type showed a strong decrease of risk of dysplasia. These findings suggest that ABO blood group can be considered a risk factor for progression towards gastric cancer in individuals infected with Hp, but the association is highly dependent on Hp cagA status.
Novelty and impact.
We have studied the impact on the risk of advanced precancerous gastric lesions of ABO blood groups and the presence of cagA in a population characterized by high prevalence of Helicobacter pylori (Hp) infection and high rates of gastric cancer.
Our findings suggest that ABO blood groups are associated with risk of advanced precancerous gastric lesions in Hp-infected individuals, but the assessment of the risk is strictly dependent on cagA status.
Acknowledgments
This work was supported by the European Community (CT90-0555 to I.K.) and the US National Cancer Institute (CA 98309 to I.K.).
Footnotes
All the authors report no conflicts of interest
References
- 1.Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96:12778–83. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 3.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Etr SH, Mueller A, Tompkins LS, Falkow S, Merrell DS. Phosphorylation-independent effects of CagA during interaction between Helicobacter pylori and T84 polarized monolayers. J Infect Dis. 2004;190:1516–23. doi: 10.1086/424526. [DOI] [PubMed] [Google Scholar]
- 5.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran AP, Gupta A, Joshi L. Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut. 2011;60:1412–25. doi: 10.1136/gut.2010.212704. [DOI] [PubMed] [Google Scholar]
- 7.Yip SP. Sequence variation at the human ABO locus. Ann Hum Genet. 2002;66:1–27. doi: 10.1017/S0003480001008995. [DOI] [PubMed] [Google Scholar]
- 8.Wolpin BM, Kraft P, Xu M, Steplowski E, Olsson ML, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, et al. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol Biomarkers Prev. 2010;19:3140–9. doi: 10.1158/1055-9965.EPI-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plummer M, van Doorn LJ, Franceschi S, Kleter B, Canzian F, Vivas J, Lopez G, Colin D, Munoz N, Kato I. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst. 2007;99:1328–34. doi: 10.1093/jnci/djm120. [DOI] [PubMed] [Google Scholar]
- 10.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, Garcia R, Buiatti E, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99:137–46. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- 11.van Doorn LJ, Figueiredo C, Rossau R, Jannes G, van Asbroek M, Sousa JC, Carneiro F, Quint WG. Typing of Helicobacter pylori vacA gene and detection of cagA gene by PCR and reverse hybridization. J Clin Microbiol. 1998;36:1271–6. doi: 10.1128/jcm.36.5.1271-1276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–31. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, Wilkening S, Drechsel M, Hemminki K. SNP_tools: A compact tool package for analysis and conversion of genotype data for MS-Excel. BMC Res Notes. 2009;2:214. doi: 10.1186/1756-0500-2-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzato C, Torres J, Plummer M, Munoz N, Franceschi S, Camorlinga-Ponce M, Fuentes-Panana EM, Canzian F, Kato I. Variations in Helicobacter pylori cytotoxin-associated genes and their influence in progression to gastric cancer: implications for prevention. PLoS One. 2012;7:e29605. doi: 10.1371/journal.pone.0029605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato I, Vivas J, Plummer M, Lopez G, Peraza S, Castro D, Sanchez V, Cano E, Andrade O, Garcia R, Franceschi S, Oliver W, et al. Environmental factors in Helicobacter pylori-related gastric precancerous lesions in Venezuela. Cancer Epidemiol Biomarkers Prev. 2004;13:468–76. [PubMed] [Google Scholar]
- 17.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Zheng W, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–90. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakao M, Matsuo K, Hosono S, Ogata S, Ito H, Watanabe M, Mizuno N, Iida S, Sato S, Yatabe Y, Yamao K, Ueda R, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102:1076–80. doi: 10.1111/j.1349-7006.2011.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gates MA, Xu M, Chen WY, Kraft P, Hankinson SE, Wolpin BM. ABO blood group and breast cancer incidence and survival. Int J Cancer. 2011;130:2129–37. doi: 10.1002/ijc.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teresa DB, Santos RA, Takahashi CS, Carrara HH, Moreira HW, Mattos LC, Lia-Neto N, Cunha LA, Bassi CL, Soares EG, Donadi EA, Mello ER, et al. Polymorphisms of Lewis and Secretor genes are related to breast cancer and metastasis in axillary lymph nodes. Tumour Biol. 2010;31:401–9. doi: 10.1007/s13277-010-0048-2. [DOI] [PubMed] [Google Scholar]
