Background: The odontopathogenic virulence factor gingipain RgpB is produced as a zymogen to prevent intracellular activity prior to secretion.
Results: The structure of the complex between the prodomain and the catalytic moiety of RgpB has been determined.
Conclusion: RgpB is kept latent by a novel molecular mechanism.
Significance: The structural details should enable to design small-molecule inhibitors to inhibit RgpB in a noncovalent manner.
Keywords: Cysteine Protease, Peptidases, Protease Inhibitor, Protein Crystallization, X-ray Crystallography, Inhibition, Latency, Pro-peptidase
Abstract
Zymogenicity is a regulatory mechanism that prevents inadequate catalytic activity in the wrong context. It plays a central role in maintaining microbial virulence factors in an inactive form inside the pathogen until secretion. Among these virulence factors is the cysteine peptidase gingipain B (RgpB), which is the major virulence factor secreted by the periodontopathogen Porphyromonas gingivalis that attacks host vasculature and defense proteins. The structure of the complex between soluble mature RgpB, consisting of a catalytic domain and an immunoglobulin superfamily domain, and its 205-residue N-terminal prodomain, the largest structurally characterized to date for a cysteine peptidase, reveals a novel fold for the prodomain that is distantly related to sugar-binding lectins. It attaches laterally to the catalytic domain through a large concave surface. The main determinant for latency is a surface “inhibitory loop,” which approaches the active-site cleft of the enzyme on its non-primed side in a substrate-like manner. It inserts an arginine (Arg126) into the S1 pocket, thus matching the substrate specificity of the enzyme. Downstream of Arg126, the polypeptide leaves the cleft, thereby preventing cleavage. Moreover, the carbonyl group of Arg126 establishes a very strong hydrogen bond with the co-catalytic histidine, His440, pulling it away from the catalytic cysteine, Cys473, and toward Glu381, which probably plays a role in orienting the side chain of His440 during catalysis. The present results provide the structural determinants of zymogenic inhibition of RgpB by way of a novel inhibitory mechanism for peptidases in general and open the field for the design of novel inhibitory strategies in the treatment of human periodontal disease.
Introduction
Periodontitis is a biofilm-associated chronic inflammatory disease of the gums caused by bacterial infection, which affects 10–15% of adults worldwide and may result in tooth loss (1, 2). Of the several hundreds of bacterial species that colonize the oral cavity, the key disease pathogens are Aggregatibacter (Actinobacillus) actinomycetemcomitans, Tannerella forsythia, Treponema denticola, and Porphyromonas gingivalis, the latter three forming the red complex, which is implicated in severe forms of the disease (3–5). P. gingivalis is an opportunistic pathogen found in up to 85% of periodontitis cases, and its presence at the infection site is indicative of disease progression (6, 7). The pathogen requires nutrients, such as heme or vitamin K, and anaerobic conditions for growth (2). As part of the process of infection, P. gingivalis invades host epithelial cells and macrophages, where it affects cell cycle pathways and suppresses apoptosis, thereby circumventing the host immune response and prolonging its survival (2, 8). The pathogen possesses several factors that participate in infection, such as the lipopolysaccharide, the capsular polysaccharide, the fimbriae, and, most importantly, cysteine proteinases, viz. gingipains K (Kgp)3 and R (RgpA and RgpB) (2, 9, 10). Although the former factors are intrinsic components of the outer membrane of the pathogen, gingipains are true cell surface-anchored or soluble virulence factors that, when secreted, account for up to 85% of the total extracellular proteolytic activity of P. gingivalis (10, 11). This activity is aimed at obtaining nutrients, cleavage of host-cell surface receptors, stimulation of protease-activated receptor expression, and inactivation of cytokines and components of the complement system. These functions contribute to resistance of the pathogen to host bactericidal activity and maintenance of the chronic inflammatory condition at the site of infection (2). In addition, gingipains contribute to bleeding and vascular permeability by activating plasma kallikrein, degrading fibrinogen, and increasing the levels of thrombin and prothrombin, thus increasing the availability of heme needed for bacterial growth (12). These functions explain why gingipains are essential for bacterial survival and the pathological outcome of periodontitis (3, 10, 13).
As occurs with most proteolytic enzymes, the activity of gingipains must be regulated to prevent undesired intracellular proteolysis yet yield full activity once secreted (14). In general, such activity control occurs at the transcriptional level, through compartmentalization or allostery, or through inhibition by specific protein inhibitors. Another regulatory mechanism is zymogenic latency (15), which is observed for gingipains and is carried out mostly by N-terminal propeptides or prodomains (PDs). These usually prevent substrates from binding to the active-site cleft of the cognate catalytic domain (CD) and are mostly removed by limited proteolysis during maturation (15–17). Such PDs often fold independently and guide on their part the folding process of the CD (18); they may also act as intramolecular chaperones or inhibitors of the mature enzymes in trans, as described for RgpB (19), and in the intracellular sorting of the zymogen (15). Therefore, the study of the molecular mechanisms by which peptidases maintain latency is indispensable to the understanding of their basic mode of action. It also paves the way for the design of inhibitors that mimic the latent state so as to modulate proteolytic activity as part of a therapeutic approach. Detailed three-dimensional structural information can contribute much to this understanding (20).
Among gingipains, Kgp is specific for peptide bonds after lysines, whereas RgpA and RgpB are arginine-specific (3, 21). These enzymes are multidomain proteins comprising at least a signal peptide, a PD, a CD, an immunoglobulin superfamily domain (IgSF), and a C-terminal domain, as found in the 736-residue RgpB spanning, respectively, 24, 205, 351, 87, and 69 residues (3). RgpA has four additional hemagglutinin/adhesion domains (termed RgpAA1–RgpAA4) inserted between the IgSF and the C-terminal domain, thus totaling 1,706 residues. Kgp may have between three and five such domains (termed KgpAA1–KgpAA5) depending on the bacterial strain, thus totaling 1,723–1,732 residues (3). The CDs of gingipains distantly resemble caspase cysteine proteinases as revealed by the crystal structure of mature RgpB of P. gingivalis strain HG66 (22) and are grouped into MEROPS database family C25 (23).
To understand the biochemical determinants of zymogenicity in gingipains, we analyzed the structure of the complex between the mature enzyme moiety (CD+IgSF domains) and the PD of RgpB from P. gingivalis strain W83. The results revealed a novel molecular mechanism of inhibition of peptidases and could thus pave the way for the design of novel inhibitory strategies that may help in palliating the effects of periodontal disease.
