Background: The role of JIP3 in axon specification and elongation in addition to axon branching remains unknown.
Results: JIP3 locally activates the JNK-cofilin pathway at axon tips and thus enhances axon elongation.
Conclusion: JIP3 is essential for axon elongation.
Significance: These results advance our understanding of the role of JIP3 in axon development.
Keywords: Axon, Cofilin, Jun NH2-terminal Kinase (JNK), Kinesin, Neurons, JIP3, Axon Elongation
Abstract
The development of neuronal polarity is essential for the establishment of the accurate patterning of neuronal circuits in the brain. However, little is known about the underlying molecular mechanisms that control rapid axon elongation during neuronal development. Here, we report that c-Jun NH2-terminal kinase (JNK)-interacting protein-3 (JIP3) is highly expressed at axon tips during the critical period for axon development. Using gain- and loss-of-function approaches, immunofluorescence analysis, and in utero electroporation, we find that JIP3 can enhance axon elongation in primary hippocampal neurons and cortical neurons in vivo. We further demonstrate that JIP3 promotes axon elongation in a kinesin- and JNK-dependent manner using several deletion mutants of JIP3. Next, we demonstrate that the successful transportation of JIP3 to axon tips by kinesin is a prerequisite for enhancing JNK phosphorylation in this area and therefore promotes axon elongation, constituting a novel mechanism for coupling JIP3 anterograde transport with JNK signaling at the distal axons and axon elongation. Finally, our immunofluorescence data suggest that the activation of JNK at axon tips facilitates axon elongation by modulating cofilin activity and actin filament dynamics. These findings may have important implications for our understanding of neuronal axon elongation during development.
Introduction
Neurons are highly polarized and compartmentalized cells. The growth of neuronal processes into an extended axon and highly branched dendrites is crucial for the development of neuronal connectivity. Axon outgrowth is essential for the proper development of the nervous system as well as for axon regeneration after injuries. Although the regulation of axon outgrowth has been a subject of intense investigation, the signaling mechanisms that mediate axon outgrowth in the mammalian brain remain to be elucidated.
JNK-interacting proteins (JIPs)2 compose a family of proteins that were first identified for their role in organizing JNK signaling cascades (1, 2). As a member of the JIPs, JIP3 has been suggested to tether specific JNK signaling modules, thereby promoting signal transmission (3–6). The JNK pathway is involved in axon formation/polarization, extension, synaptic plasticity, and dendrite development (7–11). Moreover, as a homology of UNC-16 in Caenorhabditis elegans and Sunday Driver in Drosophila, JIP3 is implicated as an adaptor protein in kinesin-dependent vesicular transport to axons (12, 13) and has recently been reported to be a mediator in TrkB anterograde axonal transport in hippocampal neurons (14). Interestingly, JIP3 is selectively expressed in the embryonic and adult mouse brains (3, 4). Furthermore, the expression of JIP3 is predominantly localized in the cell bodies and axons of developing neurons and concentrates at axon tips in cultured hippocampal neurons (5, 6). Together, these studies prompt the hypothesis that JIP3 might regulate axon development. Recently, it was reported that JIP3 could restrict axon branching via the GSK3β/DCX signaling pathway (15). However, whether and how JIP3 plays a role in other aspects of axon development such as specification and elongation remain unknown.
To address these questions, we analyzed the influence of JIP3 on axon development in primary hippocampal neurons and cortical neurons in vivo. We determined that JIP3 could enhance axon elongation but had no effect on axon specification. In addition, JIP3 enhancement of axon elongation was mediated by its facilitation of JNK activation at axon tips. Furthermore, we revealed that JIP3-enhanced JNK activation at axon tips could activate cofilin and promote actin polymerization. These findings provide a novel function of JIP3 that links actin dynamics and axon elongation.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
SP600125 was obtained from Calbiochem. Fast Green was provided by Sigma. Antibodies were purchased as follows: mouse or rabbit anti-JIP3 and goat anti-Lamin B from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-α-tubulin from Sigma; rabbit anti-KIF5 from Abcam (Cambridge, MA); mouse anti-phosphorylated JNK (pJNK), rabbit anti-JNK, rabbit anti-phosphorylated cofilin (p-cofilin) from Cell Signaling Technology (Beverly, MA); mouse or rabbit anti-GFP, Alexa Fluor 488-, 594-, or 633-conjugated goat anti-mouse or rabbit IgG (H+L) and Alexa Fluor 488- and 594-conjugated phalloidin from Invitrogen; horseradish peroxidase (HRP)-conjugated goat anti-mouse or rabbit IgG and horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG from Calbiochem. The restriction enzymes were purchased from Fermentas (Hanover, MD). Vectashield mounting medium was obtained from Vector Laboratories (Burlingame, CA). All other reagents were from Sigma except as specifically indicated.
Plasmid Constructs
EGFP-tagged mouse JIP3 and its mutants are as follows: 1–625, ΔKBDΔLZ (ΔΔ), ΔJBD, ΔCC1, and ΔCC2 were subcloned into the pCAGGS expression plasmid. FLAG-tagged Bcl-xL was cloned from rat brain cDNA by PCR and subcloned into the pcDNA3.1 expression plasmid. All of the constructs were confirmed by DNA sequencing to exclude potential PCR-introduced mutations. Oligonucleotide targeting JIP3 was inserted downstream of the U6 promoter, and the target sequence was as follows: rat and mouse JIP3, CAG GCC GAG GAG AAA TTC A. The expression levels of corresponding proteins in PC12 cells transfected with the resulting siRNA or the scramble siRNA were analyzed by Western blot. The EGFP-tagged JIP3-resistant construct was generated using site-directed mutagenesis without changing the amino acid sequence, and the JIP3 siRNA target sequence was mutated to the following: CAa GCg GAa GAG AAg TTC A. Next, pCAGIG-IRES-EGFP was chosen as the vector for the constructs used for in utero electroporation. For KIF5 knockdown, siRNA oligonucleotides against each rat KIF5 subtype were simultaneously introduced, using target sequences as follows: KIF5A, 5′-GGC GGA GAC CAA UAA CGA A-3′; KIF5B, 5′-ACA AAU CAG UAG UUU ACG A-3′; KIF5C, 5′-CAG CAG AAG AAU GGA AGA AGA-3′.
Neuronal Cultures and Transfection
Cultures of hippocampal neurons from timed-pregnant Sprague-Dawley rats were prepared as described previously (16). In brief, hippocampi were dissected from the embryos at embryonic day 18 (E18), dissociated with 0.05% trypsin/EDTA, and gently agitated with a sterile, fire-polished glass Pasteur pipette. Neurons were cultured in Neurobasal medium (Invitrogen) supplemented with 2% B27 and 0.5 mm glutamine, and an incubator with saturated humidity, 5% CO2, and invariant temperature at 37 °C was used for cell culture. For immunofluorescence staining, neurons were cultured on coverslips coated with 0.1 mg/ml poly-d-lysine (Sigma) in six-well plates (Corning Glass) in the same medium described above. Neurons were electroporated with various constructs in a Nucleofector device (Amaxa Biosystems), according to the manufacturer's instructions, before plating. To diminish the possibility that the morphological phenotypes observed with the constructs of interest were attributable to their effects on neuronal survival, an expression plasmid for the antiapoptotic protein gene Bcl-xL, which has been reported to have no effect on axon or dendrite morphology in neurons, was co-transfected (17, 18).
Immunofluorescence Analysis
Hippocampal neurons cultured for 5 days were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilized with 0.4% Triton X-100 in PBS for 10 min. After three washes, the cells were incubated with blocking solution (PBS containing 10% normal goat serum or donkey serum) for 1 h at room temperature. After incubation with the primary antibodies at 4 °C overnight, cells were washed three times and incubated with secondary antibodies for 1 h at room temperature. To visualize the F-actin in neurons, the cells were incubated with Alexa Fluor 488- or 594-phalloidin in 1% BSA in PBS for 20 min at room temperature after incubation with secondary antibodies. All images of immunostained cells were obtained with a Zeiss LSM780 confocal microscope (Microstructural Platform of Shandong University).
Microscopic Quantitative Analysis
For analyzing the length of the neurites, several morphological characteristics were used to distinguish axons from dendrites (19). For example, dendrites taper gradually and have irregular contours. In contrast, axons have a relatively even diameter along their course, display a smoother contour, and extend much longer than other neurites. Among these traits, the most valuable one in our study is that in cultures of hippocampal neurons the longest processes are the axons (19). Therefore, the lengths of axons or dendrites could be measured using MetaMorph software to trace the fibers immunostained for α-tubulin.
To quantitatively analyze the expression levels of EGFP-JIP3 or its mutants and the phosphorylation levels of JNK or cofilin in distal axons of hippocampal neurons at DIV5, GFP staining levels and the total JNK, pJNK, or p-cofilin fluorescence intensities in the cell bodies and distal 30 μm of the axons were measured with MetaMorph software. In each experiment, a consistent set of acquisition parameters was used for each set of images, and >50 cells were examined.
In Utero Electroporation
The constructs described above were transfected by in utero electroporation (20–22). Briefly, pregnant C57BL/6 mice were used. Constructs (3 mg/ml) that had been mixed with Fast Green (2 mg/ml) were injected (1–2 μl) at E15.5 into the lateral ventricle of each embryonic brain using a pulled glass micropipette. A pair of electrodes with a diameter of 7 mm (CUY650-P7, NEPA Gene) that were attached to the electroporator (CUY21SC, NEPA Gene) transmitted five square electric pulses at 30 V for 50 ms at 950-ms intervals through the uterine wall. For the analysis of neuronal polarization, labeled neurons in the IZ and SVZ were categorized according to the number of neurites as follows: single (unipolar), double (bipolar), or multiple (multipolar) morphology. Each phenotypic analysis was performed with at least three independent litters.
