Abstract
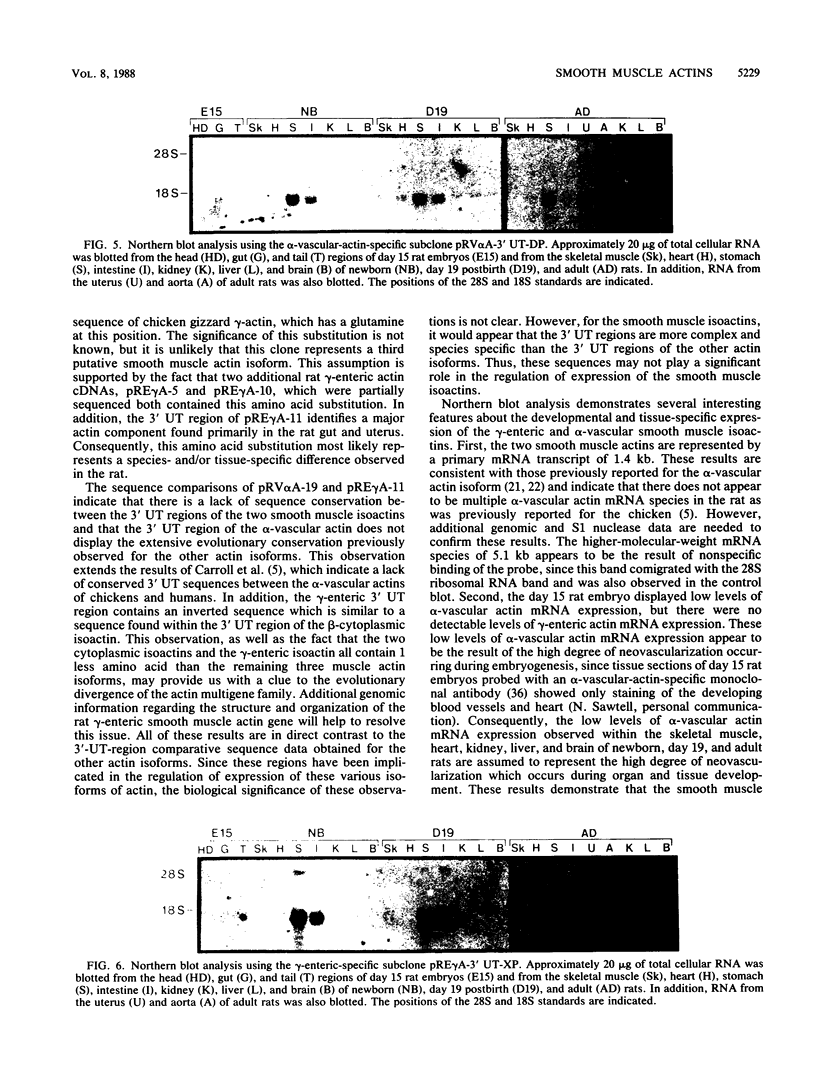
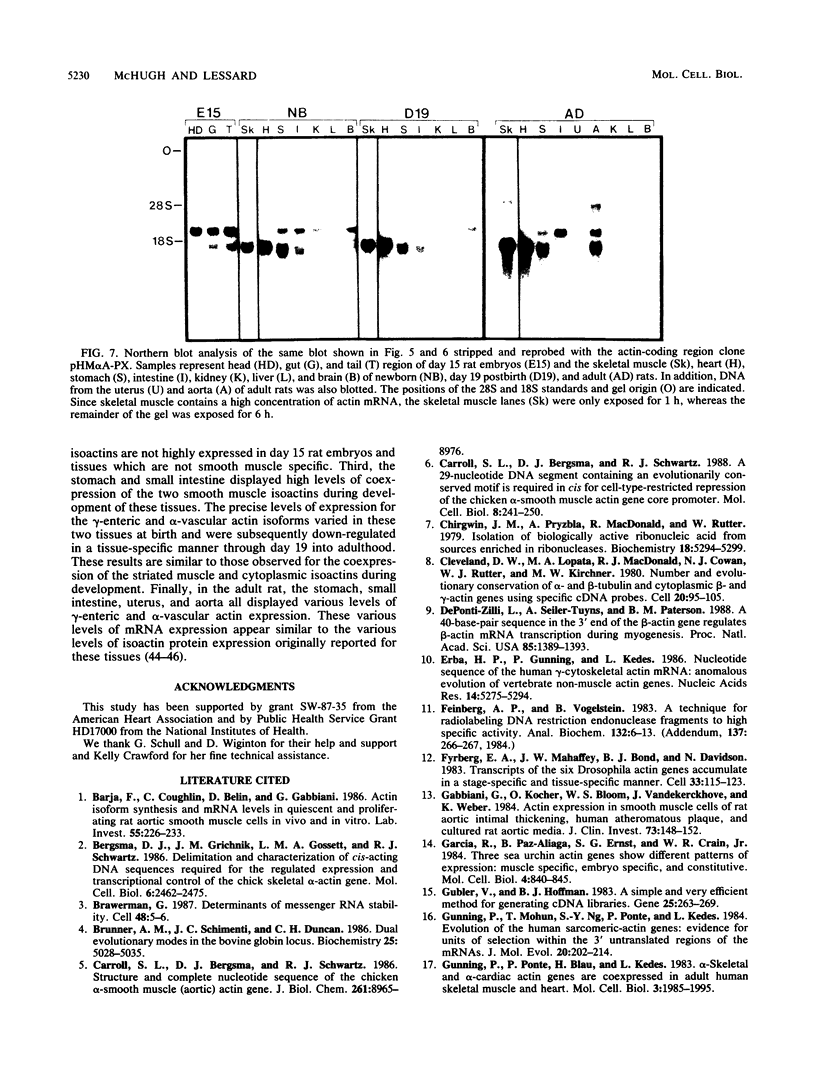
We have isolated and characterized two cDNA clones from whole rat stomach, pRV alpha A-19 and pRE gamma A-11, which are specific for the alpha-vascular and gamma-enteric smooth muscle isoactins, respectively. The rat gamma-enteric smooth muscle actin contains a single amino acid substitution of a proline for a glutamine at position 359 of the mature peptide when compared with the chicken gizzard gamma-actin sequence (J. Vandekerckhove and K. Weber, FEBS Lett. 102:219, 1979). Sequence comparisons of the 5' and 3' untranslated (UT) regions of the two smooth muscle actin cDNAs demonstrate that these regions contain no apparent sequence similarities. Additional comparisons of the 5' UT regions of the two smooth muscle actin cDNAs to all other known actin sequences reveal no apparent sequence similarities for the rat gamma-enteric isoactin within the 15 base pairs of sequence currently available, while the rat alpha-vascular isoactin contains two separate sequences which are similar to sequences within the 5' UT regions of the human and chicken alpha-vascular actin genes. A similar comparison of the 3' UT regions of the two smooth muscle actins demonstrates that the alpha-vascular isoactins do not contain the high degree of cross-species sequence conservation observed for the other isoactins and that the gamma-enteric isoactin contains an inverted sequence of 52 nucleotides which is similar to a sequence found within the 3' UT regions of the human, chicken, and rat beta-cytoplasmic isoactins. These observations complicate the apparent cross-species conservation of isotype specificity of these domains previously observed for the other actin isoforms. Northern blot analysis of day 15 rat embryos and newborn, day 19 postbirth, and adult rats demonstrates that the day 15 rat embryo displays low to undetectable levels of smooth muscle isoactin mRNA expression. By birth, the stomach and small intestine show dramatic increases in alpha-vascular and gamma-enteric actin expression. These initially high levels of expression decrease through day 19 to adulthood. In the adult rat, the uterus and aorta differ in their content of smooth muscle isoactin mRNA. These results demonstrate that the gamma-enteric and