Background: Cep57 has been shown to be a centrosome protein with microtubule-binding and microtubule-bundling activities.
Results: Cep57 localizes to the midbody and plays roles in central spindle microtubule organization to ensure the completion of cytokinesis.
Conclusion: Cep57 is required for cytokinesis.
Significance: We identified the function of Cep57 in cytokinesis as a novel factor of central spindle microtubule organization.
Keywords: Cell Cycle, Centrosome, Microtubules, Mitosis, Mitotic Spindle, Cep57, Tektin 1, Central Spindle, Cytokinesis, Midbody
Abstract
Cytokinesis is the final stage of cell division in which the cytoplasm of a cell is divided into two daughter cells after the segregation of genetic material, and the central spindle and midbody are considered to be the essential structures required for the initiation and completion of cytokinesis. Here, we determined that the centrosome protein Cep57, which is localized to the central spindle and midbody, acts as a spindle organizer and is required for cytokinesis. Depletion of Cep57 disrupted microtubule assembly of the central spindle and further led to abnormal midbody localization of MKLP1, Plk1, and Aurora B, which resulted in cytokinesis failure and the formation of binuclear cells. Furthermore, we found that Cep57 directly recruited Tektin 1 to the midbody matrix to regulate microtubule organization. Thus, our data reveal that Cep57 is essential for cytokinesis via regulation of central spindle assembly and formation of the midbody.
Introduction
Cytokinesis, the final stage of cell division, distributes the cytoplasm of a single cell into two daughter cells, which is crucial for the completion of high-fidelity transmission of genetic material (1, 2). The central spindle is composed of bundles of antiparallel interpolar microtubules between separated chromosomes and is considered to be one of the major cytokinetic machineries that ensure the completion of cytokinesis (3). At anaphase, as the ingressing cleavage furrow, the central spindle microtubules are compacted into the midbody, a narrow intercellular bridge, whereas the central distinct structure of the midbody, termed the Flemming body, is embedded in a highly electron-dense midbody matrix (4–6). In the central spindle and midbody matrix, a number of proteins, including mitotic regulators, such as mitotic kinases Plk1 (Polo-like kinase 1) (7) and Aurora B (8), and microtubule-bundling proteins, are involved in cytokinesis (4). The microtubule-bundling protein PRC1 (protein regulating cytokinesis 1) directly binds to microtubules and forms cross-bridges with a diameter of ∼35 nm between antiparallel microtubules of the central spindle, and it is required for central spindle assembly and stability (9, 10). Cep55, a microtubule-associated protein, localizes to the spindle midzone through association with the MKLP1 (mitotic kinesin-like protein 1)-male germ cell Rac GTPase-activating protein centralspindlin complex, which is essential for the midbody structure and successful cytokinesis (3, 11). Tektin 2 organizes the central spindle and midbody microtubules, and its depletion disrupts proper midbody assembly and leads to cytokinesis failure (12).
Cep57, also named Translokin, was initially identified as an intracellular trafficking mediator of FGF-2 (13). Cep57 was then found to be a centrosome component in a proteomic screen (14). The N-terminal region of Cep57 is responsible for its centrosome localization, and the C-terminal region contains a microtubule-binding domain (15). Recently, we found that Cep57 is required for spindle microtubule organization and for maintaining spindle pole integrity (16). In addition, Xenopus Cep57 (a Cep57-related protein) is involved in kinetochore-microtubule attachment (17). Loss-of-function mutations in Cep57 lead to mosaic variegated aneuploidy syndrome (18). Thus, the Cep57 family has a close relationship with the microtubule network and plays very important roles in mitosis. Here, we found that Cep57 also localizes to the central spindle and midbody and acts as a factor of central spindle microtubule organization during cytokinesis.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The full-length and truncated cDNAs of Cep57 and Tektin 1 were amplified from mouse and inserted into pEGFP-N3 (Clontech), pET-28a (Novagen), pGADT7 (Clontech), pGBKT7 (Clontech), and pCMV-Tag2B (Stratagene).
Cell Culture, Transfection, and Drug Treatment
Cells culture, transfection, and synchronization were performed as described previously (16). For blebbistatin (Sigma) treatment, cells were treated with 2.5 mm thymidine for 24 h, released to prophase, and then treated with 100 μm blebbistatin for 2 h. Cells were synchronized to post-anaphase with blebbistatin and chilled to 0 °C in the continuous presence of nocodazole to depolymerize microtubules.
Immunoprecipitation and Immunoblotting
The cells used for immunoprecipitation were washed three times with PBS and then lysed in 50 mm Hepes, 1 mm EGTA, 0.5% Triton X-100, and 150 mm NaCl (pH 7.4). The immunoprecipitation and immunoblotting assays were performed as described previously (16, 19).
