Abstract
Extensive research has been devoted to the goal of understanding how a single cell of embryonic origin can give rise to every somatic cell type and the germ cell lineage, a hallmark defined as “pluripotency.” The aggregate of this work supports fundamentally important roles for the gene transcription networks inherent to the pluripotent cell. Transcription networks have been identified that are both required for pluripotency, as well as sufficient to reprogram somatic cells to a naive pluripotent state. Several members of the nuclear receptor (NR) superfamily of transcription factors have been identified to play diverse roles in the regulation of pluripotency. The ligand-responsive nature of NRs coupled with the abundance of genetic models available has led to a significant advance in the understanding of NR roles in embryonic stem cell pluripotency. Furthermore, the presence of a ligand-binding domain may lead to development of small molecules for a wide range of therapeutic and research applications, even in cases of NRs that are not known to respond to physiologic ligands. Presented here is an overview of NR regulation of pluripotency with a focus on the transcriptional, proteomic, and epigenetic mechanisms by which they promote or suppress the pluripotent state.
In recent years much attention has been directed to the molecular characterization of the nature of cell pluripotency or “stemness”; however, the notion of pluripotency has long been recognized. One of the first pathologic illustrations came from patients who presented with large benign tumors called teratomas. These tumors comprise highly differentiated structures that often contain tissue of endodermal, mesodermal, and ectodermal origin, thus indicating that the early tumor comprised cells that were developmentally plastic (1). Such observations led the nineteenth century German pathologist Julius Cohnheim to state “all tumors owe their being to the persistence, in various organs and parts of the body, to small residues of embryonic tissue.” (2, 3) Although often cited today with regard to the cancer stem cell hypothesis, Cohnheim's hypothesis charges an embryonic contribution in the pathogenesis of tumors and indicates that the pluripotent nature of these tumors was recognized.
At present, pluripotency is understood to be a rather dynamic state, not limited to a specific subset of cells at a single stage in development. Indeed, multiple pluripotent cell types have been isolated and characterized since the isolation of embryonal carcinoma cells (EC cells); the first pluripotent cells characterized (4–6). These include embryonic stem cells (ES cells) (7–9), primordial germ cells (PGCs) (10, 11), Epi-stem cells (epi-SCs)(12, 13), and induced-pluripotent stem cells (iPS cells) (14, 15). The notion that pluripotency is a dynamic state has especially gained traction with the advent of iPS cell generation, in which terminally differentiated somatic cells are reverted back to a pluripotent state through the ectopic expression of defined factors (16). The reprogramming of somatic cells occurs through the epigenetic restructuring of the chromatin such that the epigenetic landscape resembles that of an ES cell (17) and, in part, recapitulates the physiologic reprogramming that occurs when terminally differentiated germ cells conjoin to give rise to a pluripotent embryo.
Although differences in these pluripotent cell types have been noted, they all owe their pluripotent nature to a reliance on a primary pluripotency axis composed of Oct4, Sox2, and Nanog (18–20). These transcription factors promote pluripotency through formation of heterodimeric or heterotrimeric complexes that directly regulate expression of genes that promote ES cell self-renewal (18). In a testament to their versatility, these factors also mediate transcriptional repression through the recruitment of the Polycomb Repressive Complex to the promoters of target genes that mediate differentiation (21, 22). Genetic ablation of any of the three factors triggers early embryonic lethality in the mouse, and expression of Oct4 and Sox2 is essential for ES cell self-renewal in vitro (23–25). Although Nanog expression is not regarded as essential for self-renewal in vitro (26), ectopic expression of Nanog permits self-renewal in the absence of the requisite growth factor: leukemia inhibitory factor (LIF) (27). Genome-wide binding studies of Oct4, Sox2, and Nanog identified an intriguing feature of the primary pluripotency axis. Among their many transcriptional targets, the three factors were also found to regulate each other's expression (18). This unique auto-regulatory loop reiterates the evolutionary importance of the signaling axis by ensuring a robust presence in the ES cell transcriptome to safeguard the pluripotent cell from what otherwise might be significant fluctuations in expression or environment.
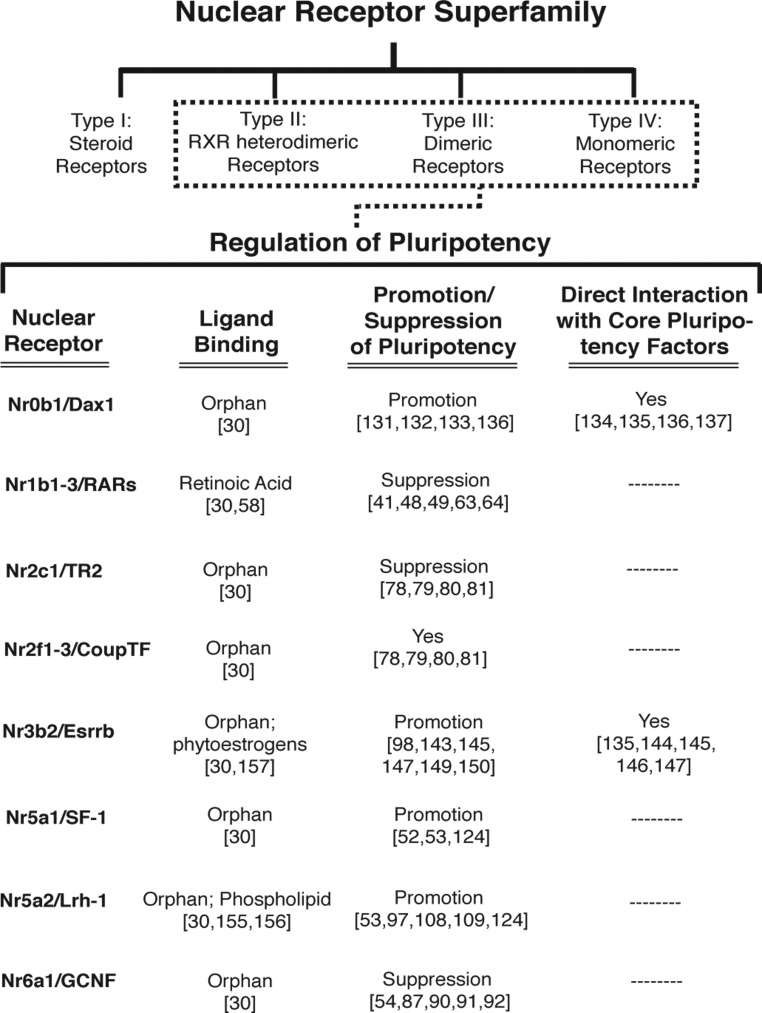
Considerable effort has been made to further identify factors that positively or negatively regulate pluripotency. In most cases, these factors do so by acting directly on the primary pluripotency axis. Detailed in this review are several factors belonging to the nuclear receptor (NR) class of transcription factors that have been implicated in the promotion or suppression of pluripotency (Figure 1). NRs and coregulators regulate myriad physiologic functions and play key pathologic roles by regulating target gene expression through site-specific chromatin remodeling (28, 29). The NR family consists of 48/49 (human/mouse) evolutionarily conserved ligand-regulated transcription factors that are genetically divided into seven groups based on homology (NR0–6), but mechanistically divided into four groups: 1) steroid receptors, 2) retinoid X receptor (RXR) heterodimers, 3) dimeric orphan receptors, and 4) monomeric orphan receptors (30, 31). Here we discuss the early findings that definitively linked NR action to the regulation of pluripotency, a presentation of the Oct4-regulatory regions as a model for NR action, individual characterizations of NRs that suppress or promote the pluripotent state, and a perspective on how NRs may be utilized in the future to benefit regenerative therapies.
Figure 1.
NR Regulation of Pluripotency. NRs comprising the RXR heterodimeric receptors, dimeric receptors, and monomeric receptor classes of the superfamily have been implicated in the regulation of pluripotency by genetic and/or cell-based assays.
Retinoid Signaling and Differentiation
The importance of NR action in the regulation of pluripotency was recognized before the isolation of the first bona fide ES cell lines, at which time pluripotency was modeled using EC cells derived from spontaneous teratomas. It was observed that some EC cell lines, among them the F9 line, were “nullipotent” insofar that conventional techniques for inducing differentiation, namely teratocarcinoma formation and in vitro embryoid body (EB) formation, were not sufficient (32, 33). The F9 EC cells were later shown to undergo differentiation, but only after retinoic acid (RA) supplementation (34). Retinoids have long been recognized as mediators of differentiation from early studies of vitamin A deficiency in rats and RA induction of embryonic endoderm in chick embryo fibroblasts (35, 36). More recent studies observed a role for RA in mediating differentiation of specific tissues in vivo and in vitro (37, 38). Although the specific NRs responsible for mediating the effects of RA were unknown at the time, RA treatment became a common and useful method to induce and, in some cases, direct differentiation in vitro (39). RA treatment of EC cells was also shown to be sufficient to suppress the expression of the pluripotency markers alkaline phosphatase and stage specific embryonic antigen-1 (39, 40). As the field transitioned from studying EC cells to ES cells during the 1980s, RA treatment remained at the forefront of in vitro differentiation. Although differences between the pluripotent cell types were observed, the capacity of RA, a NR ligand, to act as a potent inducer of differentiation was conserved in both the mouse and human ES cells (41). These pivotal studies implicated the NR class of transcription factors as fundamental regulators of pluripotency.