EXPERIMENTAL PROCEDURES
Protein Production and Complex Formation
The wild-type PD of P. gingivalis strain W83 gingipain R2 alias RgpB proteinase (see UniProt database access code P95493) was obtained by cloning the coding sequence (Gln25–Arg229) into the pGEX-6P-1 expression vector using BamHI/XhoI restriction sites, which attached an N-terminal glutathione S-transferase tag and a PreScission protease cleavage site. The vector was transformed into Escherichia coli BL21 (DE3) cells, and overexpression was induced with isopropyl-1-thio-β-d-galactopyranoside. The protein was purified in a glutathione-Sepharose high performance column, cleaved with PreScission protease, and passed again through glutathione-Sepharose. The flow-through was concentrated by ultrafiltration. The final purified protein contained an N-terminal extension of GPLGS as the result of the cloning strategy. The CD plus the IgSF (residues Tyr230–Gly662), fused to a C-terminal hexahistidine tag for purification, were purified from culture medium of the P. gingivalis strain W83 bearing a modified rgpB gene, in which a sequence encoding 6 histidine residues had been inserted in-frame at the junction between the IgSF and the C-terminal domain. This construct results in the secretion of soluble RgpB with a C-terminal His tag as described (24). For complex formation, RgpB (15 mg) was preactivated in gel filtration buffer (50 mm sodium phosphate, 0.15 m sodium chloride, pH 7.2) freshly supplemented with 10 mm l-cysteine for 10 min. The activated RgpB-His6 was then treated with 1 mm N-[5-amino-l-1-(2-chloroacetyl)pentyl]-4-methyl-benzenesulfonamide (TLCK). After a 10–15-min preincubation, the PD was added in 1.5-fold molar excess with respect to RgpB, and the reaction mixture was incubated for another 15 min. All incubations were performed at room temperature. The complex was then separated from excess PD and TLCK by gel filtration on a Superdex 75 10/60 column (GE Healthcare). The purity of the complex was evaluated by native PAGE, and the protein concentration was determined by BCA assay (Sigma). The stability of the complex was assessed by following activity over time after incubation at 37 °C. No catalytic activity was observed after 1 week. This finding correlated well with purified full-length intact pro-RgpB undergoing autocleavage at the maturation site (Arg229–Tyr230) but showing no significant catalytic activity even after 2 weeks of incubation.
Crystallization and Structure Determination
Crystallization assays were performed by the sitting-drop vapor diffusion method. Reservoir solutions were prepared by a Tecan robot, and 100-nl crystallization drops were dispensed on 96 × 2-well MRC plates (Innovadyne) by a Phoenix nanodrop robot (Art Robbins) at the High-Throughput Crystallography Platform at Barcelona Science Park. Plates were stored in Bruker steady temperature crystal farms at 4 and 20 °C. Successful conditions were scaled up to the microliter range with 24-well Cryschem crystallization dishes (Hampton Research). Best crystals were obtained at 20 °C with protein complex solution (9.1 mg/ml in 5 mm Tris·HCl, pH 7.4; 1 mm 1,4-dithio-dl-threitol (DTT); 1 mm TLCK) and 14% polyethylene glycol 6000; 0.1 m sodium acetate, pH 5.0; 0.2 m calcium chloride as reservoir solution (with barium chloride as an additive) from 1:1 μl or 2:1 μl drops. Crystals were cryo-protected by immersion in harvesting solution (21% polyethylene glycol 6000; 0.1 m sodium acetate, pH 5.0; 0.2 m calcium chloride; 20% (v/v) glycerol). A complete diffraction dataset was collected from a liquid-N2 flash-cryo-cooled crystal at 100 K (provided by an Oxford Cryosystems 700 series cryostream) on an Area Detector Systems Corp. Q315R CCD detector at beam line ID14-4 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) within the Block Allocation Group “BAG Barcelona.” This crystal was monoclinic and contained four PD-CD+IgSF complexes per asymmetric unit. Diffraction data were integrated, scaled, merged, and reduced with the programs XDS and XSCALE (25) (see Table 1).
TABLE 1.
Crystallographic data
Values in parentheses refer to the outermost resolution shell.
| Space group/cell constants (a, b, and c, in Å; β in °) | P21/84.4, 133.1, 109.8, 90.5 |
| Wavelength (Å) | 0.9393 |
| No. of measurements/unique reflections | 395,643/106,820 |
| Resolution range (Å) (outermost shell) | 47.3–2.30 (2.36–2.30) |
| Completeness (%) | 99.2 (91.2) |
| Rmergea | 0.070 (0.436) |
| Rr.i.m. ( = Rmeas)a | 0.082 (0.540) |
| Average intensity over S.D. (〈[〈I〉/σ(〈I〉)]〉) | 15.2 (2.6) |
| B-factor (Wilson) (Å2)/Average multiplicity | 40.9/3.7 (2.8) |
| Resolution range used for refinement (Å) | ∞--2.30 |
| No. of reflections in working set/in test set | 101,461 (5,323) |
| Crystallographic Rfactor (free Rfactor)b | 0.189 (0.225) |
| No. of protein atoms/solvent molecules/ligands/ions | 19,119/858/1 (CH2OH)2CHOH/14 Ca2+, 4 Ba2+, 1 Mg2+, 3 Na+, 1 Cl−,1 (OHCH2)3C (NH3+) |
| r.m.s.d. from target values | |
| bond lengths (Å)/bond angles (°) | 0.008/1.04 |
| Overall average B-factor (Å2) | 39.7 |
| Main-chain conformational angle analysisc | |
| Residues in favored regions/outliers/all residues | 2,395/9/2,438 |
a Rrmerge = ΣhklΣi |Ii(hkl) − 〈I(hkl)〉|/ΣhklΣi Ii(hkl); Rr.i.m. = Σhkl(nhkl/[nhkl−1])1/2 Σi |Ii(hkl) − 〈I(hkl)〉|/ΣhklΣi Ii(hkl); Rp.i.m. = Σhkl(1/[nhkl−1])1/2 Σi |Ii(hkl) − 〈I(hkl)〉|/ΣhklΣi Ii(hkl), where Ii(hkl) is the i-th intensity measurement and nhkl the redundancy of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 its average intensity. Rr.i.m. (alias Rmeas) and Rp.i.m. are improved multiplicity-weighted indicators of the quality of the data, the redundancy-independent merging R factor and the precision-indicating merging R factor. The latter is computed after averaging over multiple measurements (for details, see Refs. 71 and 72).
b Crystallographic Rfactor = Σhkl ‖Fobs| − k |Fcalc‖/Σhkl |Fobs|, where k is a scaling factor, and Fobs and Fcalc are the observed and calculated structure factor amplitudes, respectively. This factor is calculated for the working-set reflections; free Rfactor, same for a test-set of reflections not used during refinement.
c According to MOLPROBITY (73).
