Abstract
Estrogen-related receptor γ (ERRγ) serves a critical O2-dependent regulatory role in the differentiation of human cytotrophoblasts to syncytiotrophoblast. In this study, we investigated expression of genes encoding tissue kallikrein (KLK1) and voltage-gated K+ channels (KV7) during differentiation of human trophoblasts in culture and the roles of ERRγ and O2 tension in their regulation. Expression of KLK1 and the KV7 channel subunits, KCNQ1, KCNE1, KCNE3, and KCNE5, increased during differentiation of cultured human trophoblast cells in a 20% O2 environment. Notably, together with ERRγ, expression of KLK1, KCNQ1, KCNE1, KCNE3, and KCNE5 was markedly reduced when cells were cultured in a hypoxic environment (2% O2). Moreover, upon transduction of trophoblast cells with short hairpin RNAs for endogenous ERRγ, KLK1, KCNQ1, KCNE1, and KCNE3 expression was significantly decreased. Promoter and site-directed mutagenesis studies in transfected cells identified putative ERRγ response elements within the KLK1 and KCNE1 5′-flanking regions required for ERRγ-stimulated transcriptional activity. Binding of endogenous ERRγ to these ERRγ response elements increased during trophoblast differentiation in culture and was inhibited by hypoxia. The KV7 blocker linopirdine reduced human chorionic gonadotropin secretion and aggregation of cultured human trophoblasts, suggesting a possible role of KV7 channels in cell fusion and differentiation. Illumina gene expression arrays of cultured human trophoblast cells revealed several genes upregulated during syncytiotrophoblast differentiation and downregulated upon ERRγ knockdown involved in cell differentiation, adhesion, and synthesis of steroid and peptide hormones required for placental development and function. Collectively, these findings suggest that ERRγ mediates O2-dependent expression of genes involved in human trophoblast differentiation, function, and vascular homeostasis.
The placenta is essential for the establishment and maintenance of pregnancy. The physiological hypoxia that exists before establishment of blood flow to the intervillous space at the early stages of placental development promotes trophoblast proliferation and invasion (1, 2). However, persistent placental hypoxia is believed to contribute to the pathogenesis of pre-eclampsia and intrauterine growth restriction (IUGR) (3–5).
Estrogen-related receptor γ (ERRγ), a member of the ERR/NR3 subfamily of orphan nuclear receptors (6), controls gene networks involved in energy homeostasis (7). ERRγ was reported to be expressed at the highest levels in placenta among human reproductive tissues (8). We previously observed that ERRγ is rapidly upregulated during differentiation of human trophoblasts in primary culture and serves a critical regulatory role in the associated induction of hCYP19 (human aromatase P450), the key enzyme in estrogen biosynthesis (9). Both induction of ERRγ and aromatase gene expression were blocked when the trophoblast cells were maintained in a hypoxic (2% O2) environment. Thus, our findings suggested that ERRγ serves as a novel O2-responsive transcription factor during human trophoblast differentiation (9).
In studies of gene-targeted mice, ERRγ was found to regulate K+ homeostasis in heart, stomach, and kidney via control of a number of hypertension-associated genes (10), including the voltage-gated K+ channel, Kcne2, and tissue kallikrein, Klk1, which mediate vasodilation. Members of the potassium voltage-gated channel, KQT-like (KCNQ) subfamily (KCNQ1–5, KV7), comprised of 6 transmembrane-spanning α-subunits, are components of voltage-gated K+ channels (KV). KCNQ1 (KV7.1) has been well studied in cardiac myocytes where the KCNQ1 α-subunit assembles with the potassium voltage-gated channel subfamily (KCNE)1 β-subunit (minK) to form a channel complex that mediates the delayed rectifier current (11); dysfunction of this channel causes cardiac long QT syndrome (LQTS1). In epithelium of organs, such as lung, stomach, cochlea, intestine, and kidney, where salt and water transport are crucial for proper function, the lack of functional KCNQ1 channel expression has severe consequences (12). KCNQ1 channels associate with all 5 members of the KCNE β-subunit family (KCNE1–5), resulting in β-subunit–specific changes in current characteristics (12). KCNQ and KCNE families were found to be expressed in human placenta (13), where ion and water transport are required for synthesis and release of hormones, growth factors, and cytokines to maintain normal pregnancy.
Human kallikrein genes encode a family of 15 serine proteases (KLK1–15). Tissue kallikrein (KLK1) processes low–molecular-weight kininogen to produce vasoactive kinins (kallidin and bradykinin) (14). Upon further processing, these peptides bind to bradykinin receptors to stimulate the production of factors that promote vasodilation (15). In human placenta, KLK1 was predominantly observed in syncytiotrophoblast and was more highly expressed in first-trimester samples, suggesting that this enzyme may participate in the establishment and maintenance of placental blood flow through vasodilation, prevention of platelet aggregation, cell proliferation, and trophoblast invasion (16).
In consideration of the potential importance of ERRγ in K+ homeostasis and vascular tone in a number of tissues as well as the central role of the placenta in hypertensive disorders of pregnancy, in the present study, we investigated a role for ERRγ in the regulation of voltage-gated K+ channel genes and KLK1 during differentiation of human trophoblasts in culture. We found that KLK1 and the voltage-gated K+ channel genes, KCNQ1, KCNE1, KCNE3, were induced during human trophoblast differentiation in culture, and expression of these genes was inhibited by hypoxia and ERRγ knockdown. Moreover, KCNE1 and KLK1 were transcriptionally upregulated by ERRγ acting through ERRγ response elements (ERREs) in their promoters. Interestingly, inhibition of these voltage-gated K+ channels prevented aggregation and possible fusion of human trophoblasts in culture. Whole-genome gene expression arrays further revealed several genes aberrantly expressed upon ERRγ knockdown that are implicated in placental differentiation and synthesis and metabolism of hormones required for placental development and function.
Materials and Methods
Primary culture of human trophoblast cells
Midtrimester human placental tissues (16–24 weeks gestation) were obtained from Advanced Bioscience Resources (Alameda, California) in accordance with the Donors Anatomical Gift Act of the State of Texas. Protocols were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Cytotrophoblasts were isolated and cultured as reported previously (9). For each of the studies, at least 3 independent experiments were performed using different placentas.
To determine the effects of hypoxia on expression of K+ channel subunits and kallikrein genes, the cells were placed in an incubator (Heraeus; Thermo Scientific, Rockford, Illinois) at an atmosphere of 2% O2, 93% N2, and 5% CO2. To determine the effects of K+ channel blockers, cytotrophoblasts were plated in medium containing the K+ channel blockers or ethanol vehicle (final concentration of ethanol, 0.1%) and cultured for 72 hours. The culture medium was replaced with fresh medium with or without blocker daily. Two K+ channel blockers were chosen: 4-aminopyridine (4-AP) (0.01mM, 0.1mM, and 1mM), which blocks all voltage-gated K+ channels, and linopirdine (LP) (0.01mM and 0.1mM), which specifically blocks KV7 channels. Both K+ channel blockers were from Sigma-Aldrich (St. Louis, Missouri).
Quantitative RT-PCR
Total RNA from trophoblast cells cultured for 24, 48, or 72 hours, was extracted using the miRNeasy Mini Kit (QIAGEN, Gaithersburg, Maryland). RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 2 μg were reverse transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California). Primer sets are listed in Table 1.
Table 1.
