Abstract
Chemical-based methods have been developed for transformation of DNA into log phase cells of the budding yeast Saccharomyces cerevisiae with high efficiency. Transformation of early stationary phase cells, e.g., cells grown in overnight liquid cultures or as colonies on plates, is less efficient than log phase cells but is simpler and more adaptable to high throughput projects. In this study we have tested different approaches for transformation of early stationary phase cell cultures and identified a method utilizing polyethylene glycol (PEG), lithium acetate and dimethyl sulfoxide (DMSO) as most efficient. Plasmid DNA transformations using this method could be improved modestly by allowing cells to recover from the chemical treatment in rich broth before plating to selective media. Strong increases in transformation efficiencies were observed when cells were treated briefly with dithiothreitol (DTT). Tests using several different yeast strain backgrounds indicated that DTT treatment could enhance transformation efficiencies by up to 40-fold. Evaluation of multiple parameters affecting the efficiency of the method led to development of an optimized protocol achieving >50,000 transformants per µg DNA in most backgrounds tested.
Keywords: transformation, transfection, dithiothreitol, chemical-based
Introduction
Several approaches have been developed for the transfer of DNA molecules into cells of the budding yeast Saccharomyces cerevisiae. These methods include electroporation, enzymatic spheroplasting and various chemical-based transformation procedures (Benatuil et al., 2010; Brzobohaty and Kovac, 1986; Ito et al., 1983; Gietz and Woods, 2001; Gietz and Schiestl, 2007a, 2007b; Kawai et al., 2010). Chemical treatment methods, which are most commonly used, involve temporary destabilization of the cell wall to allow entry of DNA, which is likely to involve an endocytosis-like mechanism (Kawai et al., 2010; Pham et al., 2011a).The chemicals polyethylene glycol (PEG) and lithium acetate (LiAc), a non-specific carrier DNA such as sonicated salmon chromosomal DNA, and a brief heat shock step are frequently employed. Evidence suggests that PEG promotes association of the transforming DNA with the surface of the cell and that lithium ions and heat shock promote passage of DNA into the cell (Chen et al., 2008; Gietz and Woods, 2001; Kawai et al., 2010; Zheng et al., 2005). The precise function of the carrier DNA is unclear; recent work suggests that this DNA associates with the exterior of cells and alters the structure of the cell wall (Pham et al., 2011b).
Transformation efficiencies are greatest in log phase yeast cell cultures and high efficiency protocols have been developed for such cells (Gietz and Schiestl, 2007a; Gietz and Woods, 2001; Kawai et al., 2010). Transformation of cells harvested from patches on plates or from small overnight liquid cultures, corresponding to early stationary (post-diauxic) phase cells (Herman, 2002; Werner-Washburne et al., 1996), is less efficient than in log phase cells. However, this approach is often preferred because use of early stationary phase cells is less time-consuming, requires smaller volumes and is more adaptable to high-throughput procedures than log phase protocols (Chen et al., 1992; Elble, 1992; Gietz and Schiestl, 2007b; Gietz and Woods, 2001; Keszenman-Pereyra and Hieda, 1988; Soni et al., 1993).
In the current study we have tested multiple protocols and the impact of pretreatment and post-treatment steps on the efficiency of transformation of small overnight cultures with plasmid DNA and identified an important role for the reducing agent dithiothreitol (DTT). A new high efficiency protocol was developed and successfully tested in several different yeast strain backgrounds.
Materials and Methods
Yeast strains and plasmids
BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) (Brachmann et al., 1998) was used for all experiments except where specified otherwise. Other strain backgrounds tested were the S288c strain BY4741 (Brachmann et al., 1998), BWG1-7a (White and Haber, 1990), S1:InsE4A (Morrison et al., 1993), SK1 strain EPY214-1B (Larionov et al., 1994), the A364a-derived strain T334 (Hovland et al., 1989), and YPH102 (Sikorski and Hieter, 1989). The plasmid pRS316 (CEN/ARS URA3) (Sikorski and Hieter, 1989) was used for all transformation assays. Plasmid DNAs were purified from E. coli DH5α cells using an alkaline lysis protocol (Sambrook and Russell, 2001) in conjunction with anion exchange spin columns from USA-Qiagen, Inc. Yields of plasmid DNA were determined using a Hoefer DynaQuant 200 fluorometer and the DNA-binding fluorophore Hoechst 33258.
Cell growth and transformation reagents
Ampicillin, dimethyl sulfoxide (DMSO),D-(+)-glucose, Hoechst 33258, lithium acetate(LiAc; #L4158), and poly(ethylene glycol)-4000 (#81240) were purchased from Sigma-Aldrich Chemical Co. Stock solutions of 50% PEG-4000 and 1 M LiAc dissolved in ddH2O remained stable when stored at RT for 6–12 months. Periodically, transformation efficiencies using older solutions would decrease; fresh PEG and LiAc solutions were then prepared, which restored efficiencies to prior levels. Aliquots of DTT (2 M) were stored in a −20 °C freezer prior to use. Ethidium bromide (EtBr) was obtained from Shelton Scientific, Incorporated. Ethylene diaminetetraacetic acid (EDTA), 10X PBS and agarose were obtained from EMD Chemicals, Inc. 1 Kb DNA ladder was purchased from New England Biolabs. Sonicated salmon sperm carrier DNA was obtained from Stratagene/Agilent Technologies. Tris base was purchased from VWR International. Dithiothreitol (DTT) was purchased from either Sigma-Aldrich or Gold Biochemistry. Bacto peptone, bacto yeast extract, bacto tryptone, bacto agar, yeast nitrogen base, and LB broth mix were manufactured by Becton Dickinson Microbiological Systems.
