Abstract
In this study, we have focused on those components of Photosystem (PS) II which are significantly affected by dual stress (high salt and temperature) on wheat as measured by Plant Efficiency Analyser (PEA). It was observed that some of the chlorophyll a fluorescence parameters were temperature dominated, while some other parameters were salt dominated. We have also observed additive effects for parameters like antenna size heterogeneity. An important observation was that in high temperature alone, the K-step was observed at 40 °C, while in case of dual stress, the K-step was observed at 45 °C, while the Chl a fluorescence transient of 40 °C + 0.5 M NaCl was quite similar to 35 °C transient curve. In the presence of salt, K-step was observed at higher temperature suggesting a protection of OEC by salt. Plants are under dual stress, but effect of temperature stress is less severe in presence of salt stress. Thus, we can say that salt stress caused partial prevention from high temperature stress but it did not cause complete protection of PS II.
Keywords: Chlorophyll a fluorescence, High temperature stress, Salt stress, Wheat, Photosystem II
Introduction
Plants in field often experience multiple environmental stresses simultaneously. High temperature usually results in water deficiency which leads to increase in salt concentration in soil. In natural conditions, particularly in arid and semi-arid regions very often the plants experience high temperature and salt stress together. Salt stress leads to reduction in the growth of plant (Allakhverdiev et al. 2000) and is often associated with decreased rates of photosynthesis. Salinity affects chlorophyll content through inhibition of chlorophyll synthesis or an acceleration of its degradation (Zhao et al. 2007). PS II is known to play a key role in the response of photosynthesis to stresses (Baker 1991). Increasing temperature leads to a blockage of PS II reaction centres and then to dissociation of antennae pigment-protein complexes from the central core of the PS II light-harvesting complex. Among partial reactions of PS II, the oxygen evolving complex (OEC) is particularly more heat sensitive (Georgieva et al. 2000). However, less information is available on the responses of PS II to a combination of salt and high temperature stress on cereal crop.
The technique of chlorophyll (Chl) a fluorescence has become ubiquitous in plant ecophysiology studies. Chlorophyll fluorescence has been used as an informative tool to provide a rapid, non-destructive diagnostic method for detecting and quantifying damage to the leaf photosynthetic apparatus, particularly, about functions and activity of PSII altered under different environmental stresses. PS II is heterogeneous in nature and exhibits antenna heterogeneity and reducing side heterogeneity which vary under different environmental conditions (Mehta et al. 2010a; Mathur et al. 2011a). Any type of abiotic stress causes change in photochemical efficiency of PSII. Photochemical efficiency of PS II may be characterized by the efficiency and number of active reaction centres, net trapping of photoenergy for effective electron transport and the relative efficiency of the electron acceptors. Temperature stress causes changes in the donor as well as acceptor side of PSII. High temperature cause damage to OEC, blocks PQ pool, cause damage in membrane integrity, decrease in active reaction centers. Salt stress causes changes in electron transport. By measuring photochemical efficiency of PSII through Chl a fluorescence measurement we can determine the level of susceptibility to stress and stress tolerance in the plant.
The response of plant to a combination of various environmental stresses is very complex and it is very difficult to assign contribution of individual stress. This work focuses on those components of PS II which are significantly affected by dual stress (high salt and high temperature) on wheat. Earlier works have shown combined effects of high salt and high temperature on halophytes or salt tolerant plants (Chen et al. 2004; Wen et al. 2005). Many studies has been done on the activities of the wheat plant individually with temperature stress or with salt stress (Mathur et al. 2011b; Mehta et al. 2010b) but a few studies have been done on the biophysical status of the wheat plant with combination of both the stresses together. In this study we have used JIP test to unravel the effect of dual stress in wheat plants. The advantage of JIP test over others is that it can be used to investigate and differentiate the responses of photosynthetic apparatus to different environmental stresses. JIP test links to different steps and phases of the transient with redox states of PSII and concomitantly, with the efficiencies of electron transfer in the intersystem chain and to the end electron acceptors at the PSI acceptor side. It also considers the fraction of centers that can not reduce the secondary quinone acceptor QB and also estimates the entire probability of flow of energy among the PSII components (Misra et al. 2001; Oukarroum and Strasser 2004; Strasser et al. 2010; Kalaji et al. 2012). Wheat is a major crop all over the world, especially in tropical countries and it faces dual stress (high temperature and salt stress) quite often. In this study, we have been able to differentiate between those photochemical events of PS II that are salt sensitive, temperature sensitive or sensitive to both stresses. This information will help in strategic planning to combat salt and temperature stress on wheat.
Materials and methods
Plant material: wheat (Triticum aestivum)
Lok-1 cultivar of wheat was used. Wheat seeds were allowed to germinate and then transferred to petriplates containing Knop solution with a photosynthetically active photon flux density (PPFD) of 300 μmol m−2 s−1 at 22 °C. The plantlets were grown upto two leaf stages and then used for the stress studies.
NaCl treatment
The wheat leaves were immersed in different concentration of NaCl ranging from 0.1 M to 1 M NaCl for 24 h and measurements were taken. For all the experiments a concentration of 0.5 M NaCl was standardized and used. Wheat leaves were detached from the plants and 0.5 M NaCl treatment was given to these detached leaves for 24 h. After 24 h the leaves were removed from salt solution, wiped and Chl a fluorescence was measured. After measuring the Chl a fluorescence transients, the detached leaves were immersed in a water-bath in dark for 15 min at various temperatures (25, 35, 40, 45 °C). After 15 min of high temperature treatment in a water-bath, the leaves were removed and Chl a fluorescence was measured again. All the treatments were given in the dark.
High temperature treatment
To give temperature treatment, the wheat leaves were immersed in a water bath (Julabo F10-UC, Germany) for 15 min at temperatures of 25 °C (Control), 35 °C, 40 °C, 45 °C. The temperature stress was given in complete darkness.
Dual stress
Dual stress was given in two ways-
-
(i)
Temperature treatment was followed by salt stress—In this method leaves were given temperature stress as described above and then kept in solutions of different salt concentrations. However after temperature stress, the leaves had become too fragile and measurements could not be taken.
-
(ii)
Salt treatment followed by high temperature stress—This method was opted for giving dual stress. Initially the wheat leaves were given salt stress and fluorescence parameters were recorded which was further followed by high temperature stress.
