Abstract
In the present study, molecular (DAMD and ISSR) and chemical (α and β-asarone contents) markers were used to characterize the A. calamus genotypes procured from different parts of India. The cumulative analysis carried out for both DAMD and ISSR markers revealed 24.71 % polymorphism across all genotypes of A. calamus. The clustering patterns of the genotypes in the UPGMA tree showed that the genotypes are diverse, and did not show any specific correlation with their geographical provenances, reflecting the low level of genetic diversity and a high genetic differentiation among the genotypes from the same localities. All the 27 genotypes of A. calamus were also analyzed for α and β-asarone contents, and percentage of essential oil. The genotype (Ac13) from Kullu (Himachal Pradesh) showed maximum (9.5 %) percentage of oil, whereas corresponding minimum (2.8 %) was obtained from the genotypes from Pangthang (Sikkim). Similarly, the highest α and β-asarone contents (16.82 % and 92.12 %) were obtained from genotypes from Renuka (Himachal Pradesh) and Udhampur (Jammu & Kashmir), while lowest α and β-asarone contents (0.83 % and 65.96 %) resulted from Auranwa (Uttar Pradesh) and Pangthang (Sikkim) genotypes, respectively. A. calamus harbours tremendous economic value, and it is therefore, important to identify the genotypes with low α and β-asarone contents for its commercial utilization. Further, this study will help in evaluation and documentation of a large number of diverse genotypes for their value traits.
Keywords: Acorus calamus, Chemical constituents, DAMD, Genetic diversity, ISSR
Introduction
Acorus calamus L., is commonly known as ‘Sweet flag’, and belongs to a monotypic family Acoraceae. It is generally distributed in temperate countries like North America, Canada and Europe. In India, the plants of A. calamus are found throughout, predominantly in Himalayan and sub-himalayan regions (Karthikeyan et al. 1989; Ravindran and Balachandran 2004). A. calamus has been considered for a long time to be a member of the Araceae and only recently it has been removed from the family. There are a number of significant characters that distinguish Acorus from the Araceae: unifacial leaves, two separate vascular systems in the peduncle, absence of raphides, presence of perisperm and endosperm in the seeds (never a perisperm in Araceae), trichomes on the micropyle of the ovules, and presence of special ethereal oil cells and other anatomical characters; laticifers are also lacking but quite a number of Araceae are also without them. DNA studies show that Acorus is a sister taxon to all other monocots, which means that it is not closely related to the Araceae at all (Chang 2010). The different parts of A. calamus like rhizome, roots and leaves have been used traditionally from ancient times in India for the treatment of various ailments. The rhizome contains active ingredients and possesses insecticidal, antifungal, antibacterial and allopathic properties (Ravindran and Balachandran 2004). In the Ayurvedic system of medicine, the rhizomes are considered to posses antispasmodic, antidepressant, anxiolytic properties (Mcgaw et al. 2002; Raina et al. 2003; Bertea et al. 2005). It is also used to treat insomnia, melancholia, neurosis, epilepsy, hysteria, loss of memory, toothache and respiratory ailments (Mcgaw et al. 2002; Raina et al. 2003; Mehrotra et al. 2003). A. calamus plants exhibit tremendous variations in the chromosome numbers and chemical constitution of the essential oil. The basic chromosome number of A. calamus is n = 12, and there are generally four cytotypes viz., diploid (2× = 24), triploid (3× = 36), tetraploid (4× = 48) and hexaploid (6× = 72) found in nature (Krahulcova 2003). The A. calamus represents a geographical pattern of distribution with respect to ploidy level. The plants found in North America are generally diploid, whereas those found in Europe and temperate Asia are primarily triploid, and plants that occurs in eastern and tropical Asia are tetraploids (Raina et al. 2003; Bertea et al. 2005; Ahlawat et al. 2010). The essential oil of A. calamus contains various chemical constituents, and the proportion of each chemical constituent of the oil particularly β-asarone varied in different genotypes and corresponds to the ploidy level (Mcgaw et al. 2002). It is reported that the tetraploids have higher (70–96 %) β-asarone content, than the triploids (5–19 %), and almost negligible in diploid genotypes (Rost and Bos 1979; Todorova et al. 1995). Most of the plants found in Indian subcontinent are predominantly triploids with high β-asarone contents (Ogra et al. 2009). However, tetraploids and hexaploids are also reported from India (Janki Amal et al. 1964; Ahlawat et al. 2010).