The structure of the PD-CD+IgSF RgpB complex was solved by likelihood-scoring molecular replacement with the program PHASER (26) using the coordinates of the protein part only of mature RgpB of P. gingivalis strain HG66 (GenBankTM AAB41892; 97% sequence identity; Protein Data Bank (PDB) access code 1CVR (22)). These calculations rendered four unambiguous solutions with values for the rotation/translation function Z-scores of 18.6/18.7, 18.5/40.0, 22.6/62.3, and 19.6/73.1, respectively, and confirmed space group P21 as the correct one. Subsequent density modification with the program DM (27) under 4-fold averaging rendered an electron density map that enabled construction of most of the 205-residue PD for one of the four complexes on a Silicon Graphics Octane2 work station using the program TURBO-FRODO (28). The position and orientation of the other three copies within the asymmetric unit were determined with PHASER. Subsequent model building alternated with crystallographic refinement with the program BUSTER/TNT (29), which included translation libration screw-motion refinement and non-crystallographic symmetry restraints, until completion of the model. The final model contained four PDs (chains A (Arg31–Ser204+Thr210–Arg227), C (Gly30–Leu205+Phe211–Arg229), E (Gly30–Leu205+Phe211–Thr228), and G (Arg31–Ser204+Phe211–Glu226)) and four cognate CD+IgSF moieties (chains B (Gly239–Glu661), D, F, and H (all Asn238–Glu661)). Within each catalytic moiety, the CDs were much more rigid and better defined by proper electron density than the cognate IgSFs; within each CD, loop Lα7η3 (around residue 530) was flexible and traced based on weak electron density to preserve chain continuity. In addition to the protein chains, one barium (tentatively assigned based on the electron density map and presence in the crystallization conditions) and three calcium cations were identified for each CD+IgSF moiety. Furthermore, in addition to a single magnesium and Tris cation, two further tentatively assigned calcium and three sodium cations, as well as one chloride anion, one glycerol molecule, and 858 solvent molecules completed the model. Each of the 4 catalytic cysteine residues (Cys473) evinced extra electron density for its side chain, which we attribute to the purification strategy (see above) and conservatively interpreted as a methylsulfino group (residue type CSD). Pro532 of chain H and 2 residues of each CD (Ser449 and Val474) were the only Ramachandran outliers of the entire structure (see Table 1). The latter two were also outliers in the mature chloromethyl ketone complex structure (22) and are unambiguously defined by proper electron density. Superposition of the three complexes CD, EF, and GH onto AB, respectively, revealed 612 common Cα atoms deviating less than 3 Å, which gave rise to r.m.s.d. values of 0.75, 0.55, and 0.48 Å, respectively, and indicated close similarity of the structures. This was confirmed by an independent superposition of the four CDs, which revealed very similar orientations and positions for the cognate PDs, with just marginal displacements on the proteinase distal surface (maximum 3.8 Å at Glu197). Accordingly, the four complexes were considered equivalent, and the “Results and Discussion” section hereafter refers to complex AB, which had slightly lower overall thermal displacement parameters (A/B, 41.5 Å2/35.1 Å2; C/D, 46.2 Å2/38.1 Å2; E/F, 47.5 Å2/37.8 Å2; and G/H, 48.4 Å2/36.7 Å2), unless otherwise stated. Wherever distances, angles, etc. are mentioned, the range found in all four complexes is indicated.
Miscellaneous
Figures were prepared with the program CHIMERA (30). Interaction surfaces were calculated with CNS (31). Structure similarities were investigated with DALI (32). Model validation was performed with MOLPROBITY (33) and the WHATCHECK routine of WHATIF (34).
RESULTS AND DISCUSSION
An Experimental Model of pro-RgpB
All attempts to crystallize intact full-length pro-RgpB for structural studies failed. Accordingly, the PD (residues Gln25–Arg229) and the mature moiety (residues Tyr230–Gly662) of pro-RgpB from P. gingivalis strain W83 were produced separately and mixed to yield the zymogenic complex (see “Experimental Procedures”). Four such complexes were found in the asymmetric unit of the crystal structure, which was determined by likelihood-scoring molecular replacement and averaging techniques, and refined with diffraction data to 2.3 Å resolution (Table 1). Superposition of the respective mature moieties revealed very similar relative arrangements of the four PDs. In addition, the distances (20–26 Å) between the last residues of the PD moieties defined in the electron density (Glu226/Arg229) and the first of the respective CDs (Asn238/Gly239), both on the surface, could easily be bridged by the missing 8–11 residues running along the molecular surface. Accordingly, the present complex provides a bona fide model of the intact zymogen, which correlates well with the strong inhibitory capacity of the PD on CD+IgSF in trans. The biochemical and kinetic analysis of the interaction revealed a noncompetitive mode of interaction leading to formation of the 1:1 stoichiometric complex, which was stable in native PAGE and size-exclusion chromatography. The stability was due to an apparent inhibition constant in the low nanomolar range (Ki = 6.2 ± 1.0 nm). The very tight inhibitory interaction between the PD and the mature enzyme suggests the existence of a yet unknown mechanism facilitating dissociation of the complex and degradation of the PD in vivo. At present we can only speculate on the nature of this mechanism. It can be related to glycosylation of the gingipain during the secretion process. Alternatively, one of the components of the PorSS secretion system, which is engaged in secretion of virulence factors for periodontopathogenicity (35), could displace the PD during pro-RgpB translocation across the outer membrane and unleash activity.