Measurement of fluorescence induction kinetics
The chlorophyll a (Chl a) fluorescence induction kinetics was measured at room temperature using a Plant Efficiency Analyzer (PEA), (Hansatech, England). Excitation light of 650 nm (peak wavelength) from array of three light-emitting diodes is focused on the surface of the leaf to provide a homogenous illumination. Light intensity reaching the leaf was 3,000 μ mol m−2 s−1 which was sufficient to generate maximal fluorescence for all the treatments. The fluorescence signal is received by the sensor head during recording and is digitized in the control unit using a fast digital converter. The energy fluxes were calculated according to the equations of the JIP-test, by using Biolyzer HP3 software (the chlorophyll fluorescence analyzing program by Bioenergetics Laboratory, University of Geneva, Switzerland).
Determination of QB-reducing and QB-non-reducing centers
The fractions of QB-reducing and QB-non-reducing centers were calculated using double hit (pulse) method of (Strasser et al. 2004). According to this method, two fluorescence transients were induced by two subsequent pulses (each of 1 s duration). The first pulse was conducted after a dark period long enough to ensure the reopening of all reaction centers, followed by a second pulse. The duration of the dark interval between the two pulses was 500 ms. The dark interval between the two pulses is short enough to allow only the reopening of the QB-reducing centers (fast opening centers). Closed centers that do not open within about 500 ms were considered as QB-non-reducing centers (slow opening centers).]
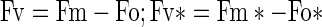 |
- Fv
Variable fluorescence of 1st pulse
- Fm
Maximal fluorescence of 1st pulse
- Fv*
Variable fluorescence of 2nd pulse
- Fm *
Maximal fluorescence of 2nd pulse
- Fo
Minimal fluorescence of 1st pulse
- Fo*
Minimal fluorescence of 2nd pulse.
QB-non-reducing centers were calculated by the following equation:
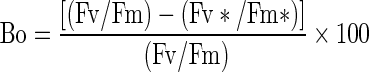 |
where Bo = Relative amount of QB-non-reducing PS II centers
Determination of antenna size heterogeneity
Determination of PS II heterogeneity from fluorescence rise (FR) curve measured with DCMU was first introduced by Melis and Homann (Melis and Homann 1976; Melis and Homann 1975). For calculation of antenna heterogeneity DCMU poisoning method was used (Strasser 1981; Hsu et al. 1989). The method is described below. The detached leaves were put into small tray filled with 100 ml DCMU solution (the DCMU concentration was 200 μM dissolved in 1 % ethanol) (Toth et al. 2005), overnight. The leaves were removed from the DCMU solution, wiped and left in the air for ~1 h to avoid possible effects of anaerobiosis. Following this, salt stress was given to the leaves in light and then parameters were recorded. Alpha (α), beta (β) and gamma (γ) centers were calculated from the complementary area growth curve (Melis 1985; Melis and Homann 1976; Melis and Homann 1975). It involved the calculation of growth of normalized complementary area, defined by the fluorescence induction curve and the line parallel with the maximum level of fluorescence (Fm), with time. The kinetics of QA accumulation was obtained by the calculation of the kinetics of complementary area [B = ∫(Fm − Ft)dt], where B is the double normalized (between 0 and 1) kinetics of complementary area (Strasser et al. 2000) and the B kinetics of the first light pulse were fitted with three exponentials that correspond to α, β and γ type centers (Toth and Strasser 2005). Their contribution to the total amplitude (A) of the kinetics of complementary area has been indicated as percentage of α, β and γ centers (Strasser 1981; Hsu et al. 1989).
The energy fluxes were calculated using Biolyzer HP3 software.
Results and discussion
Effect of dual stress on Chl a fluorescence transients
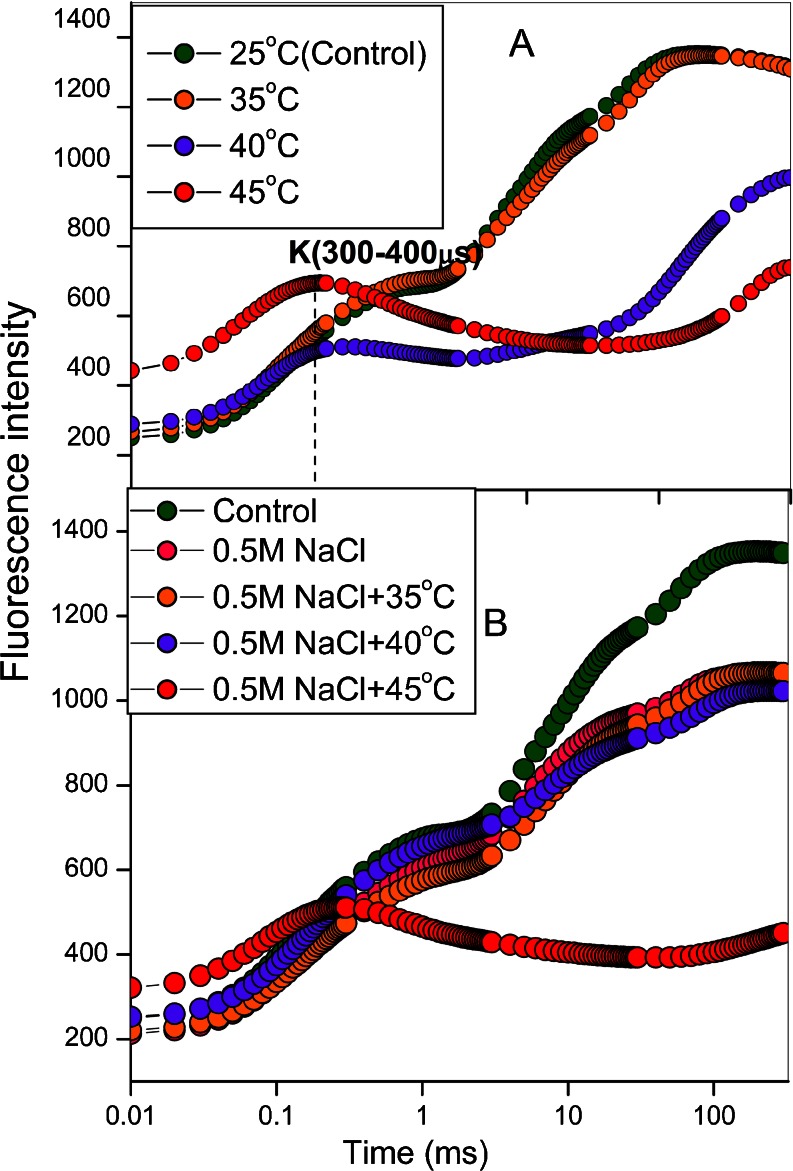
Chl a fluorescence induction kinetics was measured to evaluate effect of dual stress (high salt and high temperature) on photochemical efficiency of PS II. Leaves at 25 °C (Control) exhibited a polyphasic rise called O-J-I-P transient (Fig. 1a). The O to J phase (ends at ~2 ms), the J to I phase (ends at ~30 ms) and I to P phase (ends at ~500 ms). The JIP-test is named after the basic steps in the fluorescence transient when plotted on a logarithmic time scale (Strasser et al. 2000). The shape of the O-J-I-P fluorescence rise has been related to a major change in the photosynthetic electron transport (Joly and Carpentier 2009; Papageorgiou and Govindjee 2011). According to the recent view, O-J phase is related to the accumulation of 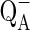 , I-P is associated with PQ pool reduction while J-I is not related with reduction in the PQ pool (Gauthier et al. 2010) while J-I phase was shown to be suppressed which affect the OEC (Schreiber and Neubauer 1987). The I-P amplitude in the transient has also been related to the relative size of the pools of final PS I electron acceptors (Kalachanis and Manetas 2010).
