Abstract
Methotrexate (MTX) is an anchor drug used to treat rheumatoid arthritis (RA), but responsiveness is variable in effectiveness and toxicity. Methotrexate and its polyglutamate conjugates (MTXPGn) in red blood cells (RBC) have been associated with patient response. In the current study, 13 collagen-induced arthritic (CIA) rats and 12 healthy rats were given subcutaneous doses of either saline or 0.3 or 1.5 mg/kg per 2 days of MTX from day 21 to 43 post-induction. Blood samples were obtained at various times to measure MTX in plasma, and MTX and MTXPGn in RBC. Effects on disease progression were indicated by body weight and paw size. After multiple-doses, RBC MTX reached steady-state (82.4 nM) within 4 days. The MTXPG2 and MTXPG3 in RBC kept increasing until the end of the study attaining 12.5 and 17.7 nM. Significant weight loss was observed after dosing of 1.5 mg/kg/2 days, whereas moderate effectiveness was observed after dosing of 0.3 mg/kg/2 days. A pharmacokinetic/ pharmacodynamic/disease (PK/PD/DIS) model with indirect mechanisms and transduction components incorporating plasma MTX, RBC MTX, and RBC MTXPGn concentrations, and paw size was developed using naïve data pooling and ADAPT 5. The PK/PD in CIA rats dosed at 0.3 mg/kg/2 days were captured well by our proposed model. MTX showed modest (Imaxd = 0.16) but sensitive (IC50d = 0.712 nM) effectiveness on paw edema. The higher dose produced toxicity. The proposed model offers improved understanding of MTX effects on rheumatoid arthritis.
Keywords: Methotrexate, rheumatoid arthritis, pharmacokinetics, pharmacodynamics, disease progression
Introduction
Rheumatoid arthritis (RA) is a systemic, autoimmune, and acute-on-chronic inflammatory disorder that primarily targets synovial joints [1]. It significantly impacts quality of life and produces joint pain, stiffness, and swelling due to synovial inflammation and effusion. In severe cases, RA leads to erosions of the inflamed sites, joint deformity, disability, and even reduced life expectancy [2]. It affects about 1.5 million US adults [3]. To treat RA, four major types of drugs are available: non-steroid anti-inflammatory drugs (NSAID), disease-modifying anti-rheumatic drugs (DMARD), biologic agents, and corticosteroids. Methotrexate (MTX) is used most frequently worldwide. It affects several targets, such as apoptosis of activated lymphocytes, and adenosine and cytokine modulation [4, 5]. Such alterations can reduce joint inflammation and bone destruction, two major RA symptoms [6, 7]. The effectiveness of MTX in RA varies from 46–65% with response as defined by American College of Rheumatology (ACR) 20 criteria [8]. It is the anchor drug among the DMARD and is accepted internationally as the first choice in management of RA [9]. In routine clinical care of patients with RA, a comparative analysis in Finland and the United States reported that the ‘early’ use of MTX (within 5 years after disease onset) increased from less than 5% in Finland and less than 25% in Nashville (TN, USA) in 1980 to more than 90% in 2004 in both countries. This was associated with substantially improved outcomes [10].
Although low-dose MTX (7.5–30 mg/week) has been used to treat RA for 30 years, its therapeutic strategy is trial-and-error partly due to an unclear relationship between plasma MTX concentrations and response [11]. There is high inter-individual variability (IIV) in PK and PD for unknown reasons, which leads to unpredictable responses and adverse events [12]. Recently, PK profiles and PD mechanisms of MTX were utilized to explore reasons for high IIV. After subcutaneous (SC) injection, MTX is absorbed rapidly in humans and rats with average bioavailability of 70–80% [13]. After entering blood, MTX is transported into red blood cells (RBC), bone marrow myeloid precursors, white blood cells, hepatocytes, fibroblasts, and synoviocytes, mainly via the reduced folate carrier (RFC) [14]. Once inside cells, MTX is further metabolized to conjugates by folylpolyglutamate synthetase (FPGS) forming up to 6 glutamates (MTXPG2 – MTXPG7) [15]. Gamma glutamyl hydrolase (GGH) removes terminal MTX glutamate to form MTX conjugates with fewer glutamates and finally returns MTXPGn to its monoglutamate form (MTX), which is rapidly transported out of cells by multidrug resistance-associated proteins. Although the mechanism of action is uncertain [16], intracellular MTXPGn may be a necessary mediator of anti-inflammatory effects [17] and was reported to be associated with MTX effectiveness [18–21]. Therefore, erythrocyte MTXPGn [22, 23] and polymorphism of those proteins transporting and forming intracellular MTXPGn [18, 24] were studied to identify responders in RA patients treated with MTX. However, genotypes of those transporters and enzymes showed inconclusive prediction performance. Non-genetic factors such as age, dosage, and renal function, were not considered [22, 25]. Theoretically, MTXPGn in RBC can relate to responses directly. Therefore, it is possible that MTXPGn concentrations in RBC may better predict therapeutic response.
The dynamic effects of MTX mediated by MTXPGn on RA disease progression and toxicology have been incompletely assessed. Use of PK/PD/Disease (PK/PD/DIS) modeling can quantitatively interpret disease progression and assess drug effects in a mechanistic manner [26]. Several physiologically-based PK, multiple-compartment PK, and PK/PD/toxicity models were utilized to describe the kinetics of MTX and MTXPGn in plasma, tissues [27], and selected cells [28] to seek relationships between PK and effectiveness and toxicity [29, 30]. However, none of these models related MTX effectiveness to intracellular MTXPGn. The collagen-induced arthritis (CIA) model in rats is an animal disease model which closely reflects major aspects of human RA such as chronic inflammation, involvement of both B and T-cells, and absence of bacteria and mycobacterium components [31, 32]. We utilized the CIA rat model to investigate the effects of MTX on disease progression and toxicology and developed a mechanistic model incorporating RBC MTXPGn.
Materials and Methods
Drugs and Compounds
Methotrexate (MTX) was purchased from Sigma Inc. (Thousand Oaks, CA). The 4-amino-10-methylpteroyldiglutamic acid (MTXPG2), 4-amino-10-methylpteroyltriglutamic acid (MTXPG3), 4-amino-10-methylpteroyltetraglutamic acid (MTXPG4), 4-amino-10-methylpteroylpentaglutamic acid (MTXPG5), 4-amino-10-methylpteroylhexaglutamic acid (MTXPG6), and 4-amino-10-methylpteroylheptaglutamic acid (MTXPG7) were obtained from Schircks Lab (Jona, Switzerland). Methotrexate-methyl-D3 (MTX-D3) was acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Dithiothreitol (DTT), citric acid, and ascorbic acid were obtained from Sigma-Aldrich Co. (St. Louis, MO).