For non-selective growth, yeast cells were grown on YPDA (rich) media (1% bacto yeast extract, 2% bacto peptone, 2% glucose, 2% bacto agar, 0.001% adenine) (Sherman, 2002). Plasmid selection used synthetic yeast growth media with drop-out mix (0.17% yeast nitrogen base without amino acids, 2% glucose, 2% bacto agar, and all essential amino acids minus the amino acids used for selection) (Sherman, 2002). E. coli cells were grown in LB broth + 0.1 mg/mL Amp or on LB + Amp plates.
Yeast DNA transformation methods
Three previously published protocols for transformation of early stationary phase cells were adapted for use in this work. These methods included the "quick-and-easy" protocol of Gietz and Schiestl (2007b), the one-step transformation protocol of Chen et al. (1992), and the DMSO-based approach of Soni et al. (1993). Four or five trials were performed for tests of each method and also during testing of modifications to the DMSO-based protocol. Averages and standard deviations were calculated and are presented in the figures. Two-tailed Student's T-test comparisons of populations were performed using Microsoft Excel in conjunction with the add-in program StatPlus.
The quick and easy protocol of Gietz and Schiestl was adapted for this study using the following steps: 1.5 mL of cells grown overnight at 30 °C in YPDA broth with shaking was pelleted for 15 s at 16,100g and the supernatant was discarded. To the pellet 240 µL 50% PEG4000, 36 µL 1 M LiAc, 50µL recently boiled sonicated 2 mg/mL single-stranded carrier DNA and 34 µL of sterile water plus pRS316 plasmid DNA (100–700 ng) were added. After mixing, tubes were incubated at 42 °C for 20 min, spun for 15 s, and the resulting cell pellets were resuspended in 200 – 500µl ddH2O and aliquots spread onto synthetic glucose plates minus uracil (Glu-Ura plates). Plates were incubated at 30 °C for 3 days.
The one-step protocol of Chen et al. was applied as follows: 1.5 mL of cells grown overnight at 30 °C in YPDA broth with shaking was pelleted for 15 s at 16,100g and the supernatant was discarded. To the pellet 100 µL of ONE-STEP buffer (40% PEG-4000 [pH 5.0], 0.2 M LiAc, 100 mM DTT), 5 µL recently boiled 10 mg/ml carrier DNA, and 2–5 µL plasmid DNA (100–700 ng) were added. After mixing, tubes were incubated at 45°C for 30 min. In this protocol, heat-shocked cells are normally spread directly onto selective plates, but to ensure that the three protocols could be readily compared, the cells were sedimented and resuspended in 200–500µl ddH2O as in each of the other methods. We note that similar colony numbers were observed using both plating procedures. The plates were then incubated at 30 °C for 3 days as above.
For the standard Soni DMSO-based protocol, 1.5 mL of cells grown overnight at 30 °C in YPDA broth with shaking was pelleted for 15 s at 16,100g and the supernatant was discarded. To the pellet 5 µL recently boiled 10 mg/ml carrier DNA, 2–5 µL pRS316 DNA (100–700 ng), and 500 µL PEG/LiAc/Tris/EDTA (PLTE) solution were added. PLTE solution was prepared by mixing 800 µL 50% PEG−4000 + 100 µL 1 M LiAc + 20 µL 50 mM EDTA + 10 µL 1 M Tris (pH 7.5) + 70 µL H2O. Enough PLTE was prepared for all transformations to be performed plus at least one more. After mixing, 56 µL DMSO was added, the solution was remixed and incubated at 30 °C for 15 min. Tubes were placed at 42 °C for 15 min, spun for 15 s, and the resulting cell pellets were resuspended in 200 – 500µl ddH2O. In the original protocol, cell pellets are normally resuspended in TE (10 mM Tris [pH 8.0] 1 mM EDTA), but to ensure that the protocols could be readily compared, cells were resuspended in ddH2O and spread to Glu-Ura plates as above.
To assess pretreatment modifications of the Soni protocol, the initial cell pellet was resuspended in 500 µL of solutions containing varying amounts of DTT. For tests of pH effects, the DTT was dissolved in 100 mM Tris buffer. For post-treatment tests, cells pelleted after the final 42 °C heat shock were resuspended in 1 ml ddH2O, YPDA broth, YP broth (same as YPDA but without glucose and without adenine), or 1 × PBS (phosphate-buffered saline). Tubes were then shaken in a Labnet model 20E microcentrifuge tube shaker inside a 30 °C incubator for 40 or 80 min. The cells were sedimented at 16,000g for 15 s and resuspended in water before plating.
The final, optimized transformation method developed here used the DMSO-based protocol described above, but with the following modifications: After centrifugation of 1.5 ml cells and removal of the supernatant, 0.5 ml 100 mM DTT dissolved in ddH2O was added. After mixing, the suspension was incubated at 42 °C for 20 min in a heating block containing conical holes, centrifuged at 16,100g for 15 s, and carrier DNA, plasmid DNA, PLTE solution and DMSO were added as in the normal protocol. When DTT was included in the mix along with the other chemicals, bypassing the preincubation step, it was added to the PLTE in place of a portion of the water, producing a final PLTE+DNA solution that contained 100 mM DTT.
