Abstract
Background
There is little information regarding gender-specific measurements of colonic transit and anorectal function in patients with defecation disorders (DD).
Aim
To compare overall colonic transit by gender in DD.
Methods
In 407 patients with constipation due to DD diagnosed by a single gastroenterologist (1994– 2012), DD was characterized by anorectal manometry, balloon expulsion test, and colonic transit by scintigraphy. The primary endpoint was overall colonic transit (geometric center, GC) at 24hours (GC24). Effects of gender in DD on colonic transit, and comparison with transit in 208 healthy controls were assessed by Mann-Whitney rank sum test. Secondary endpoints were maximum anal resting (ARP) and squeeze (ASP) pressures. We also tested association of the physiological endpoints among DD females by pregnancy history and among DD patients by colectomy history.
Results
The DD patients were 67 males (M) and 340 females (F). Significant differences by gender in DD patients were observed in GC24 (median: M: 2.2; F: 1.8; p=0.01), ARP (median: M: 87.8mmHg; F: 82.4mmHg; p=0.04), and ASP (median: M: 182.4mmHg; F: 128.7mmHg; p<0.001). GC24 was slower in DD compared to same gender healthy controls. GC24 did not differ among DD females by pregnancy history. Anorectal functions and upper GI transit did not differ among DD patients by colectomy history.
Conclusions
Patients with DD have slower colonic transit compared to gender-matched controls. Among DD patients, males have higher ARP and ASP, and females have slower colonic transit. Although the clinical significance of these differences may be unclear, findings suggest that interpretation of these tests in suspected DD should be based on same gender control data.
Keywords: anismus, dyssynergia, constipation, male, female, anal sphincter
Defecation disorder (DD) may be described by various terms including “dyssynergic defecation,” “anismus,” “pelvic floor dyssynergia,” and “outlet dysfunction,” and is characterized by paradoxical contraction or inadequate relaxation of the pelvic floor muscles during attempted defecation (1). The exact prevalence of defecation disorder (DD) in the general population is unknown1, and estimates from tertiary referral centers among patients with chronic constipation range widely from 20–81% (1–5). Although it is recognized as a frequent cause of chronic constipation with a prevalence of 2–27% in Western countries (6,7), adequate recognition and treatment remain unsatisfactory (8–10). Presence of symptomatic criteria may not be sufficient to accurately identify patients with DD (11). Furthermore, there may be significant overlap between DD and other causes of chronic constipation, most notably slow transit constipation [STC (12)]; in fact, colonic transit measured by scintigraphy was not significantly different in DD and STC (3).
Diagnosis is based on the symptoms of constipation, digital rectal examination and physiologic tests such as anorectal manometry (ARM) or electromyography (EMG) showing evidence of uncoordinated defecation with abnormal balloon expulsion test, abnormal defecography, or delay of colonic transit (3,11,13).
Though DD is more common in females (1,14), there remains a paucity of information regarding gender differences in the results of the usual physiological tests performed among patients with DD. Previous studies in normal healthy volunteers have shown that among non-elderly people, there are no significant differences between genders in overall colonic transit by scintigraphic assessment (15), resting anal sphincter pressure, rectal sensation, and balloon expulsion time (16,17). On the other hand, there were gender-based differences in maximal anal squeeze pressures and defecation indices that may be reflected in decreased likelihood of males to exhibit DD (16). The lack of gender effects on colonic transit among healthy adults (15) requires further validation with larger sample sizes, as several other studies have demonstrated gender differences; those studies used radiopaque markers (18) or scintigraphy with alternative transit endpoints (e.g. mean transit time [19] or % radioactivity retained [20]), or in different groups such as patients with lower functional gastrointestinal disorders (21).
Currently, it is not established practice to use gender-based interpretation of diagnostic tests used to identify DD. Based on observations made in a prior study (3), we hypothesized there exist important differences in the results of colonic transit and anorectal tests between males and females with DD. The primary aim of our study was to assess in DD patients, the association of overall colonic transit with gender. Three secondary aims were also addressed in this large cohort of patients with DD: first, we describe gender-based differences in anal sphincter pressures and balloon expulsion weights; second, we characterize the effect of history of pregnancy on all the physiological functions of interest; third, we characterize the anorectal functions and gastric and small bowel transit in DD patients who had previously undergone partial or total colectomy.