Antibodies
To generate antibodies to Cep57, the cDNA of mouse Cep57 was subcloned into the pET-28a vector, and the recombinant protein of Cep57 was expressed in BL21 bacteria and purified with nickel-nitrilotriacetic acid beads (Invitrogen) under denaturing conditions. Purified protein was injected into mice and rabbits to produce polyclonal antibodies. The rabbit antibodies to Cep57 was affinity-purified with the recombinant GST-Cep57(332–500) fusion protein (16). Mouse and rabbit polyclonal antibodies to Tektin 1 were generated following a similar procedure. The other antibodies used were anti-α-tubulin (DM1A, Sigma), anti-β-tubulin (9F3, Cell Signaling), anti-Tektin 2 (Abcam), anti-Aurora B (BD Transduction Laboratories), anti-MKLP1 (Santa Cruz Biotechnology), anti-Plk1 (Zymed), anti-GFP (MBL), anti-HA (Sigma), and anti-GAPDH (Biolinks).
Immunofluorescence and Microscopy
Indirect immunofluorescence was performed as described previously (16). A fluorescence microscope (Olympus TH4–200) and confocal microscopes (Leica TCS SP2 and Zeiss LSM 710 NLO) were used to observe the samples. For time-lapse microscopy, HeLa cells cotransfected with Cep57 siRNAs and α-tubulin-pCAsalGFP (20) using Lipofectamine 2000 (Invitrogen) were observed under an API DeltaVision Elite time-lapse microscope in a chamber at 37 °C under 5% CO2. Images were processed with DeltaVision softWoRx software. Immunoelectron microscopy was performed as described previously (16). The samples were observed with a transmission electron microscope (Jeol JEM 1010).
RNA Interference
The Cep57 siRNA sequences were as follows: 5′-AAGCATGCAGAAATGGAGAGG-3′, 5′-AACCATCAAGGTCTAATGGAA-3′, 5′-AACCAAATAACTAAAGTTCGA-3′ (13, 16, 21). The siRNAs were synthesized by GenePharma. The Tektin 1 siRNA sequences were 5′-GGAGTTAGATGACAAACTT-3′ and 5′-CCGTGAGGATTGAGCCAAA-3′. The negative control siRNA sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. The siRNAs were synthesized by Invitrogen and transfected with Lipofectamine 2000. The transfection concentration of each siRNA was 33.3 nm, so the total concentration was 100 nm. For siRNA-resistant Cep57 cDNA construction, PCR was used to mutate the siRNA-targeted regions to 5′-AAACACGCGGAGATGGAAAGA-3′, 5′-AGCCGTCTCGCTCAAACGGCA-3′, or 5′-AATCAGATCACAAAGGTAAGGA-3′, respectively.
Yeast Two-hybrid Screen
The yeast two-hybrid screen was based on the Cep57 N terminus (amino acids 1–265) as a bait and was performed as recommended for the Matchmaker two-hybrid system (Clontech) using a mouse embryo brain library.
Statistical Analysis
Statistical analyses were performed using SPSS software. Statistical significance was calculated by Student's t test. The fluorescence intensity was measured using Scion Image software (National Institutes of Health).
RESULTS
Cep57 Localizes at the Central Spindle and Midbody
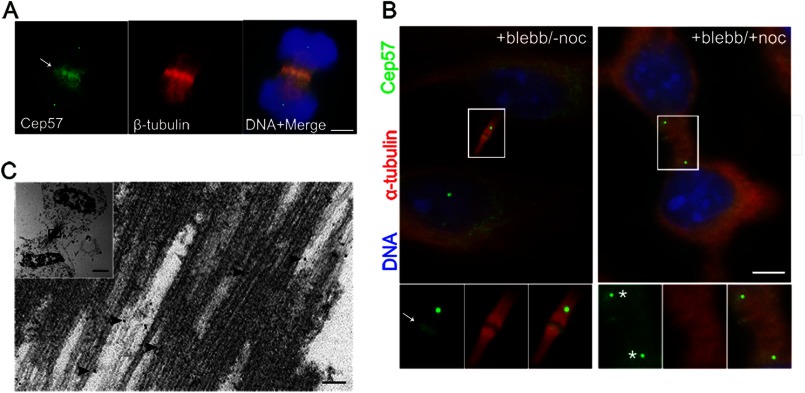
We and others have identified Cep57 as a centrosome protein (14–16). We further found that Cep57 was concentrated at the central spindle during anaphase and in the Flemming body of the midbody during cytokinesis (Fig. 1, A–C, arrows and arrowheads). Our immunoelectron microscopy data showed that Cep57 localized along microtubules at the midbody (Fig. 1C, arrowheads). As Cep57 directly binds to microtubules and facilitates central spindle microtubule organization (15, 16), we next investigated whether the central spindle and midbody localization of Cep57 is dependent on microtubules. We used blebbistatin, a non-muscle myosin II inhibitor, to synchronize HeLa cells to late anaphase and then added nocodazole to depolymerize microtubules (12, 22). After the treatment, Cep57 was absent from the Flemming body, whereas its centrosome localization was not affected (Fig. 1B, asterisks). These results suggests that Cep57 localization to the Flemming body is microtubule-dependent.
FIGURE 1.