Animals
Thirty-eight male Lewis rats, aged 6 – 9 weeks, were purchased from Harlan (Indianapolis, IN), weight-matched to approximately 150 g. Animals were housed individually in the University Laboratory Animal Facility and acclimatized for 1 week under constant temperature (22°C), humidity (72%), and 12-h light/12-h dark cycle. Rats had free access to rat chow and water. All protocols followed the Principles of Laboratory Animal Care (Institute of Laboratory Animal Resources, 1996) and were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Induction of Collagen-induced Arthritis
The induction of collagen-induced arthritis in Lewis rats followed protocols and reagents supplied by Chondrex, Inc. (Redmond, WA). Porcine collagen type II (2 mg/mL) in 0.05 M acetic acid was emulsified with incomplete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO) following procedures described in our earlier study [33].
Experimental Design
Healthy and CIA rats were utilized. In order to minimize stress to animals in the PD study, a separate group of rats were used for serial blood sampling for PK analysis. Thirty-six rats were induced by collagen injection and boosted 7 days later. Two healthy rats and additional induced rats without arthritis (paw size increases less than 15% in either paw on day 20, n=12) were randomly divided into 2 groups. Groups received SC injections of 0.3 mg/kg every two days (PKL group, n=6), or 1.5 mg/kg every 2 days (PKH group, n=6) for PK studies from day 21 (peak of disease progression) to day 43. MTX was dissolved in saline adjusted to pH 8.6. After evaluation of paw edema induction on day 20, 13 CIA rats with paw size increase of at least 50% in one or two paws were selected and randomly assigned to three groups for PD study. Animals received SC injections of saline solution (pH 8.6) (PDC group, n=4), 0.3 mg/kg (PDL group, n=4), or 1.5 mg/kg (PDH group, n=5) of MTX every two days from days 21 to 43. On day 46, PKL and PDL groups continued to be dosed with 0.3 mg/kg. In PKL and PKH groups, blood samples were collected from the saphenous vein using EDTA-coated capillary tubes and transferred to amber tubes on ice. Blood was obtained for plasma MTX and erythrocyte MTXPGn concentrations at 0.5, 1, 4, and 10 hr on day 1 of drug treatment; before dosing every two days during day 21 through 41; and 0.5, 1, 3, 6, 10, and 24 hr post-dosing on day 46. On day 46, the same blood sampling scheme was applied to the PDL group. RBC numbers were determined using a Cell-Dyn Emerald (Abbott Laboratories, Abbott Park, IL). Remaining blood (100 μL) was centrifuged at 2000 × g for 10 min at 4°C. The plasma fraction was immediately transferred into a polyethylene tube on ice and stored frozen at −80°C until assay for MTX. The RBC were washed with 3-fold volumes of ice-cold saline twice. Packed RBC were mixed with 50 μL of saline and stored frozen at −80°C until assayed for MTX and MTXPGn.
Measurement of Edema and Body Weight
Edema was indicated by size of the rat hind paws [33]. Two cross-sectional areas were determined with digital calipers (VWR Scientific, Rochester, NY), one on the rat forefoot (paw) and the other at the ankle. Two measurements were made on each section, perpendicular to each other, to define the length and height of the ellipse from which the area was determined. Measurements were made side-to-side and top-to-bottom across the paw at the base of the last food pad. Ankle measurements were made side-to-side (length a) and front-to-back (length b) at a 45° angle across the ankle. The area contained in the ellipse is: Area = π · a/2 · b/2. Edema was indicated by the sum of paw and ankle area measures for each hind foot. Paw size measurements and body weights were obtained for PDC, PDL, and PDH groups before induction (0 day) and post-induction on 3, 7, 12, 15, 17, 19, 21, 22, 23, 25, 27, 29, 31, 33, 35, 37, 41, and 43 days.
Bioanalytical Methodology
Plasma MTX, RBC MTX, and RBC MTXPG2–7 concentrations were measured using a validated HPLC-MS/MS method. Around 100 μL of plasma or suspended RBC was mixed with 25 μL of perchloric acid followed by addition of 400 μL of internal standard solution containing 2.5 nM of MTX-D3, 5% of anti-oxidation buffer (0.5% dithiothreitol (DTT) and 10% of both citric acid and ascorbic acid) in 100 mM ammonium acetate (pH 6.8). The mixed solution was vortexed for 1 min followed by centrifugation at 13000 × g for 10 min at 4°C. The supernatant was loaded on an Oasis HLB cartridge (1 cc, Waters Co., Milford, MA) equilibrated with 1.0 mL of methanol followed by 0.8 mL of 100 mM ammonium acetate buffer (pH 6.8) containing 5% of anti-oxidation buffer (buffer A). The loaded cartridge was washed with 0.8 mL of buffer A followed by elution with 0.8 mL of methanol/water (9/1, v/v). Eluted solutions were collected in a drying cartridge and evaporated by nitrogen gas at 35°C. Dried residue was reconstituted with acetonitrile/water (1/9, v/v) containing 5% of anti-oxidation buffer and stored at 8°C until analysis. Reconstitution solutions were separated on the Symmetry Shield RP8 (2.1×50 mm, 3.5 μm, Waters Co.) kept at 35°C using a gradient flow of mobile phase consisting of buffer B (water / acetonitrile = 95/5 v/v, containing 0.1% formic acid) and buffer C (acetonitrile / water = 95/5 v/v, containing 0.1% formic acid). The extracted samples were analyzed using a SIL-20A high liquid performance chromatography system (Shimadzu Co., Kyoto, JP), including autosampler, binary pumps, column oven, and controller. The analytes were determined by API 3000 tandem mass spectrometry (Applied Biosystems, Ontario, Canada). The MTXPGn were determined by multiple reaction monitoring mode with positive ionization using MTX-D3 as internal standard. The LLOQ were: MTX in plasma (20 nM), MTX and MTXPG2–4 in RBC (2 nM), MTXPG5 in RBC (5 nM), and MTXPG6–7 in RBC (10 nM).
Validation studies assessed the intra- and inter-run accuracy and precision, stability of RBC samples at room temperature for 2 hr and after 2 cycles of freeze-thaw, and stability of reconstitution samples at 8°C for 24 hr, which met the FDA bioassay guidance [34]. No significant matrix effects were seen. Results were normalized to a RBC count of 8 ×1012 cells to offset variation in RBC counts between individuals and disease periods [35].