The Oct4 Promoter: a Model for NR Regulation
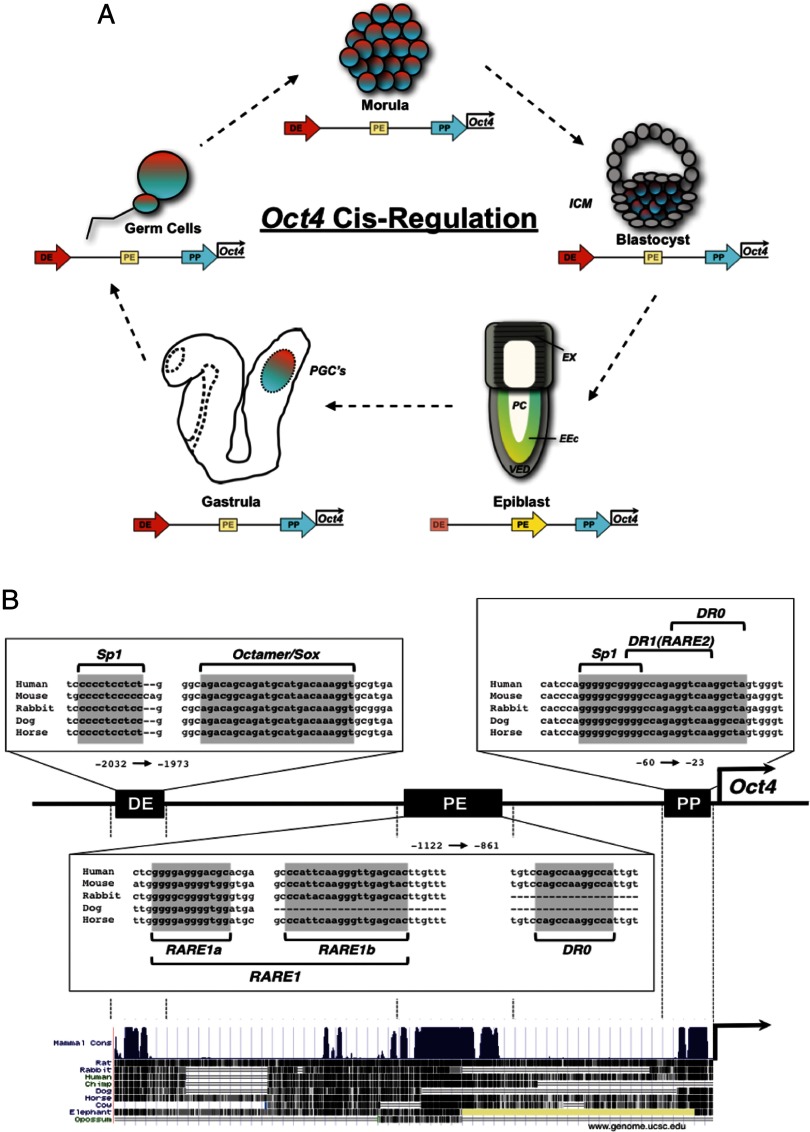
At the molecular level, NRs primarily regulate ES cell pluripotency by promoting or suppressing expression of factors that make up the primary pluripotency axis (Oct4/Sox2/Nanog). Three, evolutionarily conserved cis-regulatory regions govern Oct4 expression: the distal enhancer (DE), the proximal enhancer (PE), and the proximal promoter (PP) (42). Using transgenic reporters for each region, Yeom et al. demonstrated that although the PP is required for Oct4 expression at all stages of development, the DE and PE are differentially required for expression (Figure 2A). Specifically, until the blastocyst stage of development (E3.5), the DE is required for Oct4 expression and the PE is dispensable; however, at the late epiblast stage (E6.5) when Oct4 expression is restricted to the embryonic ectoderm, a switch occurs and the PE is required for Oct4 expression and the DE is dispensable (42). Once the embryo undergoes gastrulation, Oct4 expression then reverts back to being directed by the DE and expression is restricted to the PGCs and eventually silenced in all somatic cells (42). Importantly, these transgenic findings are largely consistent with in vitro tissue culture models. In ES cells derived from the inner-cell mass of blastocysts, Oct4 expression is maintained by the DE, whereas in epi-SCs derived from the epiblast, the PE primarily controls Oct4 expression (13).
Figure 2.
Dynamic cis and trans Regulation of Oct4 Gene[b] Expression A, cis-Regulation of the Oct4 PP, PE, and DE during embryonic development. B, The hyper-conserved Oct4-regulatory regions (PP, PE, DE) contain multiple response elements that mediate both Oct4 expression and repression upon differentiation. (ICM, inner-cell mass; Ex, extraembryonic ectoderm; EEc, embryonic ectoderm; VED, visceral endoderm; PC, proamniotic cavity; PGC, primordial germ cells).
The Oct4 DE contains no known NR response elements, but rather contains a conserved Sp1-binding site and a conserved Octamer/Sox-binding site, which is essential for auto-regulation by Oct4/Sox2 heterodimers (43, 44). In contrast, the Oct4 PE and PP are enriched with conserved binding motifs that correspond to different NRs (Figure 2B). Early studies investigating RA repression of Oct4 identified a region within the PE termed RA-repressible enhancer 1 (RARE1) for its ability to promote expression in the absence of RA, while mediating repression of Oct4 upon RA treatment (45). This region contains two response elements (RARE-1a, RARE-1b) that are important for mediating both enhancer and repressor activities. RARE-1a resembles the GC-rich Sp-1-binding motif and may mediate RAR's response through a protein-protein interaction (45). RARE-1b is unoccupied in the absence of RA, implicating it as a repressor-binding site. Later studies identified a second RA-responsive element within the Oct4 PP here named RARE2. This RARE closely resembles the classical hormone response element (HRE) binding motif (A/G)GGTCA (46, 47) and is also juxtaposed to an Sp-1 site (48–50). Analysis of these cis-elements revealed that transcription factor binding to the Sp-1 site occurs independent of binding at the RARE. As described with RARE1, RARE2 also has dual functions to promote and suppress Oct4 expression dependent on the presence of RA (48). Although not fully understood, the integration of these signals with the overlapping of NR-binding sites within the Oct4-regulatory regions is discussed below and presented in Figure 2B.
In addition to RAREs, the Oct4 PP and PE contain unique repeats of the consensus HRE. The PP exhibits three tandem repeats comprising a DR1 and a DR0 (49, 50). The DR1 element corresponds to the previously described RARE2, but is also is recognized by chicken ovalbumin upstream promoter-transcription factor (COUP-TF) during late stages of RA differentiation in P19 cells (51). The DR0 element within the PP serves as a response element for multiple NRs (52–54). Binding of the closely related receptors steroidogenic Factor-1 (SF1; Nr5a1) and liver receptor homolog (Lrh-1; Nr5a2) has been shown to promote Oct4 expression in the absence of RA (52, 53), whereas during RA differentiation the DR0 is occupied by germ cell nuclear factor (GCNF; Nr6a1) (54). Another conserved DR0 binding motif is found within the Oct4 PE. This site is bound by Lrh-1 during self-renewal and may be crucial for maintaining expression of Oct4 during the switch from the DE to the PE (53).
Ongoing unbiased approaches to investigate whole-genome binding of NRs in ES cells will surely paint a clearer picture of how the Oct4-regulatory regions are integrated and may lead to the identification of additional regulatory elements and pathways affecting Oct4 expression at different developmental stages. The enrichment of NR response elements within the PE and PP suggests an important role for NRs in regulating Oct4 expression at later stages of embryonic development. The variety of binding sites, coupled with the presence of dual-enhancer/repressor elements, positions NRs as rapid responders to induce transcriptional signaling cascades that ensure the proper restriction of Oct4 expression during the dynamic processes of embryonic development.
Suppression of Pluripotency Gene Expression by NRs
During development the pluripotent cells of the embryo endure continuous lineage restriction due to the localized specification and differentiation of cells around them (55). Within these specified regions, the primary pluripotency axis undergoes transcriptional silencing concomitant with induction of appropriate differentiation cues. Recent studies in iPS cells have detailed some effects arising from improper transcriptional silencing, which include increased tumorigenesis and defective differentiation (56, 57). These concerns are particularly relevant with regard to future therapeutic applications of pluripotent cells. Detailed below are members of the NR gene family that play an important role in suppressing pluripotency gene expression.