, I-P is associated with PQ pool reduction while J-I is not related with reduction in the PQ pool (Gauthier et al. 2010) while J-I phase was shown to be suppressed which affect the OEC (Schreiber and Neubauer 1987). The I-P amplitude in the transient has also been related to the relative size of the pools of final PS I electron acceptors (Kalachanis and Manetas 2010).
Fig. 1.
The OKJIP Chl a fluorescence transient curve (log time scale) in wheat leaves exposed to: (a) temperature stress (35 °C,40 °C, 45 °C), (b) dual stress (high temperature + salt stress)
However an additional K-step was observed at temperature 40 °C (Fig. 1a). The alteration from an OJIP curve to an OKJIP curve is an elevated temperature stress specific response as such alterations have not been recorded elsewhere in plants subjected to environmental stresses encountered in urban landscapes such as elevated ozone, salt, CO2, heavy metals, light, salt and water. The K-step was predominant at 45 °C followed by a pronounced dip and later by a slight increase to a highly suppressed P step. The appearance of K-step may be caused by an inhibition of OEC (Guissé et al. 1995a, b; Srivastava et al. 1997), by inhibition of electron transport from Pheophytin to QA and may also reflect changes in the structure of the LHC of PS II (Mathur et al. 2011b). The appearance of a K peak also is attributed to the stable formation of oxidized P680 as a consequence of the suppression of YZ re-reduction kinetics by the S-state cycle when the Mn4Ca cluster is destabilized and the Ca2+ and Mn2+ released in tandem with Cl─, the anionic ligand (Essemine et al. 2011). As shown in Fig. 1b, 0.5 M NaCl, causes changes in Chl a fluorescence curves.
However, in high temperature alone the K-step was observed at 40 °C, while in case of dual stress (high salt treated temperature stressed leaves) the K-step was observed only at 45 °C and the Chl a fluorescence transient of 40 °C + 0.5 M NaCl was more like 35 °C transient (Fig. 1a, b).
Parameters predominantly affected by salt stress
Analysis of various components of OJIP curves revealed that some of the parameters showed changes which closely resemble the changes caused by salt stress alone (Mehta et al. 2010b). In these parameters, the effects of temperature stress were not so prominent. The parameters like ΦEo, N and membrane model were predominantly affected by salt stress (Table 1). The ΦEo represents the quantum yield of electron transport i.e.
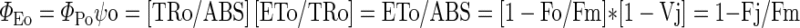 |
Table 1.
Parameters of Chl a fluorescence which predominantly changed by salt stress in wheat leaves
| Para-meters | 25 °C | 35 °C | 40 °C | 45 °C | ||||
|---|---|---|---|---|---|---|---|---|
| −NaCl | +NaCl | −NaCl | + NaCl | −NaCl | + NaCl | −NaCl | + NaCl | |
| φEo | 0.487 ± 0.001 | 0.387 ± 0.011 | 0.474 ± 0.005 | 0.356 ± 0.022 | 0.468 ± 0.015 | 0.327 ± 0.004 | 0.202 ± 0.004 | 0.140 ± 0.001 |
| N | 41 ± 1 | 25.43 ± 0.47 | 54.03 ± 2 | 37.47 ± 2 | 863 ± 2 | 38.79 ± 3 | 6199 ± 288 | 1994 ± 232 |
It also shows the probability that an absorbed photon can move an electron further than 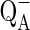 . The decreased value of ΦEo in dual stress depicted that the quantum yield of electron transport decreased resulting in the maltransferring of the electrons. In case of temperature stress upto 40 °C no significant change in ΦEo value was observed while a dramatic damage of 60 % was observed at 45 °C. When these results were compared with dual stress a decline in ΦEo value was observed. At 40 °C + 0.5 M NaCl 33 % and at 45 °C + 0.5 M NaCl 72 % damage was observed. The value of the turn over number N was calculated according to the equation of the JIP test as
. The decreased value of ΦEo in dual stress depicted that the quantum yield of electron transport decreased resulting in the maltransferring of the electrons. In case of temperature stress upto 40 °C no significant change in ΦEo value was observed while a dramatic damage of 60 % was observed at 45 °C. When these results were compared with dual stress a decline in ΦEo value was observed. At 40 °C + 0.5 M NaCl 33 % and at 45 °C + 0.5 M NaCl 72 % damage was observed. The value of the turn over number N was calculated according to the equation of the JIP test as
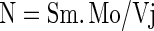 |
where Sm is the normalised total area between Fm and Ft and where Mo/Vj stands for the specific flux TRo/RC (trapping flux per reaction center of PS II) (Strasser et al. 2000). At 40 °C the value of N was increased by 20 % and it increased drastically (150 %) at 45 °C which indicated that inefficient trapping has occurred but QAˉ is not able to reduce back for efficient trapping (Mathur et al. 2011b), while in case of dual stress at 40 °C + 0.5 M NaCl a complete protection due to salt was observed. At 45 °C + 0.5 M NaCl this value has recovered upto 67 % which means that salt has protected the temperature stressed leaves. An increase in N can also occur through PS I cyclic electron transport with little contribution from PS II.