METHODS
Design and Study Population
This medical records review study was approved by the Mayo Clinic Institutional Review Board for patients who had provided unrestricted consent to use of their medical records for research purposes. We reviewed the electronic medical record (EMR) at Mayo Clinic, Rochester, MN to identify all potential cases evaluated by the senior investigator (MC) from January 1, 1994 to June 11, 2012: A previously published cohort of 1411 patients diagnosed with constipation (of whom 390 were diagnosed with DD) between January 1, 1994 and June 30, 2011 (3) was updated with the addition of a second cohort of 51 patients with constipation (of whom 17 were diagnosed with DD) evaluated from July 1, 2011 to June 11, 2012. The search queries were: “pelvic floor dysfunction,” pelvic floor dyssynergia,” obstructive defecation,” or “outlet obstruction”. Available studies including gastrointestinal and colonic transit by radioscintigraphy and anorectal manometry test were reviewed to determine study eligibility and to abstract data. We also collected information about history of pregnancy and partial or total colectomy. Diagnosis of DD was previously described (3) as constipation which was associated with: 1) either abnormal balloon expulsion test (inability to expel balloon from the rectum with <200g added weight) and/or 2) high resting anal sphincter pressure (maximum resting pressure >90mmHg). A third criterion that had been used in the original 390 patients (3) was failure of the anorectal angle to open ≥15° between resting and straining on scintigraphic defecography. However, this criterion was not applied in the recently added 51 patients as the test is no longer performed. In addition, in the prior study (3), change in the rectoanal angle <15 degrees was observed in 81 patients, all of whom had required >200 g to expel the balloon from the rectum. Thus, this third criterion was unnecessary in the current cohort since all patients with abnormal rectoanal angle change were already identified by the abnormal balloon expulsion test. Criteria for DD were previously developed from a review of published data for adults in Minnesota and Iowa (16,22). Patients with descending perineum syndrome or documented denervation were excluded from the analysis.
Healthy Normal Volunteers as Controls for Colonic Transit Comparisons
Scintigraphic data was previously obtained in 208 healthy volunteers in whom a diagnosis of DD had been excluded (3): GC24 median for both genders (n=208): 2.3 (IQR 1.8, 2.9): males (n=72): 2.6 (IQR 2.1, 3.1); females (n=136): 2.1 (IQR 1.7, 2.6).
Gastrointestinal Transit Studies
Colonic transit was evaluated by scintigraphy in patients with DD and in healthy controls with focus on images acquired at 4, 6, 24 and 48 hours. Colonic transit profile can be assessed by calculation of the geometric center, expressed as the sum of the multiplication of the proportion of 111In counts in each colonic region at a given time by the weighting factor for that region (23):
GC = [(%AC*1) + (%TC*2) + %DC*3) + (%RS*4) + (%ST*5)]/100
Performance characteristics of scintigraphic colonic transit measurements have previously been evaluated by our group in healthy patients and patients with irritable bowel syndrome with documentation of reproducibility, coefficient of variation and relationship to bowel functions; these papers have validated the use of this method for assessment of colonic transit in clinical and research settings (15,23).
Anorectal Manometry
Methods for anorectal manometry and balloon expulsion studies were conducted as previously described, including both traditional and high-resolution manometry. February 2007 marked the transition from a low compliance pneumohydraulic manometric perfusion system to a transanal solid state high-resolution probe (3). Measurements for maximum resting and squeeze anal pressures were expressed in mmHg. Balloon expulsion testing was performed with the patient in the left-lateral position and insertion into the rectum of a latex balloon filled with 50 ml water. Additional weights were added to the catheter along a traction pulley if balloon expulsion did not occur spontaneously (3,24). Added balloon weight to facilitate balloon expulsion from the rectum was censored at weights between 470 and 586g.
Study Endpoints
The primary endpoint was overall colonic transit (geometric center, GC) at 24hours (GC24). Data on GC at 48hours (GC48) was collected when available. Secondary endpoints of interest included maximum anal resting pressure (ARP), maximum anal squeeze pressure (ASP), and added weight to allow balloon expulsion (data censored if the patient was unable to expel the balloon despite addition of weights to the balloon; the lowest maximum weight was reported, with censoring [cessation of clinical test] in the range of 470–586g). Medical records were reviewed for clinical and demographic data including age, gender, body mass index (BMI), date of diagnosis, number of pregnancies, and abdominal surgeries including partial or total colectomy and hysterectomy.