Western Blot Analysis
Electroporated or untreated neurons were harvested at the indicated days in vitro with TNE buffer (10 mm Tris, pH 8.0, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 10% glycerol with protease inhibitors) or SDS lysis buffer (50 mm Tris, pH 8.0, 1% SDS). Lysates were clarified by centrifugation at 14,000 × g for 15 min at 4 °C, and the supernatants were boiled in 4× sample buffer for SDS-PAGE (Invitrogen). Finally, they were subjected to immunoblotting for analysis with the indicated antibodies.
Neuronal Culture in Microfluidic Chambers
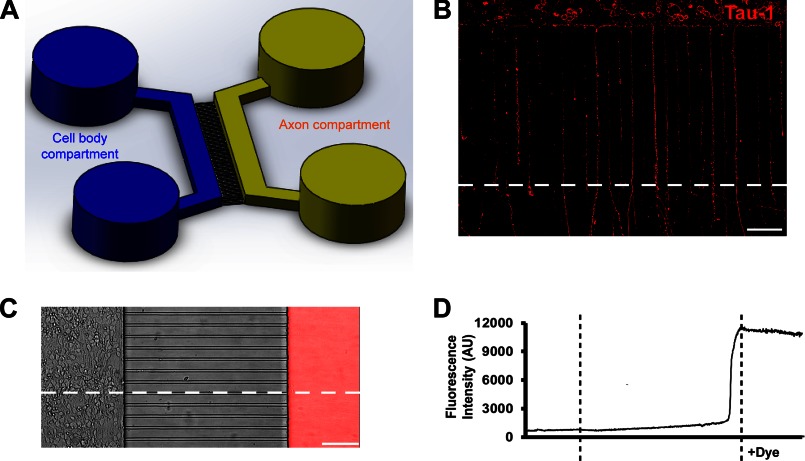
The microfluidic chambers were fabricated in poly(dimethylsiloxane) using rapid prototyping and soft lithography as published previously (23, 24). All microfluidic chambers were replica molded from the masters that were patterned by the photosensitive epoxy SU-8 (23). The microfluidic features consist of two parallel compartments (2.0 mm wide and 100 μm high), which have access ports (or wells) at both ends. A poly(dimethylsiloxane) barrier contains 400 small microgrooves (450 μm long, 10 μm wide, and 3 μm high), connecting the two compartments, as depicted in Fig. 7. The dimensions of the microgrooves are designed to guarantee that the axons are specifically isolated, allowing selective stimulation of distal axons (25).
FIGURE 7.
Microfluidic chambers direct axon growth of hippocampal neurons and fluidically isolate axons. A, schematic of a microfluidic chamber. B, image of neurons (DIV5) cultured within the microfluidic chamber, stained for tau-1 (red). Scale bar, 100 μm. C, Alexa Fluor 594 dye (20 μg/ml) was added to the axon compartment at DIV4. The image is an overlay of the phase contrast and fluorescence images after 24 h of treatment. Scale bar, 100 μm. D, fluorescence intensity change trend along the horizontal dotted line through the entire image shown in C.
The clean, sterilized, and dry microfluidic chambers were reversibly affixed to poly-d-lysine-coated 60-mm dishes (Corning Glass). Suspensions of electroporated neurons were plated into the cell body compartment, where they would attach to the dish after 10–15 min. After 4 days in culture, axons grew through the microgrooves and extended into the axon compartment. Culture medium for the microfluidic chambers was Neurobasal medium supplemented with B27 and glutamine. Neither serum nor growth factors were added to the compartments at any time.
Axon Growth Assay in Microfluidic Chambers
Axon length in the microfluidic chambers was quantified by measuring the length extended from the edge of the microgrooves to their tips (Fig. 8E). Images of the same sites under 10× objective lens were recorded immediately before SP600125 (10 μm) administration (L0) at DIV4 and at 24 h after SP600125 treatment began (L24). The changes in axon length were calculated as the average of (L24- to L0). Within one test set, the measurements from 50 to 70 GFP-positive axons were averaged for each condition.
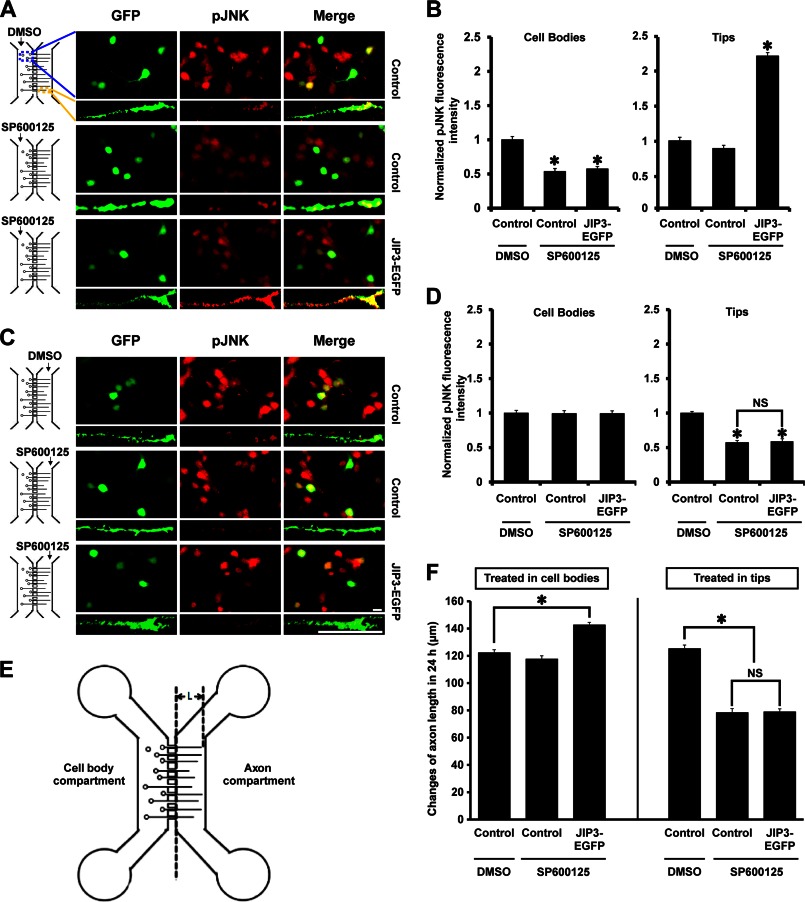
FIGURE 8.
JIP3 enhances axon elongation by locally activating JNK at axon tips. A and C, hippocampal neurons transfected with empty vector or JIP3-EGFP construct were cultured in the microfluidic chambers for 4 days. Then the JNK inhibitor SP600125 (10 μm) was applied to the cell body compartment (A) or the axon compartment (C) for 24 h. Cells were stained with anti-GFP (green) and anti-pJNK (red) antibodies. Images of cell bodies and axon tips are shown. Scale bar, 20 μm. B and D, quantification of results from A and C. The graphical data shown on the left of B and D represent the relative fluorescence intensity of pJNK in the cell bodies, and those on the right represent that at axon tips (n = 3, *, p < 0.05, versus the control + DMSO group; NS, no significance; Student's t test). E, schematic for calculation of the axon length in a microfluidic chamber; “L” represents the axon length of interest. F, statistical results for the changes in “L” in E over 24 h, during which the cell bodies or axon tips were treated with DMSO or SP600125 (n = 3, *, p < 0.05, versus the control + DMSO group; NS, no significance; Student's t test).
Statistical Analysis
Statistical significance was assessed using Student's t test or one-way analysis of variance, followed by post hoc tests. Data were presented as the mean ± S.E., and p < 0.05 was considered significant.