Parameters predominantly affected by temperature stress
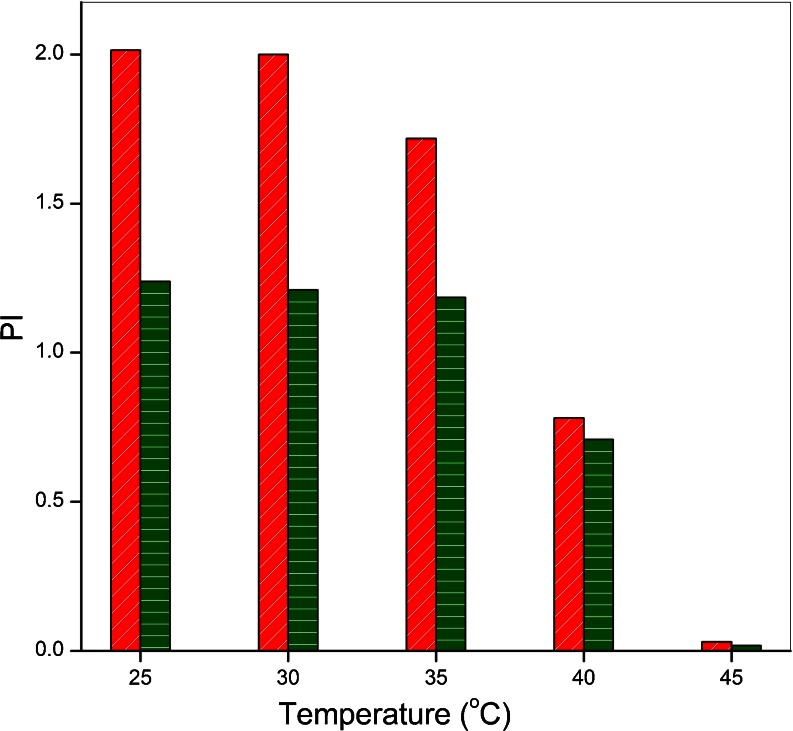
In dual-stressed leaves the parameter like PI (total) (Fig. 2) and reducing side heterogeneity were more affected by temperature stress. The Fv/Fm ratio in temperature stress alone at 40 °C and 45 °C was decreased by 14 and 58 % respectively indicating a decrease in the quantum efficiency of PS II photochemistry (data not shown). It is already known that salt stress does not affects the primary photochemistry (Mehta et al. 2010b) of the PS II apparatus so all the changes observed in Fv/Fm ratio only due to high temperature stress. The efficiencies and specific fluxes also calculate the photosynthetic Performance Index PI (total) which is a combined measure of three partial performances, namely those related with the amount of photosynthetic reaction centers (RC/ABS), the maximal energy flux which reaches the PSII reaction center (TRo), and the electron transport at the onset of illumination (ETo) (Tsimilli-Micheal and Strasser 2008). At high temperature 40 °C and 45 °C, PI(total) decreased by 62 % and 98 % respectively. PI was inhibited by 39 % in salt treatment alone (0.5 M NaCl) but in dual-stressed leaves, at 40 °C + 0.5 M NaCl 65 % and at 45 °C + 0.5 M NaCl 99 % damage was observed. This indicated that the biochemical reactions of the plant have been affected mainly due to high temperature stress.
Fig. 2.
Parameter of Chl a fluorescence which predominantly changed by high temperature stress in wheat leaves
Reducing side heterogeneity was estimated by measuring relative amounts of QB reducing and QB-non-reducing centers as described in materials and methods. The population of QB-non-reducing centers were quantified (Table 2) to about 16–20 % in control sample at 25 °C of total PS II. Exposure of leaves to temperature upto 35 °C does not affect much the relative fractions of QB-non-reducing PS II centers but as the temperature was raised to 40 °C a gradual change in the fractions of QB-non-reducing PS II centers (39 %) was observed while at temperature higher than 40 °C i.e. at 45 °C the fraction of QB-non-reducing centers increased drastically upto 50 % (Table 2) (Strasser et al. 2000; Mathur et al. 2011a). At high temperatures the fractions of QB-non-reducing centers increased which imply that these centers were unable to reduce QAˉ to PQ pool and also that the active QB-reducing centers were converted into inactive QB-non-reducing centers. In case of salt stress the QB-non-reducing centers became 32 % in 0.5 M (Mehta et al. 2010a; Mehta et al. 2011) treatment. In case of dual-stress the fractions of QB reducing and QB non-reducing centers did not increase much. At 40 °C alone the non-reducing centers were 39 %, in 40 °C + 0.5 M NaCl they were 42 %. In the same way, at 45 °C QB non-reducing centers were 50 % and in 45 °C + 0.5 M NaCl they became 54 % (Table 2). Results show that reducing side heterogeneity was affected mainly by high temperature stress and presence of salt did not protect from the damaging effects of high temperature on reducing side heterogeneity.
Table 2.
Relative amounts of QB reducing and QB non-reducing centers (in percentage) in dual-stressed (high salt and temperature) wheat leaves
| Reducing side heterogeneity | 25 °C | 35 °C | 40 °C | 45 °C | ||||
|---|---|---|---|---|---|---|---|---|
| −NaCl | +NaCl | −NaCl | + NaCl | −NaCl | + NaCl | −NaCl | +NaCl | |
| QB reducing centers | 82 ± 1 | 77 ± 2 | 80 ± 1 | 76 ± 1 | 61 ± 2 | 58 ± 1 | 50 ± 1 | 46 ± 1 |
| QB non-reducing centers | 18 ± 3 | 23 ± 1 | 20 ± 1 | 24 ± 1 | 39 ± 2 | 42 ± 1 | 50 ± 1 | 54 ± 2 |
Intermediate effects of dual stress
The leaves treated at all the temperatures have a higher Fo value as compared to the salt-stressed leaves (Fig. 1a). It is well known that the Fo value decreases with high salt concentration (Mehta et al. 2010b) while it increases in high temperature-stressed leaves (Mathur et al. 2011a). An intermediate increase in Fo (Fig. 1b) in dual-stressed leaves indicated that salt treatment might have partially protected the physical separation of LHC from the PS II core complex (Wen et al. 2005). In temperature-stressed leaves the K- step was observed at 40 °C while in case of dual stress, the K-step was observed at 45 °C (Fig. 1a, b).