Conventionally, identification of medicinal plants was based on morphological, anatomical and chemical analysis but these could be influenced by environmental factors. Among the various molecular markers employed to assess the genetic diversity in plants, PCR—based markers such as RAPD, DAMD, ISSR and AFLP are the most common, as their application does not need any prior sequence information. RAPD markers have its inherent problem of reproducibility, whereas AFLP technique is time consuming and cost intensive. Therefore in the present study, two DNA fingerprinting methods viz., Direct Amplification of Minisatellite DNA (DAMD) (Heath et al. 1993; Zhou et al. 1997) and Inter Simple Sequence Repeats (ISSRs) (Provost and Wilkinson 1999) were used to characterize the A. calamus germplasm. Although there are stray information available on the use of molecular markers like RAPD (Ahlawat et al. 2010), ISSR (Pai and McCarthy 2005; Abdul Kareem et al. 2012), Cp-SSR (Ginwal et al. 2009) to study the diversity in A. calamus, but these studies were confined to individual DNA based markers, and also the limited coverage of the geographical area occupied by the A. calamus in India. The present study was therefore, envisaged to characterize the ‘Sweet flag’ germplasm from wider geographical regions of India considering molecular (DAMD and ISSR) and chemical (α and β-asarone) markers.
Materials and methods
Plant material and isolation of genomic DNA
The plant material was collected from different localities of Jammu & Kashmir, Himachal Pradesh, Uttarakhand, Madhya Pradesh, Chhattisgarh, West Bengal, Assam, Tamil Nadu, representing four biogegraphic zones viz. Western Himalaya, Central India, North East India and Eastern Ghats (Table 1). They were planted in the herbal garden at CSIR- National Botanical Research, Institute, Lucknow (India), and conserved through clonal propagation. In the present study 27 genotypes of A. calamus were considered along with one closely allied taxon Colocasia esculanta (L.) Schott., as the out group. The detailed passport data for all the genotypes considered in the present study have also been evaluated (Table 1). Total genomic DNAs from individual accessions were isolated from fresh leaf tissue with a DNeasy Plant Mini Kit (QIAGEN, USA), according to the manufacturer’s instructions. Isolated DNA was checked for its quality and quantity by agarose gel (0.8 %) electrophoresis staining with ethidium bromide and comparison with known concentrations of EcoRI + HindIII digested lambda DNA, and also by Nanodrop ND-1,000 Spectrophotometer (Wilmington, DE 19810, USA).
Table 1.
Passport data and asarone contents of A. calamus genotypes used in the present study
| Sample code | Locality | Geographical coordinates | aLeaf | aRhizome | Chemical compound (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latitude (°′N) | Longitude (°′E) | Height (cm) | Width (cm) | Total number of leaves/plant | Number of branches/plant | Distance between nodes (cm) | Diameter (cm) | α-asarone | β-asarone | % of oil | ||
| Ac 01 | Jammu and Kashmir, Udhampur (Narsu) | 33°00.030′ | 75°12.251′ | 69.8 | 1.4 | 24.0 | 7.2 | 0.5–0.9 | 3.5–6.5 | 1.36 | 91.13 | 5.40 |
| Ac 02 | Jammu and Kashmir, Udhampur | 32°54.169′ | 75°08.167′ | 50.0 | 1.8 | 23.6 | 7.4 | 0.3–1.5 | 0.4–5.5 | 1.32 | 92.12 | 5.10 |
| Ac 03 | Jammu and Kashmir, Srinagar | 34°03.568′ | 74°50.243′ | 52 | 1.7 | 23.9 | 7.2 | 0.7–1.1 | 3.8–6.0 | 1.32 | 90.62 | 5.30 |
| Ac 04 | Himachal Pradesh, Renuka Lake | 30°36.370′ | 77°27.461′ | 40.6 | 2.2 | 24.8 | 7.2 | 0.5–0.9 | 4.1–5.7 | 16.82 | 74.99 | 4.00 |
| Ac 05 | Himachal Pradesh, Tapovan | 32°11.030′ | 76°21.577′ | 30.6 | 1.6 | 20.2 | 5.6 | 0.5–1.5 | 3.2–6.1 | 3.63 | 91.30 | 5.50 |