RESULTS
Expression of JIP3 Increases Rapidly in Hippocampal Neurons during Development
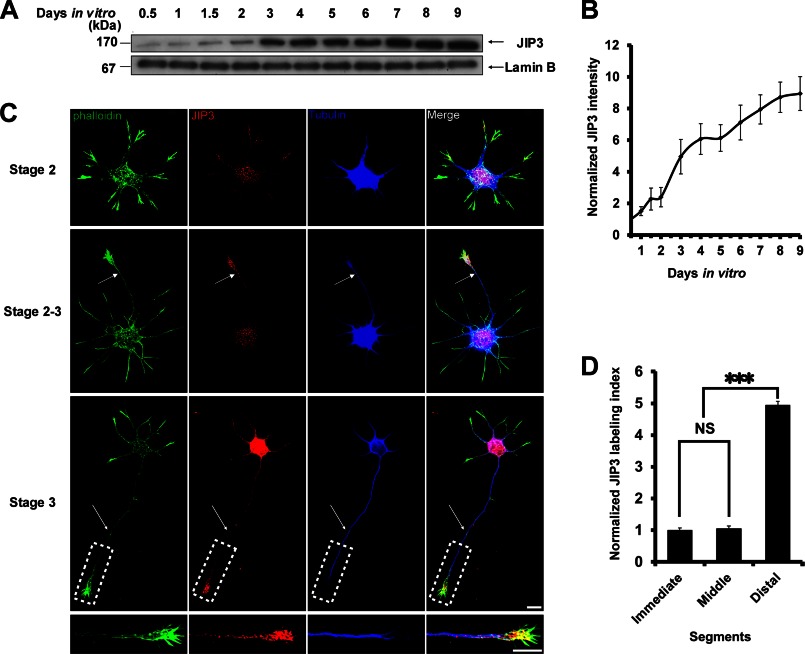
Previous studies have suggested that the expression of JIP3 is high in the developing rodent brain (5). The spatiotemporal characteristics of JIP3 expression in primary hippocampal neurons were further studied by Western blot and immunofluorescence staining. We found that the expression of JIP3 gradually increased during development, with a robust elevation from DIV2 to DIV4 (Fig. 1, A and B), a period suggested by Dotti et al. (19) to be critical for axon elongation. Next, JIP3 immunofluorescence staining was performed on hippocampal neurons at stages 2 and 3 of development (Fig. 1C). At stage 2, neurons had developed several relatively symmetric minor processes, and JIP3 was found to be evenly distributed throughout the cell body and minor neurites. However, during development through stages 2–3, JIP3 immunoreactivity accumulated considerably in the longest neurites, which had morphological characteristics of nascent axons compared with stage 2 neurons (Fig. 1C). Upon reaching stage 3, when cultured hippocampal neurons had developed a longer axon and several minor dendrites, JIP3 was mainly distributed in the cell bodies and the distal segments of the axons (Fig. 1C). Similarly to the Western blot results showing that the expression levels of JIP3 increased sharply from DIV2 to DIV4 (stage 3), JIP3 immunoreactivity of stage 3 neurons increased significantly compared with that of stage 2 or 2–3 neurons (Fig. 1C). Quantification of JIP3 labeling intensity relative to that of tubulin in stage 3 neurons confirmed that the JIP3 signal had a gradient in axons as follows: low at the proximal end and high at the distal region (Fig. 1D), suggesting that JIP3 was enriched within the axon tips. Interestingly, at the axon tips, JIP3 not only co-localized with tubulin but was also arrayed on F-actin microfilaments (Fig. 1C). Previous reports have shown that JIP3 concentrates in the axon tips (6, 26). Our results extend this finding by examining the stages of hippocampal process outgrowth. The spatiotemporal expression characteristics of JIP3 imply that JIP3 has a potential role in axon development.
FIGURE 1.
Spatiotemporal expression of JIP3 in hippocampal neurons during development. A, Western blot analysis of JIP3 in hippocampal neurons cultured for the indicated days. B, immunoreactive bands in A, quantified with ImageJ software. The values are expressed as the mean ± S.E. from three independent experiments. C, representative images of stage 2, stage 2–3, or stage 3 neurons immunostained with anti-JIP3 (red) and anti-α-tubulin (blue) antibodies. F-actin (green) was labeled with Alexa Fluor 488-conjugated phalloidin. Arrows point to the neurite that will develop into the future axon. Lower panels of the stage 3 neuron show images of the dotted box at higher magnification, which represent its axon tip. Scale bar, 10 μm. D, quantification of JIP3 immunolabeling in the axons of stage 3 neurons. The axons were subdivided into three equal parts, according to distance from the cell body as follows: immediate segment, middle segment, and distal segment. The JIP3 labeling index refers to the ratio of the JIP3 labeling intensity to the α-tubulin staining intensity; >100 cells were examined in each experiment (n = 3, ***, p < 0.001, versus the immediate or middle group; NS, no significance; Student's t test).
JIP3 Enhances Axon Elongation in Hippocampal Neurons in Vitro
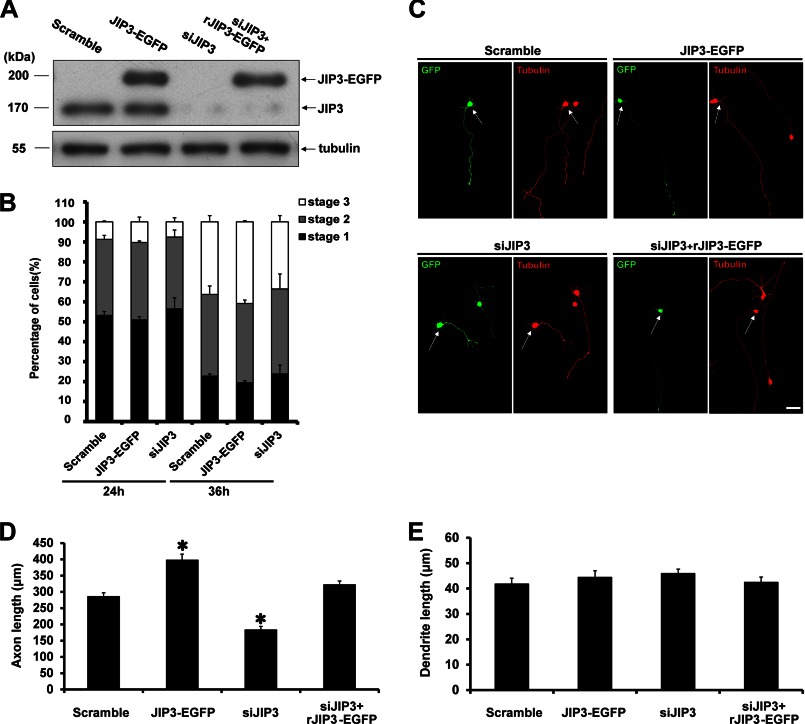
To investigate the role of JIP3 in axon development, the effects of JIP3 on axon specification and elongation were tested in gain-of-function and loss-of-function studies. Four constructs were used in our studies as follows: scrambled siRNA sequence together with EGFP (scramble); fusion protein of JIP3 and EGFP (JIP3-EGFP); JIP3 siRNA sequence (14) together with EGFP (siJIP3), and JIP3 siRNA sequence together with RNAi-resistant JIP3 fused with EGFP (siJIP3 + rJIP3-EGFP). The expression levels of JIP3-EGFP fusion protein were approximately twice the amount of endogenous JIP3 (Fig. 2A). The endogenous JIP3 expression was efficiently knocked down by siJIP3 transfection, which could be rescued by the rJIP3-EGFP construct (Fig. 2A).
FIGURE 2.
JIP3 enhances axon elongation in primary hippocampal neurons. A, PC12 cells were transiently transfected with scramble, JIP3-EGFP, siJIP3, or siJIP3 + rJIP3-EGFP construct. At 72 h after transfection, cells were lysed and immunoblotted with anti-JIP3 and anti-α-tubulin antibodies. B, percentages of electroporated hippocampal neurons in stage 1, stage 2, and stage 3 when cultured for 24 or 36 h (n = 3). C, hippocampal neurons (DIV5) transfected with the indicated construct were immunostained with anti-GFP (green) and anti-α-tubulin (red) antibodies. Arrows point to the GFP-positive neurons. Scale bar, 40 μm. D and E, quantitation of the length of axons (D) or dendrites (E) shown in C; >100 cells were examined in each experiment (n = 3, *, p < 0.05, versus the scramble group; Student's t test).
The effect of JIP3 on axon specification was assessed by examining the percentages of neurons at three different neurite development stages at 24 and 36 h in culture. We observed that neither overexpressing nor knocking down JIP3 had a significant effect on the percentages of neurons in the three stages (Fig. 2B), indicating that JIP3 had no influence on axon specification. We then investigated the role of JIP3 in neurite elongation by measuring the lengths of axons and dendrites labeled with tubulin staining at DIV5 (Fig. 2C). Statistical analysis revealed that the axon length was significantly longer in JIP3-EGFP-transfected neurons compared with that in scramble-transfected neurons (Fig. 2D). Consistently, neurons with JIP3 knockdown had shorter axons than the control cells, and this inhibiting effect was rescued by co-expressing the siRNA-resistant JIP3 construct (Fig. 2D). By contrast, manipulating JIP3 expression levels exhibited no influence on dendrite length (Fig. 2E). Together, these results suggest that JIP3 is able to enhance axon elongation but has no effect on axon specification or dendrite elongation in cultured hippocampal neurons.