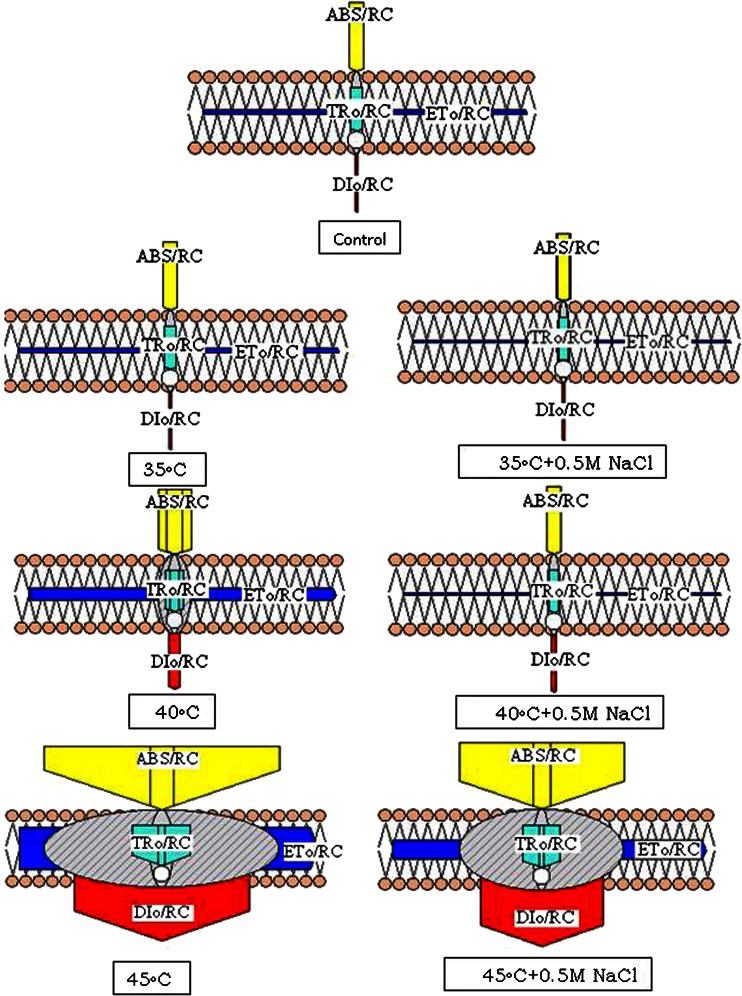
An alteration of PS II energy fluxes in response to dual stress was visualized by energy pipeline models of photosynthetic apparatus (Fig. 3). The energy fluxes ratios ABS/RC, TRo/RC, ETo/RC, DIo/RC increased in effect of dual-stress. Individually as a result of salt (0.5 M NaCl) and temperature stress the flux ratios ABS/RC, TRo/RC, ETo/RC and DIo/RC increased. These ratios also increased with dual-stress. ABS/RC demonstrates average antenna size, expresses the total absorption of PS II antenna chlorophylls divided by the number of active (in the sense of QA reducing) reaction centers (Misra et al. 2001). Increase in antenna size also represents that the number of active centers is more but since the inactive centers have also been added in the total antenna size, apparently the ratio of ABS/RC appears to be higher. However, these changes could also be due to inactivation of RCs and conversion of PS II units into heat sinks units. TRo/RC represents the maximal rate by which an exciton is trapped by the RC resulting in the reduction of QAˉ (Stirbet and Strasser 1996). An increase in this ratio indicates that all the QAˉ has been reduced but it is not able to oxidize back due to stress i.e. the reoxidation of QAˉ is inhibited so that QAˉ cannot transfer electrons efficiently to QB and also that maximum energy is lost in dissipation. ETo/RC depicts the reoxidation of reduced QAˉ via electron transport in an active RC. It reflects the activity of only the active RCs (Misra et al. 2001). An increased electron transport per active reaction center due to a thermal activation of the dark reactions is shown in Fig. 3. As ETo/RC is represented only by active centers, an increase in this ratio indicates that the inactive centers are more and the QAˉ cannot transfer electrons efficiently to QB, and there is reduced electron transfer efficiency. DIo/RC represents the ratio of the total dissipation of untrapped excitation energy from all RCs with respect to the number of active RCs. Dissipation is influenced by the ratios of active/inactive RCs. The ratio of total dissipation to the amount of active RCs increased due to the high dissipation ratio of the active RCs. As the number of inactive centers increased, the DIo/RC ratio was found to be higher because the inactive centers were unable to trap the photon so the amount of untrapped photons increased. The flux ratios of dual stressed leaves were compared with flux ratios of temperature stressed leaves and it was observed that the effect of high temperature stress was overcome by salt stress not completely but partially resulting in a lower flux ratio.
Fig. 3.