To perform a large number of transformations, a "mix" was prepared that contained the volume needed plus enough for one more transformation. For example, if 10 transformations were to be performed, a mix for 11 transformations was prepared on the day of the experiment that contained 5500 µl PLTE solution, 55 µl carrier DNA, 55 µl plasmid DNA + ddH2O, and 616 µl DMSO. Aliquots containing 566 µl of the mix were added to each cell pellet, which was then resuspended by vortexing or by scraping across the bottom of a test tube rack. Transformation efficiencies using the optimized protocol were typically ~50,000 – 100,000 per µg DNA, depending on the yeast strain employed, and these higher efficiencies allowed smaller amounts of plasmid DNA to be used (10–50 ng).
Results
Transformation of DNA into yeast cells by chemical methods is typically done using either logarithmically growing cells or cells that are in early stationary phase (the post-diauxic phase; Herman, 2002; Werner-Washburne et al., 1996). Use of log phase cells produces the highest efficiencies, but requires more time and is less easily adapted for high throughput projects. Early stationary phase protocols typically use cells grown overnight in liquid culture using tubes, flasks or microtiter dish wells, but may also employ cells grown as patches or large colonies on plates.
In an effort to improve the efficiency of transformation of early stationary phase cells, we initially examined three previously described protocols. These methods included a simple, "quick and easy" procedure (Gietz and Schiestl, 2007b) involving sedimentation of cells and resuspension in a solution containing PEG, LiAc, single-stranded carrier DNA and plasmid DNA, followed by heat shock at 42 °C, re-sedimentation and resuspension of the cells, and spreading to selective plates. The second method was the one-step protocol of Chen et al. (1992), in which cell pellets are resuspended in a solution containing PEG, LiAc, carrier DNA, plasmid DNA and the reducing agent DTT, followed by heat shock at 45 °C (a higher temperature than that used in other methods). The third procedure was that of Soni et al. (1993), which is similar to the quick and easy protocol described above except that cells are initially resuspended in a more complex mixture containing PEG, LiAc, Tris, EDTA, carrier DNA, plasmid DNA and DMSO, followed by a brief incubation at 30 °C and heat shock at 42 °C before sedimenting and spreading cells to selective plates.
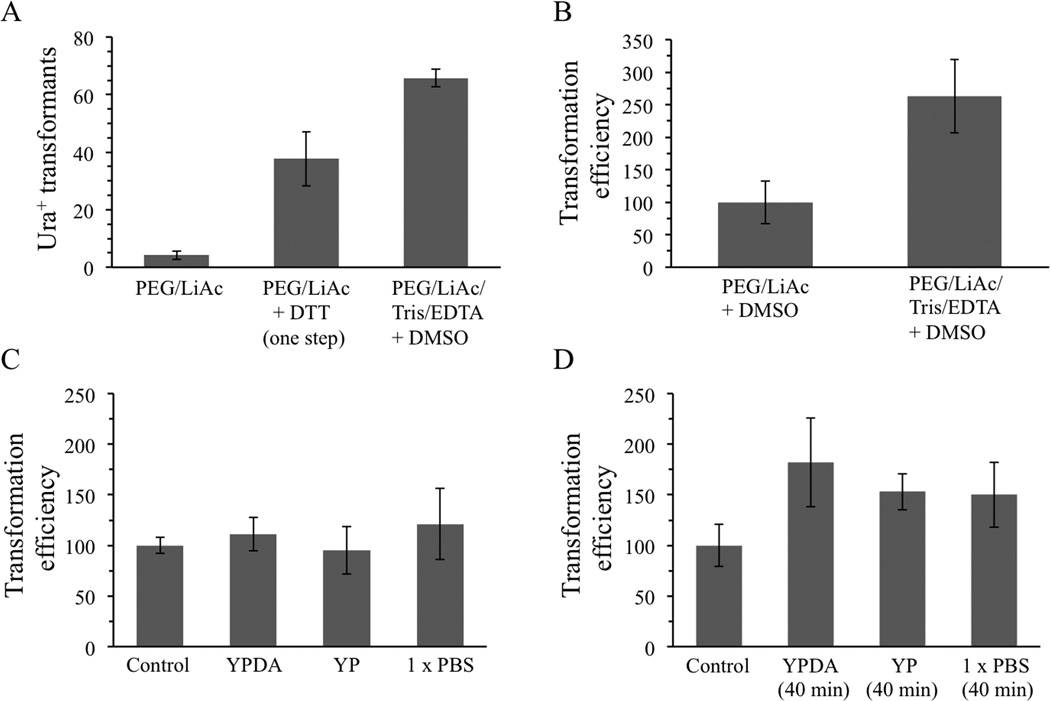
Tests were performed using the centromeric yeast shuttle vector pRS316. Haploid BY4742 cells were shaken overnight in YPDA broth in tall glass cell culture tubes prior to transformation and transformed cells were spread onto synthetic glucose plates lacking uracil. Each of the more complex protocols, combining either DTT or Tris/EDTA/DMSO with PEG and LiAc, produced much higher numbers of Ura+ transformants than the quick and easy PEG/LiAc protocol, corresponding to increases of 8-fold and 15-fold, respectively (Figure 1A). The protocol of Soni et al. utilizing PEG, LiAc, Tris, EDTA and DMSO consistently gave highest transformation efficiencies and was used for all subsequent experiments. For the experiment shown in Figure 1A, this method produced 32,000 transformants per µg DNA and reproducibly generated efficiencies of 20,000 – 40,000 per µg of DNA using BY4742 cells in repeated trials.