Cep57 is a central spindle and midbody component. A, indirect immunofluorescence of anaphase cells with anti-Cep57 (green) and anti-β-tubulin (red) antibodies. DNA was labeled with DAPI (blue). The arrow indicates Cep57 in the midzone. Scale bar = 5 μm. B, HeLa cells were treated with 100 μm blebbistatin (blebb) for 2 h. After washout of blebbistatin, cells were subjected to fresh 37 °C culture medium or to 10 μg/ml nocodazole (noc) at 0 °C for 1 h. Shown is the indirect immunofluorescence of cells with anti-Cep57 (green) and anti-α-tubulin (red) antibodies. DNA was labeled with DAPI (blue). The arrow indicates Cep57 at the midbody. The asterisks indicates Cep57 at the centrosome. Scale bar = 5 μm. C, immunoelectron microscopy images of Cep57 localization within the midbody of a HeLa cell. Arrowheads indicates antibody-conjugated gold particles. The inset shows a lower magnification image of the cell undergoing cytokinesis. Scale bars = 100 nm and 2 μm (inset).
Depletion of Cep57 Causes Cytokinesis Failure
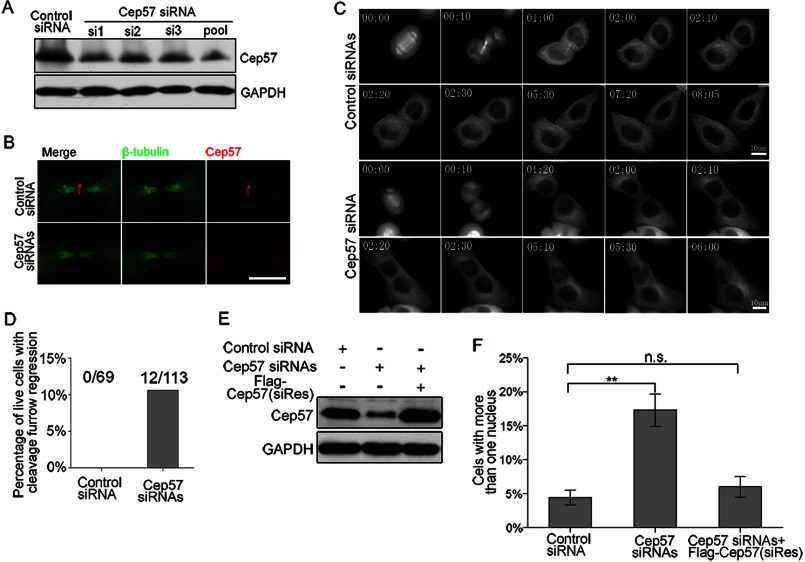
To test the role of Cep57 in cytokinesis, we performed RNAi experiments and successfully decreased the protein level of Cep57 in HeLa cells (Fig. 2, A and B). Next, we used time-lapse microscopy to record the progress of cytokinesis. Compared with control cells (Fig. 2C and supplemental Movie S1), the Cep57-depleted cells also formed a cleavage furrow and midbody but then fused back and formed a binuclear cell. Although the Cep57-depleted binuclear cell did assemble a midbody, cytokinesis was not completed (Fig. 2C and supplemental Movie S2). Twelve of 113 recorded Cep57-depleted live cells showed cleavage furrow regression, whereas none was detected in 69 control cells (Fig. 2D). Compared with the control (∼4%), the percentage of cells with more than one nucleus increased to ∼17% with Cep57 siRNA treatment (Fig. 2, E and F). Furthermore, the number of multinucleated cells could be restored by overexpressing the FLAG-tagged siRNA-resistant Cep57 mutant (Fig. 2, E and F), which confirmed siRNA target specificity of the knockdown results. Therefore, Cep57 is required for the completion of cytokinesis.
FIGURE 2.
Cep57 depletion results in incomplete cytokinesis. A, immunoblots of Cep57 from HeLa cells transfected with 100 nm control and Cep57 siRNAs for 60 h. B, immunofluorescence images of control and Cep57 siRNA-transfected HeLa cells. The cells in telophase were stained with anti-β-tubulin (green) and anti-Cep57 (red) antibodies. Scale bar = 5 μm. C, representative images of HeLa cells cotransfected with control or Cep57 siRNA recorded by time-lapse microscopy. Frames were captured at the indicated time points (hours:minutes) from early anaphase to cytokinesis. Scale bars = 5 μm. See also supplemental Movies S1 and S2. D, percentage of observed live cells that exhibited cleavage furrow regression (control siRNA, n = 69 cells; Cep57 siRNA, n = 113 cells). E, immunoblots of Cep57 from control siRNA- or Cep57 siRNA-transfected HeLa cells or HeLa cells transfected with Cep57 siRNA and FLAG-tagged siRNA-resistant Cep57 (siRes). F, percentage of cells with more than one nucleus. HeLa cells were treated with the indicated siRNAs for 60 h (mean of three separate trials). A minimum of 500 cells were counted per sample. Error bars represent ± S.E. **, p < 0.01; n.s., not statistically significant.