Statistical Analysis
Data are expressed as median (IQR) values with 10th–90th percentiles. Endpoints for gastrointestinal transit and anal sphincter pressures were assessed using the Mann-Whitney rank sum test. In the primary analysis, we assessed effects of gender on overall colonic transit. Secondary analyses included: 1) comparison of anal sphincter pressures by gender among all DD patients; 2) comparison of anal sphincter pressures by gender among all DD patients with normal colonic transit (i.e. GC24 >10th percentile from 208 healthy subjects); 3) comparison of GC24 and anal sphincter pressures by history of pregnancy among females with DD; and 4) comparison of gastric emptying at 4 hours (GE4) and small bowel transit (measured as colonic filling at 6 hours, CF6) by scintigraphy and anorectal functions between patients by history of colectomy among all DD patients.
The association between GC24 with DD vs. healthy volunteer status was assessed between groups overall and by gender using the Mann-Whitney rank sum test (SigmaPlot 12 Software 2011–2012, Systat Software Inc, Chicago, IL 60606).
ANCOVA was also used to assess outcomes after including age and BMI as covariates (SAS Version 9.1 procedures GLM, Cary, NC 27513).
Although not a primary endpoint, the potential effect of change in the method used for anorectal manometry was also considered during assessment of differences by gender in anal sphincter pressures. Since the method changed from water perfusion catheter to solid state in February 2007, we appraised the anorectal manometry in DD patients for the two genders after adjusting for time period.
A Chi-square test was used to compare the proportion of males and females who were able to expel the balloon from the rectum without the addition of any weight among the males and females..
RESULTS
Patient Cohort and Demographics
A total of 1462 patients with constipation were reviewed, and we identified 407 patients (67 male, 340 female) with a clinical diagnosis of chronic constipation secondary to DD. Median (IQR) age was 43.0 years (30.0, 58.0) for males and 37.0 (26.0, 49.0) for females. Median BMI was 23.6 kg.m−2 (21.3, 26.3) for males, and 21.4 kg.m−2 (19.1, 24.5) for females. Age (p=0.006) and BMI (p<0.001) were significantly different between males and females. Among patients with DD, clinical history including prior abdominal surgeries, hysterectomies, and pregnancies are shown in Table 1.
Table 1.
Previous Abdominal Operations and Pregnancies in Patients with DD
| Males (n=67) | Females (n=340) | |
|---|---|---|
| Pregnancies | NA | 2 (0,3) (n=158) |
| Hysterectomy | NA | 52 |
| Appendectomy | 6 | 58 |
| Cholecystectomy | 6 | 54 |
| Caesarian section | NA | 9 |
| Pelvic surgery | 1 | 60 |
| Rectocele repair | 0 | 3 |
| Colonic resection (†p/t) | 7 | 18 |
| Small bowel resection | 4 | 11 |
| Ileostomy | 0 | 5 |
| Genitourinary surgery | 4 | 7 |
| Other abdominal surgery | 5 | 21 |
partial or total
Overall Colonic Transit by Scintigraphy
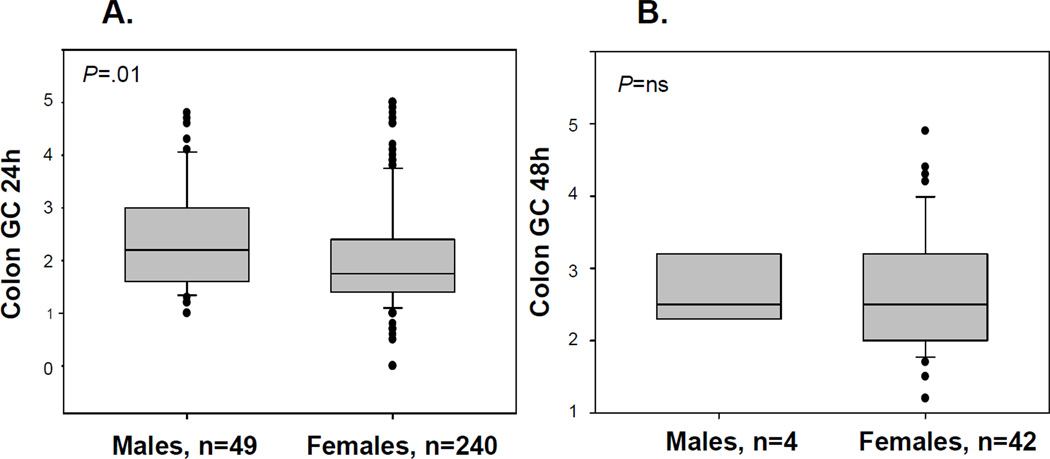
Results for GC24 were available in 49 males and 240 females and are shown in Figure 1. There were significant differences in GC24 between males (median GC24=2.2) and females (median GC24=1.8) with DD, with females having slower GC24 (p=0.01) as indicated by both the primary analysis and the supportive analysis using an ANCOVA model.