Figure 1.
A DMSO-based protocol produces highest transformation efficiencies and can be improved when cells are allowed to recover by incubation in broth. (A) Both one-step and DMSO-containing protocols generated higher transformation efficiencies than a simpler PEG/LiAc protocol. (B) Tris and EDTA are needed for maximum efficiency using the DMSO-based protocol. (C) Resuspension of cells after heat shock in YPDA broth, YP broth (lacking glucose and adenine), or phosphate-buffered saline (1×PBS) followed by immediate plating does not improve colony formation, but (D) incubation at 30 °C for 40 min in YPDA and YP media increased efficiency by 2-fold. Control, unmodified DMSO-based protocol. Error bars indicate standard deviations.
In an effort to simplify the DMSO method, the consequences of excluding Tris and EDTA (TE) from the initial resuspension solution were examined. Use of the protocol without TE caused a 2.5-fold reduction in colony formation (Figure 1B), so it was included in all subsequent experiments. Application of the Student's T-test to two repetitions of this experiment using 5 cultures each time produced similar increases but with p values of 0.06 and 0.10, indicating that the increases were significant at the 90% but not the 95% confidence level. For the graph shown in Figure 1B, the transformation efficiency of the solution lacking TE was normalized to 100 to simplify comparison. Controls in subsequent graphs, usually indicating results obtained using the standard DMSO-based protocol, were also arbitrarily set to 100 to facilitate comparisons with alternative procedures involving pre- or post-treatment with other chemicals.
Protocols developed for transformation of plasmid DNAs into bacterial cells differ from yeast protocols in that they usually require incubation of the cells in rich broth after the 42 °C heat shock step (Sambrook and Russell, 2001; Seidman et al. 2001). This final incubation is ostensibly performed to allow the cells to recover from chemical-induced alterations of the cell wall and membrane. The impact of such post-treatment on yeast cells was investigated by resuspending heat-shocked cells in nutrient or salt solutions instead of water. The solutions used were YPDA broth (containing 2% glucose), YP broth (as YPDA broth but lacking adenine and the osmoprotective effects of glucose), or phosphate-buffered saline solution (1 × PBS). Simply resuspending cells in the alternative solutions and spreading immediately to plates did not alter transformation efficiencies (Figure 1C). However, incubation in each solution at 30 °C for 40 min, which is similar to bacterial cell recovery times, improved efficiencies modestly (1.5–1.8-fold).
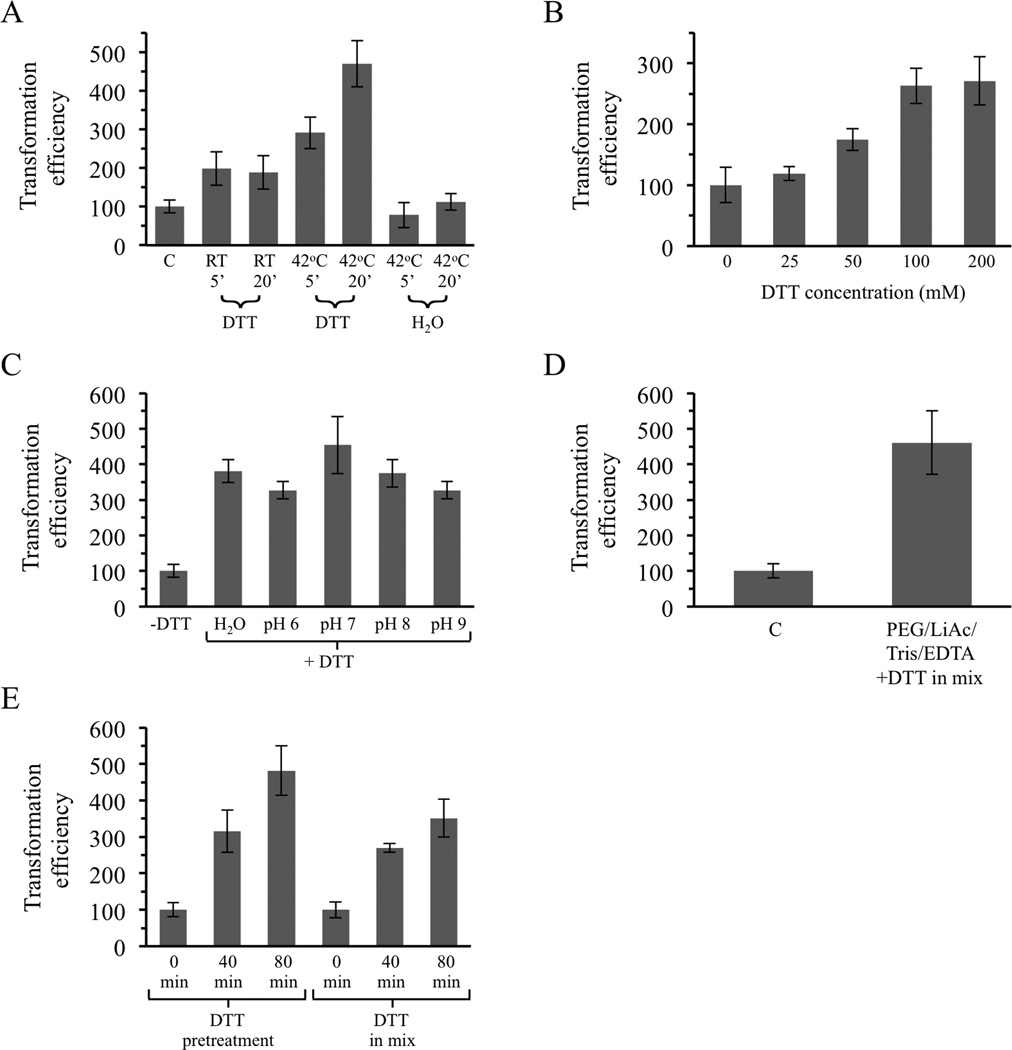