Membrane model for wheat leaves exposed to high temperature alone and dual stress (salt + high temperature). The membrane model shows the specific activities per unit reaction center (RC). The small hatched circles represent newly synthesized units. The arrows indicate fluxes for light absorbance (ABS), excitation energy trapping (TRo), energy dissipation (DIo) and electron transport (ETo) beyond QA-. The width of each arrow denotes the relative size of the fluxes or the antenna
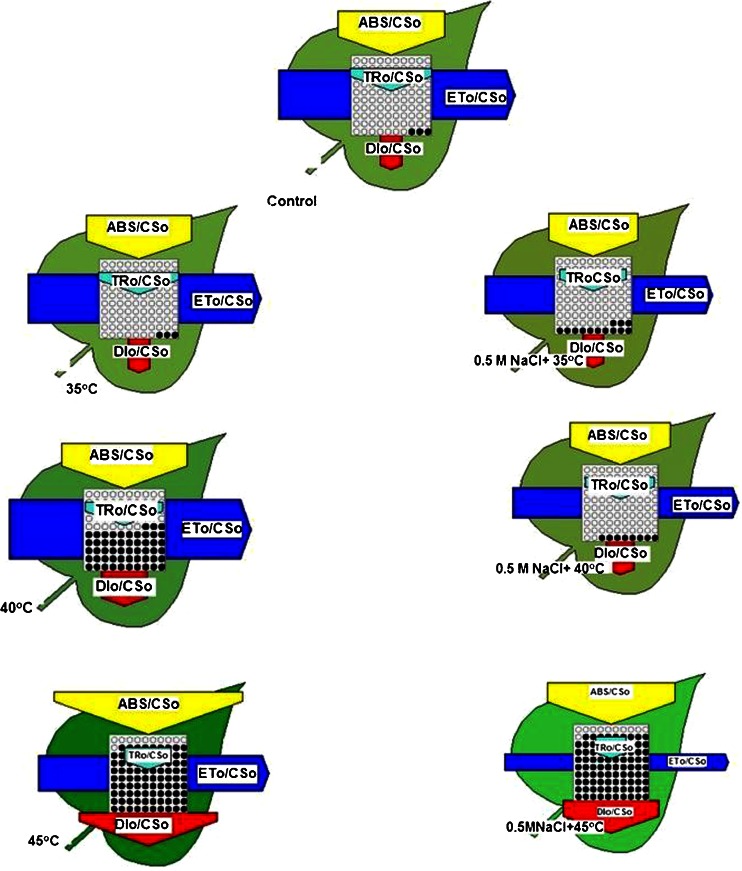
In this model the OJIP values were taken to express PS II activities in terms of the cross section unit (Leaf model Fig. 4). The decrease in ABS/CSo (Fig. 4) in dual stress treated leaves indicated a decrease of the energy absorbed per excited cross-section as compared to temperature treatment alone. The coloration in the leaf models indicates the pigment concentration per cross section (ABS/CSo). High temperature as well as high salt concentration caused a decrease in the Chl content per leaf area (ABS/CSo) (Misra et al. 2001; Tsimilli-Micheal and Strasser 2008) which is shown by the intensity of the light green color of the wheat leaves (Fig. 4). As compared to temperature treated alone more decrease in ETo/CSo ratio was observed in dual stressed leaves indicating lower energy absorption by antenna pigments (ABS/CSo), inactivation of reaction centre complexes and OEC and also suggesting that the donor side of PS II has been affected. The ratio TRo/CSo also decreased in dual stressed leaves indicating low energy trapping by reaction centers. A decrease in the density of active reaction centers (indicated as open circles) and an increase in the density of closed reaction centers (indicated as filled circles also known as inactive centers) was observed as an effect of high temperature and salt. Surprisingly same number of filled circles were observed at 45 °C and 0.5 M NaCl + 45 °C. Initially, the ratio DIo/CSo was quite similar in temperature treated as well as in dual stressed leaves but as the temperature reached 40 °C a prominent difference was observed in this ratio. The ratio DIo/CSo increased more in temperature treatment alone as compared to dual stressed treatment. The reason could be that in temperature stressed leaves excess heat dissipation has taken place at 45 °C while in dual stressed leaves salt treatment has tried to partially protect the leaves. An increase of the energy dissipation at high temperature indicated that energy available for photochemistry was less under stress condition. Thus, in case of dual stress antagonist effects was observed for DIo/CSo.
Fig. 4.
Leaf model for wheat leaves exposed to high temperature alone and dual stress (salt + high temperature). The leaf model shows phenomenological fluxes or apparent activities per cross section (CSo). The density of active photosynthetic units involved in the reduction of QA, per cross section, is shown as small open circles in the leaf model. The small closed circles demonstrate inactive photosynthetic units. The arrows indicate fluxes for light absorbance (ABS), excitation energy trapping (TRo), energy dissipation (DIo) and electron transport (ETo) beyond QA-. The width of each arrow denotes the relative size of the fluxes or the antenna
Additive effects of dual stress
The parameters that showed an additive effect of high temperature and salt stress were Mo, Fv/Fo and antenna heterogeneity (Table 3). Mo represents the net rate of PS II closure indicated by RC trapping minus electron transport and can be represented as Mo = TRo/RC- ETo/RC. The increase in Mo value depicts that the net photosynthesis has decreased in dual-stressed leaves. At 40 °C 19 % and at 45 °C, 214 % enhancement was observed in Mo as compared to control, while at 40 °C + 0.5 M NaCl, 43 % and at 45 °C + 0.5 M NaCl, 308 % increase in Mo was observed. This increase in Mo value is an additive effect of high temperature and salt stress. Decrease of Fv/Fo may be due to impairment in down-regulation of PSII photochemistry and low electron transport (Essemine et al. 2012). Fv/Fo ratio decreased in the dual stressed leaves (Table 3). The Fv/Fo ratio showed ~ 12 % decrease in salt treated leaves while 50 and 90 % damage was observed in high temperature (40 °C and, 45 °C) treated leaves (Mehta et al. 2010a and Mathur et al. 2011a), but for dual stressed leaves at 40 °C + 0.5 M NaCl 59 % damage and at 45 °C + 0.5 M NaCl, 91 % damage was observed in the leaves which indicated that a conformational change has taken place. It indicted that the structural changes in dual stressed leaves are dominated by temperature stress and salt stress has not played any role in this.
Table 3.
Parameters of Chl a fluorescence which are showing additive effects of dual stress in wheat leaves
| Parameters | 25 °C | 35 °C | 40o C | 45 °C | ||||
|---|---|---|---|---|---|---|---|---|
| −NaCl | +NaCl | −NaCl | + NaCl | −NaCl | + NaCl | −NaCl | +NaCl | |
| α centers | 72 ± 1 | 50 ± 2 | 61 ± 1 | 49 ± 2 | 57 ± 1 | 44 ± 2 | 45 ± 1 | 39 ± 2 |
| β centers | 25 ± 1 | 28 ± 1 | 26 ± 2 | 29 ± 1 | 28 ± 1 | 30 ± 3 | 42 ± 1 | 34 ± 1 |
| γ centers | 3 ± 1 | 22 ± 2 | 14 ± 1 | 22 ± 3 | 15 ± 1 | 26 ± 2 | 13 ± 3 | 27 ± 2 |
| Mo (TR/RC) | 0.97 ± 0.004 | 0.996 ± 0.001 | 1.000 ± 0.040 | 1.040 ± 0.037 | 1.152 ± 0.031 | 1.327 ± 0.019 | 3.057 ± 0.075 | 3.96 ± 0.040 |
| Fv/Fo | 3.45 ± 0.01 | 3.053 ± 0.02 | 3.16 ± 0.01 | 3.007 ± 0.004 | 1.715 ± 0.01 | 1.41 ± 0.02 | 0.347 ± 0.01 | 0.32 ± 0.001 |
By using kinetic analysis of fluorescence induction curve of DCMU poisoned chloroplast, PS II has been resolved into three components i.e. PS II α, PS II β and PS II γ. In case of dual stress the proportion of PS II β and PS II γ centers increased. In individual stress an increase in PS II β and PS II γ centers were observed (Table 3). The PS II β and γ centers seemed to increase at the cost of PS II α centers and thus these components probably are interconvertable depending on the environmental conditions. In case of high temperature at 40 °C no significant change was observed in PS II β centers (28 %). At still higher temperature (45 °C), the changes in PS II β centers were prominent (42 %) while in case of salt stress alone (0.5 M NaCl) the change in PS II γ centers were prominent (22 %) (Table 3).