PK/PD/DIS Model
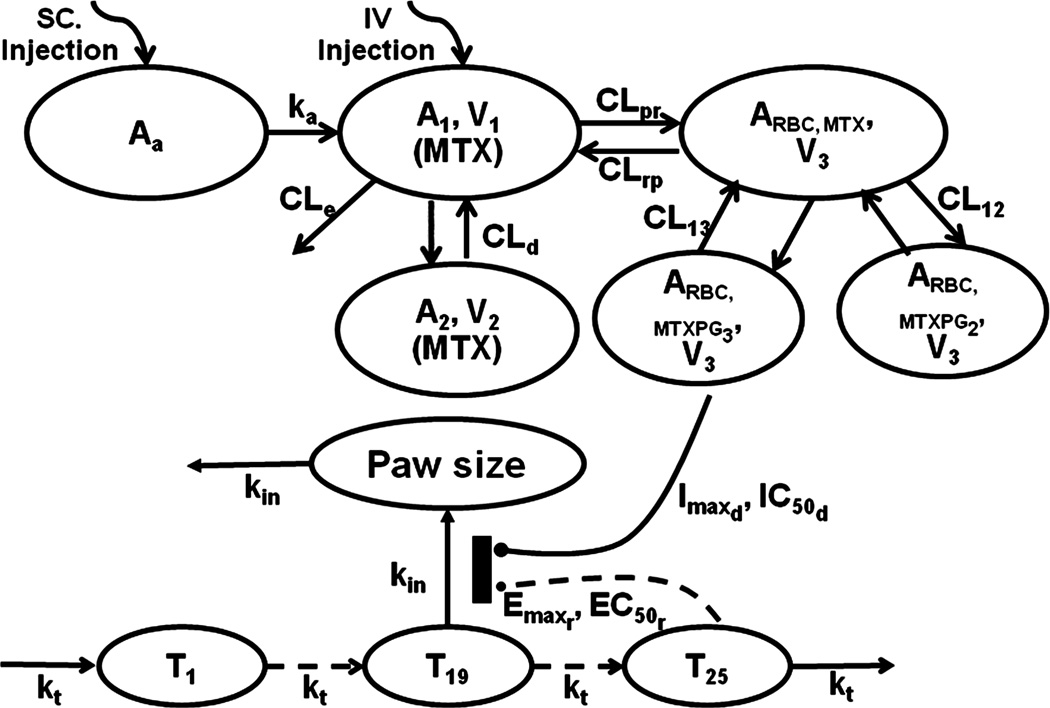
A two-compartment model (2CM) with an injection site absorption compartment was used for MTX. An additional three-compartment model with RBC volume (V3) was used to describe the transformation from MTX to MTXPG2 and MTXPG3 in RBC (Figure 1). The MTX in plasma can enter RBC (CLpr) to form MTXPG2 (CL12) and MTXPG3 (CL13), which can be metabolized back to MTX followed by return to plasma (CLrp). Because MTXPGn were only observed in RBC, the rat RBC volume, V3, was used. The model equations and initial conditions are
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where Aa, A1, and A2 are amounts of MTX in the drug absorption, central (V1) and peripheral (V2) compartments, ARBC,MTX, ARBC,MTXPG2, and ARBC,MTXPG3 are amounts of MTX, MTXPG2, and MTXPG3 in erythrocytes, CLe is elimination clearance of MTX, CLd is the distribution clearance between V1 and V2, and ka is the absorption rate constant. In order to accurately estimate distribution and elimination parameters without interference by absorption processes after SC injection, plasma MTX concentration-time data after IV injection was adapted from literature [36]. In this study, 0.1, 0.5, and 2.5 mg/kg of MTX was given to fasted rats (n=5) and blood samples collected over 8 hours. Mean concentrations of plasma MTX from the three dose groups were digitized to allow simultaneous estimation of PK parameters with our present data.
Figure 1.
Schematic of the PK/PD/DIS model for the effects of MTX on paw size and disease progression in CIA rats. Tables 1 and 2 provide definitions of parameters.
A transduction-based feedback model was used to describe disease progression and drug effects (Fig. 1) [37]. The model equations are
| (7) |
| (8) |
| (9) |
| (10) |
where T1, T2, ⋯, Tn (n=25) are paw sizes (Paw) in transduction compartments, kt is a transduction rate constant, kdis is the ratio of maximum paw size to Paw0 at disease steady-state without remission and drug effects, Imaxd and IC50d are maximum effect of MTX on paw size and RBC MTXPG3 concentration at 50% of Imaxd, the Emaxr EC50r are maximum effects of natural remission and paw size difference at 50% of Emaxr, kgrowth is the natural growth rate constant of paw size in healthy rats, and kin is the transduction rate constant between compartment T19 and Paw. The initial values for all transit steps, including T1, T2, ⋯, Tn, and Paw size (Paw), were set as Paw0.
Since no difference was observed in PK profiles between healthy and CIA rats, the two groups shared the same PK parameters. The PK and PD/DIS data were naïvely pooled firstly followed by sequential model fitting using the maximum likelihood algorithm in ADAPT 5 (Biomedical Simulations Resource, Los Angeles, CA). The variance model used in both sequential fittings is Vi = (σ1 + σ2×Yi)2, where Vi is the variance of the ith data point, σ1 and σ2 are variance model parameters, and Yi is the ith model prediction. All fitting procedures utilized Windows Vista with the Intel® Visual Fortran Compiler (Version 11.0). Graphical diagnostic analyses were performed using S-Plus (TIBCO Spotfire, Somerville, MA) software.