In some chemical-based yeast transformation protocols, cells are initially washed with water or a buffer solution prior to resuspension in PEG, LiAc and DNA (e.g., Gietz et al., 1995). We investigated the effects of brief incubation of cells in water, Tris/EDTA buffers or various chemical solutions and only observed a strong impact of preincubation with the reducing agent DTT (Figure 2 and data not shown). This reducing agent was tested because it is a normal component of the one-step protocol described above (Chen et al., 1992) and because reducing agents have been employed in some past transformation studies that used log phase yeast cells instead of early stationary phase cells (see Discussion). As shown in Figure 2A, preincubation of cells in 100mM DTT for 5 or 20 min at RT increased transformation efficiencies by 2-fold. This DTT concentration was the same as that used in the one-step procedure. Raising the temperature of the DTT preincubation to 42 °C further improved the results, producing an over 4-fold increase when cells were incubated for 20 min. No enhancement was observed when cells were simply preincubated in water at 42 °C (Figure 2A).
Figure 2.
Treatment with DTT increases efficiency of tranformation of early stationary phase cells using the DMSO-based protocol. (A) Preincubation in 100 mM DTT, but not H2O, increases colony formation. C, the standard DMSO protocol of Soni et al. without preincubation. (B) Concentration-dependence of DTT stimulation after pretreatment at 42 °C for 20 min. (C) Effect of pretreatment with DTT dissolved in H2O or 0.1 M Tris set to pH 6.0 – 9.0. (D) Addition of DTT at the same time as PEG, LiAc, Tris and EDTA, eliminating the preincubation step, also enhances transformation. (E) Colony formation is increased when cells are allowed to recover in YPDA broth at 30 °C after transformations involving either pretreatment with DTT or inclusion of DTT in the PEG/LiAc mix. Error bars indicate standard deviations.
Varying the concentration of DTT from 25 mM to 200 mM revealed that 100 – 200 mM was optimum (Figure 2B) and 100 mM was chosen for subsequent experiments. DTT is frequently used to reduce disulfide bridges within proteins in vitro. The normal mechanism of action of the chemical requires that at least one of its two sulfhydryl groups be deprotonated, a process that is pH-dependent as the two groups have pKa values of 9.2 and 10.1 (Lukesh et al., 2012). However, pretreatment of cells at 42 °C with DTT dissolved in 0.1 M Tris set to pH 6.0, 7.0, 8.0 or 9.0 did not produce results that were better than simply dissolving the DTT in ddH2O; each of the DTT pretreatments increased Ura+ colony numbers by approximately 3- to 4-fold (Figure 2C).
The possibility that adding DTT at the same time as PEG, LiAc, Tris and EDTA, eliminating the pretreatment step, might improve transformation efficiencies was also investigated. As shown in Figure 2D, this approach produced results similar to that of the pretreatment procedure, resulting in an approximately 4-fold increase in colonies (p < 0.05 using the Student's t-test).
Allowing cells to recover after the heat shock step by shaking in YPDA broth at 30 °C increased transformation efficiency with the unmodified protocol by 1.8 fold (Figure 1D). Combining recovery in YPDA broth with the new DTT-treatment protocols produced even better results. As shown in Figure 2E, allowing cells to recover in rich broth increased colony numbers by 2.5 – 3 fold when a 40 min incubation was used and by 3.5 – 4.8 fold when cells were incubated for 80 min.