JIP3 Enhances Cortical Neuronal Axon Elongation in Vivo
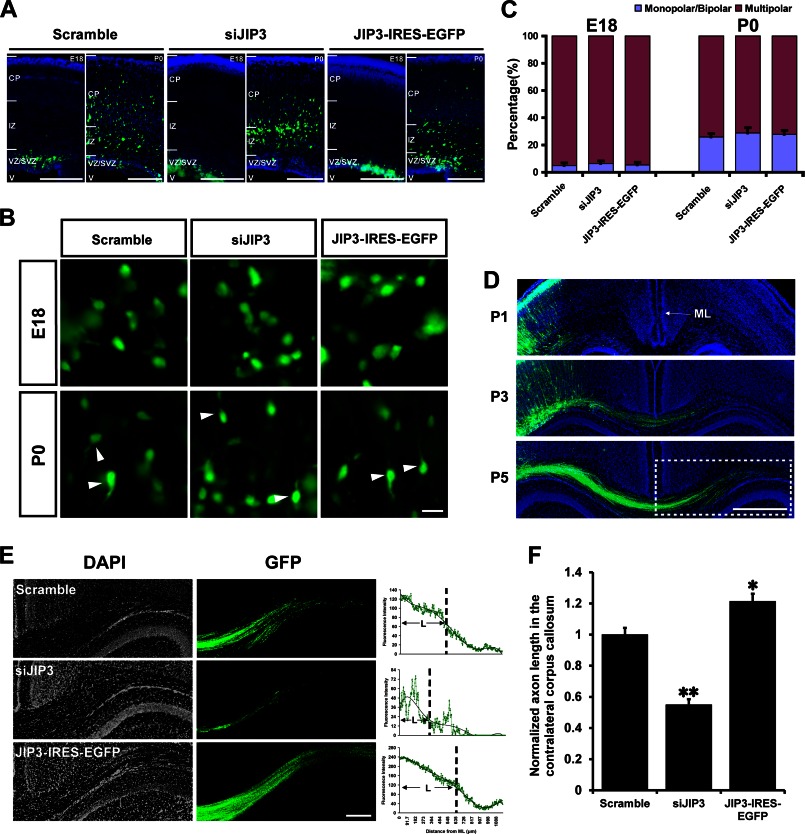
In utero electroporation was performed to examine the effect of JIP3 on axon elongation in vivo. Cortical progenitor cells of E15.5 mice were electroporated with the construct encoding scrambled siRNA with EGFP, and EGFP-positive cells were found within the ventricular zone and SVZ at E18 (Fig. 3A). These cells then migrated out of the SVZ to the IZ, and some of them had reached layers II and III of the cortical plate at P0 (Fig. 3A). These results are consistent with previous reports (27–29), indicating that our in utero electroporation system is reliable. EGFP-positive cells electroporated with siJIP3 or JIP3-IRES-EGFP also successfully migrated out of the ventricular zone and SVZ regions (Fig. 3A). As demonstrated previously, the neurons produced from cortical progenitors in the ventricular zone/SVZ migrate to the IZ and assume a multipolar morphology (30, 31). They then transform into a bipolar shape, extending a pia-directed leading process (32), and migrate radially to the cortical plate, leaving the tangential process behind (31–33). In this way, the neurons exhibiting multiple neurites (multipolar) in the IZ and SVZ represent the cells that are still at a nonpolarized state. We found that in the IZ and SVZ regions of the E18-P0 mouse cortex, there was no significant difference in the percentages of multipolar and unipolar/bipolar neurons between scramble, siJIP3, and JIP3-IRES-EGFP groups (Fig. 3, B and C). This result suggests that JIP3 has no influence on the polarization of newborn cortical neurons, consistent with our in vitro result.
FIGURE 3.
JIP3 enhances axon elongation in cortical neurons in vivo. A, representative images of coronal sections of the developing mouse cortex at E18 and P0 after in utero electroporation with scramble, siJIP3, or JIP3-IRES-EGFP constructs at E15.5. Sections with GFP-positive (green) cells were from in utero electroporation-positive brains and were counterstained with DAPI (blue) to label nuclei. Scale bar, 200 μm. B, representative images of GFP-positive neurons in the SVZ and IZ of scramble, siJIP3, and JIP3-IRES-EGFP group sections (E18 and P0). Arrowheads point to the monopolar/bipolar GFP-positive neurons. Scale bar, 20 μm. C, quantification of the proportions of monopolar/bipolar and multipolar GFP-positive neurons. Data were collected from 200 cells in three brains in parallel experiments. D, postnatal development of callosal projections in the cortices that were labeled via in utero electroporation with scramble construct. Images show the tracing of GFP-positive callosal axons into the contralateral cortex at P1, P3, and P5. Scale bar, 500 μm. E, magnified images of the region indicated by dotted box in D for scramble, siJIP3, and JIP3-IRES-EGFP-positive sections. DAPI (gray) and GFP (green) are shown. The fluorescence intensity curves on the right represent the GFP fluorescence intensity change trends in relation to the distance from the midline (ML). The corresponding values of the average fluorescence intensities on the x axis were calculated, referring to the average length of axons that cross the ML (“L” in the line graph). Scale bar, 200 μm. F, statistical result for “L” in E (n = 4, *, p < 0.05; **, p < 0.01, versus the scramble group; Student's t test).
Next, we analyzed the distance of the axon contralateral projecting from labeled cortical neurons to assess the effect of JIP3 on axon elongation in vivo. As reported previously (34), we observed that labeled callosal axons projecting from layer II/III cortical neurons appeared at P1, crossed the midline (ML) at P3, and extended to the white matter of the contralateral cortex at P5 (Fig. 3D). To quantify the axon length in vivo and to decrease the serious fluorescent background that might conceal the differences between various groups, we focused on the subsets of callosal axons that had passed the ML at P5 and measured the distance from the ML to their tips in each group (Fig. 3E). To obtain the average length of the axons that had passed the ML, we drew a fluorescence intensity curve from ML using the “Lanscan” component of MetaMorph software (Fig. 3E), so that the distance corresponding to the average fluorescence intensity would represent the average axon length. We found that, compared with the scramble group, JIP3 knockdown decreased, and JIP3 overexpression increased the length of callosal axons that invaded the contralateral cortex at P5 (Fig. 3F). We thereby confirmed that JIP3 plays a role in cortical neuronal axon elongation without affecting axon specification in vivo.
Identification of the Key Domains in JIP3 That Are Critical for Axon Elongation
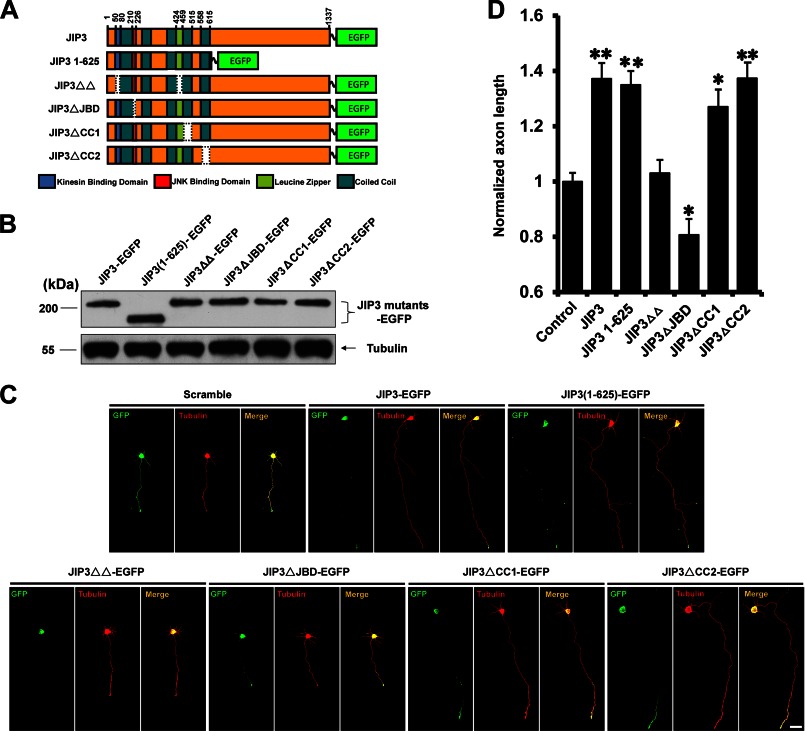
To verify which domain of JIP3 is involved in the effect on axon elongation, various JIP3 deletion mutants were constructed and successfully expressed, and their abilities to enhance axon elongation were examined (Fig. 4, A–C) (14). The JIP3(1–625) mutant had a similar capacity to enhance axon elongation compared with wild type JIP3 (Fig. 4D), suggesting that amino acids 1–625 were sufficient for JIP3 to promote axon elongation. It has been reported that several functional domains exist in the 1–625-amino acid region of JIP3, namely the kinesin binding domain (KBD) (6), JNK binding domain (JBD) (4, 6), leucine zipper-like domain (LZ) (4), coiled-coil 1 (CC1) (14), and coiled-coil 2 (CC2) (4). To more precisely define the regions required for its effect on axon elongation, four JIP3 mutants with KBD and LZ (JIP3ΔΔ), JBD (JIP3ΔJBD), and CC1 (JIP3ΔCC1) or CC2 (JIP3ΔCC2) domain deletions were constructed (Fig. 4A), and their abilities to enhance axon elongation were studied by immunofluorescence analysis (Fig. 4C). We found that deletion of KBD and LZ or JBD could abolish the enhancement of axon elongation, whereas the CC2 deletion had no effect (Fig. 4D). These data suggest that the KBD and LZ and JBD domains are critical for JIP3 to enhance axon elongation.
FIGURE 4.
Identification of the key domains in JIP3 that are critical for axon elongation. A, schematic representation of the JIP3 mutants. Amino acid numbers correspond to appropriate domains within the JIP3 deletion constructs. B, HEK293 cells were transiently transfected with the indicated constructs. At 48 h after transfection, cells were lysed and immunoblotted with anti-GFP and anti-α-tubulin antibodies. C, images of representative neurons (DIV5) transfected with empty vector, JIP3-EGFP, or JIP3 mutants described in A. Cells were stained with anti-GFP (green) and anti-α-tubulin (red) antibodies. Scale bar, 40 μm. D, quantitation of axon length from the neurons shown in C; >100 cells were examined in each experiment (n = 3, *, p < 0.05; **, p < 0.01, versus the control group; Student's t test).