Primer Sets for Real-Time RT-PCR
| Transcript | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| CYP19I.1 | ACGGAAGGTCCTGTGCTCG | GTATCGGGTTCAGCATTTCCA |
| ERRγ | CTGACGGACAGCGTCAACC | GGCGAGTCAAGTCCGTTCTG |
| RPLP0 | TGCATCAGTACCCCATTCTATCA | AAGGTGTAATCCGTCTCCACAGA |
| KCNQ1 | GACCCGCGCGTCTCCATCTAC | TGAGGAAGACGGCGAAGTGGTAA |
| KCNQ3 | CGAGTTTGCTTTGAGGATCTG | AGGCAATCAGCACAAAGATGT |
| KCNQ5 | AGTCCCACCAAAGTGCAGA | AGTGCCAAGGGCTGTGTC |
| KCNE1 | TGCTCAGGAGGAAGAGACCAGAAGG | GCAGAGGGATTTTTCCAGTTCTCGT |
| KCNE3 | TCCAGAGACATCCTGAAGAGG | GGGGAAGACTCGGTAGAAGC |
| KCNE4 | CCTCTTGGACTGGACGATTT | GTGCTGTTCAGAGGCTCCAT |
| KCNE5 | AACTCTGGGCCGTCTAACTG | AAGGCAACTGGAAGCTGGA |
| KLK1 | GGCAAAGACACCTGTGTGGGTGAT | GACAGCACTCTGACGGCGACA |
| hCGβ | ATGGAGATGTTCCAGGGGC | TTGTTGGAGGATCGGGGT |
| HSD11B2 | GACCTGACCAAACCAGGAGA | GCCAAAGAAATTCACCTCCA |
| HSD17B1 | TATGCGAGAGTCTGGCGGTT | TGCACTGGGCCGCACT |
| PLAC1 | ATTGGCTGCAGGGATGAAAG | TGCACTGTGACCATGAACCA |
For the quantitative analysis of mRNA expression, a CFX384 real-time PCR detection system (Bio-Rad) was employed using iTaq SYBR Green Supermix with ROX (Bio-Rad) for the detection of PCR products. The cycling conditions were 95°C for 3 minutes, followed by 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. The relative fold changes were calculated using the comparative cycle times (Ct) method with RPLP0 as the reference standard.
Western blot analysis
Total protein extracts were prepared from human trophoblast cells after culture using RIPA buffer (Thermo Scientific). Equivalent amounts of protein, determined by the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific), were resolved by 4%–12% Bis-Tris gel (Invitrogen, Carlsbad, California) electrophoresis and blotted to Hybond-P membranes (Amersham Biosciences, Piscataway, New Jersey). The primary antibodies were as follows: rabbit polyclonal ERRγ antibody (Abcam Inc, Cambridge, Massachusetts; catalog no. ab82319), rabbit polyclonal KCNQ1 antibody (Abcam; catalog no. ab65092), rabbit polyclonal KCNE1 antibody (Cell Signaling Technology, Danvers, Massachusetts; catalog no. 3486), anti–kallikrein-1 rabbit polyclonal antibody (Enzo Life Sciences, Plymouth Meeting, Pennsylvania; catalog no. BML-SA447), and anti-glyceraldehyde-3-phosphate dehydrogenase antibody (GAPDH, Abcam; catalog no. ab9485). Horseradish peroxidase-conjugated antirabbit IgG (GE Healthcare, Piscataway, New Jersey) was used as secondary antibody. The membranes were developed by enhanced Supersignal West Pico chemiluminescent substrate or Supersignal West Pemto maximum sensitivity substrate (Thermo Scientific).
RNA interference
Lentiviral vectors containing short hairpin RNA (shRNA) targeting ERRγ in pGIPZ vectors were obtained from a lentivirus shRNA library (Open Biosystems, Huntsville, Alabama). Two different shRNA vectors were used for silencing of ERRγ: Open Biosystems catalog no. RHS4430-98514497 and catalog no. RHS4430-98912788. Lentiviral supernatants were produced in human embryonic kidney 293T cells by Lipofectamine 2000 (Invitrogen) mediated transfection of plasmids pMD2.G, psPAX2, and human pGIPZ lentiviral shRNA for ERRγ. Lentiviral supernatants were collected after 24, 48, and 72 hours of culture. The viral particles were concentrated by ultracentrifugation, and the titers of the viral stocks, expressed as multiplicity of infection, were determined according to the protocol recommended by Open Biosystems. For control experiments, cells were infected with nonsilencing pGIPZ lentiviral shRNA control.
Reporter assays
We identified 2 putative ERREs at −816 (ERRE-1) and −49 base pairs (bp) (ERRE-2) upstream of the KCNE1 first exon, and 1 putative ERRE at −891 bp upstream of KLK1 first exon using the Genomatix MatInspector program (http://www.genomatix.de/). Genomic fragments of KCNE1 (−915 to +270) and KLK1 (−951 to +249) containing these ERREs were amplified from human placental genomic DNA using PrimeSTAR HS DNA polymerase (Takara, Foster City, California) according to the manufacturer's instructions. The primer pairs (5′–3′) are listed in Table 2. The amplified fragments were cut using restriction enzymes NheI and HindIII (New England Biolabs, Ipswich, Massachusetts) and ligated to pGL4.11[luc2P] vector (Promega, Madison, Wisconsin) to create the KCNE1- and KLK1-luciferase reporter plasmids. Site-directed mutagenesis of single ERREs within the KCNE1 (KCNE1m1 and KCNE1m2) and KLK1 5′-flanking regions was performed using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, California) system and the primers listed in Table 2. Mutation of both ERREs in the KCNE1 5′-flanking region (KCNE1m1,2) was performed using QuikChange multi-site–directed mutagenesis kit (Stratagene) system and the 4 KCNE1 primers (Table 2). All constructs were confirmed by DNA sequencing.
Table 2.
Primer Sets for Reporter Assay
| Genes | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| KCNE1 | GGGCCCGGGCTAGCTGCTTCATGTAAA GTATGCCTCA | GGGCCCGGAAGCTTATGGCTCTCCTTCGAACTCA |
| KLK1 | GGGCCCGGGCTAGCCATTGAGGCTGCAGGAATCT | GGGCCCGGAAGCTTGCCGCGCAAGATAGATAAGA |
| KCNE1m1 | GATAATGGGCAGGGGTTGGGGAGATAGGCTTAATTATCATCATATCCTTTTAA | TTAAAAGGATATGATGATAATTAAGCCTATCTCCCCAACCCCTGCCCATTATC |
| KCNE1m2 | CCCGACGCCCCAGCCTATGCATGCCACATGAGCTG | CAGCTCATGTGGCATGCATAGGCTGGGGCGTCGGG |
| KLK1m | TAATTATCTTCAGAGACCACAAAATGTGATGTTCATAGGCGCTGAATGTTGAAGTAC | GTACTTCAACATTCAGCGCCTATGAACATCACATTTTGTGGTCTCTGAAGATAATTA |
COS-7 cells (American Type Culture Collection, Manassas, Virginia) grown in DMEM (Life Technologies, Grand Island, New York) supplemented with 10% fetal bovine serum (FBS) were transfected with wild-type or mutated KCNE1:luciferase (KCNE1-LUC) or KLK1:luciferase (KLK1-LUC) reporter constructs, β-galactosidase (β-gal) expression vector, and human ERRγ expression vector or empty vector, using Lipofectamine 2000 (Invitrogen). The cells were lysed 48 hours after transfection in reporter lysis buffer (Promega) and assayed for β-gal and luciferase (Promega).
Chromatin immunoprecipitation assays
Human trophoblasts were cultured for 24 or 48 hours in DMEM with 2% FBS, in 20% or 2% O2. A chromatin immunoprecipitation (ChIP) assay using the ChIP Kit (Millipore Corp, Bedford, Massachusetts) was performed, as reported previously (9). Precleared cross-linked chromatin was immunoprecipitated using ERRγ IgG (PA1–316; Affinity Bioreagents, Golden, Colorado) or normal rabbit IgG (sc-2027; Santa Cruz Biotechnology, Inc, Santa Cruz, California), as control. Quantitative PCR was used to assess the fold enrichment of the immunoprecipitated protein-DNA complex for ERRγ. Primers were designed to amplify an 88- to 154-bp region surrounding the KCNE1 or KLK1 ERREs. Primers to amplify putative ERREs in KCNE1 and KLK1 promoters are listed in Table 3. Quantitative PCR was performed using iTaq SYBR Green Supermix with ROX (Bio-Rad) on a CFX384 real-time PCR detection system (Bio-Rad). Signals were normalized to input samples. Samples from 2 to 3 independent ChIP experiments were analyzed.
Table 3.
Primer Sets for ChIP Assay
| Genes | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| KCNE1 ERRE1 | TGCTTCATGTAAAGTATGCCTCA | AGCATCACACACTTGAAAAACG |
| KCNE1 ERRE2 | ACGCCATAAACCAGTGTTGC | ACCCACTGCTCAGCTCATGT |
| KLK1 ERRE | CATTGAGGCTGCAGGAATCT | TTAGGTCTCACGAGCCAGGT |
Whole-genome gene expression arrays
Illumina whole-genome gene expression array analysis was performed on human trophoblasts before culture or 72 hours after infection with recombinant lentiviruses expressing ERRγ shRNA or a control, nonsilencing shRNA. RNA from 3 independent experiments using placental tissues from 3 abortuses was extracted by miRNeasy Mini Kit (QIAGEN). RNA was labeled and hybridized to an Illumina HumanHT-12 v4 BeadChip, according to the manufacturer's protocol. Changes in gene expression were analyzed using GeneSpring version 12.1 and an online data analysis suite (WebGestalt) (17). Gene expression levels of ERRγ, HSD11B2, HSD17B1, and PLAC1 were validated by quantitative RT-PCR (qRT-PCR) using RNA from 3 different independent experiments.