Retinoic Acid Receptors (RAR/Nr1b1–3)
Three separate genes code for receptors that functionally bind RA: RARα(NR1B1), RARβ (NR1B2), and RARγ (NR1B3). Mechanistically RARs function as ligand-regulated transcription factors by forming heterodimers with retinoic X receptors (RXR α,β,γ/NR2B1–3) (58). RXRα is regarded as the central receptor mediating RA signaling during embryogenesis, because it is the only subtype with an embryonic lethal phenotype. RXRα-null mice die around embryonic day 12.5 (E12.5)–E16.5 exhibiting defects consistent with fetal vitamin A deficiency (59). In addition to mediating RA repression of Oct4 as detailed above, RAR promotes neural differentiation of both EC and ES cells grown in culture (60, 61). Furthermore, RAR plays a more direct role in the specification of neuronal lineages, because exposing ES cells to RA during differentiation causes them to express hindbrain and spinal cord markers, whereas cells not exposed to RA adopt a telencephalic fate (62). This finding is consistent with RA's function as a morphogen and the patterning defects that have been reported in RAR mutant mice (58).
Although our understanding of the precise transcriptional networks governed by RAR during neural differentiation is not complete, there is evidence that RAR regulation of various micro-RNAs (miRNAs) plays a significant role in suppressing pluripotency gene expression and in promoting neural differentiation. RA differentiation of ES cells promotes induction of miR-296, miR-470, and miR-134, which together posttranscriptionally suppress Oct4, Nanog, Sox2, and Lrh-1 (63, 64). The induction of miR-134 is likely specific to neural differentiation because both RA stimulation and culture in N2B27 media (a retinoid-free system that drives neural differentiation) are sufficient to induce miR-134, which is otherwise basally expressed during EB differentiation. Furthermore, transfection of miR-134 pre-miRNAs in undifferentiated ES cells promotes differentiation toward ectodermal lineages (63). Notably, the induction of miRNAs after RA treatment occurs several days after treatment, suggesting that these effects are likely secondary or tertiary to RA stimulation.
Increased focus on genome-wide binding studies for RAR will help resolve how retinoid signaling couples suppression of pluripotency with neural differentiation. One such study has been performed using an epitope-tagged RARα/γ (65). A comparison of RAR binding in mouse (m)ES cells to mouse embryonic fibroblasts (MEFs) observed few overlapping targets between the two cell types, suggesting that RARs likely play diverse roles in different tissues. Additionally, RAR binding can correspond with either transcriptional induction or repression depending on the target. In mES cells, binding was observed at the promoters of several pluripotency factors that are repressed upon RA treatment, including Oct4 and Lin28; however, binding was also found within regulatory regions of several components of the sonic hedgehog pathway, which is involved in central nervous system patterning in vivo (66). Studies carried out in ES cells found that only a minority of the RAR binding sites resemble the classic DR1, –2, –5 consensus site, but rather the loci often contained a consensus half-site with an SP-1 or AP-2 consensus site within 300 nucleotides (65). This precise arrangement is observed within the Oct4 PP and PE (see earlier section: The Oct-4 Promoter, a Model for NR Regulation). Remarkably, most reports do not support a role for RARs in the direct binding to these regions to suppress Oct4 expression, thereby exemplifying the complexity of NR signaling through the integration of nongenomic and genomic effects (50, 67).
COUP-TF I–III/Nr2f1–3)
COUP-TFs I–III are evolutionarily conserved orphan NRs that play diverse roles in regulating embryonic development, including that of the heart, vasculature (68), limbs, skeletal muscle (69), and central nervous system (70). In adults COUP-TF-II is an important regulator of metabolism through its effects on insulin expression and suppression of adipocyte differentiation (71, 72).
COUP-TFs were initially implicated in mediating RA repression of Oct4 in P19 EC cells largely by in vitro assays. Transfection of COUP-TFs is sufficient to suppress Oct4 in reporter assays, and EMSAs confirmed COUP-TF binding at the DR1 response element within the Oct4 PP (51). COUP-TF was later reported to bind the DR1 element with greater affinity than RAR, leading investigators to present a model in which COUP-TF and RAR exist in a balance to oversee Oct4 expression (52). However, the evidence for COUP-TF regulation of pluripotency in ES cells has not been definitively demonstrated, and several lines of evidence suggest its role in EC cells may be an artifact of that model system. For example, none of the COUP-TFs are appreciably induced in ES cells before Oct4 repression, and none of the genetic models support an overt role for COUP-TF in suppressing pluripotency during embryonic development (68–70). COUP-TF expression is primarily induced after gastrulation, long after Oct4 expression is restricted to the PGCs (73). However, it is possible that COUP-TF regulation of Oct4 may be physiologically relevant to other biologic processes, such as neuronal specification. Similar to the role ascribed for GCNF in neural stem cell regulation, the suppression of COUP-TFI/II impairs the temporal specification of neural stem/progenitor cells leading to a loss of gliogenic competence (74).
Testicular Orphan Receptor-2 (TR2/Nr2c1)
The TR2 orphan NR is most abundantly expressed in androgen-dependent tissues such as the testes and prostate, but is also reported in the developing embryo, germ cells, and EC cells (75, 76). Mechanistically TR2 functions as a homodimer and has primarily been implicated in promoting transcriptional repression of target genes (77, 78).
Studies in P19 cells have identified an interesting mechanism in which posttranslational modification of TR2, namely SUMOylation, underlies the regulation of Oct4 expression (79). In the un-SUMOylated state, TR2 drives Oct4 expression through the recruitment of the coactivator Pcaf, whereas SUMOylated TR2 recruits the Rip140 corepressor to the Oct4 PP to mediate transcriptional repression. When TR2 is abundant in the cell, the receptor is recruited to promyelocytic leukemia (Pml) nuclear bodies where it undergoes SUMOylation directed by ERK2 phosphorylation of the receptor (80). Furthermore, ERK2 kinase activity is reportedly induced in P19 cells by the nongenomic activity of RA, because pretreatment with a RAR panantagonist before RA treatment does not inhibit SUMOylation of TR2 (80).
Although the previous studies present an intriguing mechanism in the regulation of Oct4 expression in P19 cells, the relevance of this pathway in ES cells or iPS cells remains to be determined. Indeed, genetic ablation of TR2 in the mouse failed to reveal an embryonic phenotype (81); however compensation by TR4, a close homolog of TR2, may mask the importance of TR2 in regulating pluripotency in vivo. Nevertheless, the posttranslational regulation of receptors certainly warrants more study, Because several NRs are known to undergo SUMOylation (82), and posttranslational modifications are known to regulate Oct4 activity in ES cells (83).
GCNF/Nr6a1
In vivo, GCNF exhibits broad expression throughout the embryo at gastrulation, after which it is restricted primarily to the developing nervous system (84, 85) In adults, GCNF is restricted almost exclusively to the gonads and germ cell lineage (86), leading researchers to initially hypothesize that it may be involved in regulating meiosis. Later it was shown that GCNF expression inversely correlates with Oct4 expression both during embryogenesis and during RA differentiation of EC cells (87). GCNF is expressed in both ES and EC cells, where it functions similarly in each to counter Oct4 expression. Genetic ablation of GCNF causes embryonic lethality around E10.5 due to a number of developmental defects (84), and mutant embryos maintain expression of the pluripotency factors Oct4 and Nanog within somatic tissues after gastrulation (54).