| Ac 06 | Himachal Pradesh, Harabag | 32°03.117′ | 76°38.471′ | 39.4 | 1.4 | 25.0 | 7.6 | 0.3–1.2 | 3.1–5.8 | 2.31 | 90.00 | 6.00 |
| Ac 07 | Uttarakhand, Champawat | 29°19.180′ | 80°05.596′ | 35.6 | 2.1 | 15.4 | 4.6 | 0.5–0.7 | 5.1–6.8 | 2.46 | 88.96 | 7.50 |
| Ac 08 | Madhya Pradesh, Anuppur | 23°06.031′ | 81°41.475′ | 26.8 | 1.9 | 19.2 | 5.6 | 0.4–1.4 | 4.5–6.0 | 0.85 | 90.67 | 5.20 |
| Ac 09 | Uttarakhand, Nandaprayag | 30°19.334′ | 79°19.185′ | 51.8 | 1.8 | 28.4 | 8.2 | 0.5–0.7 | 5.0–6.3 | 13.85 | 89.36 | 5.00 |
| Ac 10 | Uttarakhand, Nainital (Bhimtal) | 29°20.575′ | 79°33.129′ | 57.2 | 1.6 | 26.0 | 7.6 | 0.3–0.4 | 5.0–6.5 | 3.85 | 89.36 | 5.00 |
| Ac 11 | Uttarakhand, Champawat (Devidhura) | 29°24.349′ | 79°51.473′ | 50.8 | 1.5 | 26.6 | 7.6 | 0.7–1.2 | 0.6–6.8 | 1.34 | 88.70 | 8.00 |
| Ac 12 | Uttarakhand, Almora (Betalghat) | 29°33.414′ | 79°20.347′ | 42.4 | 2.1 | 18.6 | 5.2 | 0.3–1.8 | 0.4–5.6 | 1.30 | 89.97 | 8.00 |
| Ac 13 | Himachal Pradesh, Kullu | 31°57.487′ | 77°06.283′ | 39.6 | 1.8 | 19.5 | 6.8 | 0.3–1.7 | 2.9–4.9 | 2.63 | 90.65 | 9.50 |
| Ac 14 | Uttarakhand, Pithoragarh | 29°34.458′ | 80°12.470′ | 41.3 | 2.1 | 24.6 | 7.6 | 0.5–1.8 | 5.1–6.8 | 2.25 | 90.61 | 5.20 |
| Ac 15 | Uttar Pradesh, Lucknow, Auranwa | 26°42.204′ | 80°50.554′ | 39.8 | 1.7 | 28.0 | 8.5 | 0.6–1.1 | 4.4–7.0 | 0.83 | 79.72 | 4.00 |
| Ac 16 | Jharkhand, Dumka | 24°15.362′ | 87°14.481′ | 45.2 | 1.8 | 26.1 | 7.9 | 0.5–1.3 | 5.0–7.5 | 1.63 | 89.10 | 4.40 |
| Ac 17 | Madhya Pradesh, Panchmarni | 22°28.067′ | 78°26.255′ | 48.1 | 1.9 | 24.5 | 8.2 | 0.4–1.9 | 3.0–5.3 | 1.63 | 88.99 | 5.00 |
| Ac 18 | Chhattisgarh, Bilaspur | 22°05.505′ | 82°07.223′ | 5.6 | 2.2 | 20.8 | 6.2 | 0.5–0.7 | 3.8–6.7 | 1.37 | 91.26 | 4.99 |
| Ac 19 | Jharkhand, Gumla | 23°02.135′ | 84°33.176′ | 52.1 | 2.3 | 19.9 | 6.2 | 1.0–1.9 | 2.8–5.9 | 4.60 | 87.56 | 5.40 |
| Ac 20 | Uttarakhand, Uttarkashi (Netwar) | 31°01.139′ | 78°02.412′ | 46.9 | 1.8 | 26.3 | 8.6 | 0.4–0.6 | 3.9–6.4 | 2.62 | 73.97 | 5.70 |
| Ac 21 | West Bengal, Darjeeling | 27°01.588′ | 88°15.434′ | 48.2 | 1.7 | 27.2 | 8.1 | 0.3–1.3 | 2.2–4.6 | 1.53 | 88.70 | 4.60 |
| Ac 22 | West Bengal, Darjeeling (Forest Nursery) | 27°18.096′ | 88°35.472′ | 52.1 | 1.4 | 24.9 | 7.9 | 0.4–0.9 | 4.3–5.9 | 0.93 | 88.38 | 5.60 |
| Ac 23 | West Bengal, Darjeeling (Krishnanagar) | 26°53.155′ | 88°11.146′ | 49.8 | 2.1 | 25.8 | 6.9 | 0.6–1.58 | 3.0–4.8 | 6.98 | 86.19 | 4.80 |
| Ac 24 | Sikkim, Pangthang | 27°31.413′ | 88°31.306′ | 48.8 | 1.6 | 27.9 | 7.3 | 0.7–1.5 | 0.3–5.2 | 0.84 | 65.96 | 2.80 |
| Ac 25 | Assam, Jorhat | 26°45.177′ | 94°12.352′ | 52.8 | 2.1 | 22.6 | 6.8 | 0.5–1.4 | 4.0–5.5 | 2.62 | 82.86 | 4.50 |
| Ac 26 | Maharashtra, Pune (Gorhe) | 18°30.114′ | 73°46.452′ | 58.1 | 1.8 | 19.8 | 6.5 | 0.5–1.2 | 5.0–5.8 | 1.84 | 85.36 | 3.20 |
| Ac 27 | Tamil Nadu,Ooty | 11°24.425′ | 76°41.561′ | 61.1 | 1.9 | 16.9 | 6.3 | 0.4–1.1 | 4.8–5.6 | 3.85 | 89.36 | 4.00 |
| Og | Uttar Pradesh, Lucknow (NBRI garden) | 26°51.304′ | 80°57.013′ | – | – | – | – | – | – | – | – | – |
aValues are the average of 10 plants/genotype
PCR amplification reactions with DAMD and ISSR primers
Six minisatellite core sequence primers for DAMD reactions were custom-synthesized from (Bangalore Genei, Bangalore, India). DNA amplification was carried out according to Zhou et al. (1997). The reaction mixture (25 μl) contained 10 mM Tris-HCl (pH 8.3), 50 mM; KCl, 2 mM MgCl2, 0.2 mM dNTP mix, 0.2 μM primer, 1 unit Taq DNA polymerase (Bangalore Genei Bangalore, India) and approximately 60 ng genomic DNA. Optimal DNA amplification was obtained through 40 cycles (92 °C for 1 min, 55 °C for 2 min and 72 °C for 2 min) in a thermal cycler (PTC 200, MJ Research, Inc. USA).