Cep57 Is Necessary for Central Spindle Microtubule Organization
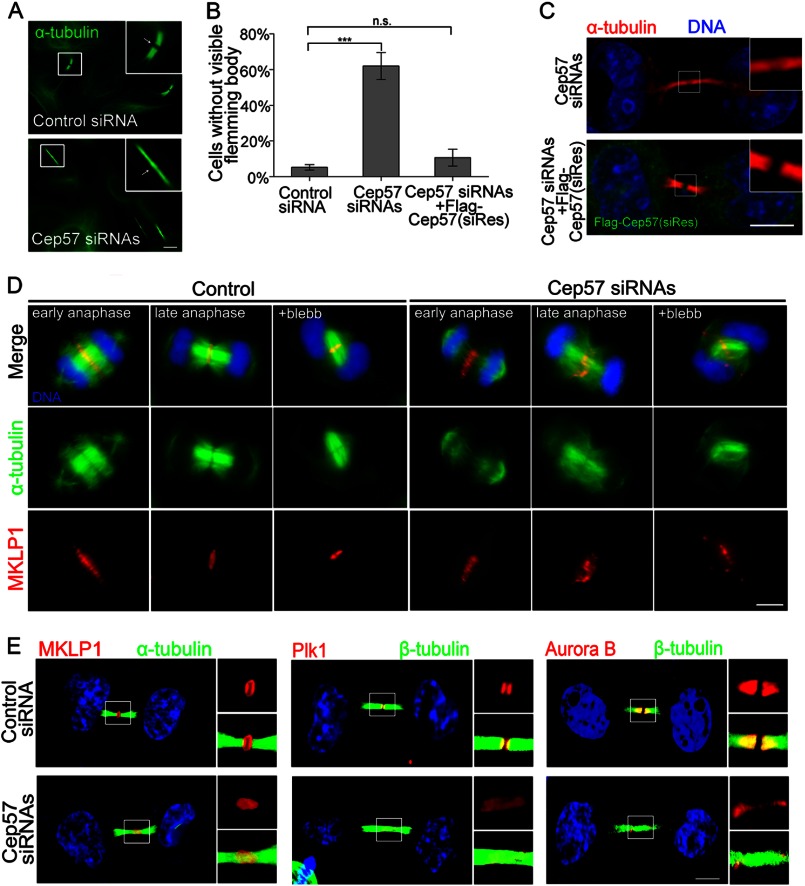
To address the functional mechanism of Cep57 in cytokinesis, we treated HeLa cells with Cep57 siRNAs and synchronized them to telophase. We found that >50% of the Cep57 knockdown cells lacked a visible Flemming body (Fig. 3, A and B). This phenotype was rescued by overexpression of siRNA-resistant FLAG-Cep57 (Fig. 3, B and C). Staining for α-tubulin did not show any split site at the midbody region in Cep57-depleted cells (Fig. 3, A–C), suggesting that the midbody microtubule organization was disrupted so that the microtubule staining was continuous throughout the intercellular bridge linking the two daughter cells.
FIGURE 3.
Depletion of Cep57 perturbs the microtubule organization of the central spindle and the localization of the midbody components MKLP1, Plk1, and Aurora B. A and B, HeLa cells transfected with control siRNA, Cep57 siRNA, or Cep57 siRNA and FLAG-tagged siRNA-resistant Cep57 (siRes) were treated with 2.5 mm thymidine for 24 h to G1/S phase and then released for 12 h to telophase. The cells were stained with anti-α-tubulin antibody (A; green). Scale bar = 5 μm. B shows the increased percentage of Cep57-depleted cells with no split site (A, arrow in lower panel) at the midbody region. A minimum of 100 cells were counted per sample in three independent experiments. Error bars represent ± S.E. ***, p < 0.001; n.s., not statistically significant. C, immunofluorescence images of HeLa cells treated with the indicated siRNAs for 60 h. The cells were stained with anti-α-tubulin (red) and anti-FLAG (green) antibodies. DNA was labeled with DAPI (blue). Scale bar = 5 μm. D, immunofluorescence images showing that depletion of Cep57 disrupts central spindle microtubule organization and MKLP1 localization. Images are shown of control and Cep57-depleted cells in anaphase with or without blebbistatin (blebb) treatment. The assembly state of the anaphase central spindle is shown by α-tubulin (green), MKLP1 (red), and DNA (blue). Scale bar = 5 μm. E, immunofluorescence images of Cep57-depleted and control cells. The cells at telophase were stained for MKLP1 (red), Plk1 (red), Aurora B (red), tubulin (green), and DNA (blue). Scale bar = 5 μm.
We further examined the central spindle to determine whether the midbody assembly defect was induced by disruption of microtubule organization. The central spindle is a transient compact structure composed of bundled microtubules at anaphase (23). In Cep57-depleted cells, the microtubules in the central spindle became jumbled and poorly bundled (Fig. 3D). Thus, Cep57 may be required for cytokinesis by playing a structural role in stabilizing the microtubules and ensuring proper central spindle organization and correct midbody assembly, which ensure the accomplishment of cytokinesis (4, 6, 12).