Unlike RAR, GCNF belongs to the class of “orphan nuclear receptors.” because no physiologic ligand has been identified for the receptor. Despite its orphan status, a great deal is known about its mode of action. GCNF mediates transcriptional repression of target genes in part through the recruitment of corepressors nuclear receptor corepressor and silencing mediator of retinoid and thyroid hormone receptor (88, 89). Mechanistically, GCNF forms a hexameric complex known as the transiently retinoid-induced factor complex which binds to direct repeats of the consensus sequence HRE (DR0 element) (87, 90). GCNF is expressed at low levels in undifferentiated ES and EC cells; however, its expression is transiently induced upon differentiation with RA, allowing GCNF to oversee the direct repression of Oct4 and Nanog (54). Chromatin-IP confirms GCNF binding to the conserved DR0 element within the Oct4 PP, whereas the repression of Nanog occurs due to binding at three DR0 elements in the 5′-promoter region and the 3′-untranslated region. To aid in the characterization of GCNF function and its role in regulating pluripotency gene expression, GCNF-null ES cells were created and uncovered an essential role for GCNF in ES cell differentiation. Specifically, pluripotency gene expression is maintained in GCNF-null ES cells upon RA differentiation (54). Furthermore, the Oct4 PP is hypomethylated in both GCNF-null embryos and ES cells during differentiation with RA (91). The loss of methylation of the Oct4 promoter indicates that, in addition to mediating transcriptional repression, GCNF functions to mediate the epigenetic silencing of the Oct4 gene. This occurs through the recruitment of methyl-DNA binding domain proteins (MBD)3/2 and DNA methyl-transferases (Dnmt)3a/3b (91, 92). Studies have confirmed that GCNF interacts with MBD3/2 in vitro, and the MBD3-null ES cells exhibit a phenotype similar to GCNF-null ES cells insofar that pluripotency gene expression is maintained in the absence of LIF (93). Investigations of Oct4 promoter occupancy by these factors have demonstrated that the recruitment of MBD and Dnmt3a is GCNF dependent (91). Surprisingly, the Dnmt3a/3b-null ES cells, which are entirely deficient in de novo methylation and therefore unable to silence the Oct4 gene, still exhibit significant repression of Oct4 upon RA treatment (91, 93–96). This indicates that GCNF and MBD3 are capable of mediating transcriptional repression of Oct4 independent of Dnmt3a/3b methylation through direct binding at the promoter (91).
Studies have shown that there is sequential recruitment of these factors to the Oct4 promoter. The first step appears to be the recruitment of MBD3 and a presumed associated Mi-Nurd complex of MBD3 (93). MBD3 is a CpG binding coregulator, as its name suggests, and binds to unmethylated DNA. The exact function of the MBD3 complex in this process is currently unknown but likely involves histone deacetylation. Next, GCNF recruits de novo DNA methyltransferases, predominantly Dnmt3A, which leads to the methylation of CpG dinucleotides in the Oct4 promoter (91, 92). There is a role for the maintenance methyltransferase Dnmt1 that has yet to be defined but likely leads to full methylation of the promoter and its maintenance during subsequent rounds of cell division. Lastly, MBD2 is recruited to the Oct4 promoter by GCNF (91). In contrast to MBD3, MBD2 is a coregulator that binds to methylated CpG dinucleotides. It is surprising that MBD2 is required after DNA methylation, at which time it is presumed that a gene is stably silenced. Significantly in MBD2−/− ES cells the Oct4 gene is repressed and methylated upon RA treatment but the repression is not stable as the promoter can be demethylated upon readdition of LIF (91). Thus, GCNF serves to link immediate gene-specific repression with long-term epigenetic silencing of Oct4, thereby making it an essential factor in the regulation of pluripotency; however there is still much to be learned about this multistep process.
Promotion of the Pluripotent State by NRs
In contrast to the suppression of pluripotency, other members of the NR superfamily have been implicated in promoting the pluripotent state. Genetic studies and novel screens have elucidated broad roles for these receptors in mediating pluripotency both in the early embryo and in ES cells grown in culture. These studies paved the way for more recent work implicating NRs as reprogramming factors capable of driving induced pluripotency (97, 98).
Steroidogenic Factor-1 (SF1/Nr5a1)
SF1 and its close relative, liver receptor homolog-1 (Lrh-1), are highly homologous orphan NRs that recognize like DNA response elements and contain a unique FTZ-F1 domain (99). Importantly, these receptors exhibit differential expression patterns, both during embryonic development and in adults, suggesting they function nonredundantly (100–102). SF1 expression is observed after gastrulation and is subsequently restricted to the adrenal glands and gonads in adults. Genetic ablation of SF1 results in mutant mice that survive in utero but die shortly after birth, exhibiting a lack of adrenal glands and gonadal agenesis (100). This phenotype is consistent with SF1's role in establishing the primary endocrine axis, which occurs, in part, through direct regulation of steroid hydroxylase gene expression (103, 104).
SF1 was demonstrated to directly regulate Oct4 expression in EC cells by binding to the conserved DR0 element within the proximal promoter (52, 53). Furthermore, its expression is rapidly lost upon RA differentiation, and specific functions in promoting pluripotency gene expression in P19 cells have been demonstrated (52–54). SF1 reportedly interacts with RAR to synergistically promote Oct4 expression in P19 cells through the RARE and DR0 response element within the Oct4 PP (52); however, this synergy has not yet been demonstrated in vivo. The extent to which SF1 mediates Oct4 expression in ES cells has been questioned by its expression profile and the genetic findings. Although cell line heterogeneity may be an underlying factor, previous reports found SF1 expression in ES cells to be only nominal by microarray analysis (106), whereas others report it being undetectable by northern blot analysis (53, 105). Furthermore, genetic ablation of SF1 does not manifest in an early embryonic phenotype, suggesting that either SF1 plays no role in regulating pluripotency in the early embryo or that significant compensatory mechanisms exist (100). Others report that ectopic expression of SF1 in ES cells does not support ES self-renewal but rather drives differentiation toward a steroidogenic lineage (106). Pinning down the precise role of SF1 in embryonic development has been difficult, likely due to compensation by Lrh-1. Whether SF1 has more specific roles, or pathologic roles, in the regulation of Oct4 beyond embryonic tissues is an intriguing possibility that ought to be investigated.
Liver Receptor Homolog-1 (Lrh-1/Nr5a2)
As questions were raised regarding SF1's role in regulating pluripotency, researchers looked to the related receptor Lrh-1. Unlike SF1, Lrh-1 exhibits broad expression localized to pluripotent cells at the morula, blastocyst, and epiblast stages of development, and genetic ablation of Lrh-1 causes embryonic lethality at the epiblast stage of development, E6.5–E7 (53).
Genetic studies of Lrh-1-null mice provided the first evidence that Lrh-1 promoted pluripotency in vivo. Despite maintaining Oct4 expression at earlier stages of development, Lrh-1-null embryos exhibit a premature loss of Oct4 expression and succumb at the epiblast stage of development (53), at which time Oct4 expression is normally restricted to the embryonic ectoderm. This finding is especially intriguing because this defect temporally coincides with the DE to PE transition that drives expression of the Oct4 gene in vivo (42).
A separate study investigating Lrh-1-null mice determined that Lrh-1 is essential for primitive streak formation and generation of embryonic and extraembryonic mesoderm (107). These mice, bred in a 129SV/MF-1 background, displayed embryonic lethality starting at E6.0, with a small fraction undergoing gastrulation and surviving until E9.5 (101). The discrepancy between the two mutant lines may be due to incomplete penetrance of the phenotype caused by breeding with an outbred MF-1 line. This is in contrast to the study described above in which perigastrulation lethality was observed for all Lrh-1-null mice in a 129SV/C57BL6 background (53). Furthermore, tetraploid-fusion complementation assays led the authors to conclude that Lrh-1 functions in a non-cell-autonomous manner in vivo (107). One caveat to this finding is that the chimeric embryos were harvested at E9.0 for analysis, despite previous reports indicating that a small fraction of the Lrh-1-null embryos survive to E9.5 (101). Therefore, it is plausible that the E9.0 harvesting of the chimeras in the tetraploid fusion assay may have selected for Lrh-1-null embryos that were already capable of undergoing gastrulation. It would be interesting to compare the two mutant backgrounds for expression levels of alternate factors, such as SF1, that might yield greater compensation in one background over another.
Lrh-1 is highly expressed in undifferentiated wild-type ES cells and is rapidly repressed upon differentiation with RA or embryoid body formation, indicative of a specific role for Lrh-1 in promoting pluripotency (53, 108). Less is known about the repression of Lrh-1 in ES cells; however, one study observed Lrh-1 to be a target of miR-134, a miRNA induced during neural differentiation (63). In undifferentiated Lrh-1-null ES cells, the primary pluripotency axis is maintained; however Oct4 and Nanog are expressed at decreased levels, and these factors are more rapidly repressed upon differentiation compared with wild-type ES cells (53, 108). Importantly, the mutant ES cells are capable of giving rise to each germ lineage during EB differentiation and teratoma formation, indicating that the cells do not exhibit a global defect in differentiation (108). Chromatin-IP confirms the direct regulation of Oct4 by Lrh-1 through direct binding to the conserved DR0 elements in the Oct4 PP and PE (53, 108). Importantly, the ability of Lrh-1-null ES cells to maintain expression of the primary pluripotency axis is consistent with in vivo observations, in which Oct4 expression is maintained in Lrh-1-null blastocysts (53), the stage from which ES cell lines are derived. Likewise, the premature loss of Oct4 expression at early stages of differentiation in the mutant cell line is reminiscent of the aberrant loss of Oct4 expression seen in Lrh-1-null epiblasts. Others have identified Lrh-1 as a statistically significant coregulator of pluripotency in a bioinformatic approach that incorporated gene expression and genomic binding data in their analysis to establish a broad gene-regulatory network in ES cells (109).