Measurement of human chorionic gonadotropin and lactate dehydrogenase
Culture medium was changed daily and collected after 24 and 72 h of trophoblast cell culture; aliquots were stored at −20°C. The medium was assayed for secreted human chorionic gonadotropin (hCG) using ELISA (DRG Diagnostics, DRG International, Marburg, Germany) following the manufacturer's instructions. Culture medium also was analyzed for lactate dehydrogenase (LDH), which is released from necrotic cells and used as a marker of cell viability, using a cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany). The cells were harvested and lysed to measure the total protein by the Pierce BCA protein assay kit (Thermo Scientific).
Morphological analysis
Cytotrophoblasts were cultured on glass chamber slides in DMEM containing 2% FBS with K+ channel blockers 4-AP (1mM) or LP (0.1mM) or ethanol, as control, and cultured for 72 hours. The cells were rinsed in PBS and fixed in 4% paraformaldehyde. Hematoxylin and eosin were used to stain nuclei and cytoplasm, respectively. Morphology was analyzed by light microscopy (×200), and ImageJ was used to quantify the percentage of syncytiotrophoblasts relative to total cell number in 8 different fields from 2 independent experiments performed in duplicate.
Data analysis
Data are expressed as mean ± SEM. Differences between groups were analyzed by Student's t test. Statistical significance was set as P < .05; each experiment was performed at least 3 times in triplicate.
Results
Expression of the K+ channel subunits, KCNQ1, KCNE1, KCNE3, and KCNE5, and the kallikrein, KLK1, are induced in concert with ERRγ in human trophoblast cells during differentiation in culture and inhibited by hypoxia
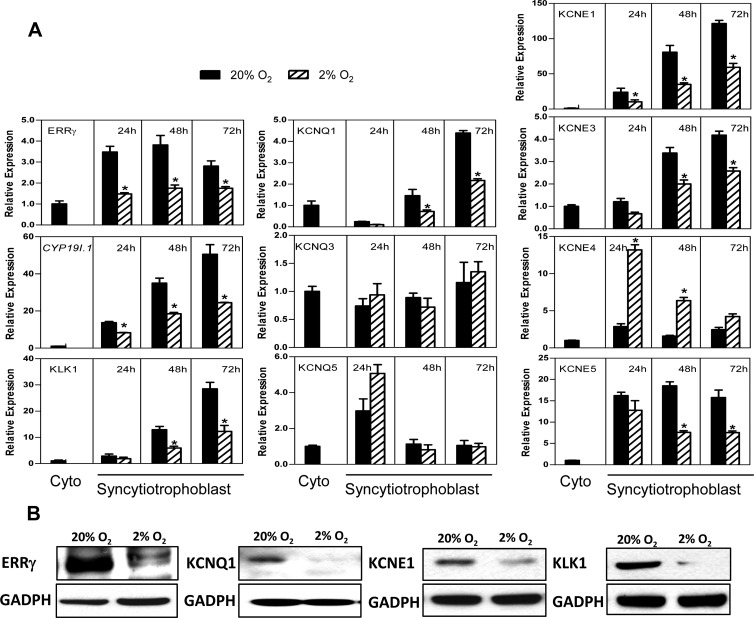
As mentioned, in studies of gene-targeted mice, ERRγ was found to regulate expression of voltage-gated K+ channel subunits in heart, stomach, and kidney (10). Because ERRγ is rapidly upregulated during differentiation of human trophoblasts in culture (9), in the present study, we analyzed expression of the voltage-gated KV7 channel α-subunit genes, KCNQ1–5, the β-subunit genes, KCNE1–5, and the kallikrein genes, KLK1, KLK5, KLK6, KLK8, and KLK13, during human trophoblast differentiation in 20% O2 and the effects of hypoxia. We observed that mRNA levels of KCNQ1, KCNE1, -3, and -5 were markedly induced during trophoblast differentiation in 20% O2 and inhibited by hypoxia (Figure 1A) in a similar pattern as CYP19I.1 and ERRγ (9). On the other hand, expression of α-subunits KCNQ3 and KCNQ5 were unaltered during culture in 20% O2 and unaffected by hypoxia (Figure 1A). By contrast, the β-subunit KCNE4 was unaltered during culture in 20% O2 and induced by hypoxia (Figure 1A). Expression of the kallikrein, KLK1, was markedly induced during trophoblast differentiation in 20% O2 and inhibited by hypoxia (Figure 1A), whereas expression of KLK5, KLK6, KLK8, and KLK13 was undetectable in human trophoblasts before and after culture under normoxic and hypoxic culture conditions. Immunoblot analysis indicated that the expression of KCNQ1, KCNE1, and KLK1 proteins also was inhibited by hypoxia (Figure 1B). Thus, KCNQ1, KCNE1, KCNE3, KCNE5, and KLK1 were regulated in a pattern that was highly similar to that of ERRγ (Figure 1), indicating their possible regulation by ERRγ during trophoblast differentiation.
Figure 1.
Expression of the K+ channel subunits, KCNQ1, KCNE1, KCNE3, and KCNE5, and the kallikrein, KLK1, are induced in concert with ERRγ in human trophoblast cells during differentiation in culture and are inhibited by hypoxia. A, Freshly isolated human placental cytotrophoblasts were cultured in a 20% O2 or 2% O2 environment for 24, 48, and 72 hours. RNA was isolated from cells either before (Cyto) or after culture, and the expression of ERRγ, CYP19I.1, KV channel α-subunits (KCNQ1, KCNQ3, and KCNQ5) and β-subunits (KCNE1, KCNE3, KCNE4, and KCNE5) and KLK1 mRNA was analyzed by qRT-PCR; RPLP0 was used as the internal reference. Data are the mean ± SEM of values from 3 independent experiments, each conducted in triplicate, and are expressed relative to expression levels in cytotrophoblasts before culture. *, Significantly different (P < .05) from values of cells cultured in 20% O2. B, Total proteins extracted from cells cultured in a 20% O2 or 2% O2 environment for 72 hours were analyzed by immunoblotting using antisera to ERRγ, KCNQ1, KCNE1, and KLK1 or GAPDH, as loading control.
Knockdown of endogenous ERRγ inhibits expression of KCNQ1, KCNE1, KCNE3, and KLK1 in human placental cells
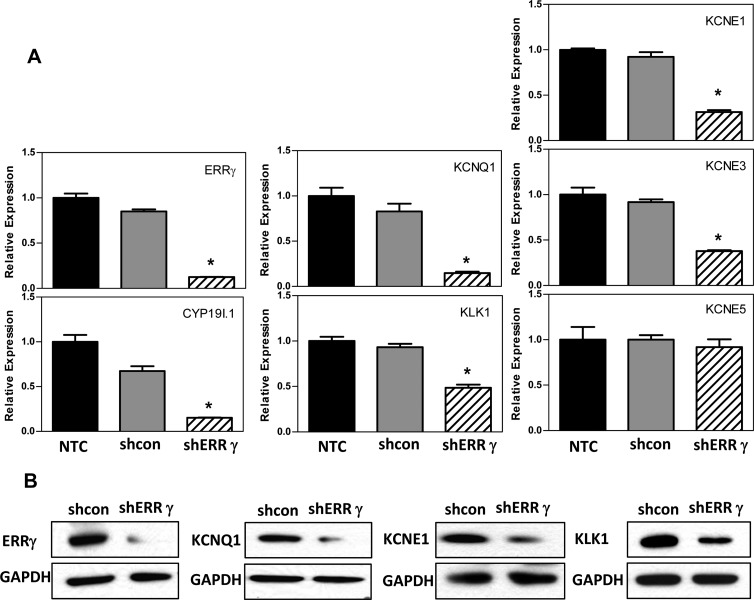
To establish a functional role of endogenous ERRγ in expression of selected K+ channel genes and KLK1 in human syncytiotrophoblast, we used an RNA interference approach. Freshly isolated cytotrophoblasts from human placenta were infected with 2 different lentiviral constructs carrying ERRγ-targeting shRNA or a control, nonsilencing shRNA at a multiplicity of infection of 0.5 for each. Nontreated cells served as another negative control. After 72 hours of culture, there was a pronounced knockdown of ERRγ mRNA (Figure 2A) and protein (Figure 2B) levels in cells infected with lentiviruses expressing ERRγ shRNA. This was associated with a corresponding marked decrease in mRNA encoding KCNQ1, KCNE1, KCNE3, KLK1, and CYP19I.1, whereas KCNE5 mRNA expression did not change (Figure 2A). Western blot analysis also revealed a comparable decrease in KCNQ1, KCNE1, and KLK1 protein expression in trophoblast cells infected with ERRγ shRNA (Figure 2B). Thus, KCNQ1, KCNE1, KCNE3, and KLK1 appear to be ERRγ target genes that are similarly inhibited by hypoxia.
