Background: Functional interaction of vitamin D (VitD) with steroid receptor superfamily receptors is poorly understood.
Results: 1,25(OH)2-VitD stimulated glucocorticoid (GC) induction of mitogen-activated protein kinase phosphatase-1 (MKP-1) in human monocytes via increased binding of the glucocorticoid receptor to the MKP-1 gene promoter, dependent on VitD receptor, GM-CSF, and MED14.
Conclusion: VitD potentiates CG through MKP-1.
Significance: This study proposes enhancement of GC responses by VitD.
Keywords: Chromatin Immunoprecipitation (ChiP), Glucocorticoid Receptor, Inflammation, Monocytes, Vitamin D
Abstract
Vitamin D (VitD) is now recognized for its pleiotrophic roles in regulating immune function. VitD interaction with other steroid receptor superfamily receptors in peripheral blood mononuclear cells is poorly understood. In the current study, we demonstrate that VitD enhanced glucocorticoid (GC) responses in human peripheral blood mononuclear cells because it stimulated GC induction of mitogen-activated protein kinase phosphatase-1 (MKP-1) and enhanced GC inhibition of LPS-induced IL-6. These VitD effects were abolished in purified CD14+ and CD14− cells but were recovered in CD14+ cells co-cultured with CD14− cells separated by tissue culture inserts. GM-CSF, found in culture supernatants from CD14- cells, was shown to mediate VitD enhancement of GC-induced MKP-1 production in monocytes via increased production of mediator complex subunit 14 (MED14). Recruitment of VitD receptor and MED14, 4.7 kbp upstream of the human MKP-1 gene transcription start site, enhanced binding of glucocorticoid receptor and histone H4 acetylation at the 4.6-kbp glucocorticoid response element of the MKP-1 promoter in the presence of GM-CSF in U937 cells. Knockdown of MED14 abolished VitD-mediated enhancement of GC-induced MKP-1 production. These data demonstrate VitD-mediated stimulation of GC anti-inflammatory effects in human monocytes and identify a role for GM-CSF and MED14 as mediators of this process.
Introduction
A growing body of scientific and medical literature supports the important anti-inflammatory functions of vitamin D (VitD)2 in health and disease beyond its known role in calcium metabolism and bone health (1, 2). VitD acts as a ligand for the VitD receptor (VDR), which belongs to the nuclear receptor superfamily (3). VDR dimerizes with the retinoid X receptor and regulates gene expression by binding to the VitD response element (VDRE) in gene promoters (4, 5). The binding of VDR to VDRE recruits co-activators and enzymes with histone acetylation activity, causing structural changes in chromatin, therefore facilitating gene transcription (6). The potential interaction of VitD with other steroid receptor superfamily receptors, including glucocorticoid receptor (GR), is poorly understood.
Glucocorticoids (GCs) are well known for their anti-inflammatory actions (7). The effects of GC are mediated by GR, which translocates to the cell nuclei once activated by binding to GC. GR dimerizes and binds to GC-responsive elements (GREs) to regulate expression of specific genes, including mitogen-activated kinase phosphatase 1 (MKP-1) (8–11). The induction of MKP-1 by GC has been well documented, and several GREs have been reported in the MKP-1 promoter (12). MKP-1 belongs to a family of phosphatases that inactivate MAPKs by dephosphorylating conserved threonine and tyrosine residues of the activated MAPKs (13, 14), thus inhibiting the production of pro-inflammatory mediators regulated by MAPKs, and this function is essential for the anti-inflammatory actions of GC (15, 16).
Cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2006 report (17) demonstrated that the odds of having VitD deficiency were 2-fold higher in those who reported GC use compared with those without steroid use (odds ratio, 2.36; 95% confidence interval, 1.25, 4.45). It was concluded that GC use is independently associated with VitD deficiency, and a need for screening and repletion in patients on chronic steroids was suggested (17). Recent publications by our research group and others (18–21) have found that asthmatics with low serum VitD have impaired lung function, increased airway hyperreactivity, and increased corticosteroid requirements. We demonstrated that dexamethasone (DEX)-induced MKP-1 expression, as a marker of GC responsiveness, is significantly increased as serum VitD levels rise (18). This suggests that VitD may enhance GC responsiveness. This study was designed to examine the mechanisms of potential steroid-enhancing effects of VitD through its regulation of the MKP-1 gene in human peripheral blood mononuclear cells (PBMCs).