Conclusion
It is concluded that both high temperature and salt stress affect the photochemical events in PS II. High temperature causes more damage and in some cases presence of salt resulted in less damage. The results indicated that some of the parameters related to photochemical efficiency of PS II like ΦEo, N and membrane model are salt dominating stress while Fv/Fm, Fo/Fm, PI and reducing side heterogeneity are high temperature dominated parameters. Parameter like Fo has intermediate effect of high temperature and salt stress. Salt and temperature stress showed additive effect for few other parameters like Mo and antenna size heterogeneity. The other point of importance is the K-step, which exhibits inhibition of OEC and impairment of electron donation from water to RC. In high temperature alone the K-step was observed at 40 °C, while in case of dual stress the K-step was observed at 45 °C. No K-step was visible in 40 °C + 0.5 M NaCl treated samples and the Chl a fluorescence transient was quite similar to 35 °C transient curve. In the presence of salt, K-step was observed at higher temperature suggesting a protection of OEC by salt. Thus treatment showed partial prevention from high temperature stress but it did not cause complete protection of PS II. Plants are under dual stress but effect of temperature stress is less severe in presence of salt stress. In all cases from 25 °C to 45 °C the maximal specific reaction of primary photochemistry = maximal Trapping per RC = TRo/RC of PS II photochemistry = Mo was higher with NaCl than without. Mo is the slope at the origin of the relative variable fluorescence V = (F-Fo) and Mo = dV/dt at the origin. These results confirm the stabilization effect of high salt conditions on reaction center complexes, while the enzyme reactions of the photosynthetic electron transport are getting inhibited for temperatures higher than 35 °C.
Acknowledgement
Financial support for the project (INT/RFBR/P-98) to AJ by Department of Science and Technology (DST), New Delhi, India is thankfully acknowledged. SM thanks Council of Scientific and Industrial Research (CSIR), India for the Senior Research Fellowship [09/301/(0119)/2010/EMR-I]. PM thanks Council of Scientific and Industrial Research (CSIR), India for the Senior Research Fellowship [09/301/(0019)09/EMR-I]. We are also thankful to Prof. Reto J. Strasser and Ronaldo Maldonado-Rodriguez for gifting Biolyzer HP 3 Software.
Abbreviations
- ABS
Absorbance
- Chl
Chlorophyll
- CSo
Cross section of tested sample at t = 0, proportional to Fo
- DCMU
3-(3, 4-dichlorophenyl)-1, 1-dimethylurea
- DIo/RC
Dissipation per reaction center
- ETo/RC
Electron transport per reaction center
- Fm
Maximal Chl a fluorescence
- Fo
Minimal Chl a fluorescence
- FR
Fluorescence rise
- Fv = Fm-Fo
Variable fluorescence
- K, J, I
Intermediate steps of Chl a fluorescence rise between Fo and P
- LHC
Light harvesting complex
- OEC
Oxygen evolving complex
- PEA
Plant efficiency analyzer
- PI
Performance index
- PS II
Photosystem II
- PQ
Plastoquinone
- QA
Primary plastoquinone
- QB
Secondary plastoquinone
- RC
Reaction center
- TRo/RC
Trapping per reaction center
References
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N. Ionic and osmotic effects of NaCl induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000;123:1047–1056. doi: 10.1104/pp.123.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol Plant. 1991;81:563–570. doi: 10.1111/j.1399-3054.1991.tb05101.x. [DOI] [Google Scholar]
- Chen HX, Li WJ, An SZ, Gao HY. Characterization of PS II photochemistry and thermostability in salt treated Rumex leaves. J Plant Physiol. 2004;161:257–264. doi: 10.1078/0176-1617-01231. [DOI] [PubMed] [Google Scholar]
- Essemine J, Govindachary S, Ammar S, Bouzid S, Carpentier R. Abolition of photosystem I cyclic electron flow in Arabidopsis thaliana following thermal-stress. Plant Physiol Biochem. 2011;49:235–243. doi: 10.1016/j.plaphy.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Essemine J, Govindachary S, Joly D, Ammar S, Bouzid S, Carpentier R. Effect of moderate and high light on photosystem II function in Arabidopsis thaliana depleted in digalactosyl-diacylglycerol. Biochim Biophys Acta. 2012;1817:1367–1373. doi: 10.1016/j.bbabio.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Gauthier A, Joly D, Boisvert S, Carpentier R. Period-four modulation of photosystem II primary quinone acceptor (QA) reduction/oxidation kinetics in thylakoid membranes. Photochem Photobiol. 2010;86:1064–1070. doi: 10.1111/j.1751-1097.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- Georgieva K, Tsonev T, Velikova V, Yordanov I. Photosynthetic activity during high temperature of pea plants. J Plant Physiol. 2000;157:169–176. doi: 10.1016/S0176-1617(00)80187-X. [DOI] [Google Scholar]
- Guissé B, Srivastava A, Strasser RJ. Effects of high temperature and water stress on the polyphasic chlorophyll a fluorescence transient of potato leaves. In: Mathis P, editor. Photosynthesis: from light to biosphere. Dordrecht: Kluwer Academic Publishers; 1995. pp. 913–916. [Google Scholar]
- Guissé B, Srivastava A, Strasser RJ. The polyphasic rise of the chlorophyll a fluorescence [O-K-J-I-P] in heat-stressed leaves. Arch Sci. 1995;48:147–160. [Google Scholar]
- Hsu BD, Lee YS, Jang YR. A method for analysis of fluorescence induction curve from DCMU-poisoned chloroplasts. Biochim Biophys Acta. 1989;975:44–49. doi: 10.1016/S0005-2728(89)80199-9. [DOI] [Google Scholar]
- Joly D, Carpentier R. Sigmoidal reduction kinetics of the photosystem II acceptor side in intact photosynthetic materials during fluorescence induction. Photochem Photobiol Sci. 2009;8:167–173. doi: 10.1039/b815070b. [DOI] [PubMed] [Google Scholar]