A set of 100 anchored microsatellite primers were procured from the University of British Columbia, (Canada). Following the screening of these microsatellite primers, PCR amplification of 50 ng DNA was carried out in a 25 μl reaction mixture containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2.0 mM MgCl2, 0.2 mM dNTPs, 0.2 μM primer, and 0.9 U Taq DNA polymerase (Bangalore Genei, Bangalore, India), using PTC 200 thermocycler (MJ Research, Inc., USA). After initial denaturation at 94 °C for 4 min, each cycle consisted of 1 min denaturation at 94 °C, 1 min annealing at 52 °C, 2 min extension at 72 °C along with 7 min extension at 72 °C at the end of 35 cycles. Details of all primers used are listed in Table 2.
Table 2.
Details of DAMD and ISSR primers used in the present study and extent of polymorphism determined with these primers.
| Sl. No. | Primer name | Sequence (5′–3′) | Bands | Percentage polymorphism | PIC | Approx. band range size (bp) | |
|---|---|---|---|---|---|---|---|
| Amplified | Polymorphic | ||||||
| DAMD | |||||||
| 1 | HVA | AGGATGGAAAGGAGGC | 12 | 2 | 16.7 % | 0.07 | 300–2,500 |
| 2 | HBV | GGTGTAGAGAGGGGT | 16 | 11 | 68.8 % | 0.21 | 350–2,000 |
| 3 | HVR | CCTCCTCCCTCCT | 13 | 2 | 15.4 % | 0.05 | 300–3,000 |
| 4 | M 13 | GAGGGTGGCGGTTCT | 08 | 0 | 00 % | 0.00 | 350–1,500 |
| 5 | 33.6 | AGGGCTGGAGG | 10 | 2 | 20 % | 0.06 | 300–1,500 |
| 6 | HVY | GCCTTTCCCGAG | 11 | 5 | 45.5 % | 0.14 | 250–1,250 |
| ISSR | |||||||
| 7 | 807 | AGAGAGAGAGAGAGAGT | 10 | 2 | 20.00 | 0.10 | 450–1,800 |
| 8 | 822 | TCTCTCTCTCTCTCTCA | 12 | 5 | 41.67 | 0.10 | 400–3,000 |
| 9 | 823 | TCTCTCTCTCTCTCTCC | 12 | 3 | 25.00 | 0.10 | 650–2,500 |
| 10 | 830 | TGTGTGTGTGTGTGTGG | 8 | 0 | 0.00 | 0.00 | 500–1,500 |
| 11 | 835 | AGAGAGAGAGAGAGAGYC | 11 | 2 | 18.18 | 0.08 | 400–2,000 |
| 12 | 845 | CTCTCTCTCTCTCTCTRG | 9 | 1 | 11.11 | 0.02 | 500–2,100 |
| 13 | 848 | CACACACACACACACARG | 15 | 1 | 6.67 | 0.01 | 450–2,000 |
| 14 | 857 | ACACACACACACACACYG | 12 | 4 | 33.33 | 0.15 | 350–2,100 |
| 15 | 859 | TGTGTGTGTGTGTGTGRC | 7 | 1 | 14.29 | 0.07 | 400–2,000 |
| 16 | 860 | TGTGTGTGTGTGTGTGRA | 8 | 2 | 25.00 | 0.08 | 450–2,500 |
Agarose gel electrophoresis
The PCR amplified DNA fragments were electrophoresed on 1.5 % agarose gel using 0.5X TBE buffer at constant voltage of 5 V/cm. After electrophoresis, the gel was stained in ethidium bromide and then visualized and archived using Alpha InnoTech Gel Documentation System (USA).