Figure 2.
Knockdown of endogenous ERRγ inhibits KCNQ1, KCNE1, KCNE3, and KLK1 expression in human placental cells. A, Freshly isolated human cytotrophoblasts were infected with lentiviral vectors carrying shRNA targeting ERRγ (shERRγ) or control shRNA (shcon). Nontreated cells (NTC) served as another negative control. RNA was isolated 72 hours later, and expression of ERRγ, CYP19I.1, K+ channel α-subunits (KCNQ1) and β-subunits (KCNE1, KCNE3, and KCNE5) and KLK1 mRNA was analyzed by qRT-PCR; RPLP0 was used as the internal reference. Data are the mean ± SEM of values from 3 independent experiments, each conducted in triplicate, and are expressed relative to expression levels in nontreated cells. *, Significantly different (P < .05) from values of cells transduced with control shRNA. B, Total proteins extracted from cells infected with lentiviral vectors carrying shRNA targeting ERRγ (shERRγ) or control shRNA (shcon) for 72 hours were analyzed by immunoblotting using antisera to ERRγ, KCNQ1, KCNE1, and KLK1 or GAPDH, as loading control.
ERRγ directly regulates KCNE1 and KLK1 promoter activity
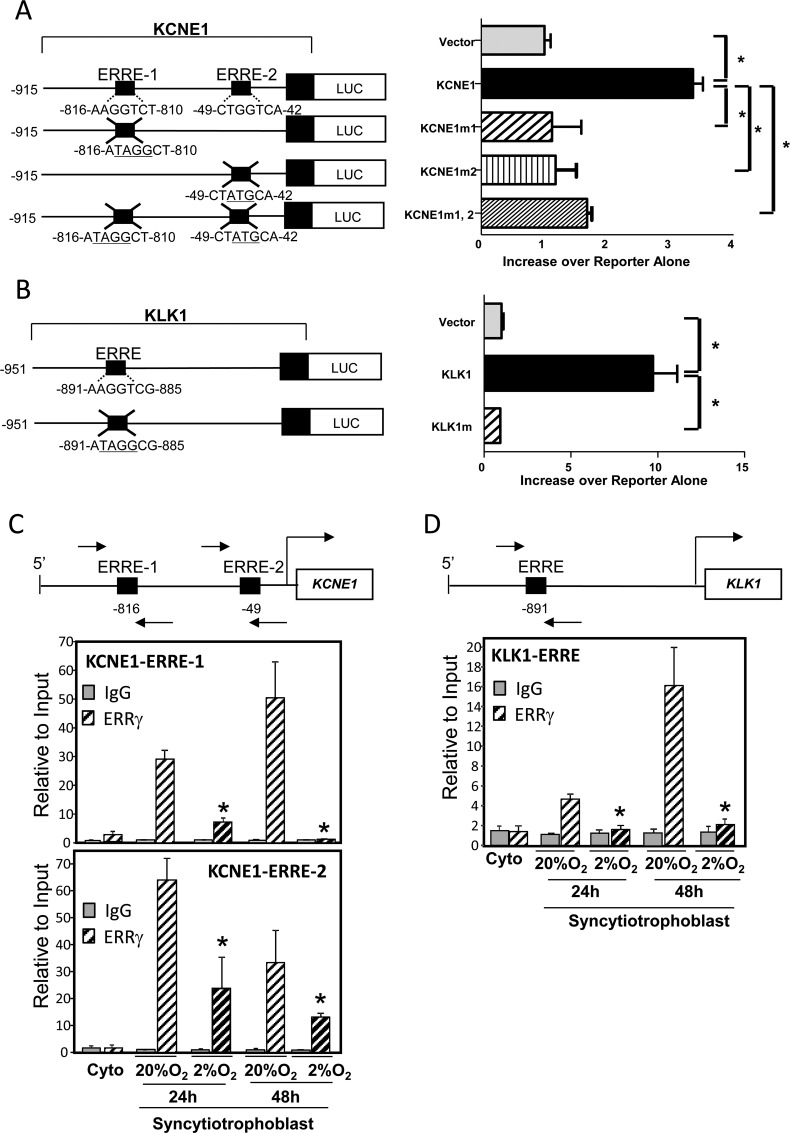
To determine whether KCNE1 and KLK1 are direct targets of ERRγ and to identify the genomic sequences that mediate its inductive effects, we examined the promoter regions of the KCNE1 and KLK1 genes for potential ERREs using the Genomatix MatInspector program. Two putative ERREs were identified at −816 and −49 bp upstream of the KCNE1 first exon, and 1 ERRE at −891 bp upstream of the KLK1 first exon. These genomic regions were cloned upstream of a luciferase reporter plasmid to determine the effects of ERRγ on KCNE1 and KLK1 promoter activity. COS-7 cells were transiently cotransfected with KCNE1 or KLK1-luciferase (Luc) reporter plasmids with or without mutations in the putative ERREs (KCNE1m1, KCNE1m2, KCNE1m1,2, or KLK1m), together with an ERRγ expression plasmid or empty control plasmid. Transcriptional activities of both KCNE1 and KLK1 promoters were significantly induced by cotransfection of the ERRγ expression vector (Figure 3, A and B). Mutation of either or both ERREs upstream of the KCNE1 first exon reduced ERRγ-stimulated KCNE1-Luc expression to levels similar to those of cells cotransfected with the empty expression vector, suggesting their cooperative interaction. Mutation of the putative ERRE at −891 bp upstream of KLK1 first exon also completely blocked ERRγ-stimulated KLK1 promoter activity. These findings suggest that ERRγ upregulates KCNE1 and KLK1 gene transcription through ERREs in their promoter regions.
Figure 3.
ERRγ directly upregulates KCNE1 and KLK1 promoter activity. A and B, COS-7 cells were transiently cotransfected with KCNE1-luciferase (A) and KLK1-luciferase (B) reporter plasmids, with or without mutation of putative ERREs (KCNE1m1, KCNE1m2, KCNE1m1,2, or KLK1m), along with either an ERRγ expression plasmid or empty control plasmid. All cells were cotransfected with β-gal expression plasmid as a control for transfection efficiency. The cells were lysed 48 hours after transfection and assayed for luciferase and β-gal activities. Data are the mean ± SEM of normalized values from 3 independent experiments, each conducted in triplicate, and are expressed as fold induction of relative luciferase activity upon ERRγ cotransfection over reporter alone. *, Significantly different (P < .05) from wild-type KCNE1-luc or KLK1-luc. C and D, Freshly isolated human placental cytotrophoblasts (0 hours) were cultured in 20% O2 or 2% O2 for 24 or 48 hours. The freshly isolated and cultured trophoblasts were treated with 1% formaldehyde and subjected to ChIP analysis using ERRγ IgG (ERRγ) or nonimmune IgG, as control. The immunoprecipitated ERRγ complexes bound to a 154-bp region (−914 to −761 bp) surrounding the KCNE1 ERRE-1 site, an 88-bp region (−107 to −20 bp) surrounding the KCNE1 ERRE-2 site (indicated by the arrows) (C) or a 107-bp region (−950 to −844 bp, indicated by the arrows) surrounding the KLK1 ERRE site (D) were quantified by qPCR using specific primers. The ChIP data were normalized to input control data and expressed relative to binding at the 0-hour time point. The data shown are the mean ± SEM of values from 3 independent experiments. Complexes immunoprecipitated with nonspecific IgG in the same experiment were quantified as a negative control. *, Significantly different (P < .05) from values of cells cultured in 20% O2.