JIP3 Must Be Transported to the Axon Tips to Exert Its Effect on Axon Elongation
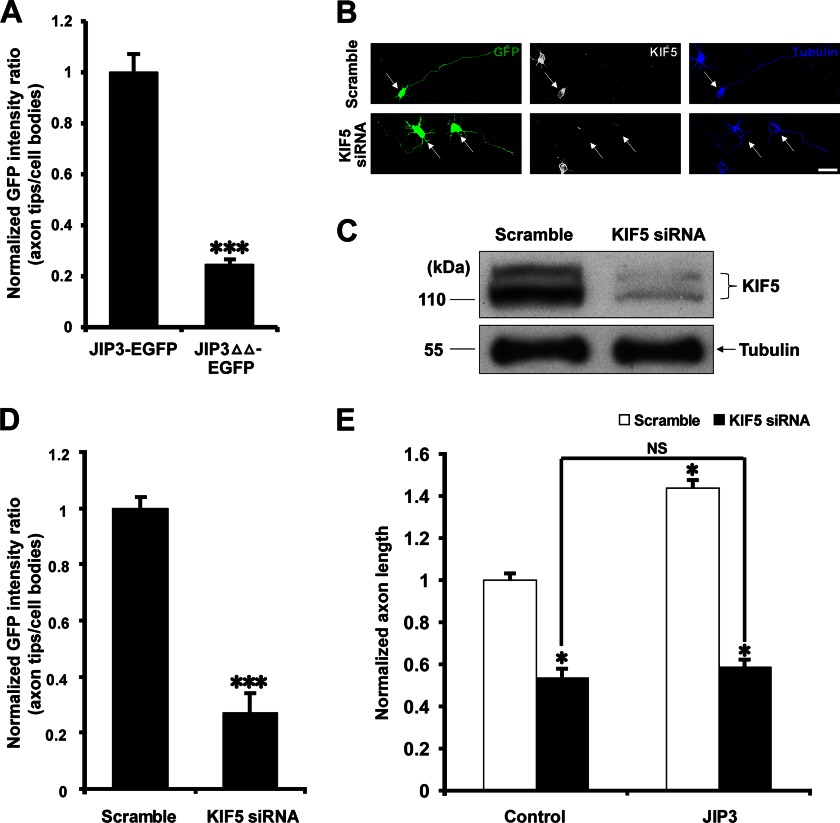
The LZ domain of JIP3 is known as a binding site for kinesin light chain (6, 14), and the KBD domain has been reported to mediate the direct interaction between JIP3 and kinesin heavy chain (6). Therefore, we hypothesized that JIP3ΔΔ lost its capacity to enhance axon elongation through its inability to be transported to the axon tips (6). To test this hypothesis, we quantified the localization of JIP3-EGFP and JIP3ΔΔ-EGFP in cultured hippocampal neurons (Fig. 4C). Compared with JIP3-EGFP, the accumulation of JIP3ΔΔ-EGFP at axon tips was significantly reduced (Fig. 5A), which is consistent with a previous report (6). It is believed that JIP3 axonal transport is mediated by kinesin 1 (14, 35). Therefore, three different siRNA oligonucleotides against each rat kinesin 1 heavy chain (KIF5) subtype were simultaneously introduced into hippocampal neurons, and the efficiency of interference was analyzed by immunocytochemistry (Fig. 5B) and Western blot (Fig. 5C). Knocking down KIF5 expression significantly decreased the localization of JIP3 at axon tips and inhibited axon elongation compared with the scramble group (Fig. 5, D and E), suggesting that KIF5 is required for JIP3 anterograde transport and axon elongation. Most interestingly, in the absence of KIF5, JIP3 lost its ability to enhance axon elongation (Fig. 5E), suggesting that kinesin-based transportation to axon tips is necessary for JIP3 to enhance axon elongation.
FIGURE 5.
JIP3 must be transported to the axon tips to exert its effect on axon elongation. A, quantitation of the distribution of JIP3-EGFP and JIP3ΔΔ-EGFP in distal axons of hippocampal neurons at DIV5 (n = 3, ***, p < 0.001, versus the JIP3-EGFP group; Student's t test). B, hippocampal neurons (DIV5) transfected with pCAGGS-EGFP and scramble or KIF5 siRNA were stained with anti-GFP (green), anti-KIF5 (gray), and anti-α-tubulin (blue) antibodies. Arrows point to the GFP-positive neurons. Scale bar, 40 μm. C, hippocampal neurons were transiently transfected with scramble or KIF5 siRNA. At 5 days after transfection, cells were lysed and immunoblotted with anti-KIF5 and anti-α-tubulin antibodies. D, quantitation of the distribution of JIP3-EGFP in distal axons of hippocampal neurons at DIV5 when co-transfected with scramble or KIF5 siRNA (n = 3, ***, p < 0.001, versus the scramble group; Student's t test). E, quantitation of the axon length of the neurons co-transfected with empty vector or JIP3-EGFP construct and scramble or KIF5 siRNA (n = 3, *, p < 0.05, versus the control + scramble group; NS, no significance; one-way analysis of variance).
JIP3 Enhances Axon Elongation by Locally Activating JNK at Axon Tips
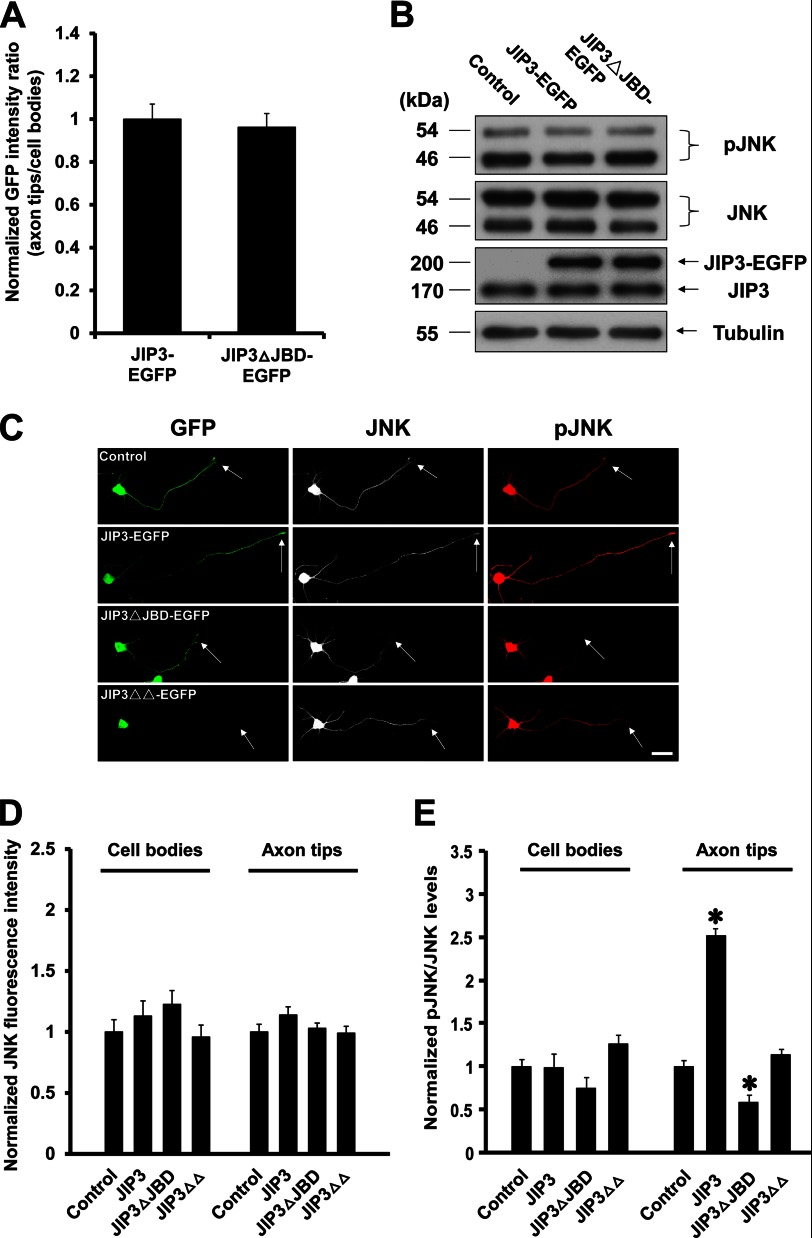
To explore the mechanism underlying the role of the JBD domain of JIP3 in axon elongation, we first quantified the localization of JIP3-EGFP and JIP3ΔJBD-EGFP at the axon tips (Fig. 4C) and found no significant difference (Fig. 6A). Previous studies indicated that the JBD domain of JIP3 could mediate the interaction between JIP3 and JNK (6, 36), so we sought to determine the effect of exogenously expressed JIP3 or JIP3ΔJBD on JNK activation. Immunoblots of lysates from hippocampal neurons transfected with the control, JIP3-EGFP, or JIP3ΔJBD-EGFP construct showed no detectable difference in the levels of total JNK and phosphorylated JNK (pJNK) (Fig. 6B), consistent with previous reports (3, 4). Immunocytochemistry was further employed to confirm the Western blot results (Fig. 6C). Overexpressing JIP3-EGFP or JIP3ΔJBD-EGFP had no effect on the total JNK levels in the cell bodies or axon tips of transfected neurons (Fig. 6D), which suggested that JIP3 had no influence on the expression and location of JNK. However, we found that the pJNK/JNK ratio at axon tips was significantly increased by overexpressing JIP3-EGFP but decreased by overexpressing JIP3ΔJBD-EGFP and that neither affected the pJNK/JNK ratio in cell bodies (Fig. 6E). On the contrary, exogenous expression of JIP3ΔΔ-EGFP, which could not be transported to axon tips, had no significant effect on pJNK/JNK ratio either in the cell bodies or the axon tips (Fig. 6E). Together, these results suggest that JIP3 is able to selectively enhance JNK activation at the axon tips but not in the cell bodies and that this effect relies on its JBD domain.
FIGURE 6.