Figure 1.
Overall colonic transit at 24 hours (A) and 48 hours (B) for males vs. females among patients with DD
Results for GC48 were available in only 4 males and 42 females and showed no significant association with gender.
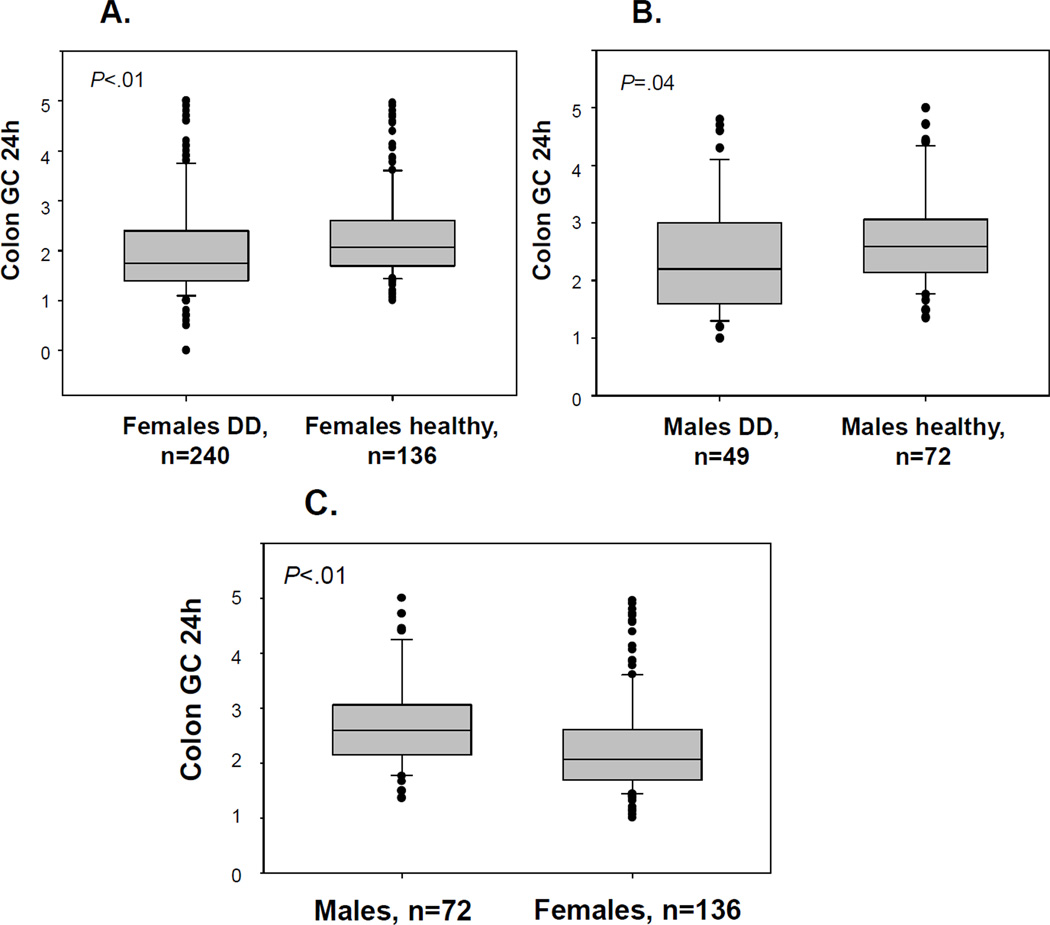
Comparison of colonic transit of DD patients with healthy volunteers (72 males and 136 females) showed significant differences in the GC24 between DD and healthy volunteer groups by gender (Figure 2A and B). Comparison of overall (both genders combined) DD and healthy volunteer groups revealed significant differences in GC24 and GC48 (both p<0.01) using unadjusted analyses. Median (IQR) GC24 for the healthy volunteer group was 2.3 (1.8, 2.9). Significant differences were observed in the GC24 between genders from healthy volunteer data (Figure 2C) with males having accelerated transit compared to females (p<0.001). Both males and females with DD had slower colonic transit GC24 than gender-matched controls.