A novel signaling axis has recently been demonstrated involving the regulation of Lrh-1 by canonical Wnt/β-catenin (108). The role of Wnt signaling in the regulation of pluripotency has been somewhat controversial, because the pathway is also essential for driving differentiation at gastrulation (110). However, Wnt signaling is known to regulate self-renewal in many adult stem cell lineages (111, 112) and has been implicated in promoting ES cell self-renewal (113–115). β-catenin-null ES cells exhibit a phenotype similar to Lrh-1-null ES cells insofar that the cell lines exhibit decreased expression of the primary pluripotency factors, compared with wild-type cells. Using molecular and pharmacologic approaches to promote or inhibit canonical Wnt signaling in mutant ES cell lines, a signaling axis was elucidated in which Wnt/β-catenin promotes Oct4 and Nanog expression in an Lrh-1-dependent manner (108). Chromatin-IP confirmed the direct regulation of Lrh-1 by β-catenin at conserved sites in the Lrh-1 full-length promoter and the previously characterized ES cell-specific promoter (116). In addition to direct regulation of Oct4, Lrh-1 was observed to directly regulate expression of Tbx3 and Nanog (108). This finding is consistent with previous reports putting Tbx3 and Nanog as downstream effectors of phosphatidylinositol 3-kinase/Akt signaling, that together constitute a single arm of the two parallel signaling axes that are driven by LIF stimulation during ES self-renewal (117). Lrh-1 regulation of these factors is an intriguing finding, because LIF is not required to maintain pluripotency in vivo (118, 119). Collectively, these findings suggest that Wnt/β-catenin regulation of Lrh-1 may be a physiologically relevant alternative during embryonic development.
Recent efforts to identify additional factors promoting somatic cell reprogramming identified Lrh-1 as a potent reprogramming factor (97). Lrh-1 and the orphan NR PXR/Nr1i2 were identified in a screen of 19 NRs examining the ability to enhance reprogramming efficiency in the presence of the classical reprogramming factors (Oct4, Sox2, Klf4, and c-Myc). Surprisingly, the study also found that Lrh-1, and to a lesser degree its close homolog SF1, are sufficient to replace Oct4 in the reprogramming of MEFs to iPS cells (97). This capability likely stems from Lrh-1 regulation of Nanog and the pluripotency axis detailed earlier in this study.
ES cells undergo self-renewal in a pluripotent ground state independent of exogenous factors; however, Nanog expression is essential for achieving ground-state pluripotency both in vivo and during iPS reprogramming (120, 121). This phenomena can be illustrated in vitro by the reprogramming of epi-SCs to inner cell mass-like ES-like cells, through the ectopic expression of Nanog or Klf4 occurring concomitantly with a transition from epi-SC culture conditions to ES culture conditions, namely LIF+2i treatment (121–123). The transition to this naïve ground state is marked by an altered gene expression profile, X-chromosome reactivation in female cells, and improved germ line transmission in chimeric mice (120, 121). A genome-wide forward genetic screen carried out in epi-SC revealed that Lrh-1 and SF1 are effective at reprogramming epi-SC to the inner cell mass-like naïve state (124). The effectiveness of Lrh-1 at driving ground-state pluripotency is not surprising when considering 1) that Lrh-1 is known to directly regulate Nanog expression in ES cells (97, 108); and 2) genetic knockout models for Lrh-1 and Nanog exhibit similar phenotypes. Chiefly, neither Lrh-1 nor Nanog is required for formation of the inner-cell mass in vivo, nor are they required for self-renewal of ES cells in vitro; however, Nanog is required for formation of the pluripotent epiblast (25), and Lrh-1-null mice succumb at the epiblast stage of development (53). The genetic linkage between Lrh-1 and Nanog in in vitro systems and the similar phenotypes exhibited in genetic models provide enough circumstantial evidence that further investigation of the role of Lrh-1 in specifying the pluripotent epiblast is warranted. Lrh-1 may play an important role in the primed epiblast through regulation of Oct4 expression through the PE and PP; this may be reflected more directly in the epi-SC as opposed to ES cell lines.
Dosage-Sensitive Sex Reversal-Adrenal Hypoplasia Congenital on the X-Chromosome Gene-1 (Dax-1/Nr0b1)
The orphan nuclear receptor Dax-1 is unique among NRs insofar that in place of the classical DNA-binding domain are three LXXLL domains termed “NR-box motifs” which mediate protein-protein interactions (125), and overlapping with the ligand-binding domain in the C terminus is a transcriptional silencing domain required for mediating transcriptional repression of target genes (126). Several genetic models exist for Dax-1 that reveal an important role in regulating steroidogenesis in endocrine tissues (126–129). Attempts to study Dax1 function before formation of the steroidal axis have been hampered by unsuccessful attempts to genetically ablate all isoforms of Dax-1; however, Dax-1 is expressed before formation of the steroidal axis at the morula and blastocyst stage of development (130) and, with the exception of the proximal visceral endoderm, exhibits broad expression throughout the preimplantation epiblast, supporting a role for Dax1 in maintaining pluripotency in vivo (131).
Dax-1 is relatively highly expressed in undifferentiated ES cells and is repressed upon differentiation (130). Early attempts at genetically ablating Dax1 in an ES cell line for the purposes of generating a Dax1-null mouse failed, which led researchers to hypothesize that Dax-1 may be important for maintaining pluripotency (131). RNA interference (RNAi) knockdown of Dax-1 in wild-type ES cells causes immediate differentiation, evident in the loss of Oct4 expression and induction of endoderm markers (131). These findings have been confirmed using a strategy to conditionally ablate Dax1 by Cre-mediated excision, which also results in spontaneous differentiation of the ES cells (105). Direct binding of Stat3 and Oct4 drives Dax1 expression to Dax1-regulatory regions (133), and withdrawal of LIF from cultures triggers rapid suppression of Dax1 due to loss of activation of the Jak/Stat pathway (130). Exactly how Dax1 promotes ES cell self-renewal is unclear, although several proteomic studies have demonstrated that Dax1 interacts with both Nanog and Oct4 in ES cells (134–137). These findings indicate that Dax1 may act as a core factor that is corecruited to target genes regulated by the primary pluripotency axis (135). This is somewhat surprising insofar as the receptor primarily functions as a transcriptional repressor. Consistent with this function, a genomic study investigating differential gene expression in ES cells revealed that most differentially expressed genes after RNAi knockdown of Dax1 were indeed up-regulated (105). This finding is supported by others who report that the interaction between Dax1 and Oct4 functions to abolish the DNA binding activity of Oct4 and mediate the subsequent repression of Oct4 target genes (136). The exact role for Dax1 in regulating pluripotency requires further study, but one possibility is that Dax1 acts directly on the primary pluripotency axis to fine tune expression of its gene targets.
Estrogen related receptor-β (Esrrb/Nr3b2)
The estrogen-related receptors comprise three orphan NRs (α,β, and γ), which exhibit close homology with estrogen receptor (ER), although they generally do not respond to estrogens as ligands (138, 139). Esrrb has the most significant function during embryonic development, in which it regulates placentation through restricted expression in a region of extraembryonic ectoderm that later gives rise to the chorion (140, 141). Esrrb is also expressed in PGCs from E11.5–E16.5 during which time the PGCs colonize the embryonic gonads. Importantly, Esrrb mutant embryos can be rescued by tetraploid fusion complementation with wild-type embryos, demonstrating that Esrrb is not required for development of the embryo proper (141). Rather, the rescued mice exhibit significantly decreased germ cell number, indicating that Esrrb regulates the proliferation of germ cells in vivo (142).
Despite reports that Esrrb expression is absent from pluripotent lineages in vivo and not required for development of the embryo proper, several studies in ES cells have identified Esrrb as an important mediator of pluripotency in vitro. RNAi knockdown of Esrrb in ES cells is sufficient to induce differentiation (143–145), and Esrrb expression in ES cells is regulated by phosphatidylinositol 3-kinase in an Oct4/Nanog-dependent manner (145, 146). Additionally, Esrrb has been identified as a core factor directly interacting with factors constituting the primary pluripotency axis (Oct4, Sox2, and Nanog) (19, 135, 145–147). The discrepancy between Esrrb function in regulating pluripotency in vivo and in vitro is puzzling. One explanation assumes the germ cell origin of ES cell lines that has previously been posited (148), in which case Esrrb has roles that are more readily attributable in ES cells because they more closely reflect the PGC cell fate than that of a blastomere.