Results
Toxicology
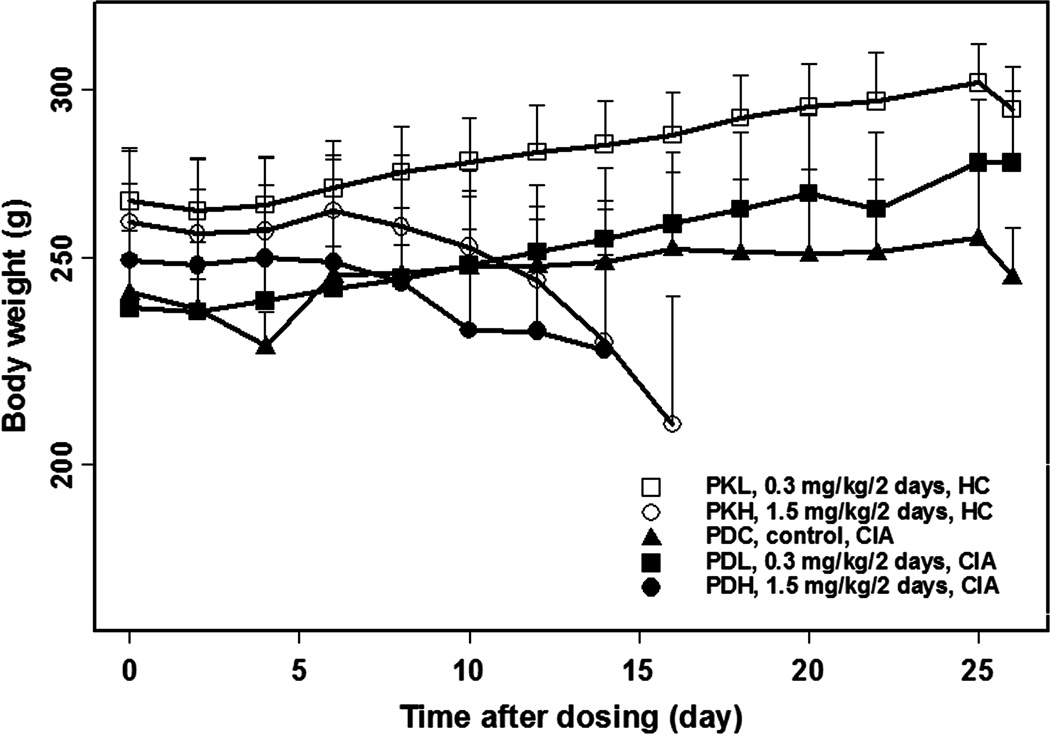
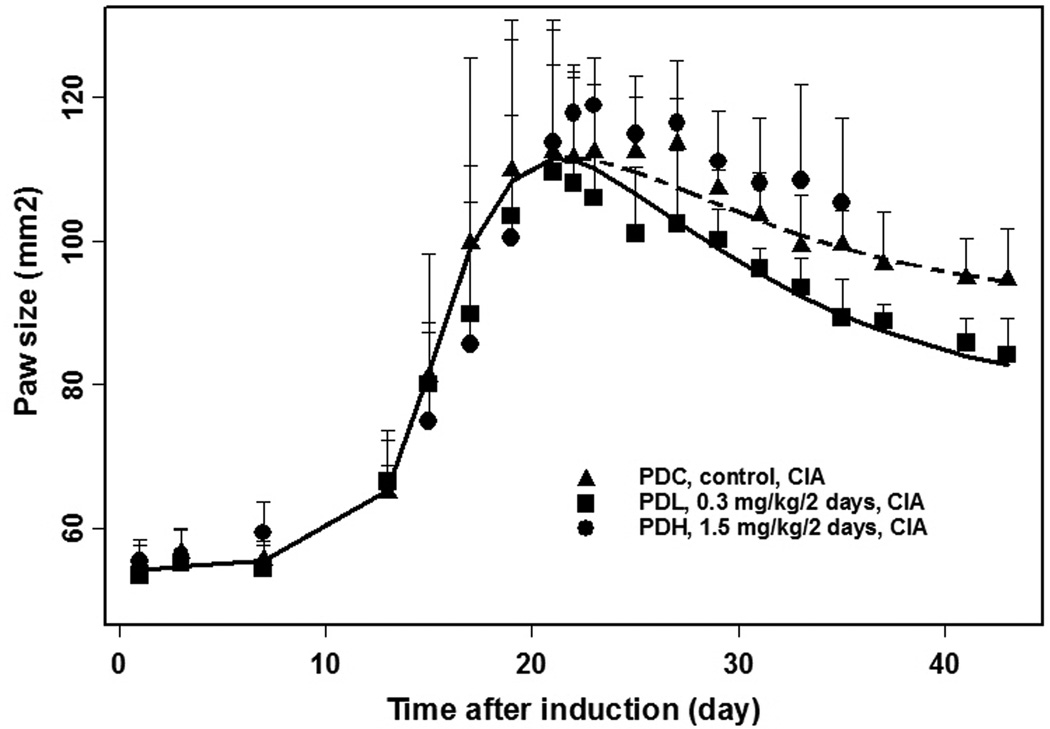
In the CIA rat study, 36 rats were induced with porcine collagen and boosted 7 days later. Thirteen of the 36 induced rats developed arthritis and were given 0, 0.3, and 1.5 mg/kg/2 days of MTX. Ten of 36 induced rats without arthritis and two additional induction-free rats were given 0.3 and 1.5 mg/kg/2 days of MTX. Body weights (Figure 2) of healthy and CIA rats receiving 1.5 mg/kg/2 days were stable over the first 8 days after dosing and then lost weight. All showed nose bleeding, diarrhea, or rapid body weight loss by day 35, consistent with MTX-induced toxicity. Therefore, CIA rats in the PKH and PDH groups were sacrificed before or on day 35. Paw size time curves of rats in the high dose group are shown in Figure 3. Body weights (Figure 2) of rats receiving 0.3 mg/kg/2 days increased faster than the control group, indicating no MTX-induced toxicity in this group. Therefore, the low dose group was utilized for modeling, whereas the high dose data were not used as being confounded by MTX toxicity.
Figure 2.
Body weight versus time curves for rats in the indicated study groups.
Figure 3.
Observed and model-fitted paw size versus time curves for CIA rats in three study groups.
Pharmacokinetics
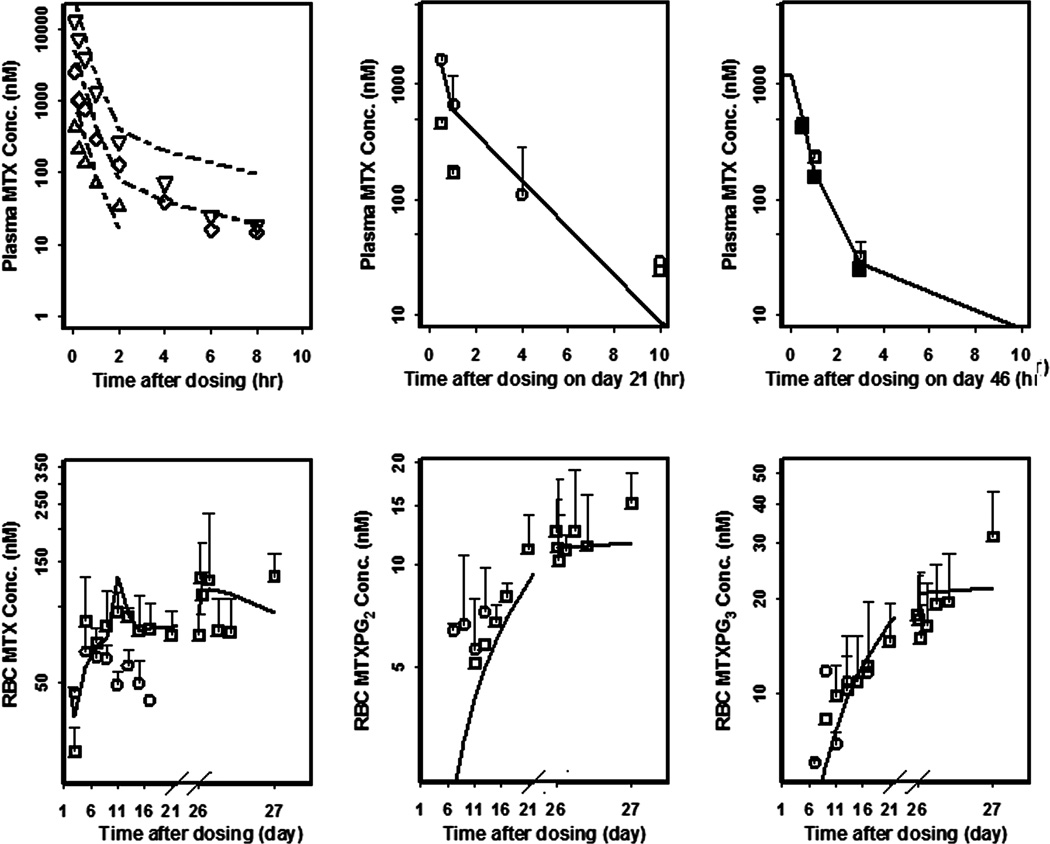
Short-term and trough concentration - time curves of MTX, MTXPG2, and MTXPG3 in healthy and CIA rats are presented in Figure 4. After SC injection, plasma MTX concentrations reached Cmax within 0.5 hour. The Cmax was 3.5-times greater in the high dose group than in low dose group. Thereafter, MTX concentrations decreased biexponentially. The terminal phase concentrations were similar to those after IV dosing [36]. Plasma MTX and RBC MTX, MTXPG2, and MTXPG3 concentrations were comparable between healthy and CIA rats at steady-state (Figure 4). For the PKL group, RBC MTX concentrations reached steady-state on day 4 whereas RBC MTXPG2 and MTXPG3 concentrations kept increasing to the end of the study. After multiple-dosing of 0.3 mg/kg/2 days of MTX, mean trough concentrations of RBC attained 82.4 nM for MTX, 12.5 nM for MTXPG2, and 17.7 nM for MTXPG3 after 23 days of dosing (Figure 4). The RBC MTX trough concentrations are lower in the high dose group than in the low dose group except for the first dosing and even decreased after multiple-dosing. The RBC MTXPG2 and MTXPG3 concentrations in the high dose group are slightly higher than or comparable to those in the low dose group.
Figure 4.
Observed (symbols) and model-fitted (lines) plasma MTX, RBC MTX, MTXPG2, and MTXPG3 concentration versus time curves of rats after IV single injection (from literature) of 0.1 (△), 0.5 (◇), and 2.5 mg/kg (▽), and SC injection of 0.3 (PKL□; PDL ■) and 1.5 mg/kg/2 days (PKH○).
Since MTX-induced toxicity in the high dose group was inexplicably accompanied by decreased RBC MTX trough concentrations, these data were not used in modeling. Only the low dose data from healthy and CIA rats were utilized. The 2CM with the ka input well captured MTX PK profiles. A physiological RBC volume was employed for all RBC concentrations using a value of 0.024 L/kg from healthy rats [38]. Biotransformation clearances were added (CL12 and CL13) along with MTX influx clearance of CLpr and efflux clearance of CLrp. Limited post-dose sampling did not support PK estimates of absorption and distribution phases. Therefore, we digitized literature data for model support. The first-order absorption rate constant (ka) was fixed to 3.69 hr−1 scaled with an exponential constant of −0.031 as calculated from monkey (ka = 2.84 hr−1, body weight = 8.8 kg) [39] and human (ka = 0.36 hr−1, body weight = 76 kg) [40] SC injection data.