A Prodomain with a Novel Fold
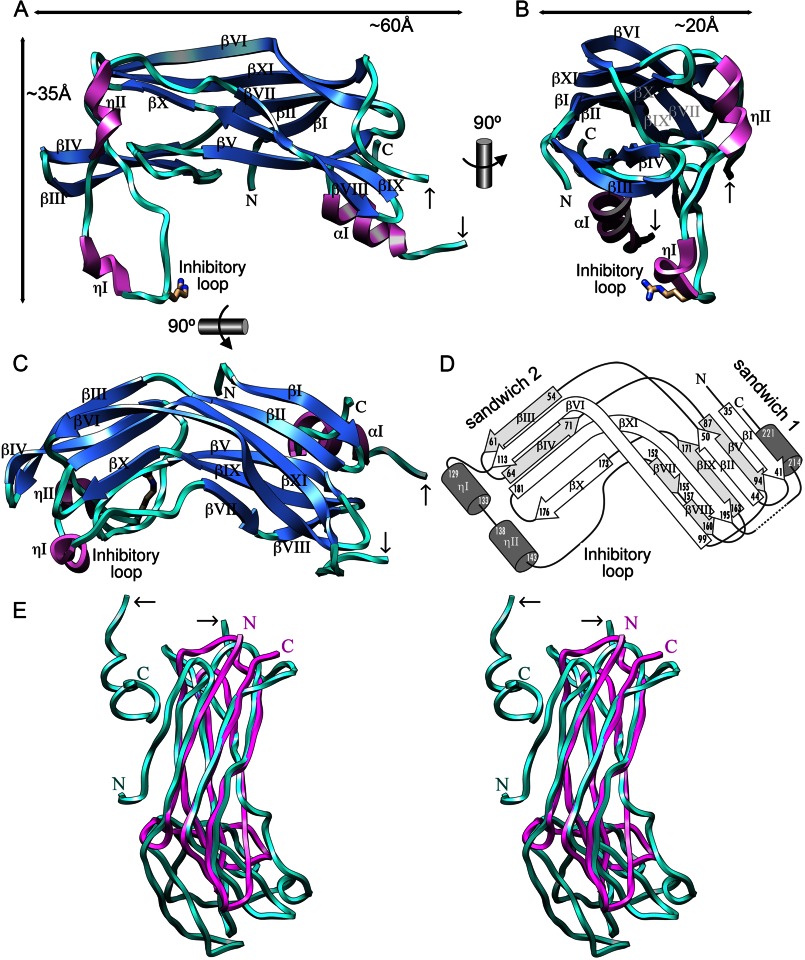
The PD is defined by the electron density for residues Gly30/Arg31–Glu226/Arg229 and has the overall shape of a croissant with maximal dimensions ∼60 × 35 × 20 Å (Fig. 1, A and B). It consists of a central 11-stranded β-core (strands βI–βXI) divided in two antiparallel β-sandwiches, 1 and 2, and decorated on the surface with an α-helix (αI) and two 310-helices (ηI and ηII; Fig. 1, C and D). The two sandwiches are held together by a continuous hydrophobic core that reaches from Leu37, Leu92, and Met223 on the right side of sandwich 1 to Tyr175, Pro177, and Lys180 on the left of sandwich 2 (view as in Fig. 1C). Sandwich 1 consists of a three-stranded back sheet (strands βV, βIX, and βVII+βVIII) and a four-stranded front sheet (strands βI, βII, βXI, and βVI; Fig. 1, C and D). Strands βXI and βVI are N- and C-terminally extended beyond the limits of the sandwich, respectively, and bent by ∼50–60°. In this manner, they also contribute to the three-stranded front sheet of sandwich 2 (strands βVI, βIX, and βX), which is packed against a two-stranded back sheet (strands βIII and βIV). The two sandwiches are roughly perpendicular to each other for the direction of both the contributing strands and the planes of the sheets (Fig. 1, C and D). The strand connectivity of the two sandwiches is such that a first β-ribbon at the top of the front sheet of sandwich 1 (ribbon βIβII) is linked to the β-ribbon that creates the back sheet of sandwich 2 (βIIIβIV). This, in turn, ends in a loop connecting strands βIV and βV (LβIVβV), which is the top strand of the back sheet of sandwich 1. After βV, the chain enters the bottom strand of the front sheet of sandwich 1, βVI (Fig. 1, C and D), the second half of which is the top strand of the front sheet of sandwich 2. After βVI, a 39-residue loop segment runs across the bottom surface of PD. This loop includes 310-helices ηI and ηII and encompasses a so-called “inhibitory loop” (see below; Fig. 1C). This long loop leads to the bottom strand of the back sheet of sandwich 1, which is split in two, βVII and βVIII. The latter contributes to a β-ribbon together with the central back sheet strand of sandwich 1, βIX. Thereafter, the polypeptide enters a β-ribbon (βXβXI), which creates the bottom of the front sheet of sandwich 2. As described for βVI, βXI is also extended and gives rise to the third strand (top to bottom) of the front sheet of sandwich 1 before leaving the β-core of the PD (Fig. 1, C and D). Thereafter, the chain passes through a flexible segment on the right surface, which is disordered at Leu205/Val206–Ser209/Thr210, and ends at the surface-located C-terminal helix αI on the back of sandwich 1, and finishes at Glu226/Arg229.
FIGURE 1.
General architecture of the RgpB prodomain. A, ribbon-type plot of RgpB PD showing the regular secondary structure elements (α- and 310-helices in magenta and labeled αI and ηI–ηII, respectively; β-strand as blue arrows and labeled βI–βXI) and the approximate overall dimensions of the molecule. The inhibitory loop, which includes the S1-intruding residue, Arg126, is also labeled. Two small black arrows pinpoint the residues flanking the disordered segment preceding the C-terminal helix αI. B and C depict orthogonal views of A. D, topology scheme of RgpB PD roughly in the same orientation as in C. Each regular secondary structure element is labeled and marked with its limiting residues. E, superposition in wall-eyed stereo image of RgpB PD (turquoise) and H. pomatia agglutinin (magenta; PDB 2CE6 (36)).
Structural similarity searches identified agglutinin from the Roman snail, Helix pomatia, as the closest structural relative of the PD (PDB 2CE6 (36); Z-score = 6.3; r.m.s.d. = 2.7 Å for 89 common residues according to the program DALI (37)). This is a hexameric sugar-binding lectin from the albumen gland of the gastropod and part of its innate immune system. It belongs to a family of sugar-binding proteins mostly from invertebrates, which also includes discoidin C-terminal domain (PDB 2W94), and is a 3+3 pure β-sandwich. Upon superposition of the PD and agglutinin, it becomes evident that the latter resembles PD sandwich 1, both in connectivity and in topology (Fig. 1E). However, the PD shows an additional β-strand, the N-terminal βI, and, most importantly, the second sandwich and the unique loop structures including the two 310-helices and the C-terminal α-helix. All-β domains similar to agglutinin are also present in glycolytic enzymes, i.e. sugar-binding proteins, such as endocellulase 9G (PDB 1G87), β-xylosidase (PDB 1W91), and endoglycoceramidase II (PDB 2OSX), as well as in the sugar-binding cellulosomal scaffolding protein A (PDB 4B9F), all of which were also identified as structurally related to the PD but which match PD sandwich 1 only. We conclude that the PD of RgpB has a novel fold, hitherto unseen in peptidase zymogens and distantly related to functionally unrelated sugar-binding proteins.
The Mature Enzyme Moiety
The structure of the mature enzyme moiety within the zymogenic complex resembles a molar, with its crown, tooth body, and root. Its superposition onto the structure of a covalent complex of a closely related mature enzyme moiety from a distinct bacterial strain with d-Phe-Phe-Arg-chloromethyl ketone (PDB 1CVR (3, 22)) reveals that, with the exception of some minor changes in side-chain conformations (see next section), the entire structure, including the active-site cleft and the surrounding moiety, is conserved, i.e. the zymogenic conformation of CD+IgSF induced by the PD reveals that the enzyme is probably in a competent conformation in the zymogen, as is often observed in peptidases, thus suggesting that inhibition is mediated by competition with the substrate (38). Briefly, the CD (Asn238/Gly239–Pro580) is subdivided into an N-terminal (or A-) subdomain (NSD; Asn238/Gly239–Glu345) and a C-terminal (or B-) subdomain (CSD; Ser346–Pro580). Both subdomains are α/β-moieties consisting of a central β-sheet, a four-stranded parallel one in the NSD and a six-stranded one parallel for all its strands except the outermost top one in the CSD (Fig. 2B; NSD on top, CSD at bottom). This strand contacts the NSD β-sheet in an approximately perpendicular manner (see also Fig. 2 in Ref. 22). NSD is decorated with one helix and two helices plus a short β-ribbon, respectively, on either side of the sheet. The CSD sheet has four helices and three helices plus a small three-stranded antiparallel β-sheet, respectively, on either side (Fig. 2B). One calcium ion is found in both the NSD and the CSD (the latter cation is most likely a barium in the present zymogenic structure due to the crystallization conditions; see “Experimental Procedures”), and a further one is present at the subdomain interface (Fig. 2B).