In addition to promoting ES cell self-renewal, Esrrb was the first NR identified to promote the reprogramming of MEFs into iPS cells (98). Esrrb transduction was sufficient to replace Klf4 in the generation of iPS cells that were both germline competent and exhibited no discernable epigenetic differences from those reprogrammed using the standard factors. The investigators went on to show that Esrrb directly regulates expression of Klf4 during reprogramming, thereby suggesting that Esrrb's potency as a reprogramming factor may stem direct regulation of other known pluripotency factors (98).
Recent studies have helped illustrate where Esrrb fits into the picture as promoter of pluripotency and reprogramming. An investigation of Nanog target genes in ES cells has identified Esrrb as a critical downstream mediator of Nanog function in ES cells (149). This characterization is highlighted by the finding that Esrrb can maintain ES self-renewal independent of cytokines even in Nanog−/− ES cells. Furthermore, Esrrb also recapitulates the function of Nanog in reprogramming, as Nanog−/− epi-SC and pre-iPS cells are readily reverted to the naïve-ground state by ectopic expression of Esrrb. Also similar to Nanog, Esrrb was shown by genome localization and transcriptome analysis to be a direct target of Tcf3 repression in ES cells (150). In the above study Esrrb was demonstrated to be necessary and sufficient for mediating ES self-renewal down stream of glycogen synthase kinase (GSK)3 inhibition insofar that elimination of Esrrb curbs the pluripotency-promoting effects of GSK3 inhibition, and overexpression of Esrrb phenocopies both that of GSK3 inhibition and Tcf3 ablation. In contrast to GSK3 inhibition, LIF stimulation can support ES self-renewal in the absence of Esrrb through the promotion of Stat3. These findings thereby support the previous findings by Niwa et al. (117) that ES pluripotency is governed by compensatory parallel signaling pathways.
Perspective
Research investigating the role for NRs in the regulation of pluripotency has relied heavily on characterizations made in mutant mouse models during development and mechanistic studies carried out in murine EC or ES cell lines. The genetic models have served as excellent tools to deconstruct complex gene-regulatory networks in the mouse; however, further implementation is limited due to both ethical and technical considerations. The future implications of the research reviewed in this study will largely depend on the degree to which these transcriptional mechanisms are conserved in human pluripotent cell lines. To address this issue, researchers have primarily investigated factors for congruous expression in comparative studies of mouse and human ES cells. In one such study, the expression of each NR was systematically profiled in H1 and H9 human ES cell lines for comparison with the CMTI-1 mouse ES cell line upon embryoid body differentiation (151). Although little heterogeneity was observed between the two hES cell lines, the NR expression profile in the mES cell line differed significantly. Among the differences reported were contrasting expression of factors in the undifferentiated state, as well as diametric expression during differentiation. One caveat to these findings is that the kinetics for induction or repression of various factors upon differentiation could differ significantly between mES and human (h)ES cell lines. Second, the variation in gene expression may reflect the differences under which each cell line was derived and propagated; thus different lines may reflect different developmental stages. Although this study effectively highlights the species-specific differences that inevitably arise when translating from one model system to another, the significance of those differences requires more detailed investigation. Future comparative studies ought to go beyond gene expression and focus on what is known mechanistically for individual factors, and ought to include human iPS cell lines as well.
The significance of NR regulation of pluripotency may ultimately lie in the potential for receptors to drive pluripotency or direct differentiation through the use of synthetic modulators. The ability of ES cells to divide indefinitely in culture makes them amenable to the type of drug screens that have enabled the identification of small molecules capable of regulating orphan receptors in other model systems. The identification of such synthetic ligands may prove especially significant in the field of induced pluripotency. One major caveat to somatic cell reprogramming is that protocols typically call for viral-mediated transduction of the ectopic factors that drive reprogramming. This results in random genomic integration of transgenes with known tumorigenic activity that may potentially become reactivated or, alternatively, the disruption of endogenous signaling axis at the site of integration. Recent reports have described methods that are effective in driving somatic cell reprogramming independent of genomic integration, such as use of episomal vectors (152), recombinant proteins (153), and piggyBac transposition to remove transgenes after reprogramming (154); however the success of these methods comes at the cost of decreased efficiency. Improving the efficiency of reprogramming by modulating NR activity is a potential application to resolve this issue. Indeed, agonists for Lrh-1 and Esrrb have been identified (132, 144, 155). Alternatively, modulating NR activity in pluripotent cell types with synthetic ligands may prove to be effective at driving differentiation toward more specific cell lineages. This is particularly relevant with regard to therapeutic applications, because a pure population of targeted differentiated cells not only yields more material for transplant, but also reduces the risk of contamination with undifferentiated cells that have tumorigenic potential. Finally, it will be interesting to determine whether NR modulation can prove effective in driving the transdifferentiation of one cell type into alternate cell lineages, currently a major benchmark in the field of regenerative medicine.
Acknowledgments
This work was supported by National Institutes of Health Grant P01 GM081627.
Disclosure Summary: Neither author has anything to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: GCNF.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- COUP-TF
- chicken ovalbumin upstream promoter-transcription factor
- DE
- distal enhancer
- DR0
- direct repeat 0
- E12.5
- embryonic day 12.5
- EB
- embryoid body
- EC
- embryonal carcinoma cell
- ES
- embryonic stem cell
- GCNF
- germ cell nuclear factor
- GSK
- glycogen synthase kinase
- HRE
- hormone response element
- iPS
- induced pluripotent stem cell
- LIF
- leukemia inhibitory factor
- Lrh-1
- liver receptor homolog
- MBD
- methyl-DNA binding domain
- MEF
- mouse embryonic fibroblast
- miRNA
- micro-RNA
- NR
- nuclear receptor
- PE
- proximal enhancer
- PGC
- primordial germ cell
- PP
- proximal promoter
- RA
- retinoic acid
- RAR
- retinoid acid receptor
- RARE
- RA-repressible enhancer
- RNAi
- RNA interference
- RXR
- retinoid X receptor.
References
- 1. Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th Edition 2005. Philadelphia: Elsevier Saunders [Google Scholar]
- 2. Cohnheim V. Congenitales, quergestreiftes muskelsarkom der nieren. Virchows Arch Pathol Anat Physiol Klin Med. 1875: 65:64–69 [Google Scholar]
- 3. Experiments on the orgin of tumors. Br Med J. 1881;2;636–637 [Google Scholar]
- 4. Kahan BW, Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. J Nat Cancer Inst. 1970;44:1015–1036 [PubMed] [Google Scholar]
- 5. Rosenthal MD, Wishnow RW, Sato GH. In vitro growth and differentiation of clonal populations of multipotential mouse cells derived from a transplantable testicular teratoma J Nat Cancer Inst. 1970;44:1001–1014 [PubMed] [Google Scholar]
- 6. Evans MJ. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morph. 1972;28:163–176 [PubMed] [Google Scholar]
- 7. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156 [DOI] [PubMed] [Google Scholar]
- 8. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 10. Labosky PA, Barlow DP, Hogan BL. Embryonic germ cell lines and their derivation from mouse primordial germ cells. Ciba Found Symp. 1994;182:157–168 [DOI] [PubMed] [Google Scholar]
- 11. Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120(11):3197–3204 [DOI] [PubMed] [Google Scholar]
- 12. Brons IG, Smithers LE, Trotter MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195 [DOI] [PubMed] [Google Scholar]
- 13. Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199 [DOI] [PubMed] [Google Scholar]
- 14. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 15. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 16. Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 2007;1:55–70 [DOI] [PubMed] [Google Scholar]
- 18. Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loh YH, Wu Q, Chew JL, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440 [DOI] [PubMed] [Google Scholar]
- 20. Masui S, Nakatake K, Toyooka Y, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635 [DOI] [PubMed] [Google Scholar]
- 21. Lee TI, Jenner RG, Boyer LA, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 2006;125:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boyer LA, Plath K, Zeitlinger J, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353 [DOI] [PubMed] [Google Scholar]
- 23. Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo dependes on the POU transcription factor Oct4. Cell. 1998;95:379–391 [DOI] [PubMed] [Google Scholar]
- 24. Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in the mouse epiblast and ES cells. Cell. 113:631–642 [DOI] [PubMed] [Google Scholar]
- 26. Chambers I, Silva J, Colby D, et al. Nanog safeguards pluripotency and mediates germline development. Nature 2007;450:1230–1234 [DOI] [PubMed] [Google Scholar]
- 27. Chambers I, Colby D, Roberson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655 [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Karimi H, Archer T. Changes in attitude, changes in latitude: nuclear receptors remodeling chromatin to regulate transcription. Mol Endocrinol. 2006;20(1):1–13 [DOI] [PubMed] [Google Scholar]
- 29. Lonard DM, Lanz RB, OMalley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28(5):575–87 [DOI] [PubMed] [Google Scholar]
- 30.Nuclear Receptor Signaling Axis on the Internet. Oct, 2010. http://www.nursa.org.