Plasma MTX concentrations (Figure 4) were captured well for the present study and all three dose groups digitized from the literature except for the terminal phase in the high dose group (2.5 mg/kg), where a slight systematic underestimation was found. This deviation was not relevant to our PK/PD/DIS model fitted with low dose data.
The final estimates of PK parameters are listed in Table 1. The systemic clearance (CLe) was 0.409 L/hr/kg. The V1 (0.174 L/kg) is higher than plasma volume (0.0312 L/kg). The V2 (0.326 L/kg) is comparable to intracellular fluid volume (0.371 L/kg). The influx and efflux rate constants are very small (0.00564 and 0.012 hr−1) which suggests that MTX takes days to enter and exit erythrocytes. The metabolic rate constants are even lower, consistent with the slow accumulation of the metabolites. Precision of all parameter estimations in this PD/DIS model are less than 40%, reflective of reliable estimates.
Table 1.
Pharmacokinetic parameters for MTX and MTXPGn in healthy and CIA rats
| Parameter (units) | Definition | Estimate | CV% |
|---|---|---|---|
| ka (hr−1) | First-order absorption rate | 3.69 | FIXEDa |
| CLe (L/hr/kg) | Systemic clearance | 0.409 | 9.05 |
| V1 (L/kg) | Volume of central compartment | 0.174 | 16.4 |
| CLd (L/hr/kg) | Distribution clearance | 0.0707 | 19.8 |
| V2 (L/kg) | Volume of peripheral compartment | 0.326 | 35.8 |
| CLpr (L/hr/kg) | Influx clearance of MTX from V1 to RBC | 0.000979 | 19.8 |
| CL12 (L/hr/kg) | Metabolic clearance of MTX to MTXPG2 in RBC | 0.00000491 | 7.04 |
| CL13 (L/hr/kg) | Metabolic clearance of MTX to MTXPG3 in RBC | 0.00000958 | 6.18 |
| CLrp (L/hr/kg) | Efflux clearance of MTX from RBC to V1 | 0.000289 | 18.5 |
| V3 (L/kg) | Volume of RBC | 0.024 | Fixedb |
| kpr (hr−1) | Influx rate constant of MTX from V1 to RBC | 0.00564 | c |
| k12 (hr−1) | Metabolic rate constant of MTX to MTXPG2 in RBC | 0.00020 | c |
| k23 (hr−1) | Metabolic rate constant of MTX to MTXPG3 in RBC | 0.0004 | c |
| krp (hr−1) | Efflux rate constant of MTX from RBC to V1 | 0.0120 | c |
Parameter fixed to value scaled from monkeys and human data in literature
Parameter fixed to value from healthy rats in literature
Secondary parameter
Pharmacodynamics and Disease Progression
The time course of paw sizes in CIA rats are displayed in Figure 3. The control group exhibited typical disease onset followed by a remission phase. After low doses (0.3 mg/kg/2 days) of MTX, paw size rapidly decreased in the first 4 days. This group also showed greater increases in body weights (Figure 2). Both profiles suggest that low dose MTX is effective in CIA rats. The final estimated PK/PD/DIS parameters are listed in Table 2. The kgrowth was fixed as 0.0130 mm2/hr as reported [33] for similar rats. The logistic growth rate constant (kdis) was 19.1 and maximum effect of natural remission (Emaxr) was 0.939, which accounts for paw sizes at the remission phase. The paw size at 50% of maximum remission (EC50r) was 24.5 mm2, which indicates that the feedback inhibition on paw growth occurs after paw sizes reach 24.5 mm2 above baseline. Maximum effect of MTX on paw size (Imaxd) was 0.160 and the RBC MTXPG3 IC50d was 0.712 nM. The former value means paw swelling is modestly relieved by MTX with the potential of 16% reduction.