- Kalachanis D, Manetas Y. Analysis of fast chlorophyll fluorescence rise [O-K-J-I-P] curves in green fruits indicates electron flow limitations at the donor side of PS II and the acceptor sides of both photosystems. Physiol Plant. 2010;139:313–323. doi: 10.1111/j.1399-3054.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- Kalaji HM, Carpentier R, Allakhverdiev SI, Bosa K. Fluorescence parameters as early indicators of light stress in barley. J Photochem Photobiol B: Biol. 2012;112:1–6. doi: 10.1016/j.jphotobiol.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Mathur S, Allakhverdiev SI, Jajoo A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem II in wheat (Triticum aestivum) Biochim Biophys Acta. 2011;1807:22–29. doi: 10.1016/j.bbabio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Mathur S, Jajoo A, Mehta P, Bharti S. Analysis of elevated temperature-induced inhibition of photosystem II by using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum) Plant Biol. 2011;13:1–6. doi: 10.1111/j.1438-8677.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- Mehta P, Allakhverdiev SI, Jajoo A. Characterization of photosystem II heterogeneity in response to high salt stress in wheat leaves (Triticum aestivum) Photosynth Res. 2010;105:249–255. doi: 10.1007/s11120-010-9588-y. [DOI] [PubMed] [Google Scholar]
- Mehta P, Jajoo A, Mathur S, Bharti S. Chlorophyll a fluorescence study revelling effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem. 2010;48:16–20. doi: 10.1016/j.plaphy.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Mehta P, Kraslavsky V, Bharti S, Allahverdiev SI, Jajoo A. Analysis of salt stress induced changes in photosystem II heterogeneity by prompt fluorescence and delayed fluorescence in wheat (Triticum aestivum) leaves. J Photochem Photobiol B: Biol. 2011;104:308–313. doi: 10.1016/j.jphotobiol.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Melis A. Functional properties of PS IIβ in spinach chloroplasts. Biochim Biophys Acta. 1985;808:334–342. doi: 10.1016/0005-2728(85)90017-9. [DOI] [Google Scholar]
- Melis A, Homann PH. Kinetic analysis of the fluorescence induction in 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea poisoned chloroplasts. Photochem Photobiol. 1975;21:431–437. doi: 10.1111/j.1751-1097.1975.tb06701.x. [DOI] [Google Scholar]
- Melis A, Homann PH. Heterogeneity of the photochemical centers in system II of choloroplasts. Photochem Photobiol. 1976;23:343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Misra AN, Srivastava A, Strasser RJ. Utilization of fast chlorophylla fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol. 2001;158:1173–1181. doi: 10.1078/S0176-1617(04)70144-3. [DOI] [Google Scholar]
- Oukarroum A, Strasser RJ. Phenotyping of dark and light adapted barey plants by the fast chlorophyll a fluorescence rise OJIP. South African J Bot. 2004;70:277–283. [Google Scholar]
- Papageorgiou GC, Govindjee Photosystem II fluorescence: slow changes-scaling from the past. J Photochem Photobiol B: Biol. 2011;104:258–270. doi: 10.1016/j.jphotobiol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Neubauer C. The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: II partial control by photosystem II donor side and possible ways of interpretation. Z Naturforsch. 1987;42c:1255–1264. [Google Scholar]
- Srivastava A, Guissé B, Greppin H, Strasser RJ. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta. 1997;1320:95–106. doi: 10.1016/S0005-2728(97)00017-0. [DOI] [Google Scholar]
- Stirbet AD, Strasser RJ. Numerical simulation of the in vivo fluorescence in plants. Math Comput Simulat. 1996;42:245–253. doi: 10.1016/0378-4754(95)00114-X. [DOI] [Google Scholar]
- Strasser RJ (1981) The grouping model of plant photosynthesis: heterogeneity of photosynthetic units in thylakoids Primary reactions of photochemistry in higher plants, in: G. Akoyunoglou, (Ed.), photosynthesis III. Structure and Molecular Organization of the Photosynthetic Apparatus, Balaban International Science Services, Philadelphia, PA, pp 727–737
- Strasser RJ, Srivastava A, Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P, editors. Probing photosynthesis: mechanisms, regulation and adaptation. London: Taylor & Francis; 2000. pp. 443–480. [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Srivastava A. Analysis of chlorophyll a fluorescence transient. In: Papageorgiou G, Govindjee, editors. Advances in photosynthesis and respiration: chlorophyll a fluorescence a signature of photosynthesis. Dordrecht, The Netherlands: Springer Publishers; 2004. pp. 321–362. [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta. 2010;1797:1313–1326. doi: 10.1016/j.bbabio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Toth SZ, Strasser RJ (2005) The specific rate of QA reduction and photosystem II antenna heterogeneity. Proceedings of the 13th International Congress on Photosynthesis. Montreal, Canada, pp 198–200
- Toth SZ, Schansker G, Strasser RJ. In intact leaves, the maximum fluorescence level (FM) is independent of the redox state of the plastoquinone pool: a DCMU-inhibition study. Biochim Biophys Acta. 2005;1708:275–282. doi: 10.1016/j.bbabio.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Tsimilli-Micheal M, Strasser RJ (2008) In Viovo assessment of stress inpact on plants vitality: Applications in detecting and evaluating the beneficial role of Mycorrhization on host plants, in: Verma A (Ed), Mycorrhiza, Springer-Verlog Berlin Heidelberg, pp 679–703
- Wen X, Qiu N, Lu Q, Lu C. Enhanced thermotolerance of PSII in salt-adapted plants of the halophyte Artemisia anthifolia. Planta. 2005;220:486–497. doi: 10.1007/s00425-004-1382-7. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Ma BL, Ren CZ. Growth, gas exchange, chlorophyll fluorescence and ion content of naked oat in response to salinity. Crop Sci. 2007;47:123–131. doi: 10.2135/cropsci2006.06.0371. [DOI] [Google Scholar]