Binding of endogenous ERRγ to ERREs upstream of KCNE1 and KLK1 genes increased during human trophoblast differentiation and was repressed by hypoxia
To determine whether endogenous ERRγ binds to these putative ERREs and is altered by hypoxia, ChIP assays were performed using freshly isolated human cytotrophoblast cells (0 hours) or cells cultured for 24 or 48 hours in 2% vs 20% O2. Recruitment of endogenous ERRγ to ERRE-1 and ERRE-2 of KCNE1 and to the ERRE upstream of KLK1 increased between 0, 24, and 48 hours of culture in 20% O2 (Figure 3, C and D). Notably, ERRγ recruitment was significantly reduced in trophoblasts cultured in hypoxia both at 24 and 48 hours (Figure 3, C and D). This hypoxia-mediated decline in ERRγ recruitment was associated with an inhibitory effect of hypoxia on ERRγ expression levels (Figure 1, A and B).
ERRγ regulates genes important for human trophoblast development and function
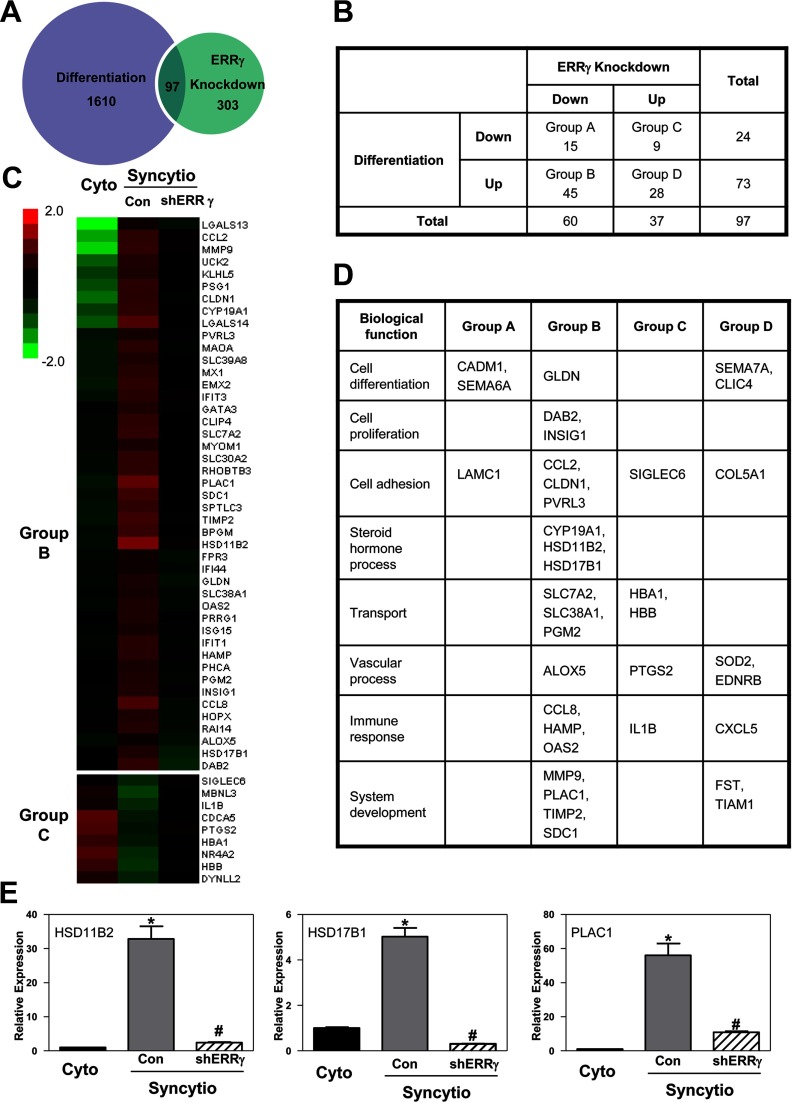
To identify genes regulated by ERRγ in human trophoblast cells, we used Illumina whole-genome gene expression arrays to compare gene expression profiles of freshly isolated cytotrophoblasts, before and after 72 hours of culture with or without ERRγ knockdown. Expression analysis was performed using GeneSpring version 12.1 and an online data analysis suite (WebGestalt) (17). In syncytiotrophoblast infected with nonsilencing shRNA, expression of 1610 genes was found to be altered more than 2-fold compared with cytotrophoblasts. When gene expression profiles were compared in cultured trophoblasts infected with ERRγ shRNA vs control nonsilencing shRNA, 303 genes were found to be altered more than 2-fold by ERRγ knockdown. We observed that 97 genes were differentially regulated both during syncytiotrophoblast differentiation and by ERRγ knockdown (Figure 4A). Among these, 15 genes were downregulated during differentiation and downregulated by ERRγ knockdown (group A); 45 genes were upregulated during differentiation and downregulated by ERRγ knockdown (group B); 9 genes were downregulated during differentiation and upregulated by ERRγ knockdown (group C); and 28 genes were upregulated during differentiation and by ERRγ knockdown (group D) (Figure 4B). Notably, we did not observe K+ channel and kallikrein transcripts in the array, which may be due to relatively low levels of expression. Genes in groups B and C are shown in the heat map (Figure 4C), whereas genes in all 4 groups were assembled into various categories of cell regulation and function in Figure 4D. As expected, CYP19A1 was upregulated during differentiation and downregulated with ERRγ knockdown. Notably, genes encoding 2 other steroidogenic enzymes, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2, encoded by HSD11B2), which metabolizes cortisol to cortisone to protect the fetus from excessive levels of glucocorticoids, and 17β-HSD1 (encoded by HSD17B1), which metabolizes estrone to estradiol-17β, were present in group B. Another gene found to be markedly upregulated during differentiation and downregulated by ERRγ knockdown was PLAC1, a paternally imprinted, X-linked gene found to be essential for normal placental and embryonic development (18). Gene expression levels of HSD11B2, HSD17B1, and PLAC1 were validated by qRT-PCR (Figure 4E).
Figure 4.
Genomic analysis of ERRγ function. Freshly isolated human cytotrophoblasts were infected with a lentiviral vector carrying shRNA targeting ERRγ (shERRγ) or control shRNA (Con). RNA was isolated from cells either before (Cyto) or 72 hours after culture (Syncytio) from 3 independent experiments, and genome-wide analysis of gene expression was performed using Illumina whole-genome gene expression arrays to uncover processes that are regulated by ERRγ during trophoblast differentiation. A, Expression analysis detected altered expression of 1610 genes before and after syncytiotrophoblast differentiation (Cyto vs Con) (differentiation group). Altered expression of 303 genes was observed when we compared ERRγ knockdown v. control (shERRγ vs Con) (ERRγ knockdown group). There were 97 genes differentially regulated both by differentiation and ERRγ knockdown. B, These 97 genes were divided into 4 groups according to their expression during differentiation and ERRγ knockdown. C, Heat map of differentially expressed genes upregulated during differentiation and downregulated by ERRγ knockdown (group B) or downregulated during differentiation and upregulated by ERRγ knockdown (group C). The heat map shows in green the genes that are downregulated and in red the genes that are upregulated. D, Biological functions of differentially expressed genes. E, Gene expression of HSD11B2, HSD17B1, and PLAC1 was validated by qRT-PCR; RPLP0 was used as the internal reference. Data are the mean ± SEM of values from 3 independent experiments, each conducted in triplicate, and are expressed relative to expression levels in cytotrophoblasts before culture. *, Significantly different (P < .05) from values of cytotrophoblasts before culture; #, significantly different (P < .05) from values of cells transduced with control shRNA.