- 31. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donovan PJ, Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97 [DOI] [PubMed] [Google Scholar]
- 33. Martin GR, Evans MJ. The differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Nat Acad Sci U S A. 1975;72:1441–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strickland S, Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978;15:393–403 [DOI] [PubMed] [Google Scholar]
- 35. Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson EL, Reich E. Plasminogen activator in chick embryo fibroblasts: induction of enzyme synthesis by retinoic acid; synergism with viral transformation and phorbol ester. Cell. 1978;15:385–392 [DOI] [PubMed] [Google Scholar]
- 37. Sabella JD, Bern HA, Kahn RH. Effects of locally applied vitamin A and estrogen on rat epidermis. Proc Soc Exp Biol Med. 1951;76:499–503 [DOI] [PubMed] [Google Scholar]
- 38. Yuspa SH, Harris CC. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974;86:95–105 [DOI] [PubMed] [Google Scholar]
- 39. Strickland S, Smith KK., Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–355 [DOI] [PubMed] [Google Scholar]
- 40. Solter D, Shevinsky L, Knowles BB, Strickland S. The induction of antigenic changes in a teratocarcinoma stem cell line (F9) by retinoic acid. Dev Biol. 1979;70:515–521 [DOI] [PubMed] [Google Scholar]
- 41. Mummery CL, Feyen A, Freund E, Shen S. Characteristics of embryonic stem cell differentiation: a comparison with two embryonal carcinoma cell lines. Cell Differ Dev. 1990;30:195–206 [DOI] [PubMed] [Google Scholar]
- 42. Yeom Y, Fuhrmann G, Ovitt CE, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996;122(3):881–94 [DOI] [PubMed] [Google Scholar]
- 43. Chew JL, Loh YH, Zhang W, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okumura-Nakanishi S, Saito M, Niwa H, et al. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;240:5307–5317 [DOI] [PubMed] [Google Scholar]
- 45. Okazawa H, Okamoto K, Ishino F, et al. The oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991;10(10):2997–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139–1146 [DOI] [PubMed] [Google Scholar]
- 47. Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pikarsky E, Sharir H, Ben-Shushan E, et al. Retinoic acid represses Oct-3/4 gene expression through several retinoic acid-responsive elements located in the promoter-enhancer region. Mol Cell Biol. 1994;14(2):1026–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schoorlemmer J, van Puijenbroek A, van den Eijnden M, et al. Characterization of a negative retinoic acid response element in the murine Oct4 promoter. Mol Cell Biol. 1994;14(2):1122–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sylvester I, Schöler H. Regulation of Oct-4 gene by nuclear receptors. Nucleic Acids Res. 1994;22(6):901–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ben-Shushan E, Sharir H, Pikarsky E, Bergman Y. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expresssion in embryonal carcinoma cells. Mol Cell Biol. 1995;15(2):1034–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barnea E, Bergman Y. Synergy of SF1 and RAR in activation of Oct-3/4 promoter. J Biol Chem. 2000;275(9):6608–19 [DOI] [PubMed] [Google Scholar]
- 53. Gu P, Goodwin B, Chung AC, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25(9):3492–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gu P, Le Menuet D, Chung AC, et al. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25(19):8507–19 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 55. Ovitt CE, Schöler HR. The molecular biology of Oct-4 in the early mouse embryo. Mol Hum Reprod. 1998;4(11):1021–31 [DOI] [PubMed] [Google Scholar]
- 56. Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–5 [DOI] [PubMed] [Google Scholar]
- 57. Stadtfeld M, Apostolou E, Akutsu H, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Germain P, Chambon P, Eichele G, et al. International union of pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58(4):760–772 [DOI] [PubMed] [Google Scholar]
- 59. Wendling O, Chambon P, Mark M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc Natl Acad Sci USA. 1999;96:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones-Villeneuve EM, Rudnicki MA, Harris JF, McBurney MW. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983;3(12):2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bain G, Ray WJ, Yao M, Gottlieb DI. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem Biophys Res Commun. 1996;223(3):691–694 [DOI] [PubMed] [Google Scholar]
- 62. Kim M, Habbiba A, Doherty JM, et al. Regulation of mouse embryonic stem cell neural differentiation by retinoic acid. Dev Biol. 2009;328:456–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tay YM, Tam WL, Ang YS, et al. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells where is causes post-transcriptional attenuation of Nanog and LRH-1. Stem Cells. 2008;26(1):17–29 [DOI] [PubMed] [Google Scholar]
- 64. Tay Y, Zhang J, Thomson AM, et al. MicroRNAs to Nanog, Oct4, and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128 [DOI] [PubMed] [Google Scholar]
- 65. Delacroix L, Moutier E, Altobelli G, et al. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol Cell Biol. 2010;30(1):231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25(2):89–96 [DOI] [PubMed] [Google Scholar]
- 67. Schoorlemmer J, Jonk L, Shen S, et al. Regulation of Oct-4 gene expression during differentiation of EC cells. Mol Biol Reports. 1995;21:129–140 [DOI] [PubMed] [Google Scholar]
- 68. Pereira FA, Qiu Y, Zhou G, et al. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13(8):1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee CT, Li L, Takamoto N, et al. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol. 2004;24(24):10835–10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qiu Y, Pereira FA, DeMayo FJ, et al. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11(15):1925–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bardoux P, Zhang P, Flamez D, et al. Essential role of chicken ovalbumin upstream promoter-transcription factor II in insulin secretion and insulin sensitivity revealed by conditional gene knockout. Diabetes. 2005;54(5):1357–1363 [DOI] [PubMed] [Google Scholar]
- 72. Xu Z, Yu S, Hsu CH. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc Natl Acad Sci USA. 2008;105(7):2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pereira FA, Qiu Y, Tsai MJ, Tsai SY. Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J Steroid Biochem Mol Biol. 1995;53:503–508 [DOI] [PubMed] [Google Scholar]
- 74. Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11(9):1014–1023 [DOI] [PubMed] [Google Scholar]
- 75. Lee CH, Chang L, Wei LN. Molecular cloning and characterization of a mouse nuclear orphan receptor expressed in embryos and testes. Mol Reprod Dev. 1996;44(3):305–314 [DOI] [PubMed] [Google Scholar]
- 76. Lee CH, Chang L, Wei LN. Dictinct expression patterns and biological activities of two isoforms of the mouse orphan receptor TR2. J Endocrinol. 1997;152(2):245–255 [DOI] [PubMed] [Google Scholar]
- 77. Lee YF, Lee HJ, Chang C. Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily. J Steroid Biochem Mol Biol. 2002;81:291–308 [DOI] [PubMed] [Google Scholar]
- 78. Lee CH, Chinpaisal C, Wei LN. Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol Cell Biol. 1998;18(11):6745–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park SW, Hu X, Gupta P, et al. SUMOylation of Tr2 orphan receptor involves Pml and fine-tunes Oct4 expression in stem cells. Nat Struct Mol Biol. 2007;14(1):68–75 [DOI] [PubMed] [Google Scholar]
- 80. Gupta P, Ho PC, Huq MM, et al. Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA. 2008;105:11424–11429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shyr CR, Collins LL, Mu XM, et al. Spermatogenesis and testis development are normal in mice lacking testicular orphan nuclear receptor 2. Mol Cell Biol. 2002;22:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee MB, Lebedeva LA, Suzawa M, et al. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol. 2005;25(5):1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wagner RT, Cooney AJ. Oct4: less is more. Cell Res. 2009;19(5):527–528 [DOI] [PubMed] [Google Scholar]
- 84. Chung AC, Katz D, Pereira FA, et al. Loss of orphan receptor germ cell nuclear factor function results in ectopic development of the tail bud and novel posterior truncation. Mol Cell Biol. 2001;21:663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Süsens U, Aguiluz JB, Evans RM, Borgmeyer U. The germ cell nuclear factor mGCNF is expressed in the developming nervous system. Dev Neurosci. 1997;19:410–420 [DOI] [PubMed] [Google Scholar]
- 86. Katz D, Niederberger C, Slaughter GR, Cooney AJ. Characterization of germ cell-specific expression of the orphan nuclear receptor, germ cell nuclear factor. Endocrinology. 1997;138:4364–4372 [DOI] [PubMed] [Google Scholar]
- 87. Fuhrmann G, Chung AC, Jackson K, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell. 2001;1(3):377–387 [DOI] [PubMed] [Google Scholar]
- 88. Lan ZJ, Chung AC, Xu X, et al. The embryonic function of germ cell nuclear factor is dependent on the DNA binding domain. J Biol Chem. 2002;277(52):50660–50667 [DOI] [PubMed] [Google Scholar]
- 89. Yan Z, Jetten AM. Characterization of the repressor function of the nuclear orphan receptor retinoid receptor-related testis-associated receptor/germ cell nuclear factor. J Biol Chem. 2000;275(45):35077–35085 [DOI] [PubMed] [Google Scholar]