FIGURE 2.
The zymogenic complex. A, ribbon-type plot in wall-eyed stereo image of the complex between the RgpB PD (in blue/magenta) and the mature RgpB moiety in front view. The latter consists of domains CD (in yellow/orange) and IgSF (in green/brown). The three calcium ions and the barium ions are depicted as red and magenta spheres, respectively. The inhibitory loop and the respective N and C termini are labeled, in turquoise for PD and in brown for CD+IgSF. Arg126 from the PD inhibitory loop and the active-site residues of CD (Cys473, His440, and Glu381) are further shown as sticks for reference of the active site. B, orthogonal view of A showing the CD in standard orientation (22, 70), i.e. with the view into the active-site cleft, which runs horizontally from left (non-primed side) to right (primed side). C, view of the complex in the orientations of A (left) and B (right) showing the regions of the PD and CD engaged in binding in dark blue and orange, respectively. The rest of each molecule is shown in turquoise and yellow, respectively. D, close-up view in wall-eyed stereo image of the area around the inhibitory loop delimited by a black rectangle in C. The CD moiety is shown as a tan ribbon, and selected residues are labeled and shown for their side chains as sticks with tan carbons. The inhibitory loop (Lys121–Tyr135) is shown as a stick model with carbons in turquoise. Selected residues are also labeled. The barium ion of the CD is depicted as a magenta sphere. Note that the catalytic cysteine, Cys473, is oxidized to 3-sulfino-l-alanine (residue name CSD).
The active-site cleft is found at the “masticating surface” (22) of the molar crown and is formed by the CSD. As with enzymes with an α/β-hydrolase or PLEES fold (39, 40), active-site residues are provided by loops and strands at the C-terminal edge of the central β-sheet, in this case that of the CSD. Cys473 is donated by the loop after the fourth strand (Fig. 2B, bottom to top), His440 is donated by the first strand of the small three-stranded sheet inserted after the third strand, and Glu381 is donated by a loop after the second strand. Although cysteine-histidine dyads are common for cysteine proteinases, including MEROPS family C25 (41), the position and distance of Glu381 to His440 Nϵ2 in the mature enzyme (22) and in the present structure (Fig. 2B) suggest a role in protonation and, thus, side-chain orientation of the catalytic histidine during catalysis in RgpB, as described for an aspartate in the foot-and-mouth disease virus leader cysteine peptidase (42). In the present structure, the catalytic cysteine displayed extra density for its side chain beyond its Sγ atom, possibly due to the purification procedure, which included covalent inhibition steps, and which we conservatively interpreted as a methylsulfino side chain (see “Experimental Procedures”). On the opposite surface to the molar crown, where the active site is located, the downstream IgSF (Thr581–Glu661) is inserted between the NSD and the CSD, thus mimicking the root of the molar. This domain is an antiparallel seven-stranded β-barrel or 3+4 β-sandwich, which contains a fourth calcium-binding site on the surface (Fig. 2A). Its fold corresponds to that of classic immunoglobulin-like domains (43) as found in e.g. α2-macroglobulin (44).
Interaction between the Prodomain and the Catalytic Domain
By contrast with other zymogens that are inhibited by large, structured, globular domains, e.g. those of the metallocarboxypeptidase class (38), the PD does not frontally cover and shield the CD active-site cleft but, rather, attaches laterally through its concave croissant surface to the enzyme moiety (Fig. 2, A and B). The interaction of the PD with the CD (no interaction is observed between the PD and the IgSF) occludes a surface of ∼1,650 Å2 (∼16% of the total PD surface), which is within the range generally described for protein-protein complexes (1,250–1,750 Å2 (45)). It shows a surface complementarity (Sc = 0.75) that is likewise within the range reported for protein oligomers and protein/protein inhibitor interfaces (0.70–0.76 (46)). The interaction results from 82 contacts (<4 Å), among them three salt bridges (Asp216–Lys536, Glu73–Lys553, and Arg126–Asp392), one protein-metal interaction, 21 hydrogen bonds, and hydrophobic interactions between 11 PD and 13 CD residues. Participating segments of the CD are provided by the interface between the subdomains and the active-site cleft: Gly239, Gly296–Thr300, Asn331–Phe338, Glu381–Asp392, Thr438–His440, Val471–Cys473, Asn510–Arg518, Asn533–Lys536, Asn545–Phe548, and Glu552–Asp557. Further interactions include the calcium ion of the NSD. The PD contributes with segments Val68–Ile77 and Ser89–Ser91 (from βIV, LβIVβV, and βV), Ser155–Arg161 and Val167–Asn169 (from βVII, βVIII, LβVIIIβIX, and βIX), and Asn201–Ile203 and Phe211–Val221 (from LβXIαI and αI). In particular, Ile159 oxygen of PD replaces a solvent molecule binding the calcium ion of the NSD in the mature inhibitor-complex structure (PDB 1CVR (22)). This ion has an overall octahedral coordination sphere and is further bound by NSD atoms Val329 oxygen, Asp332 Oδ2, Tyr334 main-chain oxygen, and, bidentately, Glu336 Oδ1 and Oδ2, as well as by a solvent molecule.
Notwithstanding, the most relevant interaction of the prodomain with the catalytic moiety is exerted by the inhibitory loop (Lys121–Tyr135), which is part of the segment flanked by strand βVI and the 310-helix ηII and is inserted like a stinger into the non-primed side of the active-site cleft, so that Arg126-Arg127 occupies the position of a potential scissile bond. The inhibitory loop has a compact structure, which is contributed to by a tight 1,4-turn of type I (Ser125–Glu128), 310-helix ηI (Asn129–Ile133), and a small hydrophobic core created by Pro130, Ile133, Ile124, and Lys121, which interacts with Trp513 of the CD (Fig. 2D). The lateral ζ-ammonium group of Lys121 also contributes to the compact structure of the inhibitory loop through a double hydrogen bond with the main chain at Pro130 and Ile133 (Fig. 2D). Superposition of the present zymogenic complex and the reported chloromethyl ketone-bound enzyme, which mimics a substrate-bound form, reveals that segment Ile124–Arg126 of the prodomain binds to the active-site cleft in a substrate-like manner, i.e. in extended conformation and in the correct orientation. This binding places Ser125 in cleft subsite S2, Ile124 in S3, and, most importantly, Arg126 in S1, thus matching the specificity of RgpB (Fig. 2, C and D). The S1 specificity pocket is lined by Gln511, Val471, Met517, His395, Thr438, and, at the bottom, Asp392, which establishes a bidentate salt bridge with Arg126. In addition, the side chain of Trp513 closes the S1 pocket like a lid. Moreover, Arg126 also interacts with the catalytic His440; the carbonyl oxygen of Arg126 establishes a very strong hydrogen bond with His440 Nδ1 (2.3–2.6 Å apart), causing the imidazole side chain to be rotated slightly around its χ1-angle, away from the catalytic cysteine and toward Glu381 (Fig. 3). This rotation, in turn, gives rise to a second strong hydrogen bond between one of the Glu381 carboxylate oxygens and His440 Nϵ2 (2.6–2.9 Å). This interference with the catalytic residues most likely prevents cleavage of the PD at Arg126-Arg127. In addition, the polypeptide chain folds back after Arg126 toward bulk solvent so that the active-site cleft is free on its primed side, and this also leads the catalytic Cys473 Sγ atom to be too far apart (3.7–3.8 Å) from Arg126-Arg127.