Figure 2.
Overall colonic transit at 24 hours for females (A) or males (B) with DD vs. healthy volunteers; and (C) transit data for healthy volunteer males vs. females
Anorectal Functions
Results of ARM and balloon expulsion tests among all patients with DD are summarized [Table 2A (n=size/group)]. There were significant differences between males and females in maximum anal resting pressure (p=0.04), and maximum anal squeeze pressure (p<0.001). Supportive analysis with an ANCOVA model (adjusting for age and BMI) confirmed significant difference between genders in ARP and ASP (data not shown). A higher proportion of males than females were successful in balloon expulsion without the addition of any weight (p<0.001). Median values for added balloon weight were slightly lower in males, but these were not subjected to statistical analysis due to censoring of weights beyond 470g.
Table 2.
| A. ARM Characteristics and Balloon Expulsion Tests in all Patients with DD [data show median (IQR), other than proportions able to expel the balloon] | |||
|---|---|---|---|
| Males; [n] | Females; [n] | p-value | |
| Maximum anal resting pressure (mm Hg) |
87.8 (67.6, 111.0); [67] | 82.4 (64, 98.6); [332] | 0.04* |
| 10–90th percentile | 56.6–131.4 | 49.3–116.5 | |
| Maximum anal squeeze pressure (mm Hg) |
182.4 (155.1, 244.1); [67] |
128.7 (102.1, 159.6); [329] |
<0.01* |
| 10–90th percentile | 114.0–299.3 | 77.2–205.0 | |
| Added balloon weight (g) | 500.0 (188.0, 564.0); [67] |
564.0 (376.0, 564.0); [323] |
censored |
| 10–90th percentile | 0.0–586.0 | 188.0–586.0 | |
| Able to expel balloon with 0g added weight (%) |
23.9; [16] | 7.4; [24] | <0.01# |
| Unable to expel balloon with 0g added weight (%) |
76.1; [51] | 92.6; [299] | |
| B. ARM Characteristics and Balloon Expulsion Tests in Patients with DD and Normal Colonic Transit at 24 Hours (GC >1.47, 10th percentile of 208 healthy volunteers) | |||
|---|---|---|---|
| Males; [n] | Females; [n] | p-value | |
| Maximum anal resting pressure (mm Hg) |
89.1 (64.3, 114.8); [40] | 83.5 (64.9, 98.2); [176] |
0.10* |
| 10–90th percentile | 55.3–133.8 | 46.8–118.7 | |
| Maximum anal squeeze pressure (mm Hg) |
205.1 (164.7, 274.9); [40] |
127.2 (98.5, 157.4); [176] |
<0.01* |
| 10–90th percentile | 136.8–330.8 | 77.0–205.1 | |
| Added balloon weight (g) | 500.0 (0, 564.0); [40] | 564.0 (340.8, 586); [172] |
censored |
| 10–90th percentile | 0.0–586.0 | 122.2–586.0 | |
| Able to expel balloon with 0g added weight (%) |
27.5; [11] | 8.7; [15] | <0.01# |
| Unable to expel balloon with 0g added weight (%) |
72.5; [29] | 91.3; [157] | |
Mann-Whitney rank sum test
Chi-square test
Results of ARM tests and balloon expulsion for DD patients with normal colonic transit are shown in Table 2B. There remained significant differences between genders in maximum ASP (p<0.01) and proportion of males vs. females who were able to expel the balloon with 0 g added weight (p<0.01). Differences between males and females in maximum ARP were not statistically significant (p=0.10).
Association of Colonic Transit, Anorectal Function and History of Pregnancy
Data on prior history of pregnancy were available in 330 female patients. Analysis of nulliparous compared to women who had at least one pregnancy (Table 3) showed the latter were older, had higher BMI, lower maximum ARP and ASP (all p<0.01); however, overall colonic transit at 24 hours was not different in the two groups. The difference in ARP remained statistically significant after adjustment for age and BMI, while the difference in ASP was not significant with these adjustments.
Table 3.