Analysis of α and β-asarone contents
The rhizomes with roots were washed, dried at 40 °C and coarsely powdered. The essential oils were obtained from 200 gm of the powdered air-dried sample by hydro-distillation using a Clevenger-type apparatus. The percentage of the oil yield was calculated. The gas chromatography of the essential oils was performed using a Perkin Elmer GC apparatus equipped with a flame ionization detector (FID) and factor four capillary column VF-5 (60 m × 0.32 mm i.d., film thickness 0.25 μm). The GC oven temperature was set at 60 °C for 3 min and then programmed to rise from 60 to 280 °C at a rate of 3 °C /min, and held isothermally for 3 min at 280 °C. Nitrogen was used as carrier gas at a flow rate of 1 ml/min a split ratio of 1:40, an injection size of 0.05 μL neat and the injector and detector temperatures were maintained at 290 °C and 300 °C respectively. The identification of α and β-asarone content was carried out by comparison of their retention indices with those of authentic compounds. The relative amounts of individual components were calculated based on GC peak areas without applying any correction factors. All the twenty seven genotypes were analyzed for α and β-asarone contents.
Data analysis
Data analysis was carried out only for those genotypes that resulted in consistent and reproducible profiles. For each primer, the molecular sizes of each band were estimated on the basis of the corresponding marker lane. Data were scored as presence (1) or absence (0) of a band. Only distinct and well-separated bands were used for further analysis. The polymorphic information content (PIC) was calculated according to Botstein et al. (1980) for each primer. A pair wise matrix of similarity between genotypes was determined for the band data using Jaccard’s similarity coefficient for UPGMA method in the FreeTree program (ver. 0.9.1.5) (Pavlicek et al. 1999). From this data, the UPGMA tree was computed after allowing a 1,000 replicate bootstrap test using the same program. The trees were viewed, annotated and printed using Tree View (ver. 1.6.5) (Page 2001). In order to determine the utility of each of the marker systems, diversity index (DI), effective multiplex ratio (EMR) and marker index (MI) were calculated according to Powell et al. (1996).
Results and discussion
In the present analysis, DAMD and ISSR markers were used to characterize the ‘Sweet flag’ germplasm. Six DAMD primers were considered in the present analysis that resulted in 70 bands ranging from 250 bp–3,000 bp in size. Out of total 70 bands obtained in DAMD analysis, 22 bands were polymorphic, revealing 31.4 % polymorphism across all the genotypes. Primer HBV produced maximum PIC value (0.21), while primer HVR resulted in minimum PIC value (0.05), and primer M13 showed all the monomorphic bands. In case of ISSR, 10 primers were considered, which resulted in to a total of 104 bands, and the size of the fragments varied from 350 bp–3,000 bp. Out of the total number of bands, 21 bands were polymorphic revealing 20.19 % polymorphism. Primer 857 produced the highest PIC value (0.15), while primer 848 produced lowest PIC value (0.01), and primer 835 revealed all monomorphic bands. The average PIC value for DAMD and ISSR were 0.09 and 0.07 respectively (Table 2).
The cumulative analysis carried out for the data generated with DAMD and ISSR methods showed 24.71 % polymorphism across all genotypes of A. calamus. The cumulative data was also used to compute pair wise distances between the pairs of genotypes. The pair wise distance matrix calculated by NJ method using Jaccard’s coefficient showed a distance range of 0.01 to 0.18 among all the 27 genotypes of A. calamus. The maximum distance (0.18) was observed between the Ac09 from Uttarakhand and Ac25 from Assam, whereas minimum genetic distance (0.01) was showed by four pairs of genotypes namely; Ac02–Ac03 (Jammu & Kashmir), Ac03–Ac04 (Jammu & Kashmir and Himachal Pradesh), Ac17–Ac18 (Madhya Pradesh), Ac18–Ac19 (Madhya Pradesh), and Ac22–Ac23 (West Bengal), suggesting very low level of genetic diversity. Analysis of individual marker system for DAMD and ISSR also showed the weak correlation between the pairs of genotypes (data not shown).
A comparative statistical analysis was carried out to determine the utility of each marker used in the present study. The diversity index (DI), the effective multiplex ratio (EMR) and the marker index (MI) were calculated for two marker systems (DAMD and ISSR) according to Powell et al. (1996). The MI value, which reveals the predictive power of a marker system in diversity studies was found maximum in DAMD (0.32) followed by ISSR (0.13). These findings were corroborating with the percentage of polymorphism (P) and mean polymorphic information content (PIC) values resulted in DAMD (P = 31.04 %, PIC = 0.09) and ISSR (P = 20.19 %, PIC = 0.07) analysis, respectively (Table 3).