EXPERIMENTAL PROCEDURES
Materials
LPS, DEX, 1,25(OH)2D3 (the active form of VitD), and monoclonal anti-β-actin antibody were purchased from Sigma. Rabbit polyclonal antibody to GR and rabbit IgG were purchased from Abcam, Inc. (Cambridge, MA). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-labeled IgG were purchased from Amersham Biosciences. Rabbit polyclonal antibodies to VDR, MKP-1, and Protein A/G PLUS-agarose beads were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Chemiluminescent reagent was purchased from PerkinElmer Life Sciences. Recombinant human GM-CSF, monoclonal anti-human GM-CSF antibody, Quantikine human GM-CSF immunoassay kit, and a human cytokine array kit were purchased from R&D Systems, Inc. (Minneapolis, MN). ON-TARGETplus SMARTpool siRNA against MED14 (mediator complex subunit 14) and ON-TARGETplus Non-targeting Pool siRNA were purchased from Dharmacon (Lafayette, CO). Amaxa Cell Line Nucleofector Kit C was purchased from Lonza (Basel, Switzerland).
Cell Culture and Treatment
The U937 cell line was purchased from the American Type Culture Collection and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). PBMCs were isolated from heparinized, venous blood of healthy donors by Ficoll-Hypaque density gradient centrifugation. CD14+ cells were separated from PBMC using the Human Monocyte Isolation Kit II purchased from Miltenyi Biotech Inc. (Auburn, CA). CD14- cells used in experiments were labeled non-monocyte cells trapped on the column during the process of negative selection of CD14+ cells and later eluted from the column. Millicell cell culture inserts purchased from Millipore Corp. (Billerica, MA) were used to co-culture CD14+ cells (or U937 cells) and CD14− cells. Cells were cultured in hormone-free medium (phenol red-free RPMI containing 5% charcoal-stripped FCS, 50 μg/ml streptomycin, and 50 units/ml penicillin) during hormone and LPS treatments.
Real-time PCR
Total RNA from cells was prepared using the RNeasy minikit (Qiagen, Valencia, CA). After reverse transcription, cDNA from each sample was analyzed by real-time PCR using the dual-labeled fluorigenic probe method on an ABI Prism 7300 real-time PCR system (Applied Biosystems). Expression of specific genes was determined using primers purchased from Applied Biosystems (Foster City, CA). Relative gene expression levels were calculated and normalized to the corresponding levels of the housekeeping gene (β-actin).
Western Blot
Protein samples were resolved on Invitrogen 4–12% BisTris gel and transferred to PVDF membranes. The membranes were incubated in PBS containing specific antibodies, 5% dry milk, and 0.1% Tween 20 at 4 °C overnight. Subsequently, membranes were washed in PBS, 0.1% Tween 20, incubated for 1 h at room temperature with HRP-labeled secondary antibodies, washed, incubated with chemiluminescent reagent, and processed for autoradiography.
ELISA Analysis
Supernatants were collected from cultured cells. IL-6 levels were tested using the human IL-6 ELISA Ready-set-go! kit from eBioscience, Inc. (San Diego, CA). GM-CSF levels were tested using the Quantikine human GM-CSF immunoassay kit from R&D Systems.
Chromatin Immunoprecipitation (ChIP) Assay
VDR binding to VDRE and GR binding to GRE were assessed by a ChIP assay as described previously (22) with modifications. Briefly, 4 × 106 cells were used in each precipitation. After sonication, 1 ml of chromatin solution was precleared with 60 μl of Protein A/G PLUS-agarose beads plus 12 μg of herring DNA at 4 °C for 2 h and incubated with specific antibody or isotype control at 4 °C overnight, followed by precipitation with 60 μl of Protein A/G PLUS-agarose beads at room temperature for 2 h. Precipitated chromatin complexes were removed from the beads through incubation at 65 °C for 30 min with 210 μl of Elution Buffer (50 mm Tris, pH 8.0, 1 mm EDTA, 1% SDS). 200 μl of eluate was mixed with 10 μl of 5 m NaCl, 1 μl of RNase A (10 mg/ml, DNase-free), and incubated at 65 °C overnight. Samples were then digested with proteinase K. DNA was purified with QIAquick PCR purification columns (Qiagen). Precipitated DNA was quantified by quantitative real-time PCR using SYBR Green (Applied Biosystems). Primers to detect GRE4.6 and GRE1.3 were synthesized according to Ref. 12; primers to detect VDRE4.7 were as follows: 5′-AGCTGGGATTCTAATCCAGGCAGT-3′ and 5′-CTTTGGAAGGTGGAGTTCCTCTCA-3′.
Re-ChIP experiments were done using anti-MED14 antibody to precipitate the chromatin complex first and then precipitate the complex again with anti-VDR or anti-GR antibodies following the ChIP procedures as above. Precipitated DNA was quantified by real-time PCR using primers to detect GRE4.6.
Inhibition of MED14 Expression
1 × 106 U937 cells were transfected with 300 nm siRNA against MED14 or non-targeting control siRNA using Amaxa Cell Line Nucleofector Kit C following the manufacturer's instructions.
Statistical Analyses
Results were expressed as the mean ± S.E. Statistical analysis was conducted using GraphPad Prism, version 5 (GraphPad Software, La Jolla, CA). These data were analyzed by the paired Student's t test, pairing by experimental conditions. Before testing, paired difference distributions were examined for outliers, which can indicate violation to the normality assumption of the t test. No outliers were apparent. Differences were considered significant at p < 0.05. A minimum of three independent experiments were conducted to allow for statistical comparisons.
