Abstract
A study was conducted to examine the physiological response of contrasting mung bean (Vigna radiata) genotypes viz., T 44 & MH–96–1 (tolerant) and Pusa Baisakhi & MH–1K–24 (sensitive) under waterlogging conditions. Plants were waterlogged at vegetative stage (30 days after sowing) for 3, 6 and 9 days. Waterlogging resulted in decreased leaf area, crop growth rate, root growth and nodules number, membrane stability index, photosynthesis rate, chlorophyll and carotenoid contents, flowering rate, pod setting, yield and altered dry matter partitioning. Sensitive genotypes showed large reductions in aforementioned physiological traits and slow recovery in photosynthesis rate. On the other hand, tolerant genotypes maintained higher photosynthetic rate, chlorophylls and carotenoids, growth rate, membrane stability and fast photosynthetic recovery under waterlogging. After 9 days of exposure to waterlogging, photosynthetic rate and yield losses in most sensitive genotype (MH-1K-24) were 83 and 85 %, respectively. On an average, photosynthetic loss at 3, 6 and 9 days of waterlogging was 43, 51, and 63 %, respectively, while grain yield loss was 20, 34 and 52 % respectively.
Keywords: Growth, Root proliferation, Photosynthetic loss, Respiration, Dry matter partitioning, Yield loss
Introduction
Mung bean [Vigna radiata (L.) Wilczek] also known as green gram, is one of the important pulse crops of India. It is rich in digestible protein (approximately 25–28 %) by virtue of N2 fixation machinery. It is extensively grown in tropical and subtropical Asia because of its wider range of adaptability (Poehlman 1991). This crop is fitted well in multi-cropping systems, because of its rapid growth and early maturity, results in the increase of small landholders’ income and improvement of soil fertility (Nsoukpoe-Kossi et al. 1999). However, its large-scale adoption is constrained by low yield potential. Various biotic and abiotic factors are responsible for low yields of mung bean (Chotechuen 1996). Among the abiotic stresses, excess moisture or soil flooding stands prominent.
Mung bean cannot withstand waterlogging, particularly during the early stages of growth (Singh and Singh 2011). Extensive grain yield losses have also been observed when the plants are young. Flooding or waterlogging reduces oxygen concentrations around the roots of the submerged plants and restricts nodule activity and nitrogen fixation. Thus, mung bean is not suited to the wet tropics, where the annual precipitation is above 1,000 mm (Fernandez and Shanmugasundaram 1988). The heavy rain damages the crop causing severe yield losses. Although, there have been a good number of reports on the excess moisture tolerance of other upland crops such as tomato (Kuo and Chen 1980), maize (Singh and Ghildyal 1980), wheat (Musgrave and Ding 1998) etc., and soil flooding in mung bean is not uncommon, but despite this fact, very little information is available on the physiological responses of mung bean to soil waterlogging. Waterlogging reduces plant growth by affecting one or several physiological processes. One of the main physiological effects of waterloggging is an inhibition of photosynthesis (Ahmed et al. 2002, 2006). Since photosynthesis is fundamentally associated with yield, therefore, the present study was carried out with an aim to analyze genotypic variability in growth, gas exchanges and yield responses of mung bean in relation to waterlogging tolerance and to estimate photosynthetic and yield losses under different levels of waterlogging at vegetative growth stages.
Materials and methods
Experimental material and growth conditions
A pot-culture experiment was conducted in complete randomized design using four genotypes of mung bean viz., two tolerant (T 44, and MH-96-1), and two sensitive (Pusa Baisakhi, and MH-1K-24) to study their response to waterlogging stress. Seeds were obtained from Division of Genetics, Indian Agricultural Research Institute, New Delhi and Indian Institute of Pulse Research, Kanpur, (UP), India and sown in 30 × 30 cm (height × diameter) earthen pots filled with clay-loam soil mixed farm yard manure in 4:1 ratio during the summer-rainy season.
Twelve kg of soil was filled in pots and fertilized with 0.264, 0.600, and 0.520 g urea, triple super phosphate, and muriate of potash corresponding to 40-60-40 kg N, P, and K per hectare, respectively. Half of the urea and other fertilizers were mixed with soil before sowing. The rest of the urea was top-dressed during the vegetative stage of plants. The plants were watered regularly to maintain optimal soil moisture until the flooding treatments were imposed. Adequate plant protection measures were taken to keep the plants free from diseases, insects, and weeds by having repeated manual hand weeding and spraying with Bavistin and Rogor @ 0.3 %. Before sowing, seeds were treated with the required Rhizobium culture following the method described elsewhere (Tripathi et al. 2012). Initially, four plants were sown in each pot, which were thinned to three plants per pot after 20 d. For waterlogging treatment, earthen pots along with 30 d old plants were transferred to polythene bags filled with water and placed in plastic troughs. The water level in polythene bags was maintained almost up to the upper surface of soil in the pot. Treatments were control, 3, 6, and 9 d of waterlogging, and recovery after 3, 6, and 9 d of termination of waterlogging. Two samples were collected from each of the four replicates (n = 8) for the estimation of growth parameters, relative water content (RWC), membrane stability index (MSI), and chlorophyll (Chl) and carotenoid contents.
Growth parameters
Plants were harvested from control and waterlogging treatments and after termination of waterlogging. Plants were dug out gently and roots were washed thoroughly for counting of root nodules. Leaf area was measured using leaf area meter (Model 3100, LI-COR, Inc Lincoln, NE, USA). Plant samples were dried in hot air oven at 80 °C till constant weight was achieved. Plant growth rate (GR) was computed following the method of Gardner et al. 1985.
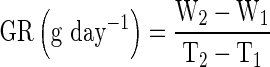 |
Where,
- W1
Total plant dry matter at time T1
- W2
Total plant dry matter at time T2
- T1
Time of first observation and
- T2
Time of second observation
Leaf relative water content (RWC)
Leaf relative water content (RWC) was determined by recording the turgid weight of 0.5 g fresh leaf samples by keeping in water for 4 h, followed by drying in hot air oven till constant weight achieved (Weatherley 1950).
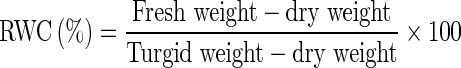 |
Membrane stability index
Membrane stability index was estimated 100-mg leaf material using two sets of test tubes containing 10 ml of double distilled water (Sairam 1994). One set was heated at 40 °C for 30 min in a water bath, and the electrical conductivity of the solution was recorded using conductivity meter (C1). Second set was boiled at 100 °C on a boiling water bath for 10 min, and its conductivity (C2) was measured as above. Membrane stability index (MSI) was calculated as:
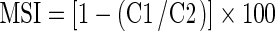 |
Estimation of chlorophylls and carotenoids
Chlorophyll content was extracted in 0.05 g leaf material in 10 ml dimethyl sulfoxide (DMSO) (Hiscox and Israelstam 1979). Samples were heated in an incubator at 65 °C for 4 h, and than after cooling to room temperature, the absorbance of extracts were recorded at 665 and 645 nm. Total chlorophyll concentration was estimated in leaf tissue using following formula given by Arnon (1949).
