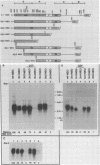
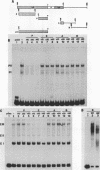
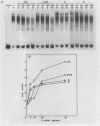
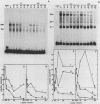
Abstract
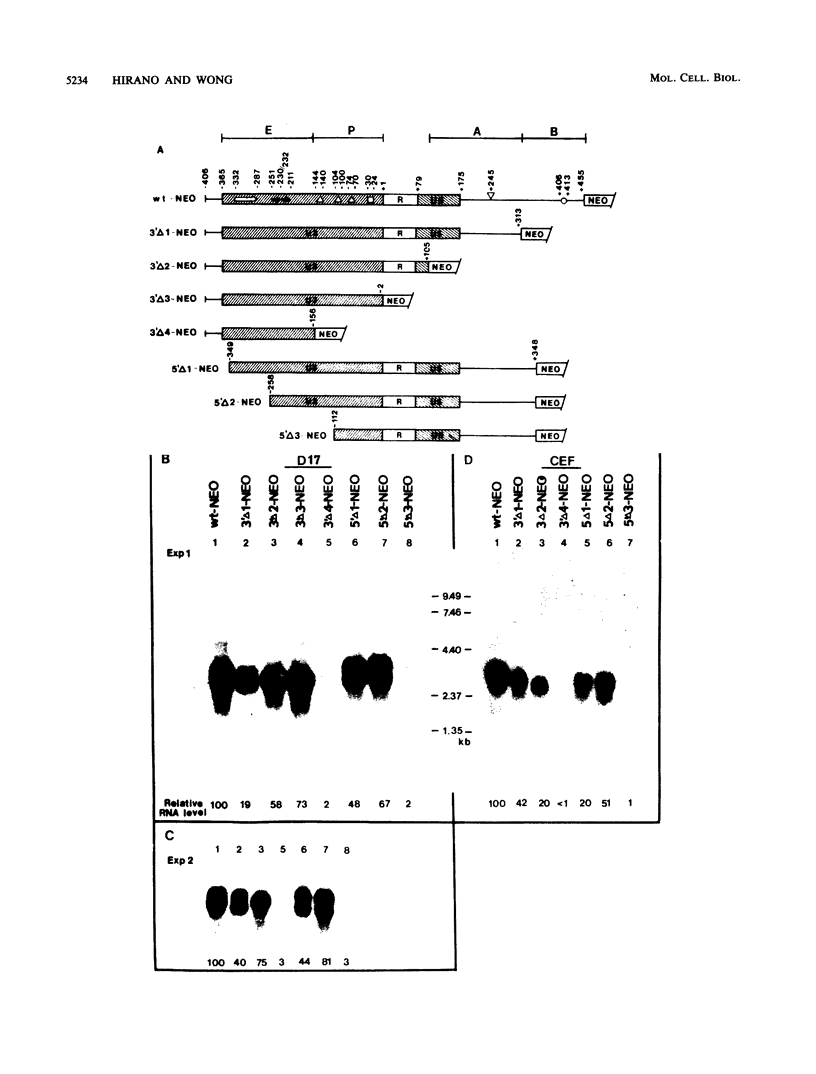
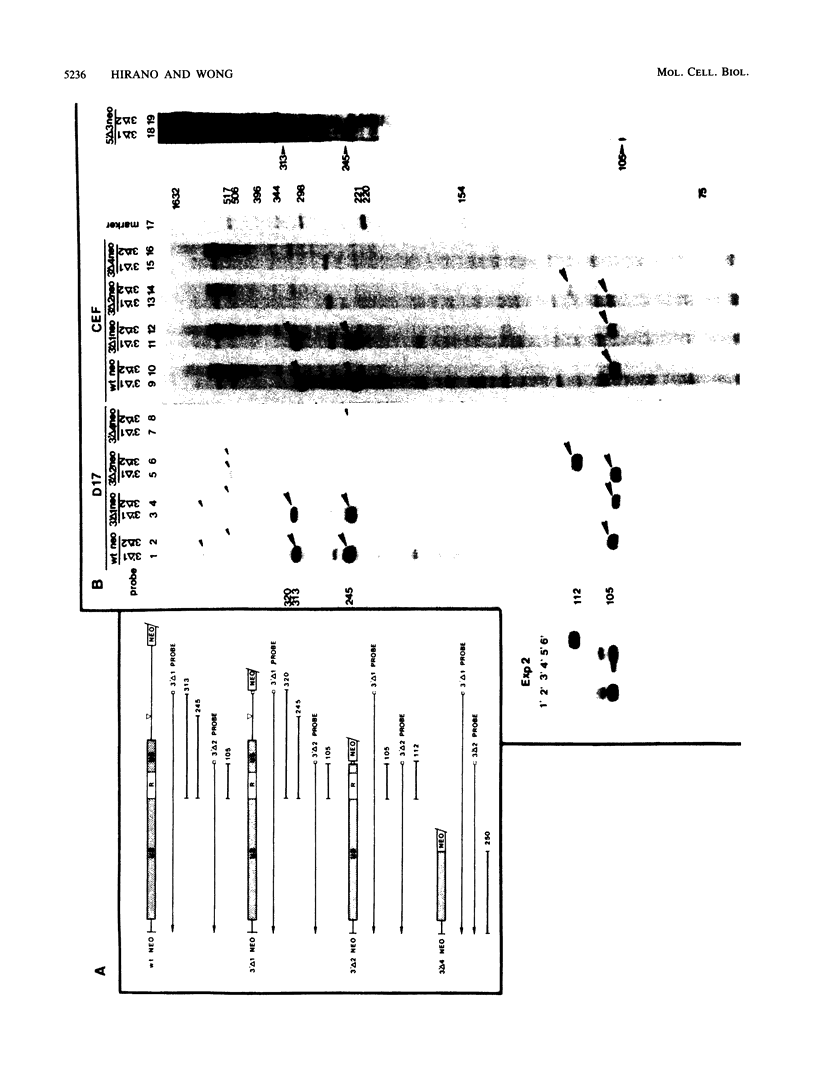
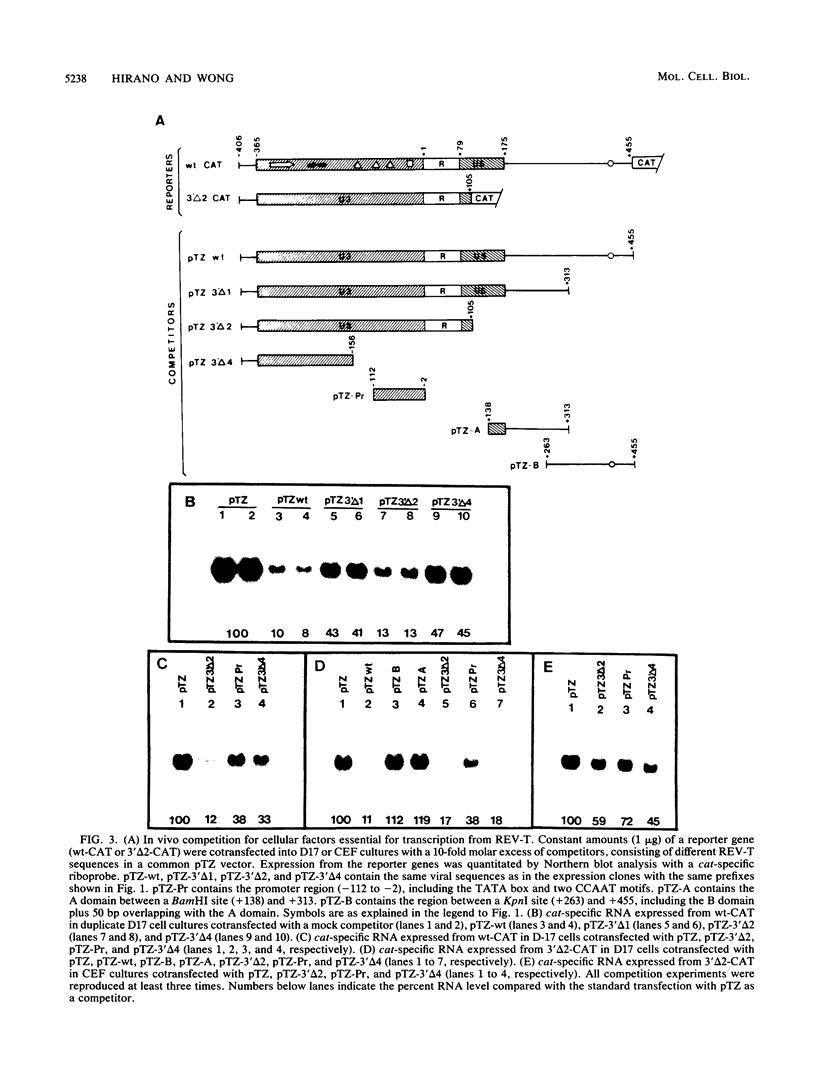
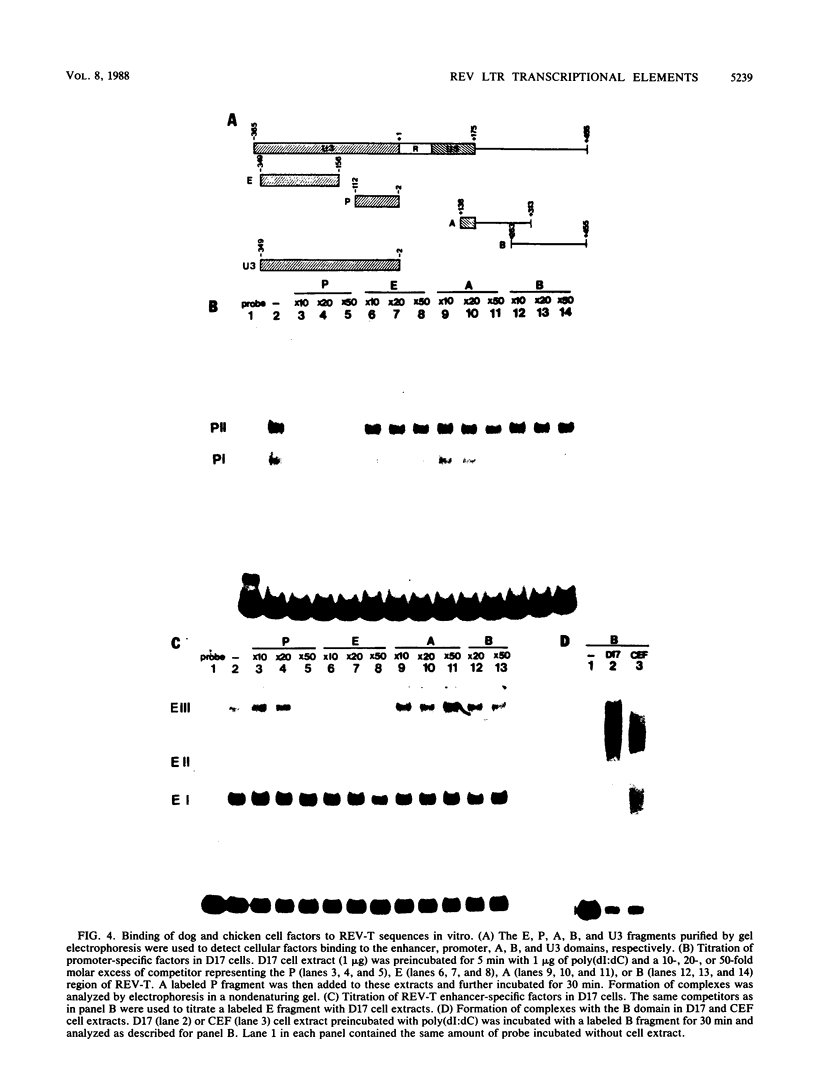
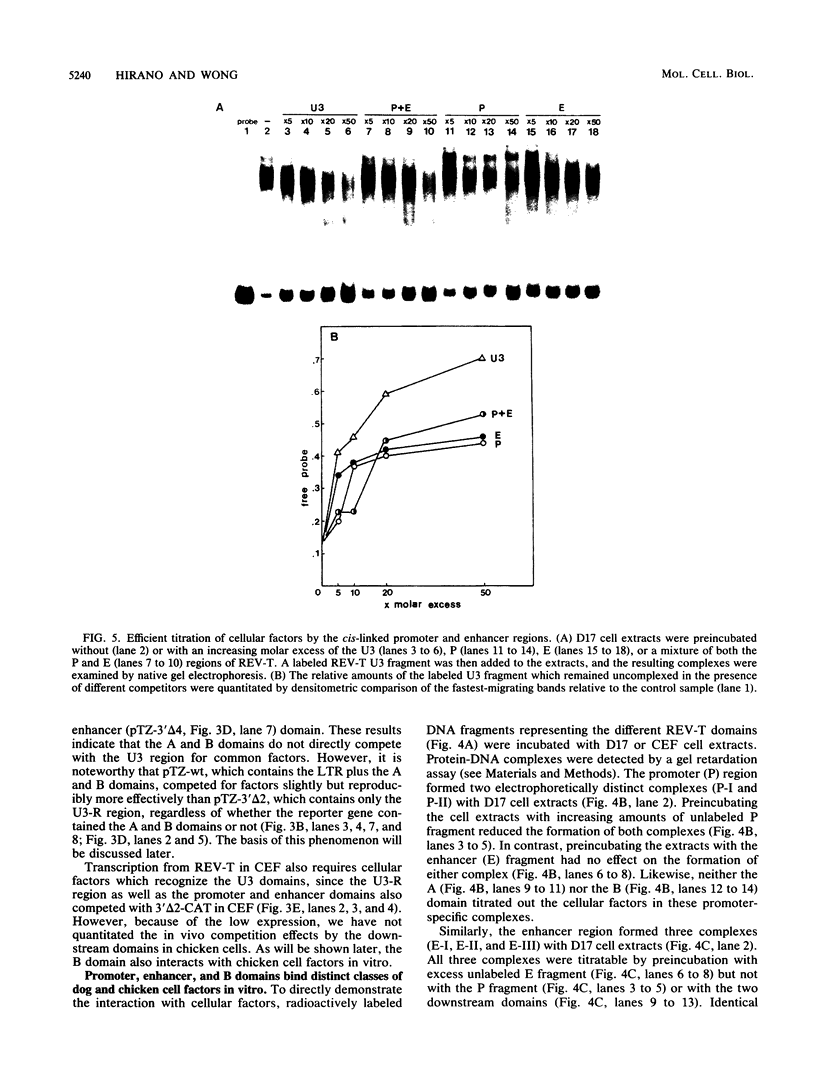
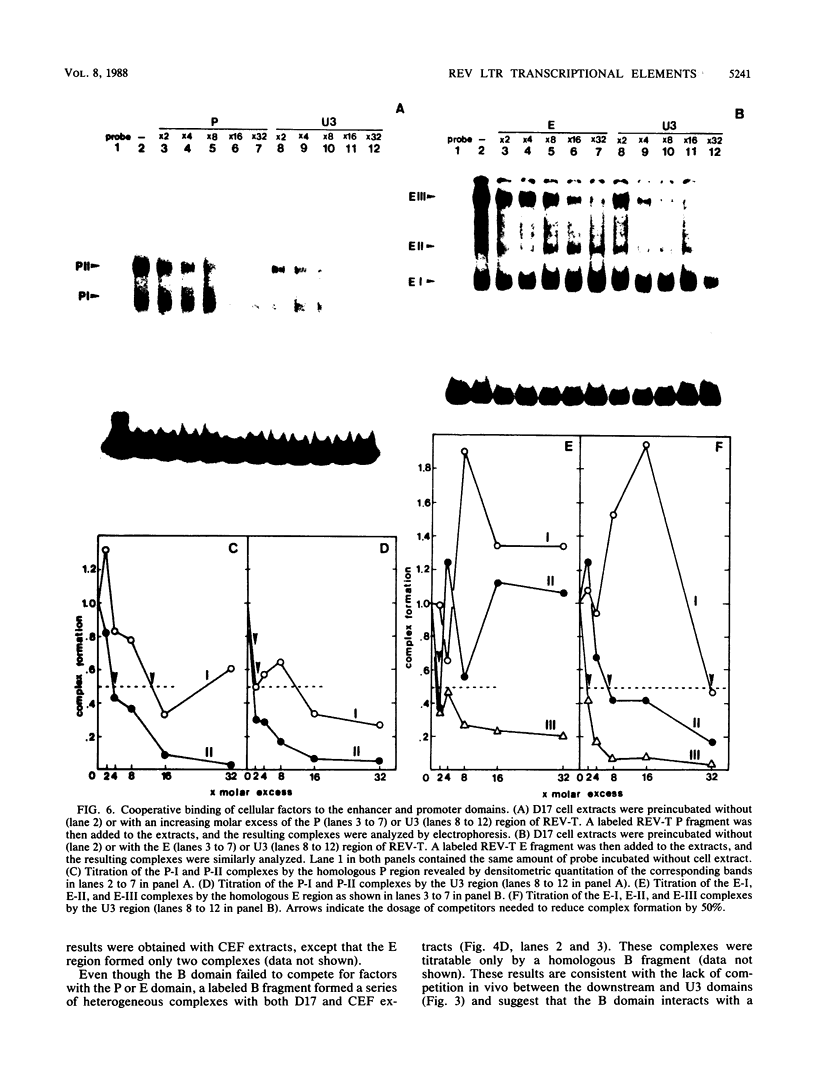
Transcription from reticuloenodotheliosis virus strain T (REV-T), an avian retrovirus unrelated to avian leukosis and sarcoma viruses, is modulated by sequences in at least five functional domains. A promoter containing a TATA and multiple CCAAT motifs in U3 of the long terminal repeat was absolutely required for transcription. Transcriptional efficiency was greatly augmented by an enhancer immediately upstream, which contained a 22-base-pair repeated sequence. Transcription was further influenced by a negative-acting domain in the 5' region of U3 and two downstream domains in the transcribed non-protein-coding region. One of these latter domains contained a consensus enhancer core sequence and positively affected transcription in both