alpha-vascular isoactin mRNAs are coexpressed to various degrees in tissues which contain smooth muscle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barja F., Coughlin C., Belin D., Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986 Aug;55(2):226–233. [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Brunner A. M., Schimenti J. C., Duncan C. H. Dual evolutionary modes in the bovine globin locus. Biochemistry. 1986 Sep 9;25(18):5028–5035. doi: 10.1021/bi00366a009. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. A 29-nucleotide DNA segment containing an evolutionarily conserved motif is required in cis for cell-type-restricted repression of the chicken alpha-smooth muscle actin gene core promoter. Mol Cell Biol. 1988 Jan;8(1):241–250. doi: 10.1128/mcb.8.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. Structure and complete nucleotide sequence of the chicken alpha-smooth muscle (aortic) actin gene. An actin gene which produces multiple messenger RNAs. J Biol Chem. 1986 Jul 5;261(19):8965–8976. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R., Paz-Aliaga B., Ernst S. G., Crain W. R., Jr Three sea urchin actin genes show different patterns of expression: muscle specific, embryo specific, and constitutive. Mol Cell Biol. 1984 May;4(5):840–845. doi: 10.1128/mcb.4.5.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. Y., Frankel F. R. Effect of estrogen on the expression of mRNAs of different actin isoforms in immature rat uterus. Cloning of alpha-smooth muscle actin message. J Biol Chem. 1987 Jul 15;262(20):9594–9600. [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Analysis of alpha-smooth-muscle actin mRNA expression in rat aortic smooth-muscle cells using a specific cDNA probe. Differentiation. 1987;34(3):201–209. doi: 10.1111/j.1432-0436.1987.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Kost T. A., Theodorakis N., Hughes S. H. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Dec 10;11(23):8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M. Change of actin isomers during differentiation of smooth muscle. Biochim Biophys Acta. 1985 Dec 13;843(3):208–213. doi: 10.1016/0304-4165(85)90141-2. [DOI] [PubMed] [Google Scholar]