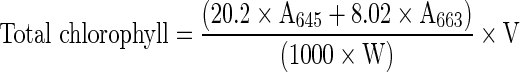 |
Where
- A
Absorbance at given wavelength
- V
final volume of DMSO in ml
- W
weight of sample in g.
Estimation of carotenoids
The chlorophyll above extract was also used for the quantification of carotenoids following the formula (Hendry and Price 1993) given below:
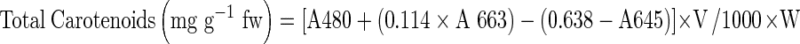 |
Gas exchange measurements
At each of the three growth stages, leaf gas exchange was measured on successive days in control and treated plants and also after the termination of flooding. Gas exchange parameters were measured at ~1,500 photosynthetic photon flux density (PPFD) light between 10.00 and 11:30 AM, using a portable photosynthesis system (LICOR-6200) which is a measure of the photosynthetic photon flux (area) density normally quantified as μmol photons/m2/s. Third leaf from the top of the plant was selected for the gas exchange measurements and data on leaf photosynthetic rate (Pn), stomatal conductance (Cs) and respiration rate were recorded. For measurement of the rate of respiration, leaf chamber of portable photosynthesis system was covered with black cloth during the recording of respiration rate. During the measurement, air temperature and relative humidity were around 33.5 °C and 72.5 (%) respectively.
Yield attributes and seed yield
At maturity, pods were harvested in three pickings, viz. at 54, 70 and 88 days after emergence (DAE). Data on pods per plant, seeds per pod, and 100-seed weight were recorded for individual treatments and at each harvest. At final harvest, plant height was recorded. Yield attributes and seed yield of each plant were determined by summing up three harvests and seed yield was adjusted at 12 % moisture content.
Data analysis
Standard statistical methods were employed to compare different parameters of waterlogged and non-waterlogged plants. All experimental data recorded were average mean values for at least three independent assays with three replicates each. The data were subjected to ANOVA for completely randomized design factorial (Gomez and Gomez 1984).
Results
Growth and development
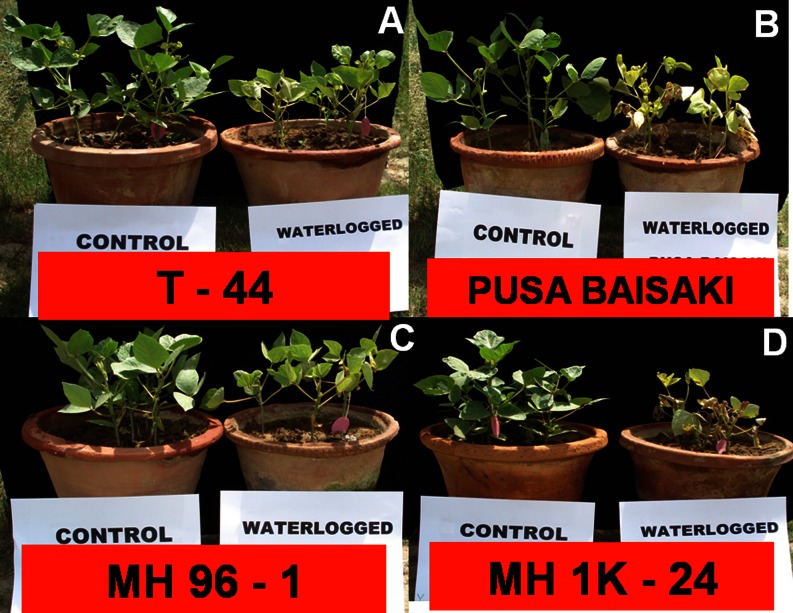
In general, 9 days waterlogging caused yellowing of leave and reduced the plant growth of all genotypes, however, reduction was significantly lower in tolerant genotypes (T- 44 and MH-96-1) compared to sensitive genotypes (Pusa Baisakhi and MH-1K-24) (Fig. 1a–d). Root growth was also affected under waterlogging and tolerant genotypes showed formation of horizontal adventitious roots at soil surface from the transition zone between root and shoot (Fig. 2a and b). These genotypes also maintained higher root nodules per plant than sensitive genotypes under waterlogging (Fig. 3a). Similarly, tolerant genotypes maintained significantly higher leaf area and growth rate under waterlogging compared with sensitive genotypes (Fig. 3b–c).
Fig. 1.
Growth of four contrasting tolerant (T-44 & MH 96-1) and sensitive (Pusa basakhi & MH 1K – 24) genotypes of mung bean after 9 days of waterlogging
Fig. 2.
Comparative adventitious root formation in tolerant (T-44) (a) and sensitive (Pusa Baisakhi) (b) genotypes under waterlogging conditions
Fig. 3.
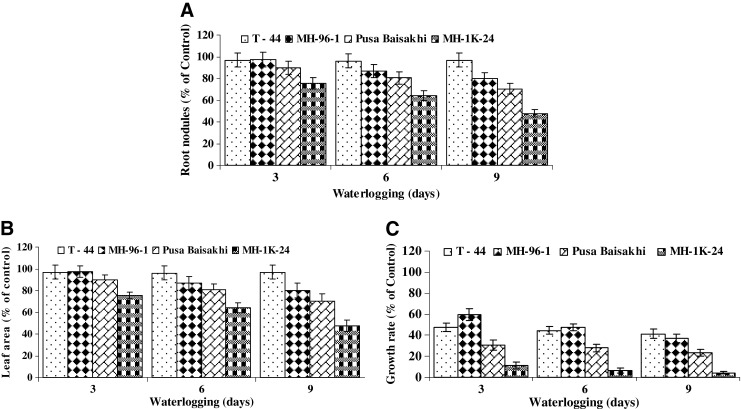
Effect of waterlogging on a root nodule/plant growth rate, b leaf area and c growth rate in tolerant (T-44 & MH-96-1) and susceptible (Pusa Baisakhi and MH-1K-24) genotypes of mung bean. For different parameter genotype wise control average values equivalent to 100 % were recorded as: Root nodules (No. of nodules/plant) 12.67 (T - 44), 17.67 (MH 96 – 1), 19.33 (Pusa Baisakhi) and 16.67 (MH 1K – 24); Leaf area (cm2) 167.92 (T - 44), 190.74 (MH 96 – 1), 222.15 (Pusa Baisakhi) and 152.32 (MH 1K – 24); Growth rate (mg/plant/day) 244.63 (T - 44), 224.17 (MH 96 – 1), 262.24 (Pusa Baisakhi) and 198.93 (MH 1K – 24). Vertical bars show ± SD of mean
MSI and RWC
Membrane stability index and relative water content decreased in all genotypes with advancing in waterlogging. Tolerant genotypes T- 44 and MH - 96-1 maintained significantly higher membrane stability and relative water content than sensitive ones (Pusa Baisakhi and MH-1K-24) under waterlogging (Fig. 4a–b).
Fig. 4.