Comparison of Nulliparous Women and Those Who Had at Least One Pregnancy [data show median (IQR)]
| Nulliparous; [n] | Women with prior pregnancy; [n] |
p-value | |
|---|---|---|---|
| Age (y) | 27.0 (21.0, 35.5); [164] | 47.0 (38.0, 55.0); [166] | <0.01* |
| BMI (kg/m2) | 20.9 (18.5, 23.2); [156] | 22.3 (19.9, 25.0); [161] | <0.01* |
| Maximum anal resting pressure (mm Hg) |
88.2 (73.7, 104.4); [159] | 72.1 (58.1, 92.6); [163] | <0.01* |
| Maximum anal squeeze pressure (mm Hg) |
132.8 (109.9, 161.7); [158] |
122.5 (90.4, 154.4); [161] |
<0.01* |
| Able to expel balloon with 0g added weight (%) |
7.7; [12] | 7.6; [12] | ns# |
| Colonic GC at 24h | 1.7 (1.5, 2.2); [114] | 1.8 (1.4, 2.5); [119] | ns* |
Mann-Whitney rank sum test
Chi-square test
Association of Gastrointestinal Transit, Anorectal Function and Colectomy
Prior colectomy was documented in 25 of 407 patients with DD. There was no significant difference (p=0.11) in the proportion of males (10.5%) and females (5.3%) with prior colectomy. Comparison between patients who underwent prior partial or total colectomy with those without colectomy among the DD cohort showed no significant difference in age, BMI, and GE4 or CF6 by scintigraphy. Similarly, analyses of anorectal measurements showed no significant difference in ARP or ASP or in the proportion of patients with successful balloon expulsion with 0 g added weight (Table 4).
Table 4.
Comparison of DD Patients with Prior Colectomy and DD Patients without Prior Colectomy [data show median (IQR)]
| Colectomy; [n] | No colectomy; [n] | p-value | |
|---|---|---|---|
| Age (y) | 38.0 (32.0, 53.5); [25] | 38.0 (26.0, 50.0); [382] | ns* |
| BMI (kg/m2) | 22.2 (18.5, 24.6); [25] | 21.7 (19.3, 24.7); [365] | ns* |
| GE4 (%) | 91.5 (84.0, 96.0); [20] | 94.0 (86.0, 98.0); [300] | ns* |
| CF6 (%) | 57.0 (28.0, 84.0); [17] | 59.0 (24.0, 87.0); [278] | ns* |
| Maximum anal resting pressure (mm Hg) |
88.3 (64.6, 112.9); [25] | 82.8 (64.7, 98.9); [374] | ns* |
| Maximum anal squeeze pressure (mm Hg) |
146.5 (101.8, 197.3); [25] |
137.5 (105.1, 172.1); [371] |
ns* |
| Able to expel balloon with 0g added weight (%) |
8.7; [2] | 10.5; [38] | ns# |
Mann-Whitney rank sum test
Chi-square test
DISCUSSION
In this retrospective review of 1462 patients evaluated by a single gastroenterologist at a tertiary referral center, 28% of patients were diagnosed with DD based on clinical evaluation and abnormal ARM findings, consistent with previously reported prevalence rates of DD in chronic constipation among tertiary referral populations (2).The majority of patients included in this cohort met at least one criterion used to identify DD based on the results of ARM or balloon expulsion test. Thus, 350 of 407 patients demonstrated impaired balloon expulsion.
There were 4 main observations in this analysis of over 400 patients with DD evaluated by a single clinical team at a single institution: First, in patients with DD, there were significant differences in colonic transit at 24 hours between genders, with females having slower overall colonic transit. The patients with DD also have slower colonic transit at 24 hours compared to gender-matched controls, and this expands on the previous data demonstrating delay of overall colonic transit in patients with DD relative to healthy volunteers without subgrouping by gender (3). Second, there were lower resting and squeeze anal sphincter pressures in women compared to men presenting with DD. Third, history of prior pregnancy is not associated with changes in overall colonic transit among female patients with DD, when compared to patients with DD who had no prior pregnancy; however, higher maximal anal resting and squeeze pressures were observed in nulliparous women compared to those with prior pregnancies. Fourth, patients with DD may present after partial or total colectomy; the anorectal functions of these patients are not significantly different from those of patients with DD who did not undergo prior colectomy.
The gender-related effect on colonic transit (slower in females than males) in patients with DD is consistent with gender-related effects on gastric emptying (25). The clinical significance of a 0.4 unit difference in GC24 observed between males and females was previously examined (23); there was a significant correlation of colonic transit with stool form in IBS and healthy subjects, with a 1 unit increase in GC24 being correlated with a 0.58 unit change in stool form. Therefore, although there is an average 0.4 GC unit difference in GC24 between 49 males and 240 females in a large cohort of DD patients, it is unclear whether the ~0.25 unit difference on the 7-point Bristol Stool Form Scale could be appreciated by patients.