- McHugh K. M., Lessard J. L. The nucleotide sequence of a rat vascular smooth muscle alpha-actin cDNA. Nucleic Acids Res. 1988 May 11;16(9):4167–4167. doi: 10.1093/nar/16.9.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Brennan S., Dathan N., Fairman S., Gurdon J. B. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984 Oct 25;311(5988):716–721. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Cooper T. A. Strong homology in promoter and 3'-untranslated regions of chick and rat alpha-actin genes. Nature. 1983 May 26;303(5915):348–349. doi: 10.1038/303348a0. [DOI] [PubMed] [Google Scholar]
- Peacock S. L., McIver C. M., Monahan J. J. Transformation of E. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta. 1981 Sep 28;655(2):243–250. doi: 10.1016/0005-2787(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Horovitch S. J., Scott M. P., Rich A., Pardue M. L. Myogenesis in primary cell cultures from Drosophila melanogaster: protein synthesis and actin heterogeneity during development. Cell. 1978 Apr;13(4):589–598. doi: 10.1016/0092-8674(78)90210-6. [DOI] [PubMed] [Google Scholar]
- Strauch A. R., Rubenstein P. A. Induction of vascular smooth muscle alpha-isoactin expression in BC3H1 cells. J Biol Chem. 1984 Mar 10;259(5):3152–3159. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Bugaisky G., Buckingham M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem. 1986 Feb 5;261(4):1838–1843. [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin typing on total cellular extracts: a highly sensitive protein-chemical procedure able to distinguish different actins. Eur J Biochem. 1981 Jan;113(3):595–603. doi: 10.1111/j.1432-1033.1981.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of actin from chicken skeletal muscle actin and chicken gizzard smooth muscle actin. FEBS Lett. 1979 Jun 15;102(2):219–222. doi: 10.1016/0014-5793(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Nudel U., Mayer Y., Neuman S. Highly conserved sequences in the 3' untranslated region of mRNAs coding for homologous proteins in distantly related species. Nucleic Acids Res. 1985 May 24;13(10):3723–3737. doi: 10.1093/nar/13.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar R. S., Sodja A. Homology between actin coding and its adjacent sequences in widely divergent species. Biochem Biophys Res Commun. 1983 Feb 28;111(1):67–73. doi: 10.1016/s0006-291x(83)80118-1. [DOI] [PubMed] [Google Scholar]