We continued our investigation of the role of Cep57 in the localization of characterized central spindle and midbody components: MKLP1 (8), Aurora B (24), and Plk1 (7). Mislocalization of these proteins could in turn exacerbate cytokinesis failure. After Cep57 knockdown, they were still localized at the midzone, but their localization pattern within the central equator and Flemming body was drastically disrupted (Fig. 3, D and E; and supplemental Fig. S1). In Cep57-depleted cells, MKLP1 was not concentrated at the central spindle equator plate, and its elliptical localization at the midbody was disrupted (Fig. 3, D and E). Plk1 was diffused and localized like a disordered stick in the midzone instead of accumulating in two narrow and separated pieces (Fig. 3E and supplemental Fig. S1). Aurora B staining also was extended along the microtubules and abnormally localized to the midbody (Fig. 3E and supplemental Fig. S1). Collectively, the depletion of Cep57 not only resulted in defective microtubule bundling but also disrupted the localization of several key midzone components.
Cep57 Interacts with Tektin 1
To explore the Cep57-interacting proteins, we performed yeast two-hybrid screening experiments using the N-terminal domain of Cep57 (amino acids (1–265) as a bait. Tektin 1, a structural component of axonemal doublet microtubules in cilia and flagella (25, 26), was identified as a Cep57-binding protein (supplemental Fig. S2A).
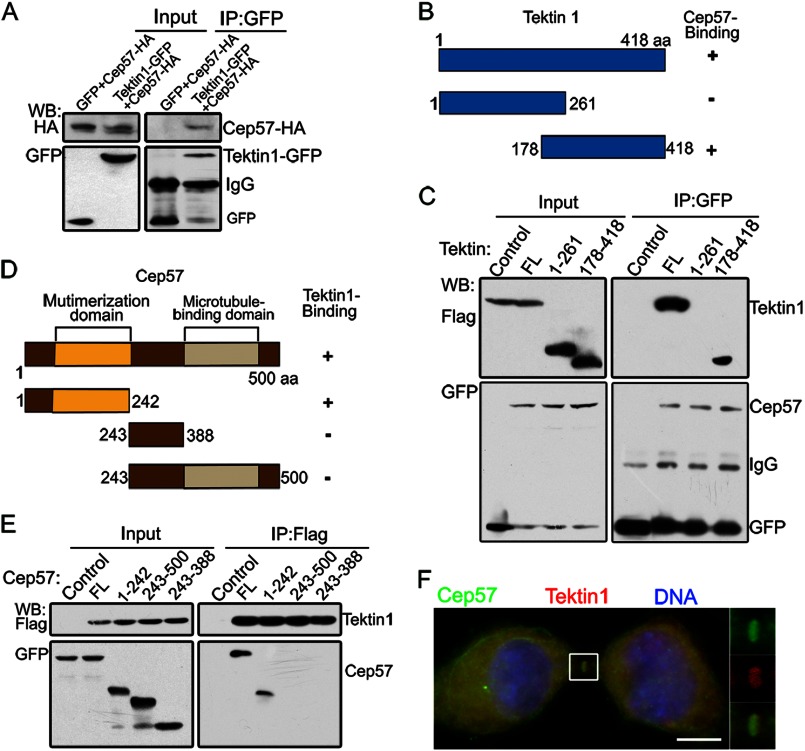
Co-immunoprecipitation assays further confirmed the interaction of Cep57 and Tektin 1 (Fig. 4A). We then constructed truncated mutants of Cep57 and Tektin 1 to map their interacting domains. The C terminus (amino acids 178–418) of Tektin 1 and the N terminus (amino acids 1–242) of Cep57 were found to be responsible for their interaction (Fig. 4, B–E). Furthermore, Tektin 1 colocalized with Cep57 at the midbody and centrosome (Fig. 4F and supplemental Fig. S2, B and C). Thus, these data reveal that Tektin 1 is a binding partner of Cep57 in the midbody.
FIGURE 4.
Cep57 interacts with Tektin 1. A, immunoblots from 293T cells transfected with Cep57-HA and Tektin 1-GFP or control vectors. Lysates were immunoprecipitated (IP) and immunoblotted with the indicated antibodies. WB, Western blotting. B and D, schematic pictures of Tektin 1 and Cep57 truncations. aa, amino acids. C and E, mapping interacting fragments between Tektin 1 and Cep57. Co-immunoprecipitation was performed with the indicated antibodies in lysates of 293T cells coexpressing Cep57-GFP and FLAG or FLAG-Tektin 1, followed by immunoblotting with the indicated antibodies. FL, full-length. F, immunofluorescence images of HeLa cells stained for Cep57 (green) and Tektin 1 (red). DNA was labeled with DAPI (blue). Scale bar = 5 μm.