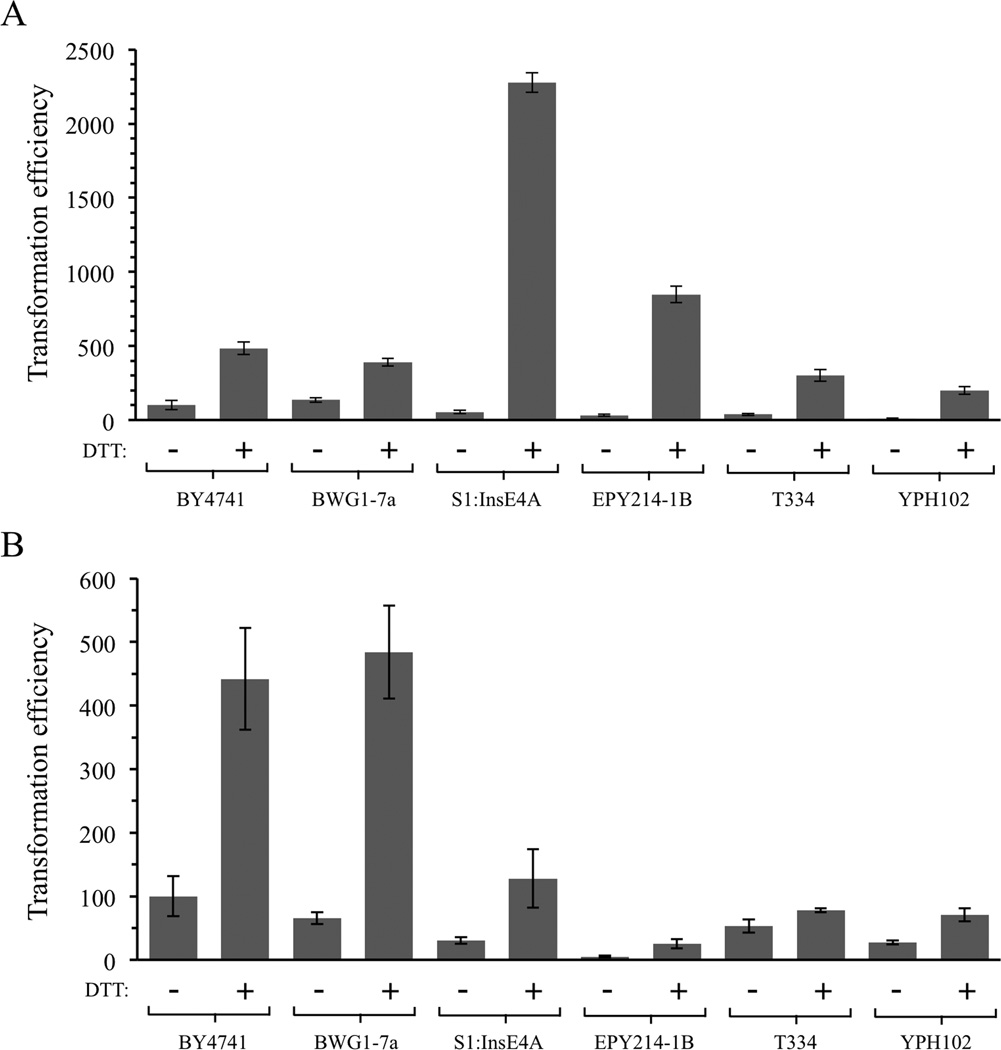
The effects of treatment with DTT were also assessed in other strain backgrounds (Figure 3). For these experiments, previously published strains BY4741, BWG1-7a, S1:InsE4A, EPY214-1B, T334, and YPH102 (including S288c, SK1 and A364a backgrounds) (Brachmann et al., 1998; Hovland et al., 1989; Larionov et al., 1994; Lewis et al., 1998; Morrison et al., 1993; Sikorski and Hieter, 1989; White and Haber, 1990) were all tested using the adapted DMSO-based protocol described here with and without DTT treatment (and without post-heat shock recovery in YPDA). Efficiencies observed among the different strains without DTT were variable and colony formation in two strains, EPY214-1B and YPH102, was only 10–30% as high as that of BY4741 cells in the experiments shown in Figure 3A and 3B. The untreated BY4741 control was arbitrarily set to 100 in the graphs. Specific efficiencies for BY4741 in Figure 3A were 16,200 transformants per µg DNA without DTT and 78,100 transformants per µg after pretreatment with 100 mM DTT. Pretreatment with DTT at 42 °C increased colony formation in all strains, with increases ranging from 2.9-fold for BWG1–7A cells to 27- and 42-fold in EPY214-1B and S1:InsE4A cells (Figure 3A). This experiment was repeated by adding DTT to the PEG-LiAc mix as in Figure 2D, without a preincubation step. DTT addition again improved results in all strains tested, with increases ranging from 1.5 fold in T334 cells to 7.4 fold for BWG1-7A cells (Figure 3B).
Figure 3.
Treatment with DTT increases colony formation in several yeast strain backgrounds. (A) Cells were preincubated in 100 mM DTT at 42 °C for 20 min. (B) Cells were treated with the standard mixture containing PEG, LiAc, Tris, EDTA and DNA supplemented with 100 mM DTT. Error bars indicate standard deviations.
Discussion
This study has examined methods to improve the relatively low DNA transformation efficiencies of early stationary (post-diauxic) phase yeast cells. The one-step procedure of Chen et al. (1992) and the DMSO-based protocol of Soni et al. (1993) achieved much higher efficiencies than a simple PEG/LiAc protocol, with a 15-fold increase seen using the DMSO method. Soni et al. observed plasmid transformation efficiencies of < 5,000 per µg DNA, but the adapted version of the protocol used in the current study routinely gave 20,000 – 40,000 transformants per µg. It is unclear at present if the higher efficiency is due to the different strain backgrounds, the DNAs employed in each study, or to some other factor. Our observation that some strains (e.g., YPH102 and EPY214-1B) gave poor results when DTT was not present (Figure 3) suggests that strain differences are likely to be important.
The DMSO-based approach was not improved further by simply resuspending cells in rich broth or phosphate-buffered saline and spreading directly to selective plates. However, incubating the cells in YPDA broth at 30 °C for 40 min increased efficiency by approximately 2-fold. The doubling time of yeast cells is 90 – 120 min, much longer than the 40 min incubation time, which suggests that the increase in Ura+ colonies was not simply due to growth of the transformed cells.