Table 2.
Pharmacodynamic/disease parameters for MTX in CIA rats
| Parameter (units) | Definition | Estimate | CV% |
|---|---|---|---|
| kdis | Ratio of max. paw size to Paw0 at disease steady-state without remission and drug effects | 19.1 | 154 |
| kt (hr−1) | Transduction rate between T1~T25 | 0.0432 | 17.4 |
| Paw0 (mm2) | Baseline of paw size | 54.2 | 2.0 |
| kin (hr−1) | Transduction rate between T19 and Paw | 0.00417 | 77.7 |
| kgrowth (mm2/hr) | Natural growth of paw size | 0.0130 | Fixeda |
| Emaxr | Maximum effect of natural remission | 0.939 | 9.85 |
| EC50r (mm2) | Paw size for 50% of Emaxr | 24.5 | 78.6 |
| Imaxd | Maximum effect of MTX on paw size | 0.160 | 49.7 |
| IC50d (nM) | RBC MTXPG3 conc. for 50% of Imaxd | 0.712 | 498 |
Parameter fixed to values from healthy rats in literature
Discussion
We measured RBC MTX polyglutamates and observed three MTX glutamates in rat RBC. The RBC MTX was associated with adverse effects earlier than body weight changes. Based on our understanding of MTX mechanism of action, we constructed a PK/PD/DIS model to relate effects to exposure of MTX metabolites. As in vitro studies reported that MTXPG3 is ten-fold more potent than MTX and MTXPG2 for inhibition of the major anti-RA target enzyme, 5-aminoimidazole-4-carbox-amide ribonucleotide (AICAR) transformylase [41], MTXPG3 was utilized in Eq. 10 to inhibit paw size in CIA rats in our PK/PD/DIS model.
MTX can temporarily elevate liver enzymes in 10–43% of RA patients. After four years of MTX treatment, mild and severe fibrosis occurs in 15.3% and 1.3% of patients [42]. Monitoring biochemical factors every 2–4 weeks was recommended in RA patients over the first 4 months followed by monitoring every 8–12 weeks in RA patients taking stable doses of MTX [43]. We observed that trough RBC MTX concentrations were higher in rats in the high dose group than in the low dose group on day 2 whereas it was opposite for the rest of the time. This inverse RBC MTX concentration was observed 4 days earlier than reduced body weight, also an indicator of MTX-induced toxicity in mice [29]. Since intracellular MTX exerts anti-folic acid action and causes side effects thereafter [44], decreased RBC MTX concentrations may predict side effects earlier than those biochemical factors produced after damage. Concentrations (mean ± SD) in liver were MTX (440 ± 215 nmol/g), MTXPG2 (600 ± 35 nmol/g), MTXPG3 (855 ± 442 nmol/g), MTXPG4 (234 ±132 nmol/g), and total MTXPGn (2128 ± 330 nmol/g) in rats with MTX-induced toxicity. After single doses of 0.2 mg/kg of MTX in CIA rats and multiple-dosing of 4 mg/kg/week of MTX in monkeys, total concentration of MTX and MTXPGn in liver were around 1000 and 2800 nmol/g tissue [45, 46], which are comparable to our values.
MTX was absorbed quickly after SC injection with a scaled ka of 3.69 hr−1 in rats. After entering blood, plasma MTX concentrations decreased biexponentially. The sum of V1 of 0.174 and V2 of 0.326 L/kg are comparable to body water volume [38], which suggests moderate distribution of the drug. Plasma MTX concentrations for the 3 dose levels merged in the terminal phase [36], which suggested saturable nonlinear distribution [47]. This might be due to active transport for influx and efflux of MTX by reduced folate carrier (RFC), ABCB1, and ABCC1 transporters on cell membranes [48], which are saturable, pH- and energy-dependent [49]. To capture the saturable distribution, we tested nonlinear influx and efflux clearance in our MTX PK model, which did not yield precise estimates. Since mature erythrocytes lose their nucleus in the last stage of erythropoiesis, it cannot supply energy for ATP-dependent transporters on RBC membranes [50]. Therefore, MTX may be taken up during formation of erythrocytes and persist throughout the RBC lifespan. This is the same fate as folate in erythrocytes [51]. However, adding a time-dependent volume of RBC in our model did not work. The estimated long turnover time of influx, efflux, and transformation of RBC MTX in our model includes rat RBC lifespans of 55 days [52]. The estimated CLe is comparable to glomerular filtration rate in healthy rats (0.314 L/hr/kg) [38] considering 70–80% bioavailability after SC injection [13] and predominantly renal excretion [53]. After 23 days of dosing, RBC MTX (82.4 nM), MTXPG2 (12.5 nM), and MTXPG3 (17.7 nM) concentrations were estimated well in our model. These concentrations are comparable to corresponding values (30, 30, and 60 nM) in RA patients [22]. The RBC MTX reached steady-state quickly, whereas MTXPG2 and MTXPG3 kept increasing. The RBC MTXPG3 concentrations are slightly higher than MTXPG2, which was also observed in patients with RA [54]. In humans, RBC MTXPG3 concentrations are even higher than RBC MTX, which suggests specific binding for RBC MTXPG3 or different conjugation and degradation rates. To model this special profile using our limited data, different metabolic rates were tested to capture the various concentration-time curves. Using different metabolic rate constants for FPGS and GGH to describe the conversions from MTX to MTXPG2 and from MTXPG2 to MTXPG3 was found to overestimate MTXPG2 concentrations. Studies of the intracellular transformation and distribution processes for MTX are warranted.