Cep57 Recruits Tektin 1 to the Midbody and Functions with It Cooperatively in Midbody Assembly
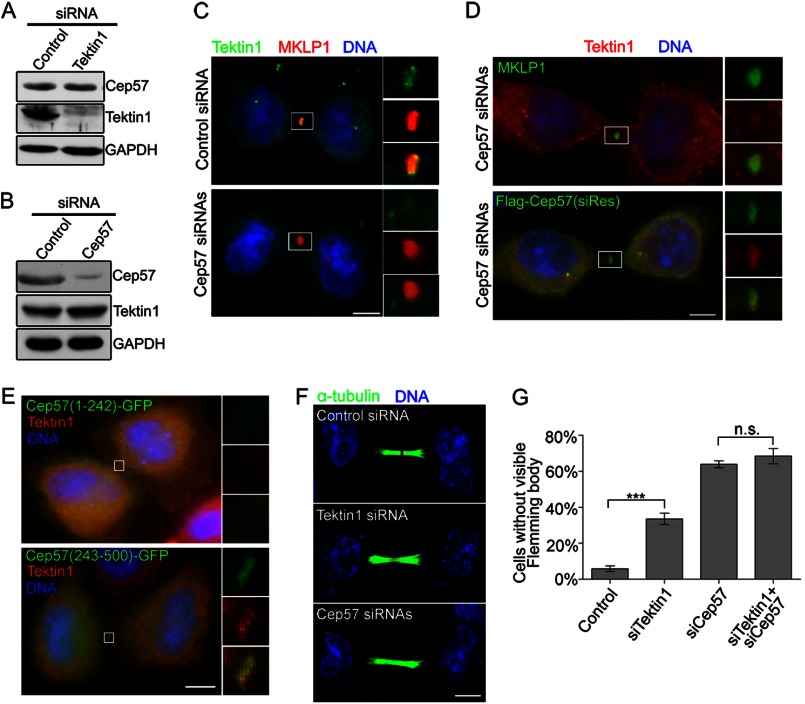
To further investigate the relationship between Cep57 and Tektin 1, we performed RNAi experiments (Fig. 5, A and B). Depletion of Tektin 1 did not change the fluorescence intensity of Cep57 in the midbody (supplemental Fig. S2B), whereas the localization of Tektin 1 at the midbody was significantly weakened after Cep57 depletion (Fig. 5C). Overexpression of siRNA-resistant FLAG-Cep57 in these Cep57-depleted cells restored Tektin 1 localization to the midbody (Fig. 5D). Furthermore, overexpressed Cep57(1–242), which acted as a dominant-negative mutant, but not Cep57(243–500), without the Tektin 1-interacting domain, decreased the midbody localization of Tektin 1 (Fig. 5E). Thus, Cep57 is necessary for the midbody localization of Tektin 1 (Fig. 5, C–E). However, Cep57 depletion did not affect the centrosome localization of Tektin 1 (supplemental Fig. S2C), which may be recruited by other centrosome proteins.
FIGURE 5.
Cep57 affects Tektin 1 midbody localization. A and B, Western blotting performed after 60 h of control and Cep57 siRNA transfection. Cep57 (A) and Tektin 1 (B) in HeLa cells were detected by immunoblotting with the indicated antibodies. C, immunofluorescence images showing synchronized telophase HeLa cells treated with Cep57 or control siRNA stained for Tektin 1 (green), MKLP1 (red), and DNA (blue). Scale bar = 5 μm. D, immunostaining of MKLP1 (green), FLAG-Cep57 (green), and Tektin 1 (red) in HeLa cells transfected with Cep57 siRNA alone or together with FLAG-tagged siRNA-resistant Cep57 (siRes). DNA was labeled with DAPI (blue). Scale bar = 5 μm. E, immunostaining of Tektin 1 (red) in HeLa cells transfected with GFP-tagged Cep57 truncated mutants (green). Scale bar = 5 μm. F, immunofluorescence images of HeLa cells stained for α-tubulin (green). The cells were transfected with Tektin 1 or Cep57 siRNA and synchronized to telophase. DNA was labeled with DAPI (blue). Scale bar = 5 μm. G, percentage of cells without a visible Flemming body at 60 h after the indicated siRNA treatment (mean of three separate trials). A minimum of 200 cells were counted per sample. Error bars represent ± S.E. ***, p < 0.001; n.s., not statistically significant. siTektin1, Tektin 1 siRNA; siCep57, Cep57 siRNAs.
Tektin 2, another member of the Tektin family, is a midbody component and is indispensable for central spindle microtubule organization (12). Our results suggested that Tektin 1 also has a similar function at the midbody during cytokinesis. About 38% of the Tektin 1 knockdown cells lacked a visible Flemming body, and ∼64% cells lacked a visible Flemming body after depletion of Cep57 (Fig. 5, F and G). After simultaneous depletion of Cep57 and Tektin 1, the percentage of cells without a visible Flemming body was ∼68%, which had no significant statistical difference compared with Cep57 depletion alone (Fig. 5G). This result indicates that Cep57 and Tektin 1 function by cooperating during midbody assembly but not through separate pathways. Because Tektin 1 and Tektin 2 belong to the same family, we further tested whether Cep57 also interacts with Tektin 2. The immunoprecipitation results did not show any detectable bands of their interaction (supplemental Fig. S2D). Sequence alignment results showed that Tektin 1 has only limited sequence similarity (∼30%) to Tektin 2. Thus, it is possible that Cep57 interacts only with Tektin 1.
DISCUSSION
Cep57 is a centrosome component recruited by NEDD1 (16) and forms a ring-like structure at the proximal end of the centriole, where it nucleates and stabilizes microtubules (27). During anaphase, the centrosome localization of Cep57 decreased. Cep57 accumulated at the central spindle and midbody instead (Fig. 1, A and B). A similar phenomenon has been reported for some centrosome proteins, such as Cep55 and centriolin (11, 28). The kinesin protein MKLP1 directly binds to Cep55 and mediates its midbody localization (11). A previous study showed that Cep57 interacts with the kinesin motor Kif3a (29), which was also confirmed by our experiments (data not shown). It will be interesting to test whether the midbody localization of Cep57 is mediated by Kif3a.