FIGURE 3.
The preformed catalytic moiety. A ribbon plot in wall-eyed stereo image showing the superposition of the CD in the present zymogenic complex (yellow) and its mature inhibitor-bound form (purple; PDB 1CVR (22)) is shown in standard orientation. The two calcium ions (red spheres) and the barium ion (magenta sphere) found in the CD correspond to the zymogenic complex structure. Selected active-site residues are shown as sticks for each structure, as is the covalent inhibitor, atom-colored with green carbons.
A Novel Mechanism of Zymogenic Inhibition
Latency maintenance in cysteine proteinases (17, 47) has been structurally studied for MEROPS family C1 members such as Carica papaya plant papain (48) and caricain (49), and mammalian cathepsins B, K, L, S, and X (50–57). In these cases, the CD and the active-site cleft are, overall, in a preformed competent conformation in the zymogens, and the ∼60–100-residue PDs, which contain a globular part laterally attached to the CD, possess a C-terminal segment that runs across the entire active-site cleft in the opposite orientation to that of a substrate, thus blocking access to the cleft and preventing autolysis. Zymogens of family C14, in turn, include the structurally studied mammalian caspases 1 (58); 3 (59); 7 (60, 61), and 8 (62, 63) and the insect Drosophila caspase-9 ortholog DRONC (64) and Spodoptera frugiperda caspase-1 ortholog (Ref. 65 and PDB 2NN3). Caspases are oligomeric functional enzymes, and activation cleavage entails major rearrangement of the loops flanking the active-site cleft from an incompetent to a competent conformation. There are no truly globular PDs but, rather, short N-terminal and/or internal peptides that are cleaved off during maturation, thus giving rise to two chains in the competent enzymes (60, 61, 66). Zymogenic activation has been also studied for Staphylococcus aureus staphopain B (67), a member of family C47. Here, a large 183-residue globular PD based on a barrel-sandwich hybrid possesses a loop in the middle of the structure that binds, in the opposite orientation to that of substrates, to the cleft of an overall competent CD but only blocks the primed side of the cleft. Streptococcus pyogenes exotoxin B (alias SpeB or streptopain) (68) and Prevotella intermedia interpain A (69) belong to family C10, and here a backing helix of an ∼115-residue α/β-sandwich PD is inserted laterally into the cleft in the zymogen but does not completely block substrate access. Activation entails major rearrangement of a zymogenic hairpin and a latency flap, which leads to a large displacement of the catalytic histidine (69).
In stark contrast, the description of the zymogenic complex of RgpB reported here demonstrates that the PD, the largest structurally characterized to date for a cysteine peptidase with 205 residues, interacts laterally through a large surface with the CD. The distance from the activation cleavage site, Arg229-Tyr230, on the top back surface of the complex in Fig. 2B, to the catalytic cysteine, on the front bottom in Fig. 2B, is >40 Å, strongly suggesting that cleavage and, thus, removal of the PD and activation of the CD occurs in trans, as suggested previously for the cysteine peptidase interpain A (69). The PD intrudes into the active-site cleft through a structurally cohered inhibitory loop in the middle of the domain, thus blocking access to the non-primed side of the cleft only. This mechanism is unlike previously reported ones and is thus unique for cysteine peptidases and peptidases in general. It will pave the way to designing small-molecule inhibitors that mimic the structure of the inhibitory loop and that inhibit RgpB in a noncovalent manner, and may contribute to the development of novel drugs to combat periodontitis.
Acknowledgments
We are grateful to the Automated Crystallography Platform for assistance during crystallization experiments. We acknowledge the help provided by local contacts at the ESRF synchrotron. Funding for data collection was provided in part by the ESRF.
This study was supported in part by grants from European, United States, Polish, Spanish, and Catalan agencies (Polish Ministry of Science and Higher Education Project 137/7.PR-EU/2011/2 (to J. P.); Foundation for Polish Science TEAM Project DPS/424-329/10 (to J. P.); National Institutes of Health Grants DE 09761 and DE 022597 from the NIDCR (both to J. P.); Polish National Science Center Grants 2011/01/B/NZ6/00268 and 2012/04/A/NZ1/00051 (both to J. P.); European Union Grants FP7-HEALTH-F3-2009-223101 “AntiPathoGN” (to F. X. G.-R.), FP7-HEALTH-2010-261460 “Gums&Joints” (to J. P. and F. X. G.-R.), and FP7-PEOPLE-2011-ITN-290246 “RAPID” (to J. P. and F. X. G.-R.); Spanish Ministry of Economy and Competitivity Grants BIO2009-10334, BFU2012-32862, and CSD2006-00015 (all to F. X. G.-R.); Fundació “La Marató de TV3” Grant 2009-100732 (to F. X. G.-R.); and Catalan National Government AGAUR Grant 2009SGR1036 (to F. X. G.-R.)).
The atomic coordinates and structure factors (code 4IEF) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- Kgp
- gingipain K
- Rgp
- gingipain R
- PD
- prodomain
- CD
- catalytic domain
- NSD
- N-terminal subdomain
- CSD
- C-terminal subdomain
- IgSF
- immunoglobulin superfamily domain
- TLCK
- N-[5-amino-l-1-(2-chloroacetyl)pentyl]-4-methyl-benzenesulfonamide
- r.m.s.d
- root mean square deviation.