JIP3 enhances the JNK activation at the axon tips but not in the cell bodies. A, quantitation of the distribution of JIP3-EGFP and JIP3ΔJBD-EGFP in distal axons of hippocampal neurons at DIV5 (n = 3). B, hippocampal neurons were transfected with empty vector, JIP3-EGFP, or JIP3ΔJBD-EGFP constructs. At 5 days after transfection, cells were lysed and immunoblotted with anti-pJNK, anti-JNK, anti-JIP3, and anti-α-tubulin antibodies. C, images of representative neurons (DIV5) transfected with empty vector, JIP3-EGFP, JIP3ΔJBD-EGFP, or JIP3ΔΔ-EGFP constructs. Cells were stained with anti-GFP (green), anti-JNK (gray), and anti-pJNK (red) antibodies. Arrows point to the axon tips. Scale bar, 40 μm. D and E, quantitation of JNK immunostaining intensities (D) or pJNK/JNK ratios (E) in the cell bodies or axon tips of the neurons shown in C (n = 3, *, p < 0.05, versus the control group; Student's t test).
We then surmised that JIP3 would stimulate axon elongation by specifically increasing JNK phosphorylation at axon tips. To test this hypothesis, we employed a microfluidic chamber containing two parallel compartments connected by microgrooves (Fig. 7A) (25). As axons grow faster and longer than dendrites, microfluidic chambers can isolate axons and allow the selective manipulation of axon microenvironments (25). We used immunocytochemistry to confirm that axons could extend into the axon compartment (Fig. 7B). To demonstrate the microfluidic isolation of the two compartments, we incubated the axon compartment with Alexa Fluor 594 hydrazide, a low molecular weight fluorescent dye, and only a tiny amount (<1%) of the dye was detected in the cell body compartment after 24 h (Fig. 7, C and D). Therefore, the microgrooves produced a tiny but sustained flow between the two compartments that counteracts diffusion. At DIV4, the JNK inhibitor SP600125 was added to the cell body compartment; 24 h later, the levels of pJNK in the cell bodies were significantly decreased in the SP600125-treated group compared with the control group. However, administration of SP600125 to the cell body compartment did not affect pJNK levels at the axon tips, and JIP3 overexpression could still enhance JNK activation at the axon tips (Fig. 8, A and B). Next, SP600125 was applied to the axon compartment, and we observed substantially decreased levels of pJNK at the tips compared with axons treated with DMSO. Furthermore, JIP3 overexpression could not increase the levels of pJNK at axon tips in the presence of SP600125 in the axon compartments (Fig. 8, C and D). However, the exposure of distal axons to SP600125 had negligible effects on the levels of pJNK in the cell bodies (Fig. 8, C and D). Together, these results indicate that the enhanced JNK activation at axon tips by JIP3 could be specifically blocked by application of SP600125 to the axon rather than the cell body.
Finally, we tested whether JIP3 stimulated axon elongation by increasing the levels of pJNK at axon tips. As it was difficult to trace a single tip back to its cell body through the microgrooves among a plenitude of axons, the axon length in the microfluidic chambers was quantified by measuring the distances between the edge of the microgrooves and the axon tips (Fig. 8E). After 24 h of SP600125 treatment in the cell body compartment, JIP3 overexpression still led to a robust increase in axon elongation (Fig. 8F). In contrast, SP600125 applied to the axon compartment significantly blocked axon elongation, whether JIP3 was overexpressed or not (Fig. 8F). Taken together, these findings support a model in which JIP3 promotes axon elongation by specifically stimulating JNK phosphorylation at axon tips.
JIP3 Regulates Actin Dynamics at Axon Tips through the JNK-Cofilin Pathway
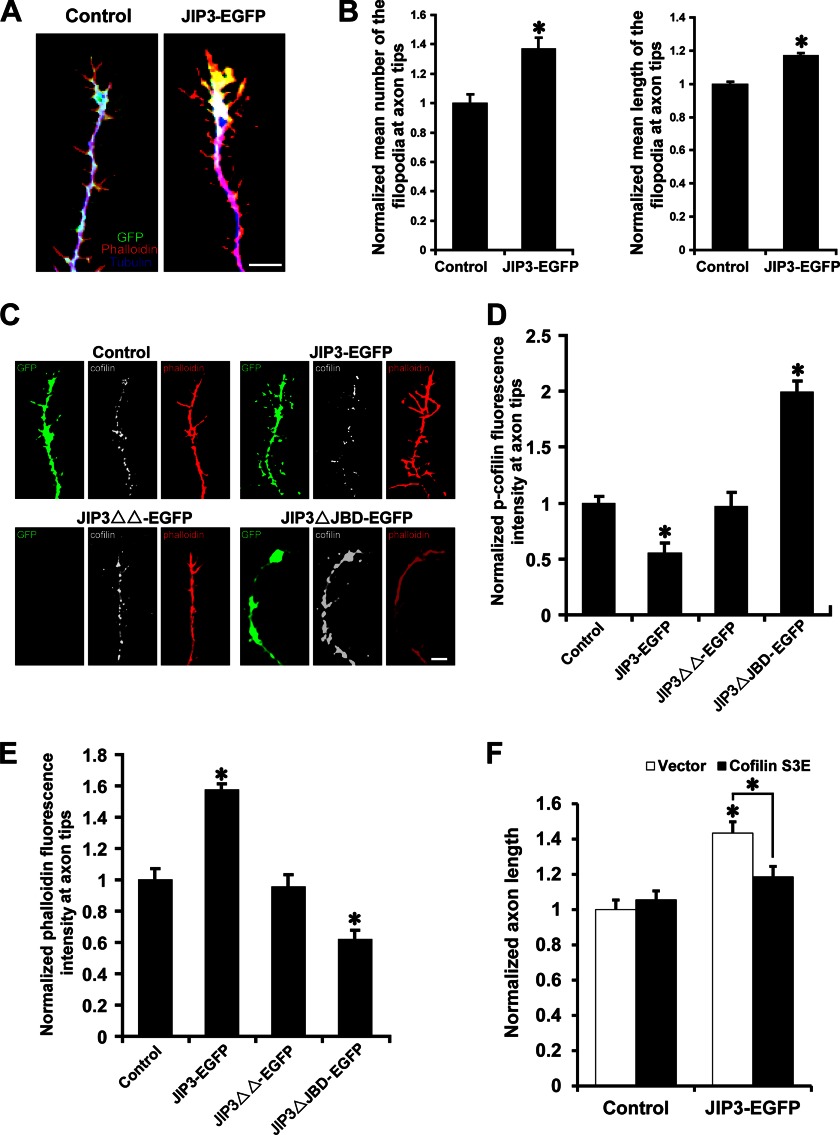
How could JIP3-enhanced JNK activation at axon tips lead to axon elongation? We found that JIP3 overexpression could increase the number and length of filopodia at axon tips (Fig. 9, A and B). Filopodia are actin-rich plasma membrane protrusions and play an important role in axon development. This result suggested that the dynamics of the actin-based filopodia might be responsible for the enhancement of axon elongation by JIP3. JNK has been reported to promote PDGF-BB-induced dephosphorylation of cofilin (37). The dephosphorylated form of cofilin is active and can influence filopodial dynamics (38, 39) and neurite outgrowth (40–42) by regulating F-actin dynamics. To investigate a more direct mechanism for the effect of JIP3 on axon elongation, we performed immunofluorescence analysis of the levels of phosphorylated cofilin (p-cofilin) in hippocampal neurons (Fig. 9C). Compared with that in the control group, the p-cofilin immunolabeling intensity was significantly weaker at the axon tips of neurons transfected with JIP3-EGFP (Fig. 9D), suggesting that JIP3 overexpression could enhance cofilin activity at the axon tips. It has been proven that activated cofilin can promote actin filament assembly (43). Accordingly, the levels of phalloidin labeling (representing F-actin levels) at axon tips were increased significantly when JIP3 was overexpressed (Fig. 9E). Furthermore, the trafficking-deficient mutant JIP3ΔΔ showed little influence on cofilin activity and F-actin levels, whereas JIP3ΔJBD, which serves as a dominant negative construct and decreases JNK activation at axon tips, significantly decreased both cofilin activity and actin polymerization (Fig. 9, D and E). Most interestingly, overexpression of the less active, pseudo-phosphorylated cofilin mutant S3E abolished the ability of JIP3 to enhance axon elongation (Fig. 9F), suggesting that cofilin activity is important for the effect of JIP3 on axon elongation. Together, these data indicate that JIP3 may regulate actin dynamics at axon tips through the JNK-cofilin pathway and thereby control axon elongation.
FIGURE 9.
JIP3 regulates actin dynamics at axon tips through the JNK-cofilin pathway. A, representative images of axon tips from neurons (DIV5) transfected with empty vector or JIP3-EGFP. Cells were stained with anti-GFP (green) and anti-α-tubulin (blue) antibodies. F-actin (red) was labeled with Alexa Fluor 594-conjugated phalloidin. Scale bar, 5 μm. B, quantitation of the relative number and length of filopodia at axon tips shown in A (n = 3, *, p < 0.05, versus the control group; Student's t test). C, representative images of axon tips from neurons (DIV5) transfected with the indicated constructs. Cells were stained with anti-GFP (green) and anti-p-cofilin (gray) antibodies. F-actin (red) was labeled with Alexa Fluor 594-conjugated phalloidin. Scale bar, 5 μm. D and E, quantitation of the staining intensities of p-cofilin (D) and F-actin (E) at axon tips shown in C (n = 3, *, p < 0.05, versus the control group; Student's t test). F, quantitation of the axon length in neurons co-transfected with empty vector or JIP3-EGFP and control or cofilin S3E constructs (n = 3, *, p < 0.05, versus the control + vector group; one-way analysis of variance).