Voltage-gated K+ channel blockers inhibit human trophoblast differentiation
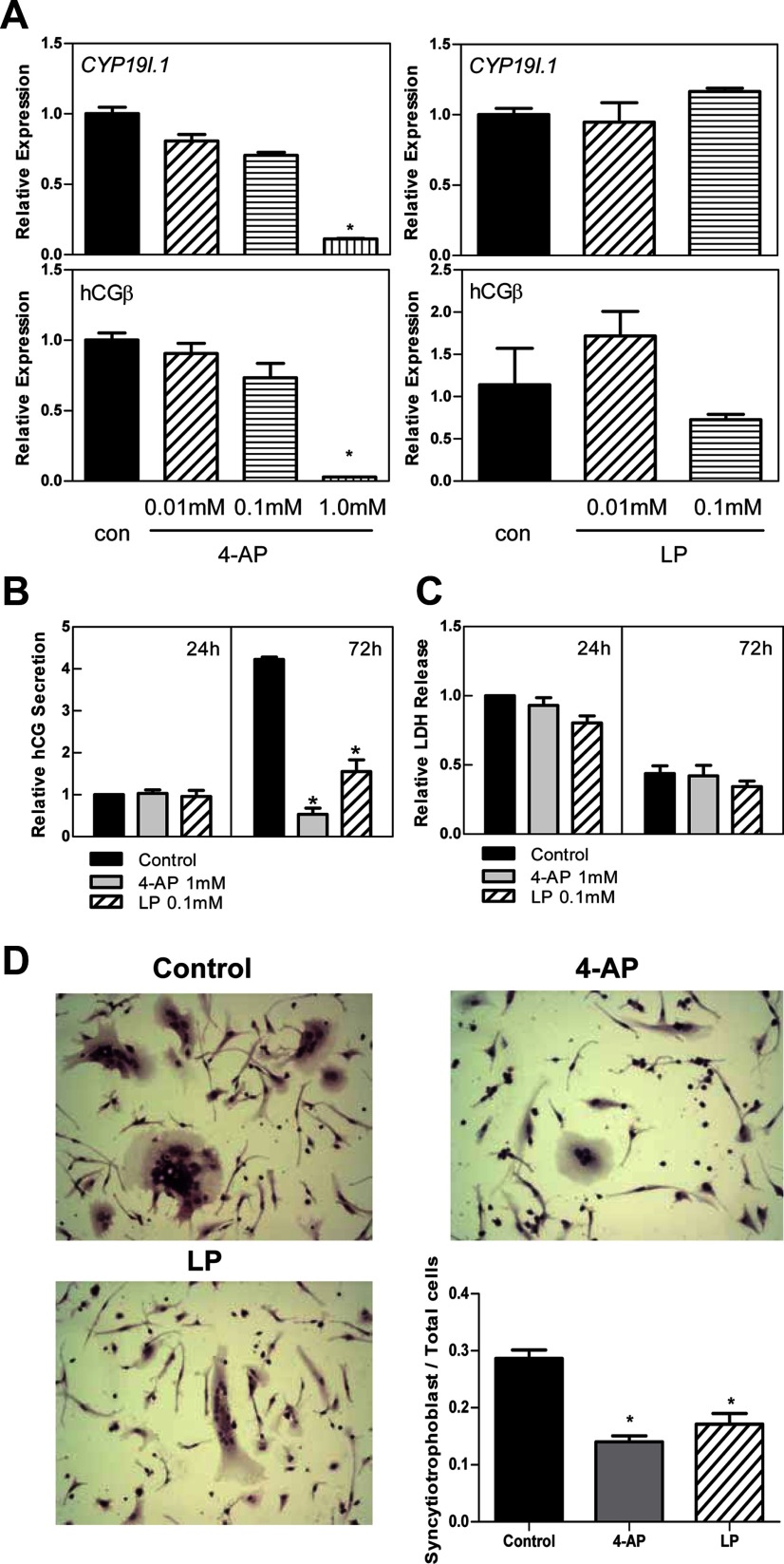
In addition to their roles in ion transport and vascular reactivity, KV7 channels have been implicated in cell proliferation and differentiation (19). To assess the role of KV7 channels in human trophoblast differentiation, freshly isolated cytotrophoblasts were treated with 2 different K+ channel blockers: 4-AP, which blocks all voltage-gated K+ channels, and LP, which specifically blocks KV7 (KCNQ) channels with ∼20-fold selectivity as compared with other K+ channels (20, 21). Treatment with 0.01mM and 0.1mM 4-AP did not alter mRNA expression of hCYP19I.1 and hCG, markers of syncytiotrophoblast differentiation, compared with control cells at 72 hours (Figure 5A); however, treatment with 1mM 4-AP reduced mRNA expression of CYP19I.1 and hCG (Figure 5A) and secretion of hCG into the culture medium (Figure 5B) and inhibited fusion of the cells to form multinucleated syncytiotrophoblast (Figure 5D). Treatment with 0.01mM and 0.1mM LP did not alter hCG mRNA expression (Figure 5A) but significantly inhibited hCG secretion and aggregation of cultured trophoblasts, a precursor of syncytia formation (Figure 5, B and D). These findings suggest that KV7 channels may serve an important role in syncytiotrophoblast differentiation. LDH, which is released from dying cells and is used as a marker of cell viability, was not altered by treatment with 1mM 4-AP or 0.1mM LP compared with vehicle-treated controls (Figure 5C). Thus, inhibitory effects of these K+ channel blockers on hCG secretion and syncytiotrophoblast differentiation are not likely due to effects on cell viability.
Figure 5.
Voltage-gated K+ channel blockers inhibit differentiation of human trophoblast cells. A, Freshly isolated human placental cytotrophoblasts were treated with voltage-gated K+ channel blocker 4-AP (0.01 mM, 0.1 mM, or 1 mM), KV7 channel blocker LP (0.01 mM or 0.1 mM), or ethanol (con) for 72 hours. RNA was isolated from cells after treatment, and the expression of CYP19I.1 and hCGβ mRNA was analyzed by qRT-PCR; RPLP0 was used as the internal reference. *, Significantly different (P < .05) from values of cells treated with ethanol. B and C, Culture medium was collected 24 and 72 hours after the initiation of treatment with 4-AP (1 mM), LP (0.1 mM), or ethanol (control), and assayed for secreted hCG using ELISA (B) or LDH release using a cytotoxicity detection kit (C). Data are the mean ± SEM of values from 3 independent experiments, each conducted in duplicate, and are expressed relative to the 24-hour control. *, Significantly different (P < .05) from control. D, After 72 hours of treatment with 4-AP (1 mM), LP (0.1 mM), or ethanol (control), the cells were stained with hematoxylin and eosin and viewed by light microscopy (×200). ImageJ was used to quantify the percentage of clustered cells relative to total cell number in 8 different fields from 2 independent experiments performed in duplicate. *, Significantly (P < .05) decreased compared with control.
Discussion
During human placental development, mononuclear cytotrophoblasts fuse to form a specialized multinucleated cell layer, the syncytiotrophoblast, which comprises the outer layer of the placental villi and performs functions essential for fetal growth and development, including nutrient transport, gas exchange, and hormone synthesis (22). Previously, we observed that ERRγ is upregulated during syncytiotrophoblast differentiation and acts as a novel O2-responsive transcription factor that contributes to the induction of aromatase/hCYP19 expression (9). ERRγ is selectively expressed in metabolically active and highly vascularized tissues such as heart, kidney, skeletal muscles, and placenta (7, 23, 24) and is known to play a regulatory role in oxidative metabolism and mitochondrial biogenesis (25–30).
In the present study, we found that specific members of the voltage-gated K+ (KV7) channel subunit KCNQ and KCNE families were markedly induced during human trophoblast differentiation and inhibited by hypoxia and by knockdown of endogenous ERRγ. Moreover, endogenous ERRγ binding to the KCNE1 promoter was significantly induced during differentiation of human trophoblasts in culture and inhibited by hypoxia. In cell transfection studies using KCNE1-luciferase vectors, we observed that KCNE1 promoter activity was upregulated by ERRγ cotransfection, suggesting that ERRγ causes transcriptional activation of K+ subunit genes. Mutation of either ERRE-1 or ERRE-2 in the KCNE1 promoter blocked ERRγ induction of KCNE1 expression, suggesting that the 2 ERREs may act cooperatively. The present findings clearly demonstrate a role for ERRγ in the regulation of potassium homeostasis in the human placenta.
KV7 channels (encoded by KCNQ1–5) mediate outwardly rectifying, voltage-dependent K+ currents, which influence membrane potential and affect vascularization by modulating secretion of angiogenic and antiangiogenic factors (31). The functional properties of a number of K+ channels have previously been reported to be regulated by O2 tension; hypoxia was observed to inhibit KV channel activity in the human placental vasculature, resulting in vasoconstriction (32–34). Combined with the present findings, we propose that, K+ channel control of placental perfusion and oxygenation may regulate placental vascular tone as well as secretion of placental hormones that influence development of the placental vasculature. Thus, placental hypoxia may contribute to the pathogenesis of pre-eclampsia and IUGR through impairment of kallikrein production and K+ channel function (5). The findings presented herein suggest that hypoxia-elicited inhibition of a number of the KV7 channel subunits and of KLK1 may be mediated, in part, by decreased expression of ERRγ.