The transit of radiolabeled solids through the colon is slower in female than in male healthy volunteers, based on the cohort of 208 healthy volunteers. These findings are in contrast to a prior observation in 37 healthy volunteers, which revealed no gender-related differences in colonic transit (15). Manabe et al. (21) also reported faster transit in males compared to females in a cohort of 287 patients with lower functional gastrointestinal disorders including irritable bowel syndrome. These differences in colonic transit between men and women suggest that control data specific to men and women should be considered when evaluating patients with DD and chronic constipation.
Previously, we reported the absence of gender differences in GC24 among patients with DD (3). Although these results initially appear contradictory to our current findings, they are in fact consistent. In our prior study, differences in the mean GC24 between males and females with DD were of borderline statistical significance. However, the differences in GC24 between males and females were numerically similar to those observed in the current study. The addition of subsequent patients with an increase in sample size now allows us to confirm significant findings that are consistent with our prior observations. The clinical relevance of these observations are yet unclear considering the large size of this DD cohort and should be interpreted in combination with symptoms and other physiologic tests during diagnostic assessment of DD. It should also be emphasized that the observation that GC24 was not a significant discriminator between DD and HV in the previous study was based on an analysis that adjusted for gender, age, and BMI, thereby removing any variability in GC24 attributable to gender. In the current study, our aim was to study the effect of gender. Thus, we performed a comparison between DD and HV groups that was not adjusted for gender to show significant differences in GC24.
Although not a primary focus of this study, analysis of anorectal functions by manometry also revealed significant differences between genders in maximal ARP and ASP among patients with DD in the current study. Males with DD had significantly higher maximal ARP and maximal ASP when compared to females, similar to gender-related differences in anal pressures in healthy controls (16,17,26,27). Normal values for healthy volunteers have recently been published for females using high-resolution ARM (28); however, there are still no male-specific parameters established to evaluate patients with suspected DD. Our results, along with previous data in normal healthy volunteers, suggest that, as with gender-specific colonic transit normal data, it may be important to develop and validate gender-based diagnostic criteria of anorectal functions.
Gender-based interpretation should also consider the potential effects of prior history of pregnancy (29–31). In our study, nulliparous women had significantly higher maximal ARP and ASP compared to women with at least one prior pregnancy. A supportive analysis adjusting for age and BMI was performed to show that ARP remained significantly different between women by prior history of pregnancy, while the difference in ASP was no longer significant. Thus, future studies need to appraise “normal values” in parous women with DD compared with data from parous women without DD.
Comparison of transit parameters and anorectal functions did not reveal significant differences between DD patients with prior colectomy and DD patients without prior colectomy, suggesting that physiological characteristics are not different between these two groups. While it is impossible from our analysis to conclude that the same anorectal functional abnormalities existed prior to colectomy, these data are certainly consistent with that hypothesis. These results also emphasize the need to exercise caution in performing colectomy among patients with suspected DD and suggest that assessment of anorectal functions is indicated prior to colectomy.
The major strengths of this study include access to a large patient cohort, standardization of clinical diagnosis based on evaluation by a single, experienced gastroenterologist, use of a validated scintigraphic method for assessment of colonic transit, as well as the large number of healthy controls of both genders to interpret colonic transit results. Limitations include the retrospective nature of the study design, inherent referral bias as the study evaluated tertiary referral patients, incomplete data on GC48, censored balloon weights >470g, and change in manometric technique after 2007. Although high-resolution manometry reports higher anal sphincter resting and squeeze pressures than traditional water-perfusion manometry, these methods are highly correlated (32). A supportive analysis adjusting for time period in which the study was performed (and the use of water-perfused vs. solid state pressure measurements) showed that differences between males and females in ARP and ASP remained statistically significant even after adjusting for this variable.
In summary, there are significant differences between genders in overall colonic transit at 24 hours and in anorectal functions, specifically resting and squeeze pressures, among patients with DD. We also showed a significant difference between genders among healthy volunteers in overall colonic transit at 24 hours. In conclusion, our findings suggest that among patients with chronic constipation and suspected DD, interpretation of diagnostic testing for colonic transit and anorectal functions should use normative data from gender-matched controls.
Acknowledgments
We thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Sources of Funding
Dr. Camilleri is supported by grant R01-DK092179 from National Institutes of Health.