DISCUSSION
JIP3 was originally identified as a scaffolding protein for the JNK cascade through binding to the MAPK (JNK), the MAPKKs (including MKK4/SEK1 and MKK7), and the MAPKKKs (including MEKK1, MLK3 and ASK1) (3, 4, 44). It was also found to interact directly with the light chain (12) or heavy chain (6) of kinesin and to be involved in axon transport (12, 13). Recently, we reported that JIP3 directly links the cytoplasmic tail of TrkB to KLC1 and mediates axonal anterograde transport of TrkB in hippocampal neurons (14). Interestingly, a recent report showed that JIP3 restricts axon branching in cerebellar granule neurons (15). In this study, we demonstrate that JIP3 is transported to the axon tips, where it enhances axon elongation by locally activating JNK and cofilin and thereby regulating actin dynamics.
Our studies provide three novel insights into the JIP3-mediated regulation of axon elongation. First, we characterized the spatiotemporal expression of JIP3 in primary hippocampal neurons. It has been widely accepted that JIP3 is selectively expressed in embryonic and adult brains (3, 4) and is mainly localized in cell bodies and axon tips (5, 6). We analyzed the time curve of JIP3 expression during development using primary cultured hippocampal neurons and found a sharp increase from DIV2 to DIV4, which is a critical period for axon elongation (19). Moreover, tight control of the asymmetric and dynamic localization of JIP3 during neuronal development appears to be a key element in shaping the neurons (Fig. 1). At stage 2–3, the localization of JIP3 changed from equal distribution among minor neurites to polarized accumulation at the distal axon. The expression levels of JIP3 increased sharply when a neuron reached stage 3, which is a critical period for axon elongation. Thus, both the temporal and the spatial characteristics of JIP3 expression in hippocampal neurons suggest its function on axon development.
Second, we found that JIP3 had no effect on axon specification but could enhance axon elongation, which depended on JIP3 being anterogradely transported to the axon tips and locally activating JNK. Recently, it was reported that JIP3 restricted axon branching in the cerebellar cortex by regulating the GSK3β/DCX signaling pathway (15). It was also found that JIP3−/− mice lack the telencephalic commissure, a major connection between the right and left hemispheres of the brain (45), suggesting a possible role for JIP3 in axon specification or elongation. Using both the in vitro primary hippocampal neuron cultures and the in vivo-in utero electroporation system, we concluded that JIP3 had no effect on axon specification but could promote axon elongation, which explained the absent telencephalic commissure phenotype observed in JIP3 knock-out mice.
What is the mechanism underlying JIP3-mediated axon elongation? We found that JIP3 must first be anterogradely transported to the axon tips as a JIP3 mutant lacking kinesin binding domains (6) and that the knockdown of kinesin 1 abolished the ability of JIP3 to promote axon elongation. Kinesin 1 can transport many different cargo items, including JIP3, APP, mitochondria, and many other vesicles (46). KIF5s are the heavy chains of kinesin 1, and mammals have three KIF5 genes: kif5a, kif5b, and kif5c. It has been reported that the KIF5A knock-out mouse lost its large caliber axons (47) and that the KIF5C knock-out mouse has a smaller brain (48), suggesting that KIF5s are important for neuronal development. Our finding that JIP3-enhanced axon elongation is dependent on the kinesin-mediated anterograde transportation of JIP3 to the axon tips could partially explain the phenotypes of KIF5 knock-out mice.
Furthermore, we found that JIP3 concentrated at the axon tips could locally activate JNK. There are controversies in the literature as to whether JIP3 can regulate JNK activation. Some reports have shown that JIP3 enhances SEK1-, MEKK1-, or MLK3-stimulated JNK activity by in vitro kinase activity assays using c-Jun as a substrate (3, 4). However, other reports used Western blot analysis of pJNK in HEK293 cells to show that JIP3 has little effect on the basal activity of JNK (36, 49). Moreover, as JIP3 is selectively expressed in neurons, whether it can regulate JNK activation in neurons remains unclear. Our present study revealed that JIP3 selectively activates JNK at axon tips but not in cell bodies, which may explain the discrepant findings in the literature. In addition, JIP3 has been proposed to mediate both the anterograde and retrograde transport of JNK in injured nerves (50). However, we found that JIP3 does not influence JNK distribution in the developing hippocampal neurons. JNKs have been proven to be important for neurite outgrowth (11, 51, 52). Our microfluidic chamber experiment demonstrated that inhibition of JNK activity in the cell body has no effect, whereas JNK activation at axon tips is essential for axon elongation. We next found that inhibition of JNK activity at axon tips totally abolished the effect of JIP3 on axon elongation, which further confirmed that JIP3 enhances axon elongation by locally activating JNK at axon tips.
Third, we found that JIP3 could activate cofilin at axon tips via local activation of JNK, thereby regulating filopodial dynamics and enhancing axon elongation. Filopodia are actin-rich plasma membrane protrusions and play key roles in exploring the local environment and in neuronal development. In mammalian cells, cofilin activity is inhibited by phosphorylation at serine 3, and it has been reported that cofilin can enhance neurite outgrowth in cortical neurons (40), Drosophila neurons (41), or PC12 cells (42) by regulating F-actin dynamics. The JIP3ΔΔ and JIP3ΔJBD constructs, which were unable to activate JNK at axon tips, failed to activate cofilin. These data suggested that JIP3 facilitates cofilin activation at axon tips via local activation of JNK, which is consistent with a previous report that JNK could promote cofilin activation (37). Interestingly, cofilin S3E, which is the less active, pseudo-phosphorylated mutant of cofilin, could abolish the effect of JIP3 on axon elongation. The result suggested that cofilin activity at axon tips is important for JIP3-enhanced axon elongation. However, many other JNK substrates, such as doublecortin (DCX), MAP2, the heavy subunit of the neurofilament protein (NFH), and superior cervical ganglia clone 10 (SCG10), have been reported to be important for neurite outgrowth (37, 51, 53–56). Whether these JNK substrates participate in the effect of JIP3 on axon elongation is worthy of further investigation.
It has been reported that JIP1, another member of the JIP protein family, promotes axon elongation via a newly described interaction with c-Abl tyrosine kinase (57). JNK-associated leucine zipper protein, a novel member of the JIP protein family, negatively regulates NGF-induced neurite outgrowth of PC12 cells by decreasing the levels of phosphorylated SCG10 (56). Combined with our results, we hypothesize that those different members of the JIP protein family could regulate different aspects of neuronal development and might coordinate to regulate neuronal development.
Taken together, our in vitro and in vivo studies have demonstrated that JIP3 enhances axon elongation by regulating actin dynamics through the JNK-cofilin pathway at axon tips, although it has no influence on axon specification. These findings provide a novel function of JIP3 that links actin dynamics and axon elongation, which may help further the understanding of the molecular mechanisms controlling axon elongation.
This work was supported by National Natural Science Foundation of China Grants 31071254, 31130026, and 51073045, National 973 Basic Research Program of China Grants 2012CB911000 and 2009CB941403, State Program of National Natural Science Foundation of China for Innovative Research Group Grant 81021001, and the Independent Innovation Foundation of Shandong University.
- JIP
- JNK-interacting protein
- KBD
- kinesin binding domain
- JBD
- JNK binding domain
- LZ
- leucine zipper
- EGFP
- enhanced GFP
- DIV
- days in vitro
- P
- postnatal day
- E
- embryonic day
- SVZ
- subventricular zone
- IZ
- intermediate zone
- ML
- midline.