Table 3.
Comparison of DNA fingerprinting methods (DAMD and ISSR) and details of their results and analyses computed for the A. calamus genotypes
| Method | Number of genotypes | No. of primers used | Total no. of bands | No. of polymorphic bands | Polymorphism (%) | Average PIC | Genetic distance | Average distance | Diversity index | Effective multiplex ratio | Marker index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DAMD | 27 | 6 | 70 | 22 | 31.4 | 0.09 | 0.00–0.23 | 0.12 | 0.28 | 1.15 | 0.32 |
| ISSR | 27 | 10 | 104 | 21 | 20.19 | 0.07 | 0.00–0.16 | 0.08 | 0.31 | 0.42 | 0.13 |
| Cumulativea | 27 | 16 | 174 | 43 | 24.71 | 0.08 | 0.01–0.18 | 0.09 | 0.32 | 0.71 | 0.23 |
aCombined data of DAMD and ISSR
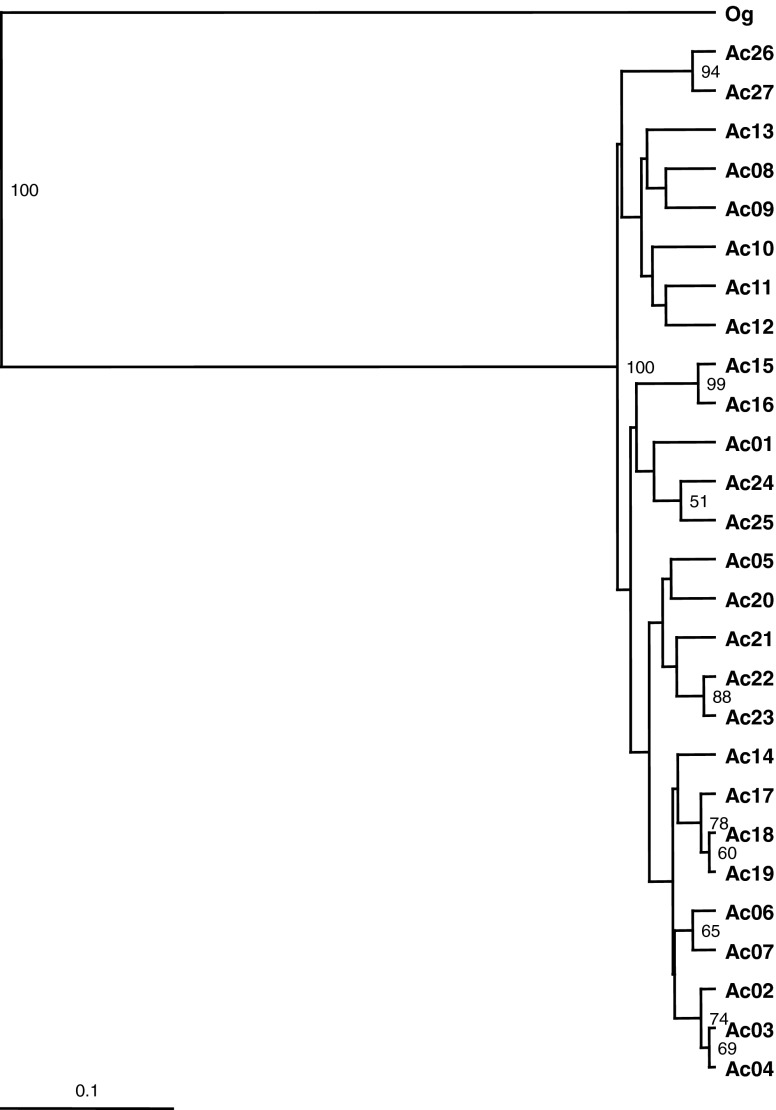
UPGMA tree was generated on the basis of Jaccard’s similarity coefficient after a 1,000 replicate bootstrap test of robustness and bootstrap values that were 50 or above have been shown on the branches of the tree (Fig. 1). It is clear from the results that clustering in the UPGMA tree was not in accordance with the geographical affiliations of the genotypes, instead two broad groups were formed. Group I consisted of majority of the genotypes from Uttarakhand with a sister group, where two genotypes from Maharashtra (Ac26) and Tamil Nadu (Ac27) clustered together showing close affinity to each other with 94 % bootstrap support. Group II comprised of heterogeneous assemblage of genotypes from states like Uttar Pradesh, Jammu & Kashmir, West Bengal, Himachal Pradesh, Chandigarh, Assam, Madhya Pradesh, Chhattisgarh and Sikkim.
Fig. 1.