In some protocols cells are washed in water or salt solution prior to mixing with PEG, LiAc and DNA. We observed that brief incubation in DTT prior to addition of PEG-LiAc-Tris-EDTA solution strongly increased Ura+ colony formation. Preincubation in 0.1 M DTT at 42 °C for 20 min yielded best results, increasing efficiencies by 3- to 5-fold in BY4742 cells. Subsequent experiments established that simply adding DTT at the same time as the PEG-LiAc-Tris-EDTA solution also elevated transformation efficiencies by 4-fold.
Adding a 40 minute post-heat shock recovery step in YPDA broth to the DTT-treated cells enhanced colony formation by an additional 3–4 fold, even more than the 1.8 fold increase seen using the unmodified protocol (Figure 2E vs Figure 1D). Chemical-based transformation methods attempt to disorganize cell membranes and/or cell walls enough to allow DNA entry but not enough to cause the death of most cells. They are highly inefficient because the vast majority of cells do not take up the DNA and/or survive the harsh chemical treatment. It is possible that DTT acts positively to modify cell surfaces and allow more cells to take up DNA, but also has a negative effect, causing more potentially lethal changes in many of the cells. The larger increase in transformation efficiencies after incubation of DTT-treated cells in YPDA broth may be due to enhanced survival of cells that had taken up the plasmid DNA but were strongly damaged by the DTT treatment.
A few past reports of DNA uptake by cells have suggested that reducing agents such as DTT may influence the process, but results have been contradictory. For example, Ito et al. (1983) and Brzobohaty and Kovac (1986) observed that uptake of DNA into log phase yeast cells was modestly increased by 2-mercaptoethanol in the presence of LiCl. However, Ito et al. noted that other reducing agents such as DTT, L-cysteine and reduced glutathione were not effective in their system. Another study described a procedure for log phase cells that achieved modest efficiencies using solutions containing CaCl2 and DTT (Reddy and Maley, 1993). In addition, Hayama et al. (2002) demonstrated DNA uptake after incubation of early log phase cells with PEG and oxidized glutathione (but not reduced glutathione), though overall transformation efficiencies using this method were quite low (< 1,000 transformants per µg DNA). Finally, we note that the one-step procedure of Chen et al. used here also included DTT in the PEG/LiAc/DNA mixture in conjunction with a higher heat shock temperature. Unlike the studies cited above, this one-step procedure employed early stationary phase cells and reported efficiencies of 10,000 – 20,000 tranformants per µg DNA, which is similar to the efficiencies achieved in the current study using the one-step method.
DTT is a 4-carbon molecule containing 2 sulfhydryl groups that actively reduces disulfide bridges within proteins, suggesting the possibility that it may disrupt disulfide bonds in one or more cell surface proteins to facilitate entry of DNA. The chemical reaction mediated by DTT requires that at least one of the two sulfhydryl groups, which have pKa values of 9.2 and 10.1, be deprotonated (Lukesh et al., 2012). Although the active deprotonated forms are favored at higher pH values, experimental outcomes were not affected by varying the pH of the DTT solution from 6.0 – 9.0 in the current study.
Several previous reports have demonstrated that DTT and other reducing agents can affect disulfide bridges within proteins associated with cell surfaces, including receptor proteins, ion channel proteins and other complexes (e.g., Kurata et al., 1998; Osawa et al., 2003, Vilardaga et al., 1997). It is currently unclear what step in the process is being affected by DTT, e.g., the reducing agent may alter proteins to affect association of DNA with the cell exterior, passage of DNA into cells, and/or the stability of the DNA after it has entered cells. Brzobohaty and Kovac (1986) determined that another reducing agent, 2-mercaptoethanol, causes intracellular RNAs to leak out of yeast cells. If DTT acts similarly, this result suggests that the chemical may increase simple diffusion of biomolecules across the cell wall and outer cell envelope.
In summary, we have demonstrated that a DMSO-based method for transformation of early stationary phase yeast cells produces relatively high efficiencies that can be boosted further by addition of DTT and by allowing cells to recover from the chemical treatment in rich broth. A rapid procedure for transformation of overnight cultures was developed and optimized, producing efficiencies of approximately 50,000 – 100,000 transformants per µg of plasmid DNA. The method was shown to work well in several different yeast strain backgrounds, including S288c, A364a and SK1 strains.
Acknowledgements
The authors wish to thank Leasha Schaub and Lesli Rogers for their expert assistance during the initiation of this project. LKL was supported in part by National Institutes of Health grant 1R15GM09904901 and a departmental grant from the Welch Foundation.