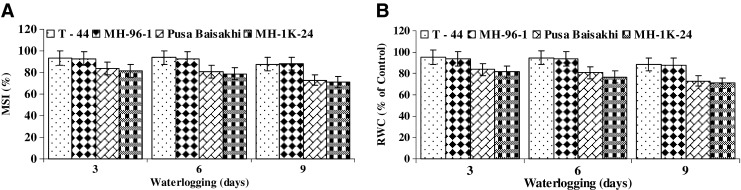
Effect of waterlogging on a membrane stability index and b relative water content of leaf tissues in tolerant (T-44 & MH-96-1) and susceptible (Pusa Baisakhi and MH-1K-24) genotypes of mung bean. For different parameter genotype wise control average values equivalent to 100 % were recorded as: MSI (%) 90.56 (T - 44), 92.22 (MH 96 – 1), 90.23 (Pusa Baisakhi) and 88.29 (MH 1K – 24); RWC (%) 89.23 (T - 44), 87.33 (MH 96 – 1), 86.67 (Pusa Baisakhi) and 83.43 (MH 1K – 24). Vertical bars show ± SD of mean
Chlorophylls and carotenoids
Under waterlogging, all genotypes showed chlorosis and yellowing of leaves and reduction in photosynthetic pigments (Fig. 5a–b). However, sensitive genotypes (Pusa Baisakhi and MH-1K-24) exhibited relatively higher chlorosis and drastic reduction in the level of chlorophylls and carotenoids as compared to tolerant ones viz. T 44 and MH- 96-1. The level of photosynthetic pigments reduced with increasing level of waterlogging. Amongst all the genotypes, T 44 maintained the highest levels of total chlorophylls and total carotenoids under waterlogging. Both the sensitive genotypes showed drastic reduction in the levels of total chlorophylls and carotenoids under waterlogging. Ratio of total carotenoids and total chlorophylls showed the genotypic variation during waterlogging (Fig. 5c). Tolerant genotypes namely T- 44 and MH -96-1 exhibited an increase in the relative ratio of total carotenoids and chlorophylls under waterlogging. However sensitive genotype MH-1K-24 and Pusa Baisakhi showed the reduction in the relative ratio of total chlorophylls and carotenoids particularly after 9 days waterlogging (Fig. 5c).
Fig. 5.
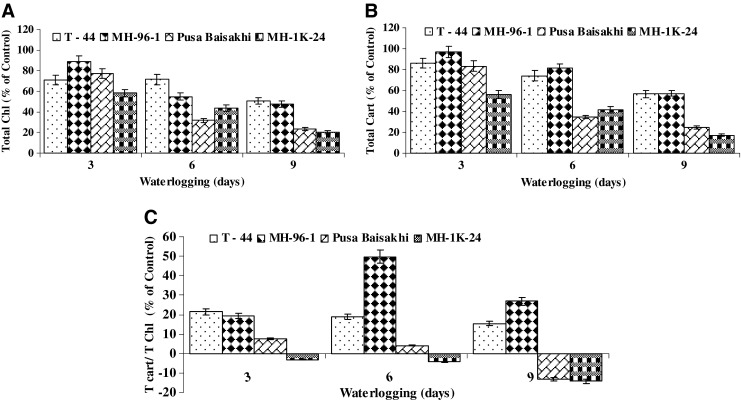
Effect of waterlogging on a total chlorophyll, b total carotenoid and c relative ratio of total chlorophyll/total carotenoids in leaf tissues of tolerant (T-44 & MH-96-1) and sensitive (Pusa Baisakhi and MH-1K-24) genotypes of mung bean. For different parameter genotype wise control average values equivalent to 100 % were recorded as: Total chlorophyll (mg/g f wt) 2.041 (T - 44), 2.078 (MH 96 – 1), 1.814 (Pusa Baisakhi) and 1.441(MH 1K – 24); Total carotenoids (mg/g f wt) 0.345 (T - 44), 0.392 (MH 96 – 1), 0.340 (Pusa Baisakhi) and 0.260 (MH 1K – 24); Total carotenoids/Total chlorophyll (Ratio) 0.1698 (T - 44), 0.1895 (MH 96 – 1), 0.1872 (Pusa Baisakhi) and 0.1804 (MH 1K – 24). Vertical bars show ± SD of mean
Leaf photosynthesis and respiration
Waterlogging inhibited the rate of photosynthesis in all genotypes. Inhibition of photosynthesis increased with the advancing of waterlogging duration. Moreover, photoinhibition was comparatively higher in sensitive genotypes (Pusa Baisakhi and MH-1K-24) than tolerant ones (T - 44 and MH-96-1) (Fig. 6a). Tolerant genotypes not only maintained the higher rate of photosynthesis during waterlogging but also showed faster recovery after termination of waterlogging (Fig. 6d). Furthermore, after 9 days waterlogging termination, almost 100 % recovery in terms of rate of photosynthesis was recorded in tolerant genotypes. Amongst sensitive genotypes, photosynthetic recovery was observed slower than tolerant (Fig. 6d). Photosynthetic loss among mung bean genotypes increased with increase in the level of waterlogging and was estimated up to 80 % at 9th day. However, photosynthetic losses among tolerant genotypes were quite lower than sensitive genotypes (Fig. 6a). Stomatal conductance also showed the similar pattern as observed in for photosynthesis rate (Fig. 6b and e).
Fig. 6.
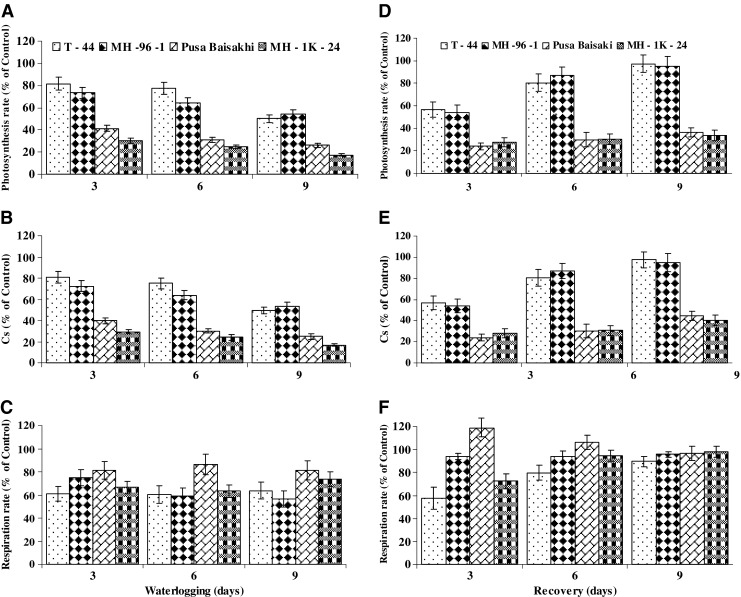
Leaf photosynthesis, stomatal conductance (Cs) and respiration rate in mung bean genotypes during waterlogging. a Photosynthetic rate (% of control), b Stomatal conductance (% of control), c respiration rate (% of control) and after waterlogging termination during recovery, d photosynthetic rate (% of control), e Stomatal conductance (% of control f respiration rate (% of control). For different parameter genotype wise control average values equivalent to 100 % were recorded as: Photosynthesis rate (μmole CO2/m2/s) 21.07 (T - 44), 22.23 (MH 96 – 1), 21.97 (Pusa Baisakhi) and 19.77 (MH 1K – 24); Stomatal Conductance (cm/s) 3.434 (T - 44), 3.623 (MH 96 – 1), 3.581 (Pusa Baisakhi) and 3.223 (MH 1K – 24); Respiration rate (μmole CO2/m2/s) 8.82 (T - 44), 8.85 (MH 96 – 1), 8.61 (Pusa Baisakhi) and 6.17 (MH 1K – 24). Vertical bars show ± SD of mean
On an average, leaf respiration increased at 3rd day of waterlogging, as compared to normal conditions. MH- 96-1 and Pusa Baisakhi exhibited maximum enhancement of leaf respiration rate than other genotypes. At 6th and 9th day of waterlogging, slight reduction in rate of respiration was recorded. Leaf respiration rate in T-44 remained unaffected throughout waterlogging treatment. Thus, in general, leaf respiration rate, did not decrease and maintained normal level even during last phase of waterlogging (Fig. 6c). After 3 days of waterlogging termination Pusa Baisakhi had the highest CO2 liberation (respiration rate) and after 9 days of waterlogging termination all genotypes of mung bean showed almost complete respiration recovery (Fig. 6f).