UPGMA dendrogram generated for cumulative band data after 1,000 replicate bootstrapping. Accessions names are as in Table 1. Numbers at the nodes are bootstrap scores
All the 27 genotypes were also analyzed for its chemical constituents like α and β-asarone contents, The genotype (Ac13) from Kullu (Himachal Pradesh) showed maximum (9.5 %) percentage of oil, whereas, genotype from Pangthang (Sikkim) showed minimum (2.8 %). Similarly, highest α and β-asarone contents (16.82 % and 92.12 %) were obtained from genotypes from Renuka (Himachal Pradesh) and Udhampur (Jammu & Kashmir) while, minimum α and β-asarone contents (0.83 % and 65.96 %) were obtained from Auranwa (Uttar Pradesh) and Pangthang (Sikkim), respectively. Almost similar level of β-asarone content was also reported in the earlier study from Himachal Pradesh, Uttarakhand and Jammu & Kashmir (Ahlawat et al. 2010).
The earlier studies on genetic diversity among and within population of A. calamus revealed that the individuals within a population were homogenous in terms of their genetic composition. Low level of genetic variation was found among the populations, and high genetic differentiation between geographical regions, this could be attributed to the clonal nature of A. calamus (Pai and McCarthy 2005; Abdul Kareem et al. 2012). Similarly, in A. gramineus another species of the genus Acorus showed the similar kind of genetic variations with RAPD markers (Liao and Hsiao 1998). In the present study also very low level of genetic variability (24.71 %) was revealed across the different genotypes of A. calamus, and the clustering patterns in the UPGMA tree showed that genotypes are diverse, and did not show any specific correlation with their geographical provenances (Fig. 1). This indicates that the A. calamus has very narrow genetic base, and this could also be attributed to the fact that the plants of A. calamus proliferate through rhizomes and grow gregariously, and very seldom a solitary plant is found in the nature. The pollination of the A. calamus species is also not known, but it is presumed that plants are entomophilic, because the pollens are sticky in nature (Chang 2010).
Though the calamus oil obtained from A. calamus has several medicinal, aromatic and industrial properties, the Food and Drug Administration (FDA) banned its use in the food formulations and therapeutic preparations due to the carcinogenic and toxic properties of the β-asarone compounds; a major and active constituent in the oil (Riaz et al. 1995; Ravindran and Balachandran 2004). The European Union (EU) has also recommended the limits of calamus oil utilization i.e. 0.1 mh/kg in food and beverages and 1 mg/kg in spirits and spices used for snacks. Its use is prohibited by the United States and Canada (Mittal et al. 2009). In the present study, the genotype (Ac24) from Sikkim resulted in to a reasonably low level of α (0.84 %) and β (65.96 %) asarone contents respectively. Our results are in congruence with the earlier studies wherein β-asarone contents were very low in the genotypes found in the temperate regions of India (Ogra et al. 2009). The European Commission (EC) have also suggested that only the plants of A. calamus with no β-asarone or having very low quantity of this compound should be used in the food stuffs, alcoholic beverages as well as in the medicines due to the highly carcinogenic and toxic properties of the β-asarone compounds (Mittal et al. 2009).
The multifarious properties of A. calamus make it a potential plant species for various industrial applications. So, the identification and evaluation of low α and β-asarone contents containing germplasm is therefore, pre-requisite for its commercial utilization. This study contributes to the evaluation of large number of diverse germplasm from different parts of India in order to identify the genotypes with low α and β asarone contents.
Acknowledgements
The authors are thankful to the Director, CSIR-National Botanical Research Institute, Lucknow for facilities and encouragements. The authors (MMP, SKS and AKSR) are also thankful to the NAIP (ICAR, New Delhi) for financial support.