- 90. Gu P, Morgan DH, Sattar M, et al. Evolutionary trace-based peptides identify a novel asymmetric interaction that mediates oligomerization in nuclear receptors. J Biol Chem. 2005;280(36):31818–31829 [DOI] [PubMed] [Google Scholar]
- 91. Gu P, Xu X, Le Menuet D, Chung AC, Cooney AJ. Differential recruitment of methyl CpG-binding factors and DNA methyltransferases by the orphan receptor germ cell nuclear factor initiates the repression and silencing of Oct4. Stem Cells. 2011;29(7):1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sato N, Kondo M, Arai K. The orphan nuclear receptor GCNF recruits DNA methyltransferase for Oct-3/4 silencing. Biochem Biophys Res Commun. 2006;344(3):845–851 [DOI] [PubMed] [Google Scholar]
- 93. Kaji K, Caballero IM, MacLeod R, et al. The NuRD component of Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol. 2006;8(3):285–292 [DOI] [PubMed] [Google Scholar]
- 94. Okano M, Bell DW, Haber DA, et al. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylationand mammalian development. Cell. 1999;99:247–257 [DOI] [PubMed] [Google Scholar]
- 95. Chen T, Ueda Y, Dodge JE, et al. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003;23(16): 5594–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li JY, Pu MT, Hirasawa R, et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol. 2007;27(24):8748–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Heng JC, Feng B, Han J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174 [DOI] [PubMed] [Google Scholar]
- 98. Feng B, Jiang J, Kraus P, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11(2):197–203 [DOI] [PubMed] [Google Scholar]
- 99. Fayard E, Auxerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism, and steroidogenesis. Trends Cell Biol. 2004;14(5):250–260 [DOI] [PubMed] [Google Scholar]
- 100. Luo X, Ikeda Y, Lala DS, et al. A cell-specific nuclear receptor plays essential roles in adrenal and gonadal development. Endocr Res. 1995;21:517–524 [DOI] [PubMed] [Google Scholar]
- 101. Pare JF, Malenfant D, Couremanche C, et al. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J Biol Chem. 2004;279:21206–21216 [DOI] [PubMed] [Google Scholar]
- 102. Hinshelwood MM, Repa JJ, Shelton JM, et al. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45 [DOI] [PubMed] [Google Scholar]
- 103. Ikeda Y, Lala DS, Luo X, et al. Characterization of the mouse FTZ-F1 gene which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993;7(7):852–860 [DOI] [PubMed] [Google Scholar]
- 104. Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor-1 acts at multiple levels of the reproductive axis. Genes Dev. 1994; 8(19):2302–2312 [DOI] [PubMed] [Google Scholar]
- 105. Sun C, Nakatake Y, Ura H, et al. Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2008;372:91–96 [DOI] [PubMed] [Google Scholar]
- 106. Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor-1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17(7):3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Labelle-Dumais C, Jacob-Wagner M, Pare JF, et al. Nuclear receptor NR5A2 is required for proper primitive streak morphogenesis. Dev Dyn. 2006;235:3359–3369 [DOI] [PubMed] [Google Scholar]
- 108. Wagner RT, Xu X, Yi F, et al. Canonical Wnt/β-catenin regulation of liver receptor homolog-1 (Lrh-1) mediates pluripotency gene expression. Stem Cells. 2010;28(10):1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:16438–16443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Haegel H, Larue L, Ohsugi M, et al. Lack of β-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537 [DOI] [PubMed] [Google Scholar]
- 111. Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383 [DOI] [PubMed] [Google Scholar]
- 112. Reya T, Duncan AW, Ailles L, et al. A role for Wnt signaling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414 [DOI] [PubMed] [Google Scholar]
- 113. Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63 [DOI] [PubMed] [Google Scholar]
- 114. Ogawa K, Nishinakamura R, Iwamatsu Y, et al. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166 [DOI] [PubMed] [Google Scholar]
- 115. Cai L, Ye Z, Zhou BY, et al. Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res. 2007;17:62–72 [DOI] [PubMed] [Google Scholar]
- 116. Gao DM, Wang LF, Liu J, et al. Expression of mouse liver receptor homologue 1 in embryonic stem cells is directed by a novel promoter. FEBS Lett. 2006;580:1702–1708 [DOI] [PubMed] [Google Scholar]
- 117. Niwa H, Ogawa K, Shimosato D, et al. A parallel circuit of LIF signaling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122 [DOI] [PubMed] [Google Scholar]
- 118. Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992; 359:76–79 [DOI] [PubMed] [Google Scholar]
- 119. Nichols J, Chambers I, Taga T, et al. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339 [DOI] [PubMed] [Google Scholar]
- 120. Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Silva J, Nichols J, Theunissen TW, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Guo G, Yang J, Nichols J, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009;136:1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yang J, van Oosten AL, Theunissen TW, et al. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol. 2003;17:1445–1453 [DOI] [PubMed] [Google Scholar]
- 126. Lalli E, Bardoni B, Zazopoulos E, et al. A transcriptional silencing domain in DAX-1 whose mutation causes adrenal hypoplasia congenital. Mol Endocrinol. 11:1950–1960 [DOI] [PubMed] [Google Scholar]
- 127. Swain A, Narvaez V, Burgoyne P, et al. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767 [DOI] [PubMed] [Google Scholar]
- 128. Zanaria E, Muscatelli F, Bardoni B, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641 [DOI] [PubMed] [Google Scholar]
- 129. Muscatelli F, Strom TM, Walker AP, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–676 [DOI] [PubMed] [Google Scholar]
- 130. Clipsham R, Niakan K, McCabe ER. Nr0b1 and its network partners are expressed early in murine embryos prior to steroidogenic axis organogenesis. Gene Expr Patterns. 2004;4:3–14 [DOI] [PubMed] [Google Scholar]
- 131. Niakan KK, Davis EC, Clipsham RC, et al. Novel role for the orphan nuslear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab. 2006;88:261–271 [DOI] [PubMed] [Google Scholar]
- 132. Lee JM, Lee YK, Mamrosh JL, et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sun C, Nakatake Y, Ura H, et al. Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2008;372:91–96 [DOI] [PubMed] [Google Scholar]
- 134. Wang J, Rao S, Chu J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368 [DOI] [PubMed] [Google Scholar]
- 135. Kim J, Chu J, Shen X, et al. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sun C, Nakatake Y, Akagi T, et al. Dax1 binds to Oct3/4 and inhibits its transcriptional activity in embryonic stem cells. Mol Cell Biol. 2009;29:4574–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van den Berg DL, Snoek T, Mullin NP, et al. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptor. Nature. 1988;331:91–94 [DOI] [PubMed] [Google Scholar]
- 139. Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6:203–215 [DOI] [PubMed] [Google Scholar]
- 140. Pettersson K, Svensson K, Mattsson R, et al. Expression of a novel member of estrogen response element-binding nuclear receptors is restricted to the early stages of chorion formation during mouse embryogenesis. Mech Dev. 54:211–223 [DOI] [PubMed] [Google Scholar]
- 141. Luo J, Sladek R, Bader JA, et al. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature. 1997;388:778–782 [DOI] [PubMed] [Google Scholar]
- 142. Mitsunaga K, Araki K, Mizusaki H, et al. Loss of PGC-specific expression of the orphan nuclear receptor ERR-β results in reduction of germ cell number in mouse embryos. Mech Dev. 2004;121:237–246 [DOI] [PubMed] [Google Scholar]
- 143. Ivanova N, Dobrin R, Lu R, et al. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538 [DOI] [PubMed] [Google Scholar]
- 144. Suetsugi M, Su L, Karlsberg K, et al. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res 2003;1:981–991 [PubMed] [Google Scholar]
- 145. van den Berg DL, Zhang W, Yates A, et al. Estrogen-related receptor β interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008;28:5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Storm MP, Bone HK, Beck CG, et al. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 282:6265–6273 [DOI] [PubMed] [Google Scholar]
- 147. Zhang X, Zhang J, Wang T, et al. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2008;283:35825–35833 [DOI] [PubMed] [Google Scholar]
- 148. Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233 [DOI] [PubMed] [Google Scholar]
- 149. Festuccia N, Osorno R, Halbritter F, et al. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell 2012;11(4):477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Martello G, Sugimoto T, Diamanti E, et al. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell. 2012;11(4): 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Xie CQ, Jeong Y, Fu M, et al. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol. 2009;23:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim D, Kim CH, Moon JL, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Woltjen K, Michael IP, Mohseni P, et al. piggy Bac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Whitby RJ, Dixon S, Maloney PR, et al. Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J Med Chem. 2006;49:6652–6655 [DOI] [PubMed] [Google Scholar]