KV7 channels regulate hormone secretion, vesicle cycling, and cell excitability. KCNQ1 channels, expressed in secretory epithelia, such as the distal colon and airways, participate in secretion of salt and water (35). It was previously observed that 4-AP– and tetraethylammonium-sensitive K+ channels (ie, voltage-gated and Ca2+-activated, respectively) regulate hCG secretion by human trophoblast cells in culture (36). Inhibition of KV channels caused membrane depolarization resulting in inhibition of Ca2+ influx and of hCG secretion (37–39). LP has ∼20-fold selectivity for KV7 channels as compared with other K+ channels (21). In the present study, specific inhibition of KV7 channels using LP inhibited hCG secretion as well as aggregation and possible syncytia formation by the cultured trophoblast cells. The hCG produced by terminally differentiated syncytiotrophoblast has been suggested to exert an autocrine/paracrine feedback effect on cytotrophoblast cells to regulate their differentiation and fusion (40). Therefore, ERRγ may promote syncytiotrophoblast formation, in part, by upregulating expression of KV7 channels, resulting in increased hCG expression and secretion.
In this study, we also observed that tissue kallikrein, KLK1, was induced during human trophoblast differentiation and inhibited by hypoxia. As mentioned above, KLK1 processes low–molecular-weight kininogen to produce vasoactive kinins (kallidin and bradykinin) (14), which are further processed and bind to bradykinin receptors to stimulate the production of factors that promote vasodilation (15). In addition to its role in vasodilation, the kallikrein-kinin system has been suggested to regulate angiogenesis (41). KLK1-knockout mice had impaired muscle neovascularization in response to hind limb ischemia, whereas injection of adenovirus expressing KLK1 was able to rescue this defect (42). Alterations in the kallikrein-kinin system are associated with pre-eclampsia, a condition of impaired angiogenesis (43–46). Kininogen levels were found to be significantly lower in chorion laeve, placental plate chorion, and placentas of women with pregnancy-induced hypertension when compared with tissues from normotensive pregnant women (43). Reduced urinary kallikrein levels were also observed in hypertensive and IUGR pregnancies (44, 45). Moreover, at 16 weeks gestation, the ratio of kallikrein to creatinine is one of the best predictors of pre-eclampsia (46). In the present study, expression levels of both KLK1 and ERRγ were repressed by hypoxia in cultured human trophoblasts; endogenous ERRγ binding to the KLK1 promoter was significantly reduced in trophoblasts cultured in a hypoxic environment. Furthermore, ERRγ knockdown and cotransfection studies of wild-type and mutant KLK-Luc reporter constructs with ERRγ expression vectors indicated that ERRγ directly regulates KLK1 gene expression. Thus, ERRγ, through regulation of KLK1 expression, may be a critical regulator of placental vascular tone.
Gene expression arrays of cultured human trophoblast cells with or without ERRγ knockdown revealed that ERRγ also regulates a number of important placental genes, including those that affect cell differentiation, proliferation, adhesion, ion and nutrient exchange, and the synthesis of steroid and peptide hormones required for fetal growth and development. Notably, expression of the genes encoding 11β-HSD2 (HSD11B2), which metabolizes cortisol to cortisone, and 17β-HSD1 (HSD17B1), which metabolizes estrone to estradiol, were induced during trophoblast differentiation and inhibited by ERRγ knockdown. HSD11B2 has been reported to be upregulated during human syncytiotrophoblast differentiation and to be downregulated by hypoxia (47, 48). Importantly, decreased expression of HSD11B2 was observed in placentas of women with pre-eclampsia, as compared with gestation-matched normotensive controls (48). 11β-HSD2 is postulated to protect the developing fetus and placenta from potentially hypertensive effects of elevated maternal glucocorticoids. The findings that placental expression of HSD17B1 was significantly decreased in placentas of pre-eclamptic women as compared with controls (49) and that reduced plasma levels of 17β-HSD1 were an independent risk factor for pre-eclampsia (50) suggests an important role of estradiol in the placental vasculature. Furthermore, as mentioned, estradiol-17β, acting through estrogen receptor-α, mediates a positive feedback action on human syncytiotrophoblast differentiation (51).
In conclusion, our findings suggest that the O2-dependent nuclear receptor, ERRγ, serves a critical role in placental K+ homeostasis and vascular health through regulation of genes encoding KV7 channel subunits and KLK1. K+ channels also may positively impact trophoblast differentiation through stimulatory effects on aromatase and hCG expression (Figure 6). Importantly, our data also suggest that disruption of these novel regulatory functions of ERRγ may alter expression of several key genes for placental development and steroid hormone metabolism and, consequently, contribute to the pathogenesis of pre-eclampsia and IUGR.
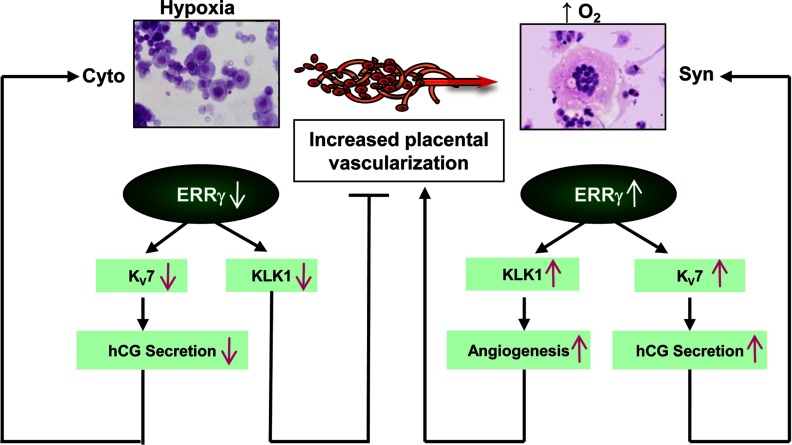
Figure 6.
Proposed mechanisms for ERRγ regulation of K+ channels and KLK1 during human trophoblast differentiation. In cytotrophoblasts and in cultured trophoblasts maintained in a hypoxic environment, decreased ERRγ levels prevent expression of selected KV7 channels and KLK1. This prevents dilation of the vascular bed and inhibits vascularization, causing further hypoxia. The inhibition of KV7 expression results in decreased hCG secretion, which suppresses trophoblast differentiation. On the other hand, increased placental vascularization and increased oxygen tension after 8–10 wk gestation promotes increased ERRγ expression, which enhances KLK1 expression, resulting in vasodilation and increased angiogenesis, further increasing O2 tension and ERRγ expression. This, in turn, enhances expression of selected KV7 channels resulting in increased hCG expression, which acts to further promote syncytiotrophoblast differentiation.
Acknowledgments
We thank Ms Jo Smith, University of Texas Southwestern Medical Center, for her expert assistance in preparing the primary human trophoblast cultures for these studies. We also thank the postdoctoral trainee exchange program between University of Texas Southwestern Medical Center and the First Affiliated Hospital of Sun Yat-sen University for providing Dr Yanmin Luo the opportunity to perform this research at University of Texas Southwestern. We are grateful to Dr Charles R. Rosenfeld (Department of Pediatrics, University of Texas Southwestern) for insightful discussions regarding this work.
This work was supported by National Institutes of Health Grant 5 R01 DK031206 (to C.R.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 4-AP
- 4-aminopyridine
- ChIP
- chromatin immunoprecipitation
- ERRγ
- estrogen-related receptor γ
- ERRE
- ERRγ response element
- FBS
- fetal bovine serum
- β-gal
- β-galactosidase
- hCG
- human chorionic gonadotropin
- hCYP19I.1
- human CYP19 placenta-specific transcript
- 11β-HSD2
- 11β-hydroxysteroid dehydrogenase type 2
- IUGR
- intrauterine growth restriction
- KCNE
- potassium voltage-gated channel subfamily
- KCNQ
- potassium voltage-gated channel, KQT-like
- LDH
- lactate dehydrogenase
- LP
- linopirdine
- qRT-PCR
- quantitative RT-PCR
- shRNA
- short hairpin RNA.