Total dry matter production and Dry matter partitioning
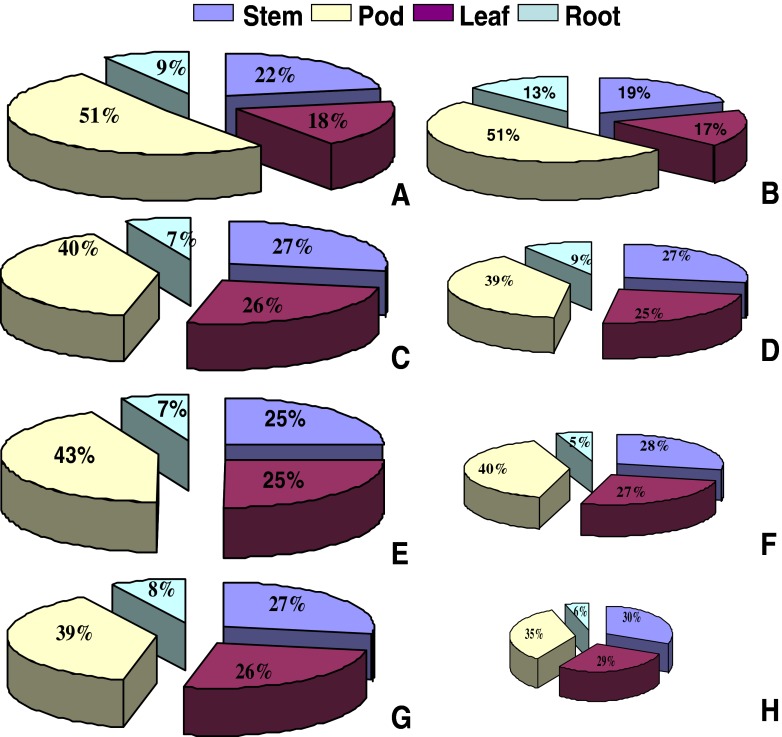
Waterlogging in mungbean reduced total dry matter production and also affected the dry matter partitioning (Fig. 7). Relative reduction in dry matter was more pronounced in sensitive genotypes than tolerant ones. At harvest, under waterlogged condition tolerant genotypes i.e. T- 44 and MH- 96-1 exhibited relatively higher dry matter accumulation in root and slight reduction in stem and leaf over its control (Fig. 7a–d). However, waterlogging sensitive genotypes Pusa Baisakhi and MH-1K-24 showed poor dry matter accumulation in root and higher dry matter accumulation in stem and leaf under waterlogging than its control (Fig. 7e–h). In T- 44, proportion of dry matter partitioning in pod under waterlogging remained exactly similar to its control and very slightly reduced in MH- 96-1 (Fig. 7a–d). However, in sensitive mung bean genotypes Pusa Baisakhi and MH-1K-24, proportion of dry matter partitioning in pod under waterlogging was recorded lower than their respective controls (Fig. 7e–h).
Fig. 7.
Dry matter partitioning in leaves, stem, roots and pods in tolerant (T-44 & MH 96-1) and sensitive (Pusa Baisakhi & MH-1K 24) mung bean genotypes under control and waterlogged conditions. a T-44 (control), b T-44 (waterlogged), c MH 96-1 (control) and d MH 96-1 (waterlogged) e Pusa Baisakhi (control), f Pusa Baisakhi (waterlogged), g MH-1K 24(control) and h MH-1K 24 (waterlogged). Genotype wise total dry matter (g/plant) average values of control (non-waterlogged) equivalent to 100 % were recorded as 17.68 (T - 44), 33.72 (MH 96 – 1), 39.67 (Pusa Baisakhi) and 23.22 (MH 1K – 24) while under waterlogged conditions average values of total dry matter (g/plant) were 15.18 (T - 44), 26.36 (MH 96 – 1), 13.21 (Pusa Baisakhi) and 7.32 (MH 1K – 24)
Flowering and podding patterns
Both tolerant and sensitive genotypes showed the inhibition of flowering, pod setting and enhanced the dropping of flowers and pods under waterlogging (Fig. 8a–h). However, number of floral buds and pods per plant were most affected under waterlogging only in sensitive genotypes (Fig. 8e–h). There was severe reduction in pod setting in sensitive genotypes viz. Pusa Baisakhi & MH-1K-24 and this reduction was mainly associated with the dropping of floral buds and pods (Fig. 8e–h). In contrast, tolerant genotypes T- 44 and MH-96 -1 maintained fairly good pod setting even in waterlogged plant (Fig. 8a–d).
Fig. 8.