REFERENCES
- 1. Whitmarsh A. J., Davis R. J. (1998) Structural organization of MAP kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem. Sci. 23, 481–485 [DOI] [PubMed] [Google Scholar]
- 2. Yasuda J., Whitmarsh A. J., Cavanagh J., Sharma M., Davis R. J. (1999) The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19, 7245–7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito M., Yoshioka K., Akechi M., Yamashita S., Takamatsu N., Sugiyama K., Hibi M., Nakabeppu Y., Shiba T., Yamamoto K. I. (1999) JSAP1, a novel Jun N-terminal protein kinase (JNK)-binding protein that functions as a scaffold factor in the JNK signaling pathway. Mol. Cell. Biol. 19, 7539–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelkar N., Gupta S., Dickens M., Davis R. J. (2000) Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Mol. Cell. Biol. 20, 1030–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akechi M., Ito M., Uemura K., Takamatsu N., Yamashita S., Uchiyama K., Yoshioka K., Shiba T. (2001) Expression of JNK cascade scaffold protein JSAP1 in the mouse nervous system. Neurosci. Res. 39, 391–400 [DOI] [PubMed] [Google Scholar]
- 6. Sun F., Zhu C., Dixit R., Cavalli V. (2011) Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J. 30, 3416–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliva A. A., Jr., Atkins C. M., Copenagle L., Banker G. A. (2006) Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26, 9462–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosso S. B., Sussman D., Wynshaw-Boris A., Salinas P. C. (2005) Wnt signaling through Dishevelled, Rac, and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 [DOI] [PubMed] [Google Scholar]
- 9. Sanyal S., Sandstrom D. J., Hoeffer C. A., Ramaswami M. (2002) AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416, 870–874 [DOI] [PubMed] [Google Scholar]
- 10. Srahna M., Leyssen M., Choi C. M., Fradkin L. G., Noordermeer J. N., Hassan B. A. (2006) A signaling network for patterning of neuronal connectivity in the Drosophila brain. PLoS Biol. 4, e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waetzig V., Zhao Y., Herdegen T. (2006) The bright side of JNKs–Multitalented mediators in neuronal sprouting, brain development, and nerve fiber regeneration. Prog. Neurobiol. 80, 84–97 [DOI] [PubMed] [Google Scholar]
- 12. Bowman A. B., Kamal A., Ritchings B. W., Philp A. V., McGrail M., Gindhart J. G., Goldstein L. S. (2000) Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103, 583–594 [DOI] [PubMed] [Google Scholar]
- 13. Byrd D. T., Kawasaki M., Walcoff M., Hisamoto N., Matsumoto K., Jin Y. (2001) UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32, 787–800 [DOI] [PubMed] [Google Scholar]
- 14. Huang S. H., Duan S., Sun T., Wang J., Zhao L., Geng Z., Yan J., Sun H. J., Chen Z. Y. (2011) JIP3 mediates TrkB axonal anterograde transport and enhances BDNF signaling by directly bridging TrkB with kinesin-1. J. Neurosci. 31, 10602–10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bilimoria P. M., de la Torre-Ubieta L., Ikeuchi Y., Becker E. B., Reiner O., Bonni A. (2010) A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J. Neurosci. 30, 16766–16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao L., Sheng A. L., Huang S. H., Yin Y. X., Chen B., Li X. Z., Zhang Y., Chen Z. Y. (2009) Mechanism underlying activity-dependent insertion of TrkB into the neuronal surface. J. Cell Sci. 122, 3123–3136 [DOI] [PubMed] [Google Scholar]
- 17. Gaudillière B., Konishi Y., de la Iglesia N., Yao Gl, Bonni A. (2004) A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron 41, 229–241 [DOI] [PubMed] [Google Scholar]
- 18. Konishi Y., Stegmüller J., Matsuda T., Bonni S., Bonni A. (2004) Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 [DOI] [PubMed] [Google Scholar]
- 19. Dotti C. G., Sullivan C. A., Banker G. A. (1988) The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito T., Nakatsuji N. (2001) Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237–246 [DOI] [PubMed] [Google Scholar]
- 21. Saito T. (2006) In vivo electroporation in the embryonic mouse central nervous system. Nat. Protoc. 1, 1552–1558 [DOI] [PubMed] [Google Scholar]
- 22. Tabata H., Nakajima K. (2008) Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev. Growth Differ. 50, 507–511 [DOI] [PubMed] [Google Scholar]
- 23. Taylor A. M., Rhee S. W., Tu C. H., Cribbs D. H., Cotman C. W., Jeon N. L. (2003) Microfluidic multicompartment device for neuroscience research. Langmuir 19, 1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y., Yuan B., Ji H., Han D., Chen S., Tian F., Jiang X. (2007) A method for patterning multiple types of cells by using electrochemical desorption of self-assembled monolayers within microfluidic channels. Angew Chem. Int. Ed. Engl. 46, 1094–1096 [DOI] [PubMed] [Google Scholar]
- 25. Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) A microfluidic culture platform for CNS axonal injury, regeneration, and transport. Nat. Methods 2, 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki A., Arikawa C., Kuwahara Y., Itoh K., Watanabe M., Watanabe H., Suzuki T., Funakoshi Y., Hasegawa H., Kanaho Y. (2010) The scaffold protein JIP3 functions as a downstream effector of the small GTPase ARF6 to regulate neurite morphogenesis of cortical neurons. FEBS Lett. 584, 2801–2806 [DOI] [PubMed] [Google Scholar]
- 27. Gupta A., Tsai L. H., Wynshaw-Boris A. (2002) Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet. 3, 342–355 [DOI] [PubMed] [Google Scholar]
- 28. Marín O., Rubenstein J. L. (2003) Cell migration in the forebrain. Annu Rev. Neurosci 26, 441–483 [DOI] [PubMed] [Google Scholar]
- 29. Zheng W., Geng A. Q., Li P. F., Wang Y., Yuan X. B. (2012) Robo4 regulates the radial migration of newborn neurons in developing neocortex. Cereb. Cortex 22, 2587–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tabata H., Nakajima K. (2003) Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 23, 9996–10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noctor S. C., Martínez-Cerdeño V., Ivic L., Kriegstein A. R. (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 [DOI] [PubMed] [Google Scholar]
- 32. Hatanaka Y., Yamauchi K. (2013) Excitatory cortical neurons with multipolar shape establish neuronal polarity by forming a tangentially oriented axon in the intermediate zone. Cereb. Cortex 23, 105–113 [DOI] [PubMed] [Google Scholar]
- 33. Hatanaka Y., Murakami F. (2002) In vitro analysis of the origin, migratory behavior, and maturation of cortical pyramidal cells. J. Comp. Neurol. 454, 1–14 [DOI] [PubMed] [Google Scholar]
- 34. Wang C. L., Zhang L., Zhou Y., Zhou J., Yang X. J., Duan S. M., Xiong Z. Q., Ding Y. Q. (2007) Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci. 27, 11334–11342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hammond J. W., Griffin K., Jih G. T., Stuckey J., Verhey K. J. (2008) Co-operative versus independent transport of different cargoes by Kinesin-1. Traffic 9, 725–741 [DOI] [PubMed] [Google Scholar]
- 36. Takino T., Nakada M., Miyamori H., Watanabe Y., Sato T., Gantulga D., Yoshioka K., Yamada K. M., Sato H. (2005) JSAP1/JIP3 cooperates with focal adhesion kinase to regulate c-Jun N-terminal kinase and cell migration. J. Biol. Chem. 280, 37772–37781 [DOI] [PubMed] [Google Scholar]
- 37. Won K. J., Park S. H., Park T., Lee C. K., Lee H. M., Choi W. S., Kim S. J., Park P. J., Jang H. K., Kim S. H., Kim B. (2008) Cofilin phosphorylation mediates proliferation in response to platelet-derived growth factor-BB in rat aortic smooth muscle cells. J. Pharmacol. Sci. 108, 372–379 [DOI] [PubMed] [Google Scholar]
- 38. Gehler S., Shaw A. E., Sarmiere P. D., Bamburg J. R., Letourneau P. C. (2004) Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin. J. Neurosci. 24, 10741–10749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fass J., Gehler S., Sarmiere P., Letourneau P., Bamburg J. R. (2004) Regulating filopodial dynamics through actin-depolymerizing factor/cofilin. Anat. Sci. Int. 79, 173–183 [DOI] [PubMed] [Google Scholar]
- 40. Meberg P. J., Bamburg J. R. (2000) Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J. Neurosci. 20, 2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng J., Luo L. (2004) Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 44, 779–793 [DOI] [PubMed] [Google Scholar]
- 42. Endo M., Ohashi K., Mizuno K. (2007) LIM kinase and slingshot are critical for neurite extension. J. Biol. Chem. 282, 13692–13702 [DOI] [PubMed] [Google Scholar]
- 43. Andrianantoandro E., Pollard T. D. (2006) Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23 [DOI] [PubMed] [Google Scholar]
- 44. Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A., Margolis B. (2001) Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelkar N., Delmotte M. H., Weston C. R., Barrett T., Sheppard B. J., Flavell R. A., Davis R. J. (2003) Morphogenesis of the telencephalic commissure requires scaffold protein JNK-interacting protein 3 (JIP3). Proc. Natl. Acad. Sci. U.S.A. 100, 9843–9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirokawa N., Niwa S., Tanaka Y. (2010) Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610–638 [DOI] [PubMed] [Google Scholar]
- 47. Xia C. H., Roberts E. A., Her L. S., Liu X., Williams D. S., Cleveland D. W., Goldstein L. S. (2003) Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanai Y., Okada Y., Tanaka Y., Harada A., Terada S., Hirokawa N. (2000) KIF5C, a novel neuronal kinesin enriched in motor neurons. J. Neurosci. 20, 6374–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsuura H., Nishitoh H., Takeda K., Matsuzawa A., Amagasa T., Ito M., Yoshioka K., Ichijo H. (2002) Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the ASK1-JNK signaling pathway. A new mode of regulation of the MAP kinase cascade. J. Biol. Chem. 277, 40703–40709 [DOI] [PubMed] [Google Scholar]
- 50. Cavalli V., Kujala P., Klumperman J., Goldstein L. S. (2005) Sunday Driver links axonal transport to damage signaling. J. Cell Biol. 168, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gdalyahu A., Ghosh I., Levy T., Sapir T., Sapoznik S., Fishler Y., Azoulai D., Reiner O. (2004) DCX, a new mediator of the JNK pathway. EMBO J. 23, 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barnat M., Enslen H., Propst F., Davis R. J., Soares S., Nothias F. (2010) Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J. Neurosci. 30, 7804–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Björkblom B., Ostman N., Hongisto V., Komarovski V., Filén J. J., Nyman T. A., Kallunki T., Courtney M. J., Coffey E. T. (2005) Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: Role of microtubule-associated protein 2 as an effector. J. Neurosci. 25, 6350–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chang L., Jones Y., Ellisman M. H., Goldstein L. S., Karin M. (2003) JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4, 521–533 [DOI] [PubMed] [Google Scholar]
- 55. Shmueli O., Gdalyahu A., Sorokina K., Nevo E., Avivi A., Reiner O. (2001) DCX in PC12 cells: CREB-mediated transcription and neurite outgrowth. Hum. Mol. Genet. 10, 1061–1070 [DOI] [PubMed] [Google Scholar]
- 56. Xu H., Dhanasekaran D. N., Lee C. M., Reddy E. P. (2010) Regulation of neurite outgrowth by interactions between the scaffolding protein, JNK-associated leucine zipper protein, and neuronal growth-associated protein superior cervical ganglia clone 10. J. Biol. Chem. 285, 3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dajas-Bailador F., Jones E. V., Whitmarsh A. J. (2008) The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr. Biol. 18, 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]