References
- 1. Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Red-Horse K, Zhou Y, Genbacev O, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laresgoiti-Servitje E, Gomez-Lopez N. The pathophysiology of preeclampsia involves altered levels of angiogenic factors promoted by hypoxia and autoantibody-mediated mechanisms. Biol Reprod. 2012;87:36. [DOI] [PubMed] [Google Scholar]
- 4. Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest. 2007;64:187–192 [DOI] [PubMed] [Google Scholar]
- 5. Wareing M, Greenwood SL. Potassium channels in the human fetoplacental vasculature. Placenta. 2011;32(Suppl 2):S203–S206 [DOI] [PubMed] [Google Scholar]
- 6. Giguère V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94 [DOI] [PubMed] [Google Scholar]
- 7. Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696 [DOI] [PubMed] [Google Scholar]
- 8. Takeda Y, Liu X, Sumiyoshi M, Matsushima A, Shimohigashi M, Shimohigashi Y. Placenta expressing the greatest quantity of bisphenol A receptor ERRγ among the human reproductive tissues: predominant expression of type-1 ERRγ isoform. J Biochem. 2009;146:113–122 [DOI] [PubMed] [Google Scholar]
- 9. Kumar P, Mendelson CR. Estrogen-related receptor γ (ERRγ) mediates oxygen-dependent induction of aromatase (CYP19) gene expression during human trophoblast differentiation. Mol Endocrinol. 2011;25:1513–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alaynick WA, Way JM, Wilson SA, et al. ERRγ regulates cardiac, gastric, and renal potassium homeostasis. Mol Endocrinol. 2010;24:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80 [DOI] [PubMed] [Google Scholar]
- 12. Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda). 2005;20:408–416 [DOI] [PubMed] [Google Scholar]
- 13. Mistry HD, McCallum LA, Kurlak LO, et al. Novel expression and regulation of voltage-dependent potassium channels in placentas from women with preeclampsia. Hypertension. 2011;58:497–504 [DOI] [PubMed] [Google Scholar]
- 14. Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol. 2009;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355 [DOI] [PubMed] [Google Scholar]
- 16. Valdés G, Chacón C, Corthorn J, Figueroa CD, Germain AM. Tissue kallikrein in human placenta in early and late gestation. Endocrine. 2001;14:197–204 [DOI] [PubMed] [Google Scholar]
- 17. Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackman SM, Kong X, Fant ME. Plac1 (placenta-specific 1) is essential for normal placental and embryonic development. Mol Reprod Dev. 2012;79:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roura-Ferrer M, Solé L, Martínez-Mármol R, Villalonga N, Felipe A. Skeletal muscle Kv7 (KCNQ) channels in myoblast differentiation and proliferation. Biochem Biophys Res Commun. 2008;369:1094–1097 [DOI] [PubMed] [Google Scholar]
- 20. Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997;9:605–616 [DOI] [PubMed] [Google Scholar]
- 22. Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392 [DOI] [PubMed] [Google Scholar]
- 24. Narkar VA, Fan W, Downes M, et al. Exercise and PGC-1α-independent synchronization of type I muscle metabolism and vasculature by ERRγ. Cell Metab. 2011;13:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Ma K, Sadana P, et al. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906 [DOI] [PubMed] [Google Scholar]
- 26. Kim DK, Ryu D, Koh M, et al. Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. J Biol Chem. 2012;287:21628–21639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alaynick WA, Kondo RP, Xie W, et al. ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24 [DOI] [PubMed] [Google Scholar]
- 28. Dufour CR, Wilson BJ, Huss JM, et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab. 2007;5:345–356 [DOI] [PubMed] [Google Scholar]
- 29. Poidatz D, Dos Santos E, Brulé A, De Mazancourt P, Dieudonné MN. Estrogen-related receptor γ modulates energy metabolism target genes in human trophoblast. Placenta. 2012;33:688–695 [DOI] [PubMed] [Google Scholar]
- 30. Kamarajugadda S, Stemboroski L, Cai Q, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong XZ, Harhun MI, Olesen SP, et al. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588:3277–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corcoran J, Lacey H, Baker PN, Wareing M. Altered potassium channel expression in the human placental vasculature of pregnancies complicated by fetal growth restriction. Hypertens Pregnancy. 2008;27:75–86 [DOI] [PubMed] [Google Scholar]
- 33. Kiernan MF, Barrie A, Szkolar J, Mills TA, Wareing M. Functional evidence for oxygen-sensitive voltage-gated potassium channels in human placental vasculature. Placenta. 2010;31:553–555 [DOI] [PubMed] [Google Scholar]
- 34. Hampl V, Bíbová J, Stranák Z, et al. Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am J Physiol Heart Circ Physiol. 2002;283:H2440–2449 [DOI] [PubMed] [Google Scholar]
- 35. Grunnet M, Jespersen T, MacAulay N, et al. KCNQ1 channels sense small changes in cell volume. J Physiol. 2003;549:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams JL, Fyfe GK, Sibley CP, Baker PN, Greenwood SL. K+ channel inhibition modulates the biochemical and morphological differentiation of human placental cytotrophoblast cells in vitro. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1204–R1213 [DOI] [PubMed] [Google Scholar]
- 37. Meuris S, Polliotti B, Robyn C, Lebrun P. Ca2+ entry through L-type voltage-sensitive Ca2+ channels stimulates the release of human chorionic gonadotrophin and placental lactogen by placental explants. Biochim Biophys Acta. 1994;1220:101–106 [DOI] [PubMed] [Google Scholar]
- 38. Sharma SC, Rao AJ. Effect of calcium ion channel antagonists on chorionic gonadotropin secretion. Biochem Mol Biol Int. 1997;43:1101–1106 [DOI] [PubMed] [Google Scholar]
- 39. Polliotti B, Lebrun P, Robyn C, Meuris S. The release of human chorionic gonadotrophin and placental lactogen by placental explants can be stimulated by Ca2+ entry through a Na(+)-Ca2+ exchange process. Placenta. 1994;15:477–485 [DOI] [PubMed] [Google Scholar]
- 40. Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387–1395 [DOI] [PubMed] [Google Scholar]
- 41. Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38 [DOI] [PubMed] [Google Scholar]
- 42. Stone OA, Richer C, Emanueli C, et al. Critical role of tissue kallikrein in vessel formation and maturation: implications for therapeutic revascularization. Arterioscler Thromb Vasc Biol. 2009;29:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohamed M, Larmie ET, Singh HJ, Othman MS. Tissue kallikrein and kininogen levels in fetoplacental tissues from normotensive pregnant women and women with pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol. 2007;134:15–19 [DOI] [PubMed] [Google Scholar]
- 44. Elebute OA, Mills IH. Urinary kallikrein in normal and hypertensive pregnancies. Perspect Nephrol Hypertens. 1976;5:329–338 [PubMed] [Google Scholar]
- 45. Salas SP, Rosso P, Espinoza R, Robert JA, Valdés G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol. 1993;81:1029–1033 [PubMed] [Google Scholar]
- 46. Millar JG, Campbell SK, Albano JD, Higgins BR, Clark AD. Early prediction of pre-eclampsia by measurement of kallikrein and creatinine on a random urine sample. Br J Obstet Gynaecol. 1996;103:421–426 [DOI] [PubMed] [Google Scholar]
- 47. Hardy DB, Yang K. The expression of 11β-hydroxysteroid dehydrogenase type 2 is induced during trophoblast differentiation: effects of hypoxia. J Clin Endocrinol Metab. 2002;87:3696–3701 [DOI] [PubMed] [Google Scholar]
- 48. Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. Oxygen regulation of placental 11 β-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metab. 2002;87:4797–4805 [DOI] [PubMed] [Google Scholar]
- 49. Ishibashi O, Ohkuchi A, Ali MM, et al. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59:265–273 [DOI] [PubMed] [Google Scholar]
- 50. Ohkuchi A, Ishibashi O, Hirashima C, et al. Plasma level of hydroxysteroid (17-β) dehydrogenase 1 in the second trimester is an independent risk factor for predicting preeclampsia after adjusting for the effects of mean blood pressure, bilateral notching and plasma level of soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertens Res. 2012;35:1152–1158 [DOI] [PubMed] [Google Scholar]
- 51. Kumar P, Kamat A, Mendelson CR. Estrogen receptor α (ERα) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol Endocrinol. 2009;23:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]