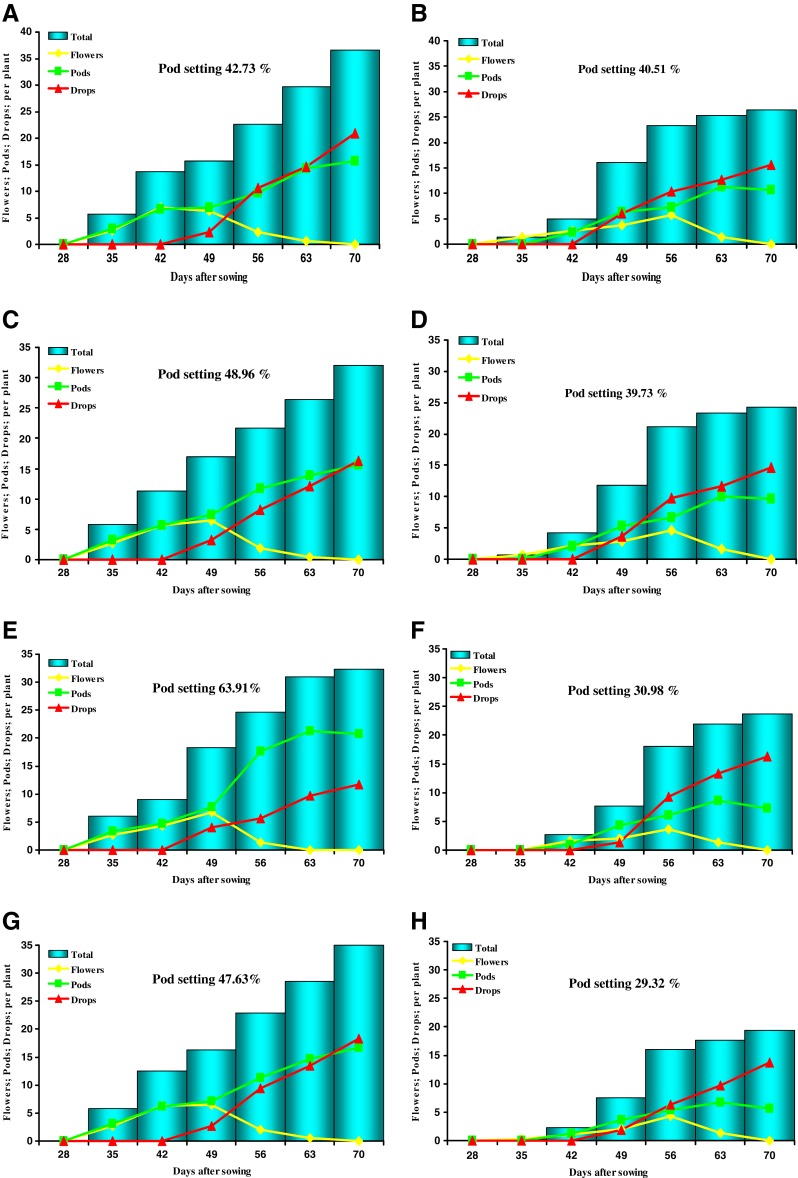
Comparative account of flowering, podding and droppings patterns in tolerant (T-44 & MH 96-1) and sensitive (Pusa Baisakhi & MH-1K 24) genotypes under control and waterlogging. a T-44 (control), b T-44 (waterlogged), c MH 96-1 (control), d MH 96-1 (waterlogged), e Pusa Baisakhi (control), f Pusa Baisakhi (waterlogged), g MH-1K 24 (control) and h MH-1K 24 (waterlogged). Total includes sum of flowers, pods and dropped ones
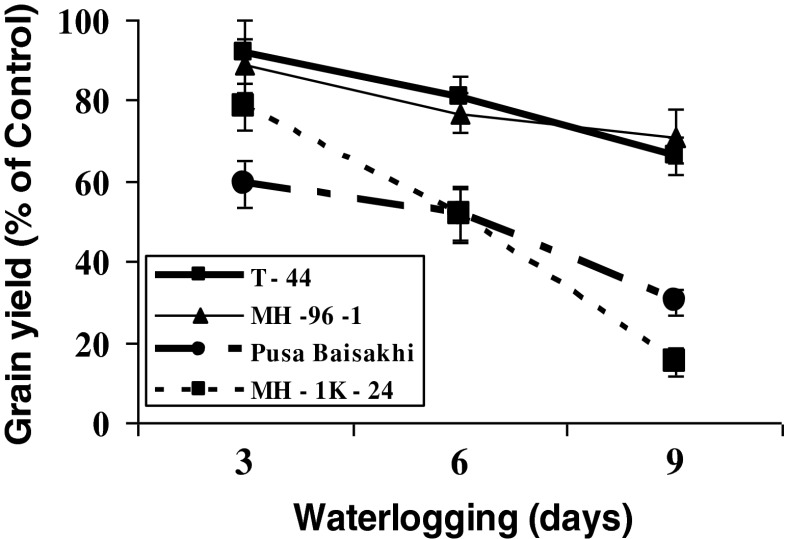
Yield loss
The yield was affected by waterlogging in all the genotypes. Yield losses increased with the increase in waterlogging duration at vegetative stage. On an average, grain yield losses in all four mung bean genotypes at 3, 6 and 9 days of waterlogging were 20.01, 33.79 and 51.88 %, respectively. Tolerant genotypes almost recovered the grain yield losses caused by 3 days waterlogging. However, for sensitive genotypes even 3 days waterlogging reduced the yield upto 20 %. Grain yield losses in sensitive genotypes after 9 days waterlogging at were estimated 70.0 (Pusa Baisakhi) to 84.9 % (MH – 1K – 24) as compared to their respective controls. Tolerant genotypes showed comparatively lesser yield reduction even after 9 days of waterlogging (Fig. 9).
Fig. 9.
Relative reduction in grain yield of mung bean tolerant (T-44 & MH 96-1) and sensitive (Pusa Baisakhi & MH-1K 24) genotypes under varying durations of waterlogging at vegetative stage. Genotype wise control average values of grain yield (g/plant) equivalent to 100 % were 7.37 (T - 44), 8.96 (MH 96 – 1), 11.15 (Pusa Baisakhi) and 6.96 (MH 1K – 24)
Discussion
During the study waterlogging induced several physiological disturbances, including reduction in growth, dry matter, photosynthesis and pod formation that resulted in low yield similar to that in other beans (Solaiman et al. 2007; Pociecha et al. 2008; Celik and Turhan 2011). Waterlogging treatment caused reduction in plant growth in terms of leaf area and growth rate in all the genotypes and the level of reduction was more pronounced in sensitive genotypes. For acclimation in waterlogging environment, avoidance of water loss through reduction in leaf area and the induction of adventitious roots proliferation have been reported in soybean by Bacanamwo and Purcell (1999). In our study, waterlogging resulted in increased adventitious root proliferation in tolerant genotypes. This in turn indicated significance of adventitious roots proliferation as an important trait. It provides an early and fast root growth recovery. Similarly, low degree of root decay and formation of adventitious roots along with aerenchyma has been reported as important characteristics to confer tolerance under waterlogging in cowpea (Takele and McDavid 1994) and faba bean (Solaiman et al. 2007). Similar to our observations inhibition of growth has been reported in sensitive genotypes in field bean (Pociecha et al. 2008), tomato (Else et al. 2009) and common bean (Celik and Turhan 2011).
The formation of new roots at the upper most part of the tap root (transition zone between root and shoot) might have occurred as a consequence of the death of existing root tips (Palta 2007). These newly formed roots under waterlogging represent not only the losses of previously-invested carbon, but an investment of new carbon (Palta et al. 2010). The malfunctioning of root systems under anoxia and enhanced production of adventitious roots was also reported earlier in several plant species like maize (Wenkert et al. 1981), Rumex spp. (Visser et al. 1996) and mungbean (Islam et al. 2010). Visser et al. (1996) reported that accumulation of ethylene has a role in the formation of flooding-induced adventitious roots formation. The production of new thick roots reflects the death and decay of existing roots (Malik et al. 2001). Formation of adventitious roots is viewed as an indicator of the presence of adaptive mechanism in plants tolerant to excess soil water (Jackson and Drew 1984). This trait allows the root system to obtain oxygen directly from the air because the adventitious roots formed in the soil and even at the soil surface. We observed reduction in number of nodules per plant in all genotypes of mung bean at 9 days of waterlogging but tolerant genotypes maintained higher number of nodules per plant. Similar observations have been reported in cowpea (Hong et al. 1977) and soybean (Matsunami et al. 2007).