In human RA, a latent phase of years with only immune responses evident is followed by the emergence of several pathological inflammatory symptoms such as joint destruction, infections, or osteoporosis [2]. Many cells (e.g. T cells, B cells, and macrophages) and their products (e.g. cytokines, rheumatoid factors, and chemokines) are involved. This disease progression can be mimicked in a short time frame by the CIA model in rats [31, 32], in whom four phases of disease progression were observed: a long delay to develop rheumatoid arthritis after induction, rapid disease worsening with a sharp increase of paw size, a natural remission, and new disease steady-state caused partly by irreversible damage. Gamma functions and an empirical biexponential equation can be utilized to capture the long delay of inflammatory disease. However, in comparison of transduction models, they are less flexible when dose-response relationships, receptor dynamics, and efficiency of the transduction process are considered [37]. The present number of transit steps provided optimal fittings of disease progression. In order to capture all four phases in a natural way, a transduction-based feedback model with logistic growth rate (Figure 1) was applied. Natural growth (kgrowth) in healthy rats was also incorporated. Briefly, 19 transduction steps (T1-T19) were selected to account for the time delay to sharp increase of paw size and 5 more transduction steps (T21-T25) were used to account for the time delay of natural remission after disease onset. A different transduction rate (from T19 to paw size, kin) was needed to fit paw sizes. A logistic growth rate constant (kdis) was used to capture rapid increases in paw size followed by a new disease steady-state. Without the natural remission feedback, paw size was estimated to increase by 19.1-fold above baseline, which is similar to our previous study [37]. A nonlinear negative feedback linking the paw size difference between baseline and disease was utilized to capture the natural remission. A larger increase of paw size will produce greater feedback, but the negative feedback function cannot obviate the entire paw swelling (0.939 of Emaxr) due to irreversible damage of the paw. MTX dosing with 0.33–2.5 mg/kg on alternate days was reported to improve arthritic symptoms by 70% in CIA rats [55, 56]. In those studies, MTX was given shortly after disease onset. A moderate but obvious effectiveness (Imax = 0.16) was observed in the present study. Lesser effects were caused by the later treatment in the present study than in reported studies because timing of drug administration appears to be of considerable importance [37, 57]. The IC50d of RBC MTXPG3 was estimated to be 0.712 nM, which means arthritis is sensitive to MTX.
A sensitivity analysis was performed to assess the reliability of IC50d and Imaxd by assessing differences in fittings with higher and lower values. In accordance with the high CV%, the fittings are insensitive to a 100-fold range of IC50d values. In contrast, the more critical parameter, Imaxd, was reliably estimated as indicated by the low CV% (49.7%) and divergence in fittings with 5-fold changes in assigned Imaxd values.
Conclusions
The PK/PD and toxicity of MTX in CIA rats were investigated. Multiple doses of 1.5 mg/kg/2 days of MTX produced, significant toxicity. These animals exhibited lower RBC MTX concentrations than after dosing of 0.3 mg/kg/2 days. After multiple doses of 0.3 mg/kg/2 days of MTX in CIA rats, RBC MTX reached steady-state (82.4 nM) within 4 days. The RBC MTXPG2 and MTXPG3 kept increasing to the end of the study. Our PK/PD/DIS model incorporating plasma MTX, RBC MTX and MTXPGn, and paw size captured the PK/PD profiles of MTX and disease progression. MTX showed moderate (Imaxd = 0.16) but sensitive (IC50d = 0.712 nM) effectiveness on paw edema in CIA rats.
Acknowledgments
This study was supported by NIH Grant GM24211 and Fellowship support from Hoffman La Roche Inc.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 3.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van VRF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5:531–541. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 5.Colmegna I, Ohata BR, Menard HA. Current understanding of rheumatoid arthritis therapy. Clin Pharmacol Ther. 2012;91:607–620. doi: 10.1038/clpt.2011.325. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM. Toward a better understanding of methotrexate. Arthritis Rheum. 2004;50:1370–1382. doi: 10.1002/art.20278. [DOI] [PubMed] [Google Scholar]
- 7.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 8.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 9.Schipper LG, Kievit W, den Broeder AA, et al. Treatment strategies aiming at remission in early rheumatoid arthritis patients: starting with methotrexate monotherapy is cost-effective. Rheumatology (Oxford) 2011;50:1320–1330. doi: 10.1093/rheumatology/ker084. [DOI] [PubMed] [Google Scholar]
- 10.Sokka T, Pincus T. Ascendancy of weekly low-dose methotrexate in usual care of rheumatoid arthritis from 1980 to 2004 at two sites in Finland and the United States. Rheumatology (Oxford) 2008;47:1543–1547. doi: 10.1093/rheumatology/ken316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafforgue P, Monjanel-Mouterde S, Durand A, Catalin J, Acquaviva PC. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1995;22:844–849. [PubMed] [Google Scholar]
- 12.Dawson AH, Grygiel JJ. Variability of methotrexate pharmacokinetics and pharmacodynamics. Agents Actions Suppl. 1988;24:226–235. doi: 10.1007/978-3-0348-9160-8_21. [DOI] [PubMed] [Google Scholar]
- 13.Grim J, Chladek J, Martinkova J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet. 2003;42:139–151. doi: 10.2165/00003088-200342020-00003. [DOI] [PubMed] [Google Scholar]
- 14.Chabner BA, Allegra CJ, Curt GA, et al. Polyglutamation of methotrexate. Is methotrexate a prodrug. J Clin Invest. 1985;76:907–912. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panetta JC, Sparreboom A, Pui CH, Relling MV, Evans WE. Modeling mechanisms of in vivo variability in methotrexate accumulation and folate pathway inhibition in acute lymphoblastic leukemia cells. PLoS Comput Biol. 2010;6:e1001019. doi: 10.1371/journal.pcbi.1001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- 17.Chan ES, Cronstein BN. Methotrexate--how does it really work. Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 18.Dervieux T, Furst D, Lein DO, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005;64:1180–1185. doi: 10.1136/ard.2004.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung N, Ellingsen T, Attermann J, Stengaard-Pedersen K, Poulsen JH. Patients with rheumatoid arthritis treated with methotrexate (MTX): concentrations of steady-state erythrocyte MTX correlate to plasma concentrations and clinical efficacy. J Rheumatol. 2008;35:1709–1715. [PubMed] [Google Scholar]
- 20.Angelis-Stoforidis P, Vajda FJ, Christophidis N. Methotrexate polyglutamate levels in circulating erythrocytes and polymorphs correlate with clinical efficacy in rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:313–320. [PubMed] [Google Scholar]
- 21.Brooks AJ, Begg EJ, Zhang M, Frampton CM, Barclay ML. Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit. 2007;29:619–625. doi: 10.1097/FTD.0b013e31811f39bb. [DOI] [PubMed] [Google Scholar]