mammalian and avian cells; the other acted negatively in a dog cell line. Transcription from REV-T in vivo required cellular factors which could be competed for specifically by the promoter or enhancer domain. The downstream domains competed with reporter genes containing these domains, but not directly with the U3 sequences. The promoter, enhancer, and the positive-acting downstream domains formed multiple complexes with distinct classes of cellular factors in both avian and mammalian cell extracts. Binding of factors to the promoter and enhancer domains was cooperative when these domains were joined in cis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K., Ryden T. A., Beemon K. Localization and footprinting of an enhancer within the avian sarcoma virus gag gene. J Virol. 1988 May;62(5):1617–1624. doi: 10.1128/jvi.62.5.1617-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Substitution of 5' helper virus sequences into non-rel portion of reticuloendotheliosis virus strain T suppresses transformation of chicken spleen cells. Cell. 1982 Nov;31(1):111–120. doi: 10.1016/0092-8674(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Cohen R. B., Sheffery M., Kim C. G. Partial purification of a nuclear protein that binds to the CCAAT box of the mouse alpha 1-globin gene. Mol Cell Biol. 1986 Mar;6(3):821–832. doi: 10.1128/mcb.6.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich R., Maguire J. E., Singer D. S. Identification of negative and positive regulatory elements associated with a class I major histocompatibility complex gene. Mol Cell Biol. 1988 Feb;8(2):695–703. doi: 10.1128/mcb.8.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J. E., Temin H. M. Lack of competition results in efficient packaging of heterologous murine retroviral RNAs and reticuloendotheliosis virus encapsidation-minus RNAs by the reticuloendotheliosis virus helper cell line. J Virol. 1987 Sep;61(9):2675–2683. doi: 10.1128/jvi.61.9.2675-2683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J. E., Temin H. M. Pseudotyped retroviral vectors reveal restrictions to reticuloendotheliosis virus replication in rat cells. J Virol. 1986 Nov;60(2):662–668. doi: 10.1128/jvi.60.2.662-668.