REFERENCES
- 1. Petersen P. E., Ogawa H. (2005) Strengthening the prevention of periodontal disease: the WHO approach. J. Periodontol. 76, 2187–2193 [DOI] [PubMed] [Google Scholar]
- 2. Bostanci N., Belibasakis G. N. (2012) Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333, 1–9 [DOI] [PubMed] [Google Scholar]
- 3. Yongqing T., Potempa J., Pike R. N., Wijeyewickrema L. C. (2011) The lysine-specific gingipain of Porphyromonas gingivalis : importance to pathogenicity and potential strategies for inhibition. Adv. Exp. Med. Biol. 712, 15–29 [DOI] [PubMed] [Google Scholar]
- 4. Paster B. J., Olsen I., Aas J. A., Dewhirst F. E. (2006) The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol. 2000 42, 80–87 [DOI] [PubMed] [Google Scholar]
- 5. Socransky S. S., Haffajee A. D., Cugini M. A., Smith C., Kent R. L., Jr. (1998) Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144 [DOI] [PubMed] [Google Scholar]
- 6. Yang H. W., Huang Y. F., Chou M. Y. (2004) Occurrence of Porphyromonas gingivalis and Tannerella forsythensis in periodontally diseased and healthy subjects. J. Periodontol. 75, 1077–1083 [DOI] [PubMed] [Google Scholar]
- 7. van Winkelhoff A. J., Loos B. G., van der Reijden W. A., van der Velden U. (2002) Porphyromonas gingivalis, Bacteroides forsythus, and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 29, 1023–1028 [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz O. (2008) The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium, and their interplay. Microbiology 154, 2897–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamont R. J., Jenkinson H. F. (1998) Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62, 1244–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo Y., Nguyen K. A., Potempa J. (2010) Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000 54, 15–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Potempa J., Pike R., Travis J. (1997) Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 378, 223–230 [DOI] [PubMed] [Google Scholar]
- 12. Schifferle R. E., Shostad S. A., Bayers-Thering M. T., Dyer D. W., Neiders M. E. (1996) Effect of protoporphyrin IX limitation on Porphyromonas gingivalis. J. Endod. 22, 352–355 [DOI] [PubMed] [Google Scholar]
- 13. O'Brien-Simpson N. M., Paolini R. A., Hoffmann B., Slakeski N., Dashper S. G., Reynolds E. C. (2001) Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect. Immun. 69, 7527–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holzer H., Heinrich P. C. (1980) Control of proteolysis. Annu. Rev. Biochem. 49, 63–91 [DOI] [PubMed] [Google Scholar]
- 15. Khan A. R., James M. N. (1998) Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 7, 815–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryan P. N. (2002) Prodomains and protein folding catalysis. Chem. Rev. 102, 4805–4816 [DOI] [PubMed] [Google Scholar]
- 17. Wiederanders B. (2003) Structure-function relationships in class CA1 cysteine peptidase propeptides. Acta Biochim. Pol. 50, 691–713 [PubMed] [Google Scholar]
- 18. Tao K., Stearns N. A., Dong J., Wu Q. L., Sahagian G. G. (1994) The proregion of cathepsin L is required for proper folding, stability, and ER exit. Arch Biochem. Biophys 311, 19–27 [DOI] [PubMed] [Google Scholar]
- 19. Mikolajczyk J., Boatright K. M., Stennicke H. R., Nazif T., Potempa J., Bogyo M., Salvesen G. S. (2003) Sequential autolytic processing activates the zymogen of Arg-gingipain. J. Biol. Chem. 278, 10458–10464 [DOI] [PubMed] [Google Scholar]
- 20. Mittl P. R., Grütter M. G. (2006) Opportunities for structure-based design of protease-directed drugs. Curr. Opin. Struct. Biol. 16, 769–775 [DOI] [PubMed] [Google Scholar]
- 21. Pike R., McGraw W., Potempa J., Travis J. (1994) Lysine- and arginine-specific proteinases from Porphyromonas gingivalis: isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269, 406–411 [PubMed] [Google Scholar]
- 22. Eichinger A., Beisel H. G., Jacob U., Huber R., Medrano F. J., Banbula A., Potempa J., Travis J., Bode W. (1999) Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 18, 5453–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rawlings N. D., Barrett A. J., Bateman A. (2012) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 40, D343–D350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skottrup P. D., Leonard P., Kaczmarek J. Z., Veillard F., Enghild J. J., O'Kennedy R., Sroka A., Clausen R. P., Potempa J., Riise E. (2011) Diagnostic evaluation of a nanobody with picomolar affinity toward the protease RgpB from Porphyromonas gingivalis. Anal. Biochem. 415, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabsch W. (2001) Chapter 25.2.9: XDS. in International Tables for Crystallography. Volume F: Crystallography of Biological Macromolecules. (Rossmann M. G., Arnold E. eds.), 1st Ed., pp 730–734, Kluwer Academic Publishers (for The International Union of Crystallography), Dordrecht, The Netherlands [Google Scholar]
- 26. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowtan K. (2010) Recent developments in classical density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carranza C., Inisan A.-G., Mouthuy-Knoops E., Cambillau C., Roussel A. (1999) Turbo-Frodo. in AFMB Activity Report 1996–1999, pp. 89–90, CNRS-UPR 9039, Marseille, France [Google Scholar]
- 29. Blanc E., Roversi P., Vonrhein C., Flensburg C., Lea S. M., Bricogne G. (2004) Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D Biol. Crystallogr. 60, 2210–2221 [DOI] [PubMed] [Google Scholar]
- 30. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 31. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J.-S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 32. Holm L., Kaariainen S., Wilton C., Plewczynski D. (2006) Using Dali for structural comparison of proteins. Curr. Protoc. Bioinformatics 14, 5.5.1–5.5.24 [DOI] [PubMed] [Google Scholar]
- 33. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vriend G. (1990) WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 8, 52–56 [DOI] [PubMed] [Google Scholar]
- 35. Sato K., Naito M., Yukitake H., Hirakawa H., Shoji M., McBride M. J., Rhodes R. G., Nakayama K. (2010) A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanchez J. F., Lescar J., Chazalet V., Audfray A., Gagnon J., Alvarez R., Breton C., Imberty A., Mitchell E. P. (2006) Biochemical and structural analysis of Helix pomatia agglutinin: a hexameric lectin with a novel fold. J. Biol. Chem. 281, 20171–20180 [DOI] [PubMed] [Google Scholar]
- 37. Holm L., Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gomis-Rüth F. X. (2008) Structure and mechanism of metallocarboxypeptidases. Crit. Rev. Biochem. Mol. Biol. 43, 319–345 [DOI] [PubMed] [Google Scholar]
- 39. Ollis D. L., Cheah E., Cygler M., Dijkstra B., Frolow F., Franken S. M., Harel M., Remington S. J., Silman I., Schrag J., et al. (1992) The α/β hydrolase fold. Protein Eng. 5, 197–211 [DOI] [PubMed] [Google Scholar]