- 22.Stamp LK, O'Donnell JL, Chapman PT, et al. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum. 2009;60:2248–2256. doi: 10.1002/art.24653. [DOI] [PubMed] [Google Scholar]
- 23.Dervieux T, Zablocki R, Kremer J. Red blood cell methotrexate polyglutamates emerge as a function of dosage intensity and route of administration during pulse methotrexate therapy in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:2337–2345. doi: 10.1093/rheumatology/keq216. [DOI] [PubMed] [Google Scholar]
- 24.Stamp LK, Roberts RL. Effect of genetic polymorphisms in the folate pathway on methotrexate therapy in rheumatic diseases. Pharmacogenomics. 2011;12:1449–1463. doi: 10.2217/pgs.11.86. [DOI] [PubMed] [Google Scholar]
- 25.Stamp LK, Chapman PT, O'Donnell JL, et al. Polymorphisms within the folate pathway predict folate concentrations but are not associated with disease activity in rheumatoid arthritis patients on methotrexate. Pharmacogenet Genomics. 2010;20:367–376. doi: 10.1097/FPC.0b013e3283398a71. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Bhattaram AV, Jadhav PR, et al. Leveraging prior quantitative knowledge to guide drug development decisions and regulatory science recommendations: impact of FDA pharmacometrics during 2004–2006. J Clin Pharmacol. 2008;48:146–156. doi: 10.1177/0091270007311111. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff KB, Dedrick RL, Zaharko DS, Longstreth JA. Methotrexate pharmacokinetics. J Pharm Sci. 1971;60:1128–1133. doi: 10.1002/jps.2600600803. [DOI] [PubMed] [Google Scholar]
- 28.Panetta JC, Yanishevski Y, Pui CH, et al. A mathematical model of in vivo methotrexate accumulation in acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2002;50:419–428. doi: 10.1007/s00280-002-0511-x. [DOI] [PubMed] [Google Scholar]
- 29.Lobo ED, Balthasar JP. Pharmacokinetic-pharmacodynamic modeling of methotrexate-induced toxicity in mice. J Pharm Sci. 2003;92:1654–1664. doi: 10.1002/jps.10431. [DOI] [PubMed] [Google Scholar]
- 30.Johansson AM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF. A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit. 2011;33:711–718. doi: 10.1097/FTD.0b013e31823615e1. [DOI] [PubMed] [Google Scholar]
- 31.Wooley PH. The usefulness and the limitations of animal models in identifying targets for therapy in arthritis. Best Pract Res Clin Rheumatol. 2004;18:47–58. doi: 10.1016/j.berh.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Hegen M, Keith JC, Jr, Collins M, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis. 2008;67:1505–1515. doi: 10.1136/ard.2007.076430. [DOI] [PubMed] [Google Scholar]
- 33.Earp JC, DuBois DC, Molano DS, et al. Modeling corticosteroid effects in a rat model of rheumatoid arthritis I: mechanistic disease progression model for the time course of collagen-induced arthritis in Lewis rats. J Pharmacol Exp Ther. 2008;326:532–545. doi: 10.1124/jpet.108.137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2001
- 35.Stamp LK, O'Donnell JL, Chapman PT, et al. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis Rheum. 2010;62:359–368. doi: 10.1002/art.27201. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda T, Namba K, Torimaru T, Kawashima K, Hayashi M. Species differences in oral bioavailability of methotrexate between rats and monkeys. Biol Pharm Bull. 2000;23:334–338. doi: 10.1248/bpb.23.334. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Lon HK, DuBois DC, Almon RR, Jusko WJ. Population pharmacokinetic-pharmacodynamic-disease progression model for effects of anakinra in Lewis rats with collagen-induced arthritis. J Pharmacokinet Pharmacodyn. 2011;38:769–786. doi: 10.1007/s10928-011-9219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 39.Balis FM, Mirro J, Jr, Reaman GH, et al. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol. 1988;6:1882–1886. doi: 10.1200/JCO.1988.6.12.1882. [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, de Laar Mv. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645–648. [PubMed] [Google Scholar]
- 41.Allegra CJ, Drake JC, Jolivet J, Chabner BA. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci U S A. 1985;82:4881–4885. doi: 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albrecht K, Muller-Ladner U. Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S95–S101. [PubMed] [Google Scholar]
- 43.Yazici Y. Long-term safety of methotrexate in the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S65–S67. [PubMed] [Google Scholar]
- 44.Shahin AA, Ismail MM, Saleh AM, Moustafa HA, Aboul-Ella AA, Gabr HM. Protective effect of folinic acid on low-dose methotrexate genotoxicity. Z Rheumatol. 2001;60:63–68. doi: 10.1007/s003930170075. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman CL, Franz TJ, Slattery JT. Pharmacokinetics of the poly-gamma-glutamyl metabolites of methotrexate in skin and other tissues of rats and hairless mice. J Pharmacol Exp Ther. 1984;231:242–247. [PubMed] [Google Scholar]
- 46.Winick NJ, Kamen BA, Balis FM, Holcenberg J, Lester CM, Poplack DG. Folate and methotrexate polyglutamate tissue levels in rhesus monkeys following chronic low-dose methotrexate. Cancer Drug Deliv. 1987;4:25–31. doi: 10.1089/cdd.1987.4.25. [DOI] [PubMed] [Google Scholar]
- 47.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–532. doi: 10.1023/a:1014414520282. [DOI] [PubMed] [Google Scholar]
- 48.Bansard C, Lequerre T, Daveau M, et al. Can rheumatoid arthritis responsiveness to methotrexate and biologics be predicted. Rheumatology (Oxford) 2009;48:1021–1028. doi: 10.1093/rheumatology/kep112. [DOI] [PubMed] [Google Scholar]
- 49.Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. [PubMed] [Google Scholar]
- 50.Tavassoli M. Red cell delivery and the function of the marrow-blood barrier: a review. Exp Hematol. 1978;6:257–269. [PubMed] [Google Scholar]
- 51.Carmel R. Megaloblastic Anemias: Disorders of Impaired DNA Synthesis. In: John P, Greer JF, Lukens aJN, editors. Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins Publishers; 2003. [Google Scholar]
- 52.Allison AC. Turnovers of erythrocytes and plasma proteins in mammals. Nature. 1960;188:37–40. doi: 10.1038/188037a0. [DOI] [PubMed] [Google Scholar]
- 53.Bannwarth B, Pehourcq F, Schaeverbeke T, Dehais J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin Pharmacokinet. 1996;30:194–210. doi: 10.2165/00003088-199630030-00002. [DOI] [PubMed] [Google Scholar]
- 54.Dalrymple JM, Stamp LK, O'Donnell JL, Chapman PT, Zhang M, Barclay ML. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:3299–3308. doi: 10.1002/art.24034. [DOI] [PubMed] [Google Scholar]
- 55.Williams A, Goodfellow R, Topley N, Amos N, Williams B. The suppression of rat collagen-induced arthritis and inhibition of macrophage derived mediator release by liposomal methotrexate formulations. Inflamm Res. 2000;49:155–161. doi: 10.1007/s000110050575. [DOI] [PubMed] [Google Scholar]
- 56.Xinqiang S, Fei L, Nan L, et al. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed Pharmacother. 2010;64:463–471. doi: 10.1016/j.biopha.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Lon HK, Liu D, Zhang Q, DuBois DC, Almon RR, Jusko WJ. Pharmacokinetic-pharmacodynamic disease progression model for effect of etanercept in lewis rats with collagen-induced arthritis. Pharm Res. 2011;28:1622–1630. doi: 10.1007/s11095-011-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]