Microtubules in the midzone are stable and highly compacted, which requires many microtubule-binding proteins to bundle the overlapping microtubule plus-ends (30). PRC1, as a microtubule cross-linker, can form homodimers that selectively bind to antiparallel microtubules and that accumulate at the plus-ends of the midzone microtubules. It forms cross-bridges of ∼35 nm between overlapping microtubules of the central spindle (31). Cep57 also localized to the overlapping microtubule plus-ends at the midbody (Fig. 1, B and C). Similar to PRC1, Cep57 has a C-terminal microtubule-binding domain, and its N-terminal domain can perform oligomerization (15), which would allow it to cross-link and bundle central spindle microtubules. Furthermore, except for its direct binding to microtubules, we found that Cep57 interacted with and recruited Tektin 1 to the midbody (Figs. 4 and 5). Tektin 1 belongs to a conserved Tektin protein family, which includes several coiled-coil proteins and which is involved in many developmental processes, such as fertilization (25). Studies on sperm flagella from sea urchin suggested that Tektin proteins may interact with the plus-ends of doublet microtubules and be related to the control of axonemal length (25). Therefore, Cep57 and Tektin 1 might have a cooperative function in the organization of the overlapping microtubule plus-ends at the central spindle and midbody, suggesting that the function of Cep57 in microtubule-bundling activity also could be related to its interacting proteins. However, the precise mechanism by which Cep57 bundles microtubules in the midbody remains to be elucidated. Depletion of Cep57 caused central spindle microtubules to be poorly bundled and disrupted, which resulted in the mislocalization of central spindle components, such as MKLP1, Aurora B, and Plk1 (Fig. 3, D and E; and supplemental Fig. S1). The mislocalization was considered to be indirectly achieved by disorganization of microtubules because we did not find that Cep57 interacted directly with these proteins.
∼10% of the Cep57-depleted cells showed cleavage furrow regression and failed to complete cell abscission (Fig. 2, C and D). Cep57 has also been reported to be required for spindle pole integrity (16). In addition, a recent study reported that Cep57 is related to prostate cancer and is required for centriole duplication (32). Therefore, Cep57 regulates the cell cycle at multiple stages to ensure correct segregation of the genetic material during mitosis. In Cep57-depleted cells, cytokinesis failure leads to an increase in chromosome number. The chromosome instability may be related to diseases. Indeed, mutations in Cep57 have been identified as a cause of the rare genetic disease mosaic variegated aneuploidy syndrome, which is characterized by mosaic aneuploidies, trisomies, and monosomies (18). The function of Cep57 in cytokinesis may provide a new clue for the pathogenesis of mosaic variegated aneuploidy syndrome or prostate cancer.
In conclusion, Cep57, as a previously identified centrosome protein, also localizes to the central spindle and midbody. Depletion of Cep57 disrupts central spindle microtubule assembly, midzone component localization, and midbody formation. Furthermore, the Tektin family member Tektin 1 localizes to the midbody and is required for midbody assembly. Cep57 interacts with Tektin 1 and regulates its midbody localization. In this study, we have shown that the Cep57-dependent microtubule organization is crucial for central spindle and midbody microtubule assembly, which ensures the completion of cytokinesis.
Acknowledgments
We thank Profs. M. Takeichi and W. Meng for the α-tubulin-pCAsalGFP plasmid and Prof. I. C. Bruce for reading the manuscript.
Footnotes
This work was supported by National Natural Science Foundation of China Grants 30971433 and 31171283 and Major State Basic Research Development Program of China 973 Program Grant 2010CB833705.

This article contains supplemental Figs. S1 and S2 and Movies S1 and S2.