Cell membrane stability has been widely used to express stress tolerance in plants and higher membrane stability is correlated with stress tolerance by Premachandra et al. (1992). Membrane disintegration as a result of oxygen deprivation and solute leakage upto 40 times has been reported in 4 days waterlogged pea plants (Jackson et al. 1982; Rawyler, et al. 2002). In our study, waterlogging significantly reduced the relative water content (RWC) and membrane stability particularly in sensitive mungbean genotypes. Similar reduction in relative water content (RWC) has been reported under flooding stress in pineapple by Min and Bartholomew (2005). Wilting in plants under excess of water during flooding has been attributed to higher resistance to mass flow of water through the roots (Jackson and Drew 1984). Flooding-tolerant plant species develop adaptive mechanisms to maintain better water relationship by means of stomatal conductance (Malik et al. 2001).
Yellowing of the plants and reduction in total chlorophyll content in the leaves of mung bean plants was observed during waterlogging. Similarly, reduction in total chlorophyll content as a result of flooding has been reported in wheat (Collaku and Harrison 2002), maize (Prasad et al. 2004), sesame (Mensah et al. 2006) and onion (Yiu et al. 2008). Under waterlogging, yellowing of the plant might be due to reduction in leaf nitrogen (Bacanamwo and Purcell 1999), nodulation and N fixation and production of toxic substances such as nitrites and sulphides which move from the soil through roots to the leave if carried upward in large quantities (Ezin et al. 2010). In addition, waterlogging results in reduced soil nitrogen through rapid volatilization and denitrification (Ali Rasaei et al. 2012). During waterlogging tolerant genotypes maintained relatively higher level of carotenoids and higher ratio of total carotenoids and total chlorophylls indicated the protective role of carotenoids in waterlogging tolerance.
Waterlogging has been reported to severely affect the process of photosynthesis in plants (Li et al. 2011a, b). We observed reduction in rate of photosynthesis in mung bean genotypes under waterlogging stress. Reduction in photosynthesis within a day after waterlogging was also reported earlier in snap bean (Lakitan et al. 1992). Decrease in rate of photosynthesis under waterlogging has been attributed to stomatal closure (Yordanova et al. 2005), decrease in leaf chlorophyll concentration (Bradford 1983), production of ethylene (Ahmed et al. 2006), reductions in sink demand (Robert and Robert 1984), and disruption of the translocation of photosynthates (Chen et al. 2005). In Spinacia oleracea photosynthesis decreased due to disruption of PSII and reductions in chlorophyll pigments under waterlogging (Schnettger et al. 1994). Damage to light-harvesting complex has also been reported in flooded tomato (Janowiak et al. 2002) and mung bean (Ahmed et al. 2006). The rate of photosynthesis under flooding may decrease due to increased photorespiration and reduced ribulose bisphosphate carboxylase (RuBisCO) activity (Yordanova and Popova 2007). Mung bean tolerant genotypes (T 44 and MH- 96-1) showed faster recovery after waterlogging termination probably due lesser damage to photosynthetic machinery was caused by waterlogging treatment.
In our study, waterlogging initially enhanced or maintained normal rate of leaf respiration in mung genotypes. Liao and Lin 2001 reported the significant enhancement in leaf respiration during flooding. This might be related to the additional energy consumption for homeostasis maintenance and adaptation of the plants under waterlogging. Maintenance respiration is known to accelerate during adaptation, which indicates additional energy consumption (Bragina et al. 2001). Further, leaf respiration was suppressed, which might be related to the fact that the genotype is already adapted to hypoxia. Maintenance of normal leaf respiration in mung bean genotypes T 44 throughout waterlogging, suggested its better adaptability to excess water environment. Rate of respiration in mung bean genotypes was increased after waterlogging termination probably due to the need of ATP for recovery and availability of photosynthates.
Waterlogging generally reduced the growth of plant components resulting in lesser total dry weight (TDW). Waterlogging reduced relative TDW as a result of reduced dry weight of plant components. Tolerant genotypes had more dry matter because they were lesser affected by waterlogging. The tolerant genotypes maintained greater root, shoot and leaf dry matter under waterlogging than the sensitive cultivars. Therefore, tolerant genotypes with vigorous shoot and root growth were better able to tolerate transient waterlogging (Hartley et al. 1993). The reduction in root dry matter is probably due to reduction in dry matter of both tap root and adventitious root as a result of a reduction in root length and branching. Earlier studies also showed the decline of both plant growth and accumulation and redistribution of dry matter by waterlogging after anthesis in wheat (Li et al. 2011a, b; Setter et al. 2009). It was shown earlier that plants invest a large proportions of carbon in their root system (Hooda et al. 1990) and the production of new roots after waterlogging, represent not only losses of a previously-invested carbon, but also an investment in new carbon (Palta et al. 2010). An alternative explanation is that transpiration flow drawn through the waterlogged roots is partially replaced by that through well-aerated adventitious roots, thereby sweeping fewer phytohormones out of waterlogged roots and into the leaves. This could reduce delivery of stomatal closing factors from the oxygen-deficient root system (Else et al. 2006), but only if water flow rate is the driving force behind its entry into xylem sap of the waterlogged roots.
In present study, waterlogging reduced seed yield primarily by reducing the number of pods per plant and pod setting. Similar reductions in plant yield have been reported in snap bean (Lakitan et al. 1992) and mung bean (Ahmad et al. 2003; Ahmed et al. 2002) grown under waterlogging. Genotypic sensitivity to waterlogging could be related to the level of endogenous plant hormones, which increase dropping of flowers and/or the loss of pod setting, as also observed in other crops (Lakitan et al. 1992; Umaharan et al. 1997) and induced by ethylene (Zhou and Lin 1995). The higher number pods in tolerant cultivars was probably due to greater availability of the source to the reproductive sinks. Higher yield in tolerant cultivars resulted with increases in the number of pods, higher rate of photosynthesis and availability of plant nitrogen under waterlogging (Palta et al. 2010). On the other hand large reduction in root nodule number and dry matter in the sensitive genotypes indicated that subsurface waterlogging might have reduced nitrogen fixation (Matsunami et al. 2005). Quick recovery of photosynthesis and leaf growth in tolerant genotypes might also have resulted in small reduction of seed yield.
Conclusions
The study concludes that contrasting mung bean genotypes responded differently to excess water in the soil, due to variability and their growth, physiological responses to waterlogging. Maintenance of normal leaf respiration by tolerant genotypes under waterlogging stress was associated with their better adaptability to excess water environment. Transient subsurface waterlogging at vegetative stage caused a severe reduction in root growth in sensitive mung bean genotypes and root proliferation in tolerant ones. The vigorous early growth and faster recovery after termination of waterlogging treatment in tolerant genotypes was associated with faster rates of adventitious root growth. However, further research is needed to evaluate the tolerance in mung bean for long term flooding conditions.