- 21.Khalili H, Wolpin BM, Huang ES, Giovannucci EL, Kraft P, Fuchs CS, Chan AT. ABO blood group and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1017–20. doi: 10.1158/1055-9965.EPI-10-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gates MA, Wolpin BM, Cramer DW, Hankinson SE, Tworoger SS. ABO blood group and incidence of epithelial ovarian cancer. Int J Cancer. 2010;128:482–6. doi: 10.1002/ijc.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–66. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 24.Dall’olio F. Protein glycosylation in cancer biology: an overview. Clin Mol Pathol. 1996;49:M126–35. doi: 10.1136/mp.49.3.m126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 26.Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9–31. doi: 10.1111/j.1600-0463.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu M, Wang F, Gildersleeve JC, Achilefu S. MAb L9E10 to blood group H2 antigen binds to colon cancer stem cells and inhibits tumor cell migration and invasion. Hybridoma (Larchmt) 2010;29:355–9. doi: 10.1089/hyb.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–43. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 29.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A, Lu C, Tracy RP, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–72. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR, Paolisso G, Rafiq S, Simon-Sanchez J, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4:e1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pare G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S, Miletich JP, Ridker PM. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4:e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE, Shen E, Mirea L, Bharaj B, Sun L, Bull SB. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958–67. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, Boren T, Haas R, Sasakawa C, Mimuro H. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256–64. doi: 10.1074/jbc.M111.233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, Lundberg C, Arnqvist A, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–22. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Kiga K, Kersulyte D, Cok J, Hooper CC, Mimuro H, Sanada T, Suzuki S, Oyama M, Kozuka-Hata H, Kamiya S, Zou QM, et al. Attenuated CagA oncoprotein in Helicobacter pylori from Amerindians in Peruvian Amazon. J Biol Chem. 2011;286:29964–72. doi: 10.1074/jbc.M111.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kersulyte D, Lee W, Subramaniam D, Anant S, Herrera P, Cabrera L, Balqui J, Barabas O, Kalia A, Gilman RH, Berg DE. Helicobacter Pylori’s plasticity zones are novel transposable elements. PLoS One. 2009;4:e6859. doi: 10.1371/journal.pone.0006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhuiyan TR, Qadri F, Saha A, Svennerholm AM. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J. 2009;28:79–85. doi: 10.1097/INF.0b013e31818a5d9d. [DOI] [PubMed] [Google Scholar]
- 38.Klaamas K, Kurtenkov O, Ellamaa M, Wadstrom T. The Helicobacter pylori seroprevalence in blood donors related to Lewis (a,b) histo-blood group phenotype. Eur J Gastroenterol Hepatol. 1997;9:367–70. doi: 10.1097/00042737-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Jafarzadeh A, Ahmedi-Kahanali J, Bahrami M, Taghipour Z. Seroprevalence of anti-Helicobacter pylori and anti-CagA antibodies among healthy children according to age, sex, ABO blood groups and Rh status in south-east of Iran. Turk J Gastroenterol. 2007;18:165–71. [PubMed] [Google Scholar]
- 40.Wu TC, Chen LK, Hwang SJ. Seroprevalence of Helicobacter pylori in school-aged Chinese in Taipei City and relationship between ABO blood groups. World J Gastroenterol. 2003;9:1752–5. doi: 10.3748/wjg.v9.i8.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson MS, Cade JF, Savoia HF, Clancy RL. Helicobacter pylori infection in the Australian community: current prevalence and lack of association with ABO blood groups. Intern Med J. 2003;33:163–7. doi: 10.1046/j.1445-5994.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 42.Loffeld RJ, Stobberingh E. Helicobacter pylori and ABO blood groups. J Clin Pathol. 1991;44:516–7. doi: 10.1136/jcp.44.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hook-Nikanne J, Sistonen P, Kosunen TU. Effect of ABO blood group and secretor status on the frequency of Helicobacter pylori antibodies. Scand J Gastroenterol. 1990;25:815–8. doi: 10.3109/00365529008999220. [DOI] [PubMed] [Google Scholar]
- 44.Sasidharan S, Uyub AM. Prevalence of Helicobacter pylori infection among asymptomatic healthy blood donors in Northern Peninsular Malaysia. Trans R Soc Trop Med Hyg. 2009;103:395–8. doi: 10.1016/j.trstmh.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Shibata A, Hamajima N, Ikehara Y, Saito T, Matsuo K, Katsuda N, Tajima K, Tatematsu M, Tominaga S. ABO blood type, Lewis and Secretor genotypes, and chronic atrophic gastritis: a cross-sectional study in Japan. Gastric Cancer. 2003;6:8–16. doi: 10.1007/s101200300001. [DOI] [PubMed] [Google Scholar]
- 46.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrovic M, Artiko V, Novosel S, Ille T, Sobic-Saranovic D, Pavlovic S, Jaksic E, Stojkovic M, Antic A, Obradovic V. Relationship between Helicobacter pylori infection estimated by 14C-urea breath test and gender, blood groups and Rhesus factor. Hell J Nucl Med. 2011;14:21–4. [PubMed] [Google Scholar]
- 48.Yei CJ, Chang JG, Shih MC, Lin SF, Chang CS, Ko FT, Lin KY, Liu TC. Lewis blood genotypes of peptic ulcer and gastric cancer patients in Taiwan. World J Gastroenterol. 2005;11:4891–4. doi: 10.3748/wjg.v11.i31.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]