Abbreviations
- ARM
anorectal manometry
- ARP
anal resting pressure
- ASP
anal squeeze pressure
- DD
defecation disorder
- EMG
electromyography
- GC
geometric center
Footnotes
DISCLOSURES
Authors’ Contributions
A. Shin: research fellow, collection of data; writing manuscript; M. Camilleri: study conceptualization, data analysis, writing manuscript; A. Nadeau: collection of data, data analysis; S. Nullens: collection of data; J.C. Rhee: study conceptualization and discussion of results; I.D. Jeong: study conceptualization and discussion of results; D. Burton: scintigraphic studies and analysis
Competing Interests
The authors have no conflicts of interest.
REFERENCES
- 1.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Surrenti E, Rath DM, Pemberton JH, Camilleri M. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–1475. [PubMed] [Google Scholar]
- 3.Nullens S, Nelsen T, Camilleri M, et al. Regional colon transit in patients with dys-synergic defaecation or slow transit in patients with constipation. Gut. 2012;61:1132–1139. doi: 10.1136/gutjnl-2011-301181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald A, Caruana BJ, Freimanis MG, Bauman DH, Hinds JP. Contributions of evacuation proctography and anorectal manometry to evaluation of adults with constipation and defecatory difficulty. Dig Dis Sci. 1990;35:481–487. doi: 10.1007/BF01536923. [DOI] [PubMed] [Google Scholar]
- 5.Shahid S, Ramzan Z, Maurer AH, Parkman HP, Fisher RS. Chronic idiopathic constipation: more than a simple colonic transit disorder. J Clin Gastroenterol. 2012;46:150–154. doi: 10.1097/MCG.0b013e318231fc64. [DOI] [PubMed] [Google Scholar]
- 6.Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 7.Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol. 1999;94:3530–3540. doi: 10.1111/j.1572-0241.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–338. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. doi: 10.1111/j.1365-2036.2006.03238.x. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Müller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation--a European perspective. Neurogastroenterol Motil. 2011;23:697–710. doi: 10.1111/j.1365-2982.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 12.Adams MA, Chey WD. Biofeedback training for dyssynergic defecation: an approach whose time has come? Gastroenterology. 2011;140:1682–1685. doi: 10.1053/j.gastro.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Tantiphlachiva K, Rao P, Attaluri A, Rao SS. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Rao SS. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol. 2010;8:910–919. doi: 10.1016/j.cgh.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 17.Schuld J, Kollmar O, Schlüter C, Schilling MK, Richter S. Normative values in anorectal manometry using microtip technology: A cohort study in 172 subjects. Int J Colorectal Dis. 2012 doi: 10.1007/s00384-012-1499-2. in press. [DOI] [PubMed] [Google Scholar]
- 18.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 19.Graff J, Brinch K, Madsen JL. Gastrointestinal mean transit times in young middle-aged healthy subjects. Clin Physiol. 2001;21:253–259. doi: 10.1046/j.1365-2281.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 20.McLean RG, Smart RC, Lubowski DZ, et al. Oral colon transit scintigraphy using indium-111 DTPA: variability in healthy subjects. Int J Colorectal Dis. 1992;7:173–176. doi: 10.1007/BF00341215. [DOI] [PubMed] [Google Scholar]
- 21.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293-e82. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49:1726–1735. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 23.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991;214:403–411. doi: 10.1097/00000658-199110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012 Jul 2; doi: 10.1111/j.1365-2982.2012.01972.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loening-Baucke V, Anuras S. Effects of age and sex on anorectal manometry. Am J Gastroenterol. 1985;80:50–53. [PubMed] [Google Scholar]
- 27.Sun WM, Read NW. Anorectal function in normal human subjects: effect of gender. Int J Colorectal Dis. 1989;4:188–196. doi: 10.1007/BF01649702. [DOI] [PubMed] [Google Scholar]
- 28.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012 doi: 10.1038/ajg.2012.221. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connell PR. The effects of age and parity on anorectal function. Dis Colon Rectum. 2012;55:235–236. doi: 10.1097/DCR.0b013e31823fe810. [DOI] [PubMed] [Google Scholar]
- 30.Boyle DJ, Knowles CH, Murphy J, et al. The effects of age and childbirth on anal sphincter function and morphology in 999 symptomatic female patients with colorectal dysfunction. Dis Colon Rectum. 2012;55:286–293. doi: 10.1097/DCR.0b013e31823fe7f1. [DOI] [PubMed] [Google Scholar]
- 31.Kepenekci I, Keskinkilic B, Akinsu F, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011;54:85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 32.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007;102:850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]