References
- Abdul Kareem VK, Rajasekharan PE, Ravish BS, Mini S, Sane A, Kumar TV. Analysis of genetic diversity in Acorus calamus populations in South and North East India using ISSR markers. Biochem Syst Ecol. 2012;40:156–161. doi: 10.1016/j.bse.2011.09.012. [DOI] [Google Scholar]
- Ahlawat A, Katoch M, Ram G, Ahuja A. Genetic diversity in Acorus calamus L. as revealed by RAPD markers and its relationship with b-asarone content and ploidy level. Sci Hortic. 2010;124:294–297. doi: 10.1016/j.scienta.2009.12.035. [DOI] [Google Scholar]
- Bertea CM, Azzolin CMM, Bossi S, Doglia G, Maffei ME. Identification of an EcoRI restriction site for a rapid and precise determination of b-asarone-free Acorus calamus cytotypes. Phytochemistry. 2005;66:507–514. doi: 10.1016/j.phytochem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Chang PS (2010) Flora of China. 23:1–2. Available from: http://www.efloras.org
- Ginwal HS, Mittal N, Barthwal S. Development and characterization of polymorphic chloroplast microsatellite markers in sweet flag (Acorus calamus L.) Indian J Genet. 2009;69:256–259. [Google Scholar]
- Heath DD, Iwama GK, Devlin RH. PCR primed with the VNTR core sequences yields species specific patterns and hypervariable probes. Nucleic Acids Res. 1993;21:5782–5785. doi: 10.1093/nar/21.24.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janki Amal EK, Sobti SN, Handa KL. The interrelationship between polyploidy, altitude and chemical composition in Acorus calamus. Curr Sci. 1964;33:500. [Google Scholar]
- Karthikeyan S, Jain SK, Nayar MP, Sanjappa M. Florae Indicae Enumeratio Monocotyledonae. Calcutta: Botanical Survey of India; 1989. [Google Scholar]
- Krahulcova A. Chromosome numbers in selected monocotyledons. Perslia Praha. 2003;75:97–113. [Google Scholar]
- Liao LC, Hsiao JY. Relationship between population genetic structure and riparian habitat as revealed by RAPD analysis of the rheophyte Acorus gramineus Soland. (Araceae) in Taiwan. Mol Ecol. 1998;7:1275–1281. doi: 10.1046/j.1365-294x.1998.00438.x. [DOI] [Google Scholar]
- Mcgaw LJ, Jager AK, Staden J. Isolation of b-asarone, an antibacterial and anthelmintic compound, from Acorus calamus in South Africa. S Afr J Bot. 2002;68:31–35. [Google Scholar]
- Mehrotra S, Mishra KP, Maurya R, Srimal RC, Yadav VS, Pandey R, Singh VK. Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome. Int Immunopharmacol. 2003;3:53–61. doi: 10.1016/S1567-5769(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Mittal N, Ginwal HS, Varshney VK. Pharmaceutical and biotechnological potential of Acorus calamus Linn.: an indigenous highly valued medicinal plant species. Pharmacogn Rev. 2009;3:93–103. [Google Scholar]
- Ogra RK, Mahapatra P, Sharma UK, Sharma M, Sinha AK, Ahuja PS. Indian calamus (Acorus calamus L.): not a tetraploid. Curr Sci. 2009;97:1644–1647. [Google Scholar]
- Page RDM (2001) TreeView (Win32), Ver. 1.6.5. Available from: http://taxonomy.zoology.gla.ac.uk/rod/treeview.html
- Pai A, McCarthy BC. Variation in shoot density and rhizome biomass of Acorus calamus L. with respect to environment. Castanea. 2005;70:263–275. doi: 10.2179/0008-7475(2005)070[0263:VISDAR]2.0.CO;2. [DOI] [Google Scholar]
- Pavlicek A, Hrda S, Flegr J. Free Tree - Freeware program for construction of phylogenetic trees on the basis of distance data and bootstrapping / jackknife analysis of the tree robustness. Application in the RAPD analysis of the genus Frenkelia. Folia Biol (Praha) 1999;45:97–99. [PubMed] [Google Scholar]
- Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed. 1996;2:225–238. doi: 10.1007/BF00564200. [DOI] [Google Scholar]
- Provost A, Wilkinson MJ. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet. 1999;98:107–112. doi: 10.1007/s001220051046. [DOI] [Google Scholar]
- Raina VK, Srivastava SK, Syamasunder KV. Essential oil composition of Acorus calamus L. from the lower region of the Himalayas. Flav Frag J. 2003;18:18–20. doi: 10.1002/ffj.1136. [DOI] [Google Scholar]
- Ravindran PN, Balachandran I. Underutilized medicinal spices (1) Spice India. 2004;17:1–14. [Google Scholar]
- Riaz M, Qamar S, Chaudhary FM. Chemistry of the medicinal plants of the genus Acorus (family Araceae) Hamdard Med. 1995;38:50–62. [Google Scholar]
- Rost LCM, Bos R. Biosystematic investigation with Acorus L. 3 Communication. Constituents of essential oils. Planta Med. 1979;36:350–361. doi: 10.1055/s-0028-1097281. [DOI] [Google Scholar]
- Todorova MN, Ognyanov IV, Shatar S. Chemical composition of essential oil from Mongolian Acorus calamus L. rhizomes. J Essent Oil Res. 1995;7:191–193. doi: 10.1080/10412905.1995.9698498. [DOI] [Google Scholar]
- Zhou Z, Bebeli PJ, Somers DJ, Gustafson JP. Direct amplification of minisatellite-region DNA with VNTR core sequences in the genus Oryza. Theor Appl Genet. 1997;95:942–949. doi: 10.1007/s001220050645. [DOI] [Google Scholar]