REFERENCES
- 1. Glotzer M. (2005) The molecular requirements for cytokinesis. Science 307, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 2. Gruneberg U., Neef R., Honda R., Nigg E. A., Barr F. A. (2004) Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr F. A., Gruneberg U. (2007) Cytokinesis: placing and making the final cut. Cell 131, 847–860 [DOI] [PubMed] [Google Scholar]
- 4. Steigemann P., Gerlich D. W. (2009) Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol. 19, 606–616 [DOI] [PubMed] [Google Scholar]
- 5. Mullins J. M., Biesele J. J. (1977) Terminal phase of cytokinesis in D-98s cells. J. Cell Biol. 73, 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otegui M. S., Verbrugghe K. J., Skop A. R. (2005) Midbodies and phragmoplasts: analogous structures involved in cytokinesis. Trends Cell Biol. 15, 404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petronczki M., Lénárt P., Peters J. M. (2008) Polo on the Rise–from mitotic entry to cytokinesis with Plk1. Dev. Cell 14, 646–659 [DOI] [PubMed] [Google Scholar]
- 8. Guse A., Mishima M., Glotzer M. (2005) Phosphorylation of ZEN-4/MKLP1 by Aurora B regulates completion of cytokinesis. Curr. Biol. 15, 778–786 [DOI] [PubMed] [Google Scholar]
- 9. Mollinari C., Kleman J. P., Jiang W., Schoehn G., Hunter T., Margolis R. L. (2002) PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subramanian R., Wilson-Kubalek E. M., Arthur C. P., Bick M. J., Campbell E. A., Darst S. A., Milligan R. A., Kapoor T. M. (2010) Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 142, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao W. M., Seki A., Fang G. (2006) Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol. Biol. Cell 17, 3881–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durcan T. M., Halpin E. S., Rao T., Collins N. S., Tribble E. K., Hornick J. E., Hinchcliffe E. H. (2008) Tektin 2 is required for central spindle microtubule organization and the completion of cytokinesis. J. Cell Biol. 181, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bossard C., Laurell H., Van den Berghe L., Meunier S., Zanibellato C., Prats H. (2003) Translokin is an intracellular mediator of FGF-2 trafficking. Nat. Cell Biol. 5, 433–439 [DOI] [PubMed] [Google Scholar]
- 14. Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570–574 [DOI] [PubMed] [Google Scholar]
- 15. Momotani K., Khromov A. S., Miyake T., Stukenberg P. T., Somlyo A. V. (2008) Cep57, a multidomain protein with unique microtubule and centrosomal localization domains. Biochem. J. 412, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Q., He R., Zhou H., Yu A. C., Zhang B., Teng J., Chen J. (2012) Cep57, a NEDD1-binding pericentriolar material component, is essential for spindle pole integrity. Cell Res. 22, 1390–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emanuele M. J., Stukenberg P. T. (2007) Xenopus Cep57 is a novel kinetochore component involved in microtubule attachment. Cell 130, 893–905 [DOI] [PubMed] [Google Scholar]
- 18. Snape K., Hanks S., Ruark E., Barros-Núñez P., Elliott A., Murray A., Lane A. H., Shannon N., Callier P., Chitayat D., Clayton-Smith J., Fitzpatrick D. R., Gisselsson D., Jacquemont S., Asakura-Hay K., Micale M. A., Tolmie J., Turnpenny P. D., Wright M., Douglas J., Rahman N. (2011) Mutations in CEP57 cause mosaic variegated aneuploidy syndrome. Nat. Genet. 43, 527–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H., Guo Y., Teng J., Ding M., Yu A. C., Chen J. (2006) 14-3-3γ affects dynamics and integrity of glial filaments by binding to phosphorylated GFAP. J. Cell Sci. 119, 4452–4461 [DOI] [PubMed] [Google Scholar]
- 20. Meng W., Mushika Y., Ichii T., Takeichi M. (2008) Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135, 948–959 [DOI] [PubMed] [Google Scholar]
- 21. Graser S., Stierhof Y. D., Lavoie S. B., Gassner O. S., Lamla S., Le Clech M., Nigg E. A. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, 1743–1747 [DOI] [PubMed] [Google Scholar]
- 23. Mishima M., Pavicic V., Grüneberg U., Nigg E. A., Glotzer M. (2004) Cell cycle regulation of central spindle assembly. Nature 430, 908–913 [DOI] [PubMed] [Google Scholar]
- 24. Kaitna S., Mendoza M., Jantsch-Plunger V., Glotzer M. (2000) Incenp and an Aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10, 1172–1181 [DOI] [PubMed] [Google Scholar]
- 25. Amos L. A. (2008) The tektin family of microtubule-stabilizing proteins. Genome Biol. 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amos W. B., Amos L. A., Linck R. W. (1986) Studies of tektin filaments from flagellar microtubules by immunoelectron microscopy. J. Cell Sci. Suppl. 5, 55–68 [DOI] [PubMed] [Google Scholar]
- 27. Lukinavičius G., Lavogina D., Orpinell M., Umezawa K., Reymond L., Garin N., Gönczy P., Johnsson K. (2013) Selective chemical crosslinking reveals a Cep57-Cep63-Cep152 centrosomal complex. Curr. Biol. 23, 265–270 [DOI] [PubMed] [Google Scholar]
- 28. Gromley A., Yeaman C., Rosa J., Redick S., Chen C. T., Mirabelle S., Guha M., Sillibourne J., Doxsey S. J. (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123, 75–87 [DOI] [PubMed] [Google Scholar]
- 29. Meunier S., Navarro M. G., Bossard C., Laurell H., Touriol C., Lacazette E., Prats H. (2009) Pivotal role of translokin/CEP57 in the unconventional secretion versus nuclear translocation of FGF2. Traffic 10, 1765–1772 [DOI] [PubMed] [Google Scholar]
- 30. Glotzer M. (2009) The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat. Rev. Mol. Cell Biol. 10, 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu C., Lau E., Schwarzenbacher R., Bossy-Wetzel E., Jiang W. (2006) Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc. Natl. Acad. Sci. U.S.A. 103, 6196–6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cuevas R., Korzeniewski N., Tolstov Y., Hohenfellner M., Duensing S. (2013) FGF-2 disrupts mitotic stability in prostate cancer through the intracellular trafficking protein CEP57. Cancer Res. 73, 1400–1410 [DOI] [PubMed] [Google Scholar]