Acknowledgements
The authors thank to the Head of the Institute for providing necessary facilities and to Mr. S. N. Rai, for technical field assistance during the course of investigation.
References
- Ahmad R, Ikraam M, Ullah E, Mahmood A. Influence of different fertilizer levels on the growth and productivity of three mungbean cultivars. Int J Agric Biol. 2003;5:335–338. [Google Scholar]
- Ahmed S, Higuchi H, Nawata E, Sakuratani T. Effects on exogenous ABA and ethylene application and waterlogging on photosynthesis in mungbean (Vigna radiata (L.) Wilczak) Jpn J Trop Agric. 2002;46:166–174. [Google Scholar]
- Ahmed S, Nawata E, Sakuratani T. Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata L. Wilczak cv. KPS1) during waterlogging. Environ Exp Bot. 2006;57:278–284. doi: 10.1016/j.envexpbot.2005.06.006. [DOI] [Google Scholar]
- Ali Rasaei, Ghobadi ME, Jalali-Honarmand S, Ghobadi M, Saeidi M. Waterlogging and its effects on nitrogen of soil and plant. Ann Biol Res. 2012;3(1):119–124. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplast: polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacanamwo M, Purcell LC. Soybean dry matter and N accumulation responses to flooding stress, N sources and hypoxia. J Exp Bot. 1999;50:689–696. [Google Scholar]
- Bradford KJ. Effects of soil flooding on leaf gas exchange of tomato plants. Plant Physiol. 1983;73:475–479. doi: 10.1104/pp.73.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina TV, Drozdova IS, Alekhin VI, Ponomareva YV, Grineva GM. The rates of photosynthesis, respiration, and transpiration in young maize plants under hypoxia. Doklady Biol Sci. 2001;380:482–485. doi: 10.1023/A:1012387708429. [DOI] [PubMed] [Google Scholar]
- Celik G, Turhan E. Genotypic variation in growth and physiological responses of common bean (Phaseolus vulgaris L.) seedlings to flooding. Afr J Biotechnol. 2011;10:7372–7380. [Google Scholar]
- Chen H, Qualls RG, Blank RR. Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. Aquat Bot. 2005;82:250–268. doi: 10.1016/j.aquabot.2005.02.013. [DOI] [Google Scholar]
- Chotechuen S (1996) Breeding of mungbean for resistance to various environmental stresses. In: Proceedings of the workshop on mungbean germplasm. Bangkok
- Collaku A, Harrison SA. Loses in wheat due to waterlogging. Crop Sci. 2002;42:444–450. doi: 10.2135/cropsci2002.0444. [DOI] [PubMed] [Google Scholar]
- Else MA, Taylor JM, Atkinson CJ. Anti-transpirant activity in xylem sap from flooded tomato (Lycopersicon esculentum Mill.) plants is not due to pH-mediated redistributions of root- or shoot-sourced ABA. J Exp Bot. 2006;57:3349–3357. doi: 10.1093/jxb/erl099. [DOI] [PubMed] [Google Scholar]
- Else MA, Janowiak F, Atkinson CJ, Jackson MB. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Ann Bot. 2009;103:313–323. doi: 10.1093/aob/mcn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezin V, Pena RDL, Ahanchede A. Flooding tolerance of tomato genotypes during vegetative and reproductive stages. Braz J Plant Physiol. 2010;22(1):131–142. [Google Scholar]
- Fernandez GCJ, Shanmugasundaram S. The AVRDC mungbean improvement program. The past, present and future. In: Fernandez J, Shanmugsundaram S, editors. Mungbean. Shanhua: Asian Vegetable Research and Development Centre; 1988. pp. 58–70. [Google Scholar]
- Gardner FP, Pearce RB, Mitchel R. Physiology of crop plants. Ames: The Iowa state University Press; 1985. [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2. New York: John Wiley and Sons; 1984. [Google Scholar]
- Hartley R, Lawn R, Byth D. Genotypic variation in growth and seed yield of soybean (Glycine max L. Merr.) in saturated soil culture. Aust J Agric Res. 1993;44:689–702. doi: 10.1071/AR9930689. [DOI] [Google Scholar]
- Hendry GAF, Price AH. Stresss indications: Chlorophylls and carotenoids. In: Henry GAF, Grime JP, editors. Methods in comparative plant ecology—a laboratory. Manual. London: Chapman & Hall; 1993. pp. 148–152. [Google Scholar]
- Hiscox JD, Israelstam GF. A method for extraction of chloroplast from leaf tissue without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Hong TD, Minchin FR, Summerfield RJ. Recovery of nodulated cowpea plants (Vigna unguiculata (l.) walp.) from waterlogging during vegetative growth. Plant Soil. 1977;48:661–672. doi: 10.1007/BF00145776. [DOI] [Google Scholar]
- Hooda RS, Sheoran IS, Singh R. Partitioning and utilization of carbon and nitrogen in nodulated roots and nodules of chickpea (Cicer arietinum) grown at two moisture levels. Ann Bot. 1990;65:111–120. [Google Scholar]
- Islam MR, Hamid A, Khaliq QA, Haque MM, Ahmed JU, Karim MA. Effects of soil flooding on roots, photosynthesis and water relations in mungbean (Vigna radiata (l.) Wilczek) Bang J Bot. 2010;39:241–243. [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on the growth and metabolism of herbaceous plants. In: Kozlowski TT, editor. Flooding and plant growth. London: Academic; 1984. pp. 47–128. [Google Scholar]
- Jackson MB, Herman B, Goodenogh A. An examination of the importance of ethanol in causing injury to flooded plants. Plant Cell Environ. 1982;5:163–172. [Google Scholar]
- Janowiak F, Else MA, Jackson MB. A loss of photosynthetic efficiency does not explain stomatal closure in flooded tomato plants. Adv Agric Sci Probl Issues (Warsaw) 2002;481:229–234. [Google Scholar]
- Kuo CG, Chen BW. Physiological responses of tomato cultivars to flooding. J Am Soc Hortic Sci. 1980;105:751–755. [Google Scholar]
- Lakitan B, Wolfe DB, Zobel RW. Flooding affects snap bean yild and genotypic variation in leaf gas exchange and root growth response. J Amer Soc Hort Sci. 1992;117:711–716. [Google Scholar]
- Li C, Jianga D, Wollenweber B, Li Y, Dai T, Cao W. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 2011;180:672–678. doi: 10.1016/j.plantsci.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Li C, Jianga D, Wollenweber B, Li Y, Dai T, Cao W. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 2011;180:672–678. doi: 10.1016/j.plantsci.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Liao CT, Lin CH. Physiological adaptation of crop plants to flooding stress. Proc Natl Sci Counc Roc. 2001;25:148–157. [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. Changes in the physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Aust J Agric Res. 2001;28:1121–1131. [Google Scholar]