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Bich-Thuy L. T., Stafford J., Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986 Jul 24;322(6077):383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Gilmartin G. M., Parsons J. T. Identification of transcriptional elements within the long terminal repeat of Rous sarcoma virus. Mol Cell Biol. 1983 Oct;3(10):1834–1845. doi: 10.1128/mcb.3.10.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Eisenman R. N., McKnight S. L. Delineation of transcriptional control signals within the Moloney murine sarcoma virus long terminal repeat. Mol Cell Biol. 1985 Aug;5(8):1948–1958. doi: 10.1128/mcb.5.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirano A., Vogt P. K. Avian sarcoma virus PRCII: conditional mutants temperature sensitive in the maintenance of fibroblast transformation. Virology. 1981 Feb;109(1):193–197. doi: 10.1016/0042-6822(81)90486-4. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. Insertion of several different DNAs in reticuloendotheliosis virus strain T suppresses transformation by reducing the amount of subgenomic mRNA. J Virol. 1986 Apr;58(1):75–80. doi: 10.1128/jvi.58.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Characterization of Rous sarcoma virus sequences essential for viral gene expression. J Virol. 1987 Apr;61(4):1171–1179. doi: 10.1128/jvi.61.4.1171-1179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway A. A., Swift R. A., Kung H. J., Fujita D. J. In vitro transcription analysis of the viral promoter involved in c-myc activation in chicken B lymphomas: detection and mapping of two RNA initiation sites within the reticuloendotheliosis virus long terminal repeat. J Virol. 1985 Apr;54(1):161–170. doi: 10.1128/jvi.54.1.161-170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Swift R. A., Boerkoel C., Ridgway A., Fujita D. J., Dodgson J. B., Kung H. J. B-lymphoma induction by reticuloendotheliosis virus: characterization of a mutated chicken syncytial virus provirus involved in c-myc activation. J Virol. 1987 Jul;61(7):2084–2090. doi: 10.1128/jvi.61.7.2084-2090.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Williams G. T., Morimoto R. I. Detection of three protein binding sites in the serum-regulated promoter of the human gene encoding the 70-kDa heat shock protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2203–2207. doi: 10.1073/pnas.84.8.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]