References
- Benatuil L, Perez JM, Belk J, Hsieh CM. An improved yeast transformation method for the generation of very large human antibody libraries. Protein Eng Des Sel. 2010;23:155–159. doi: 10.1093/protein/gzq002. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brzobohatý B, Kovác L. Factors enhancing genetic transformation of intact yeast cells modify cell wall porosity. J Gen Microbiol. 1986;132:3089–3093. doi: 10.1099/00221287-132-11-3089. [DOI] [PubMed] [Google Scholar]
- Chen DC, Yang BC, Kuo TT. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- Chen P, Liu HH, Cui R, et al. Visualized investigation of yeast transformation induced with Li+ and polyethylene glycol. Talanta. 2008;77:262–268. doi: 10.1016/j.talanta.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007a;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007b;2:35–37. doi: 10.1038/nprot.2007.14. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Genetic transformation of yeast. Biotechniques. 2001;30:816–826. doi: 10.2144/01304rv02. [DOI] [PubMed] [Google Scholar]
- Hayama Y, Fukuda Y, Kawai S, Hashimoto W, Murata K. Extremely simple, rapid 20 and highly efficient transformation method for the yeast Saccharomyces cerevisiae using glutathione and early log phase cells. J Biosci Bioeng. 2002;94:166–171. doi: 10.1263/jbb.94.166. [DOI] [PubMed] [Google Scholar]
- Herman PK. Stationary phase in yeast. Curr Opin Microbiol. 2002;5:602–607. doi: 10.1016/s1369-5274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- Hovland P, Flick J, Johnston M, Sclafani RA. Galactose as a gratuitous inducer of GAL gene expression in yeasts growing on glucose. Gene. 1989;83:57–64. doi: 10.1016/0378-1119(89)90403-4. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Hashimoto W, Murata K. Transformation of Saccharomyces cerevisiae and other fungi: methods and possible underlying mechanism. Bioeng Bugs. 2010;1:395–403. doi: 10.4161/bbug.1.6.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszenman-Pereyra D, Hieda K. A colony procedure for transformation of Saccharomyces cerevisiae . Curr Genet. 1988;13:21–23. doi: 10.1007/BF00365751. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Hisatome I, Tsuboi M, et al. Effect of sulfhydryl oxidoreduction on permeability of cardiac tetrodotoxin-insensitive sodium channel. Life Sci. 1998;63:1023–1035. doi: 10.1016/s0024-3205(98)00364-6. [DOI] [PubMed] [Google Scholar]
- Larionov V, Kouprina N, Eldarov M, Perkins E, Porter G, Resnick MA. Transformation-associated recombination between diverged and homologous DNA repeats is induced by strand breaks. Yeast. 1994;10:93–104. doi: 10.1002/yea.320100109. [DOI] [PubMed] [Google Scholar]
- Lewis LK, Kirchner JM, Resnick MA. Requirement for end-joining and checkpoint functions, but not RAD52-mediated recombination, after EcoRI endonuclease cleavage of Saccharomyces cerevisiae DNA. Mol Cell Biol. 1998;18:1891–1902. doi: 10.1128/mcb.18.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukesh JC, 3rd, Palte MJ, Raines RT. A potent, versatile disulfide-reducing agent from aspartic acid. J Am Chem Soc. 2012;134:4057–4059. doi: 10.1021/ja211931f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, Johnson AL, Johnston LH, Sugino A. Pathway correcting DNA 21 replication errors in Saccharomyces cerevisiae . EMBO J. 1993;12:1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa T, Feng XY, Nozaka Y. Scanning electron microscopic observations of the basement membranes with dithiothreitol separation. Med Electron Microsc. 2003;36:132–138. doi: 10.1007/s00795-003-0214-3. [DOI] [PubMed] [Google Scholar]
- Pham TA, Kawai S, Kono E, Murata K. The role of cell wall revealed by the visualization of Saccharomyces cerevisiae transformation. Curr Microbiol. 2011a;62:956–961. doi: 10.1007/s00284-010-9807-y. [DOI] [PubMed] [Google Scholar]
- Pham TA, Kawai S, Murata K. Visualization of the synergistic effect of lithium acetate and single-stranded carrier DNA on Saccharomyces cerevisiae transformation. Curr Genet. 2011b;57:233–239. doi: 10.1007/s00294-011-0341-7. [DOI] [PubMed] [Google Scholar]
- Reddy A, Maley F. Dithiothreitol improves the efficiency of yeast transformation. Anal Biochem. 1993;208:211–212. doi: 10.1006/abio.1993.1031. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Third Edition. NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Seidman CE, Struhl K, Sheen J, Jessen T. Molecular Biology: Introduction of plasmid DNA into cells. Wiley-Blackwell; 2001. Current Protocols. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Murray JA. Parameters affecting lithium acetate mediated transformation of Saccharomyces cerevisiae and development of a rapid and simplified procedure. Curr Genet. 1993;24:455–459. doi: 10.1007/BF00351857. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Di Paolo E, Bialek C, et al. Mutational analysis of extracellular cysteine residues of rat secretin receptor shows that disulfide bridges are essential for receptor function. Eur J Biochem. 1997;246:173–180. doi: 10.1111/j.1432-1033.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M, Braun EL, Crawford ME, Peck VM. Stationary phase in Saccharomyces cerevisiae . Mol Microbiol. 1996;19:1159–1166. doi: 10.1111/j.1365-2958.1996.tb02461.x. [DOI] [PubMed] [Google Scholar]
- White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae . EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RA, Gietz RD. High-efficiency transformation of plasmid DNA into yeast. Methods Mol Biol. 2001;177:85–97. doi: 10.1385/1-59259-210-4:085. [DOI] [PubMed] [Google Scholar]
- Zheng HZ, Liu HH, Chen SX, et al. Yeast transformation process studied by fluorescence labeling technique. Bioconjug Chem. 2005;16:250–254. doi: 10.1021/bc049833v. [DOI] [PubMed] [Google Scholar]