- Matsunami T, Jung GH, Oki Y, Zhang WH, Kokubun M. Effects of waterlogging on nitrogen fixation of a supernodulating soybean genotype, Sakukei 4. In: Wang YP, Lin M, Tian Z, Elmerich C, Newton WE, editors. Biological nitrogen fixation, sustainable agriculture and the environment. Netherlands: Springer; 2005. pp. 283–284. [Google Scholar]
- Matsunami T, Jung GH, Oki Y, Kokubun M. Effect of waterlogging during vegetative stage on growth and yield in supernodulating soybean cultivar sakukei-4. Plant Prod Sci. 2007;10:112–121. doi: 10.1626/pps.10.112. [DOI] [Google Scholar]
- Mensah JK, Obadoni BO, Eruotor PG, Onome-Irieguna F. Simulated flooding and drought effects on germination, growth, and yield parameters of sesame (Sesamumu indicum L.) Afr J Biotechnol. 2006;5:1249–1253. [Google Scholar]
- Min XJ, Bartholomew DP. Effects of flooding and drought on ehtylene metabolism, titratable acidity and fruiting of pineapple. Acta Horticult. 2005;666:135–148. [Google Scholar]
- Musgrave ME, Ding N. Evaluating wheat cultivars for waterlogging tolerance. Crop Sci. 1998;38:90–97. doi: 10.2135/cropsci1998.0011183X003800010016x. [DOI] [Google Scholar]
- Nsoukpoe-Kossi CN, AgIvanov AG, Veeranjaneyulu K, Leblanc RM. Protective action of abscisic acid against the inhibition of photosynthesis of barley leaves by bisulphate. Photosynthetica. 1999;36:51–60. doi: 10.1023/A:1007062519107. [DOI] [Google Scholar]
- Palta JA. Unravelling the roots of waterlogged wheat. Farming Ahead. 2007;180:61–63. [Google Scholar]
- Palta JA, Ganjeali A, Turner NC, Siddique KHM. Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agric Water Manag. 2010;97:1469–1476. doi: 10.1016/j.agwat.2010.05.001. [DOI] [Google Scholar]
- Pociecha E, Koscielniak J, Filek W. Effect of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor) Acta Physiol Plant. 2008;30:529–535. doi: 10.1007/s11738-008-0151-9. [DOI] [Google Scholar]
- Poehlman JM. The Mungbean. New Delhi: Oxford & IBH; 1991. p. 375. [Google Scholar]
- Prasad S, Ram PC, Uma S. Effect of waterlogging duration on chlorophyll content, nitrate reductase activity, soluble sugars and grain yield of maize. Ann Plant Physiol. 2004;18:1–5. [Google Scholar]
- Premachandra GS, Saneoka H, Fujita K, Ogata S. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in Sorghum. J Exp Bot. 1992;43:1569–1576. doi: 10.1093/jxb/43.12.1569. [DOI] [Google Scholar]
- Rawyler A, Arpagaus S, Braendle R. Impact of oxygen stress and energy availability on membrance stability of plant cells. Ann Bot. 2002;90:499–507. doi: 10.1093/aob/mcf126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert LW, Robert KT. Differences in the response of sunflower (Helianthus annuus) subjected to flooding and drought stress. Physiol Plant. 1984;61:611–616. doi: 10.1111/j.1399-3054.1984.tb05178.x. [DOI] [Google Scholar]
- Sairam RK. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol. 1994;32:594–597. [Google Scholar]
- Schnettger B, CritchleyC SUJ, Graf M, Krause GH. Relationship between photoinhibition of photosynthesis, D1 protein turnover and chloroplast structure: effects of protein synthesis inhibitors. Plant Cell Environ. 1994;17:55–64. doi: 10.1111/j.1365-3040.1994.tb00265.x. [DOI] [Google Scholar]
- Setter TL, Waters I, Sharma SK, Singh KN, Kulshreshtha N, Yaduvanshi NP, Ram PC, Singh BN, Rane J, McDonald G, Khabaz-Saberi H, Biddulph TB, Wilson R, Barclay I, McLean R, Cakir M. Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Ann Bot. 2009;103:221–235. doi: 10.1093/aob/mcn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Ghildyal BP. Soil submergence effects on nutrient uptake, growth, and yield of five corn cultivars. Agron J. 1980;72:737–741. doi: 10.2134/agronj1980.00021962007200050011x. [DOI] [Google Scholar]
- Singh DP, Singh BB. Breeding for tolerance to abiotic stresses in mungbean. J Food Legumes. 2011;24(2):83–90. [Google Scholar]
- Solaiman Z, Colmer TD, Loss SP, Thomson BD, Siddique KHM. Growth responses of cool-season grain legumes to transient waterlogging. Aust J Agric Res. 2007;58:406–412. doi: 10.1071/AR06330. [DOI] [Google Scholar]
- Takele A, McDavid CR. Effects of short-term waterlogging on cultivars of cowpea (Vigna unguiculata (L.) Walp Trop Agric. 1994;71:275–280. [Google Scholar]
- Tripathi PK, Singh MK, Singh J, Singh ON. Effect of rhizobial strains and sulphur nutrition on mungbean (Vigna radiata (l.) wilczek) cultivars under dryland agro-ecosystem of Indo-Gangetic plain. Afr J Agric Res. 2012;7(1):34–42. [Google Scholar]
- Umaharan P, Ariyanayagam RP, Haque SQ. Effect of short-term waterlogging applied at various growth phases on growth, development and yield in Vigna unguiculata. J Agric Sci. 1997;128:189–198. doi: 10.1017/S0021859696004121. [DOI] [Google Scholar]
- Visser EJW, Bogemann GM, Blom CWPM, Voesenek LACJ. Ethylene accumulation in waterlogged Rumex plants promotes formation of adventitious roots. J Exp Bot. 1996;47:403–410. doi: 10.1093/jxb/47.3.403. [DOI] [Google Scholar]
- Weatherley PE. Studies in water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol. 1950;49:81–87. doi: 10.1111/j.1469-8137.1950.tb05146.x. [DOI] [Google Scholar]
- Wenkert W, Fausey NR, Watters HD. Flooding response to Zea mays L. Plant Soil. 1981;62:351–366. doi: 10.1007/BF02374133. [DOI] [Google Scholar]
- Yiu JC, Liu CW, Kuo CT, Tseng MJ, Lai YS, Lai WJ. Changes in antioxidant properties and their relationship to paclobutrazol-indued flooding tolerane in Welsh Onion. J Sci Food Agric. 2008;88:1222–1230. doi: 10.1002/jsfa.3209. [DOI] [Google Scholar]
- Yordanova R, Popova LP. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plant. 2007;29:535–541. doi: 10.1007/s11738-007-0064-z. [DOI] [Google Scholar]
- Yordanova RY, Uzunova AN, Popova LP. Effects of short-term soil flooding on stomata behaviour and leaf gas exchange in barley plants. Biol Plant. 2005;49:317–319. doi: 10.1007/s10535-005-7319-6. [DOI] [Google Scholar]
- Zhou W, Lin X. Effects of waterlogging at different growth stages on physiological characteristics and seed yield of winter rape (Brassica napus L.) Field Crops Res. 1995;44:103–110. doi: 10.1016/0378-4290(95)00075-5. [DOI] [Google Scholar]