- 40. Puente X. S., López-Otín C. (1997) The PLEES proteins: a family of structurally related enzymes widely distributed from bacteria to humans. Biochem. J. 332, 947–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen J. M., Rawlings N. D., Stevens R. A., Barrett A. J. (1998) Identification of the active site of legumain links it to caspases, clostripain, and gingipains in a new clan of cysteine endopeptidases. FEBS Lett. 441, 361–365 [DOI] [PubMed] [Google Scholar]
- 42. Guarné A., Hampoelz B., Glaser W., Carpena X., Tormo J., Fita I., Skern T. (2000) Structural and biochemical features distinguish the foot-and-mouth disease virus leader proteinase from other papain-like enzymes. J. Mol. Biol. 302, 1227–1240 [DOI] [PubMed] [Google Scholar]
- 43. Bork P., Holm L., Sander C. (1994) The immunoglobulin fold. Structural classification, sequence patterns and common core. J. Mol. Biol. 242, 309–320 [DOI] [PubMed] [Google Scholar]
- 44. Marrero A., Duquerroy S., Trapani S., Goulas T., Guevara T., Andersen G. R., Navaza J., Sottrup-Jensen L., Gomis-Rüth F. X. (2012) The crystal structure of human α2-macroglobulin reveals a unique molecular cage. Angew. Chem. Int. Ed. Engl. 51, 3340–3344 [DOI] [PubMed] [Google Scholar]
- 45. Janin J., Chothia C. (1990) The structure of protein-protein recognition sites. J. Biol. Chem. 265, 16027–16030 [PubMed] [Google Scholar]
- 46. Lawrence M. C., Colman P. M. (1993) Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 [DOI] [PubMed] [Google Scholar]
- 47. Potempa J., Golonka E., Filipek R., Shaw L. N. (2005) Fighting an enemy within: cytoplasmic inhibitors of bacterial cysteine proteases. Mol. Microbiol. 57, 605–610 [DOI] [PubMed] [Google Scholar]
- 48. Roy S., Choudhury D., Aich P., Dattagupta J. K., Biswas S. (2012) The structure of a thermostable mutant of pro-papain reveals its activation mechanism. Acta Crystallogr. D Biol. Crystallogr. 68, 1591–1603 [DOI] [PubMed] [Google Scholar]
- 49. Groves M. R., Taylor M. A., Scott M., Cummings N. J., Pickersgill R. W., Jenkins J. A. (1996) The prosequence of procaricain forms an α-helical domain that prevents access to the substrate-binding cleft. Structure 4, 1193–1203 [DOI] [PubMed] [Google Scholar]
- 50. Coulombe R., Grochulski P., Sivaraman J., Ménard R., Mort J. S., Cygler M. (1996) Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 15, 5492–5503 [PMC free article] [PubMed] [Google Scholar]
- 51. Podobnik M., Kuhelj R., Turk V., Turk D. (1997) Crystal structure of the wild-type human procathepsin B at 2.5 Ä resolution reveals the native active site of a papain-like cysteine protease zymogen. J. Mol. Biol. 271, 774–788 [DOI] [PubMed] [Google Scholar]
- 52. Cygler M., Sivaraman J., Grochulski P., Coulombe R., Storer A. C., Mort J. S. (1996) Structure of rat procathepsin B: model for inhibition of cysteine protease activity by the proregion. Structure 4, 405–416 [DOI] [PubMed] [Google Scholar]
- 53. Sivaraman J., Lalumière M., Ménard R., Cygler M. (1999) Crystal structure of wild-type human procathepsin K. Protein. Sci. 8, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sivaraman J., Nägler D. K., Zhang R., Ménard R., Cygler M. (2000) Crystal structure of human procathepsin X: a cysteine protease with the proregion covalently linked to the active site cysteine. J. Mol. Biol. 295, 939–951 [DOI] [PubMed] [Google Scholar]
- 55. LaLonde J. M., Zhao B., Janson C. A., D'Alessio K. J., McQueney M. S., Orsini M. J., Debouck C. M., Smith W. W. (1999) The crystal structure of human procathepsin K. Biochemistry 38, 862–869 [DOI] [PubMed] [Google Scholar]
- 56. Kaulmann G., Palm G. J., Schilling K., Hilgenfeld R., Wiederanders B. (2006) The crystal structure of a Cys25 → Ala mutant of human procathepsin S elucidates enzyme-prosequence interactions. Protein Sci. 15, 2619–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Turk V., Stoka V., Vasiljeva O., Renko M., Sun T., Turk B., Turk D. (2012) Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim. Biophys. Acta 1824, 68–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elliott J. M., Rouge L., Wiesmann C., Scheer J. M. (2009) Crystal structure of procaspase-1 zymogen domain reveals insight into inflammatory caspase autoactivation. J. Biol. Chem. 284, 6546–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walters J., Pop C., Scott F. L., Drag M., Swartz P., Mattos C., Salvesen G. S., Clark A. C. (2009) A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem. J. 424, 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chai J., Wu Q., Shiozaki E., Srinivasula S. M., Alnemri E. S., Shi Y. (2001) Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substrate binding. Cell 107, 399–407 [DOI] [PubMed] [Google Scholar]
- 61. Riedl S. J., Fuentes-Prior P., Renatus M., Kairies N., Krapp S., Huber R., Salvesen G. S., Bode W. (2001) Structural basis for the activation of human procaspase-7. Proc. Natl. Acad. Sci. U.S.A. 98, 14790–14795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu J. W., Jeffrey P. D., Shi Y. (2009) Mechanism of procaspase-8 activation by c-FLIPL. Proc. Natl. Acad. Sci. U.S.A. 106, 8169–8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keller N., Mares J., Zerbe O., Grütter M. G. (2009) Structural and biochemical studies on procaspase-8: new insights on initiator caspase activation. Structure 17, 438–448 [DOI] [PubMed] [Google Scholar]
- 64. Yan N., Huh J. R., Schirf V., Demeler B., Hay B. A., Shi Y. (2006) Structure and activation mechanism of the Drosophila initiator caspase Dronc. J. Biol. Chem. 281, 8667–8674 [DOI] [PubMed] [Google Scholar]
- 65. Forsyth C. M., Lemongello D., LaCount D. J., Friesen P. D., Fisher A. J. (2004) Crystal structure of an invertebrate caspase. J. Biol. Chem. 279, 7001–7008 [DOI] [PubMed] [Google Scholar]
- 66. Fuentes-Prior P., Salvesen G. S. (2004) The protein structures that shape caspase activity, specificity, activation, and inhibition. Biochem. J. 384, 201–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Filipek R., Szczepanowski R., Sabat A., Potempa J., Bochtler M. (2004) Prostaphopain B structure: a comparison of proregion-mediated and staphostatin-mediated protease inhibition. Biochemistry 43, 14306–14315 [DOI] [PubMed] [Google Scholar]
- 68. Kagawa T. F., Cooney J. C., Baker H. M., McSweeney S., Liu M., Gubba S., Musser J. M., Baker E. N. (2000) Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. U.S.A. 97, 2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mallorquí-Fernández N., Manandhar S. P., Mallorquí-Fernández G., Usón I., Wawrzonek K., Kantyka T., Solà M., Thøgersen I. B., Enghild J. J., Potempa J., Gomis-Rüth F. X. (2008) A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A. J. Biol. Chem. 283, 2871–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schechter I., Berger A. (1967) On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 27, 157–162 [DOI] [PubMed] [Google Scholar]
- 71. Weiss M. S. (2001) Global indicators of X-ray data quality. J. Appl. Crystallogr. 34, 130–135 [Google Scholar]
- 72. Evans P. (2006) Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 [DOI] [PubMed] [Google Scholar]
- 73. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]