RESULTS
VitD Reduces the Concentration of GC Required for MKP-1 Induction and Enhances the Activity of GC to Suppress Proinflammatory Cytokine Production by Human PBMCs
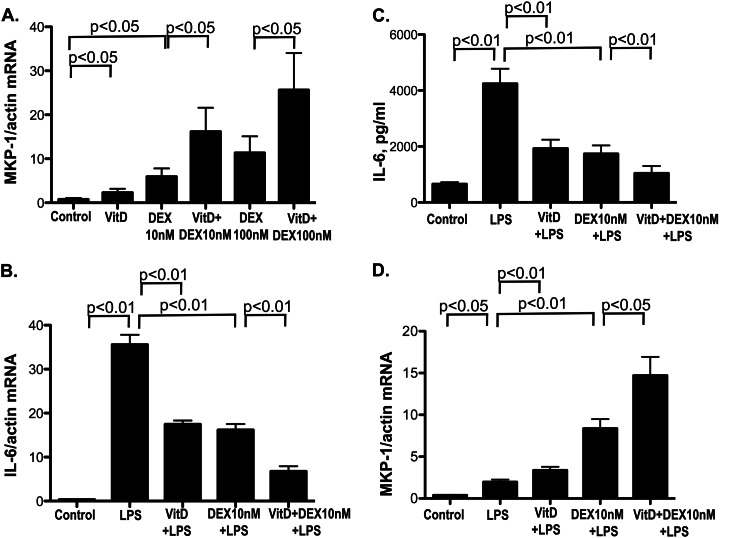
To determine whether VitD affects the induction of MKP-1 by GC, human PBMCs were preincubated with 10 nm 1,25(OH)2D3 (the biologically active form of VitD) or vehicle control (ethanol) for 21 h, followed by treatment with 10 or 100 nm DEX, a synthetic GC, for 3 h. We observed that DEX alone induced MKP-1 mRNA by 9.4 ± 1.9-fold (p < 0.05 as compared with medium-treated cells) and 16.6 ± 3.9-fold (p < 0.05) when the cells were treated with 10 and 100 nm DEX, respectively, and the expression of MKP-1 mRNA was enhanced 2.9 ± 0.4-fold (p < 0.05 as compared with DEX alone) and 2.6 ± 0.3 fold (p < 0.05), respectively, if the cells were preincubated with VitD prior to treatment with DEX. VitD alone induced MKP-1 by 3.2 ± 0.4-fold (p < 0.05 as compared with media-treated cells) (Fig. 1A).
FIGURE 1.
VitD enhances MKP-1 induction by DEX (A), stimulates DEX inhibition of the LPS-induced IL-6 production (B and C), and increases MKP-1 induction by DEX in the presence of LPS in human PBMCs (D). A, PBMC from healthy donors were preincubated with VitD for 21 h, followed by DEX treatment at 10 and 100 nm, respectively, for 3 h. MKP-1 mRNA levels were detected by real-time PCR and were normalized to β-actin mRNA (n = 6). B and C, PBMCs were preincubated with VitD (10 nm) for 24 h, followed by LPS (10 ng/ml) stimulation for 6 h with or without DEX (10 nm). IL-6 (B) and MKP-1 (D) mRNA levels in PBMC and IL-6 protein levels in the culture supernatants (C) were detected by real-time PCR and ELISA, respectively (n = 4). Values represent mean ± S.E. (error bars).
We recently reported that VitD up-regulated MKP-1 inhibits LPS-induced IL-6 and TNFα production in monocytes/macrophages (23). To examine whether changes in MKP-1 expression by VitD and GC combination enhanced GC suppression of proinflammatory cytokine production, human PBMCs were preincubated with VitD for 24 h, followed by stimulation with 10 ng/ml LPS for 6 h. IL-6, TNFα, and MKP-1 mRNA expression were examined in the cells for all treatment conditions. LPS treatment stimulated IL-6 mRNA production by the cells (p < 0.01). VitD and DEX significantly inhibited LPS-induced IL-6 mRNA expression by 51% (n = 4, p < 0.01) and 54% (n = 4, p < 0.01), respectively; the combination of VitD and DEX resulted in 81% inhibition of LPS-induced IL-6 mRNA levels (n = 4, p < 0.01) (Fig. 1B). Upon stimulation with LPS, the amounts of IL-6 protein in culture supernatants increased from 656 ± 67 to 4244 ± 534 pg/ml (p < 0.01). VitD and DEX inhibited LPS-induced IL-6 protein production by 63% (n = 4, p < 0.01) and 67% (n = 4, p < 0.01), respectively; the combination of VitD and DEX enhanced this inhibition to 80% (n = 4, p < 0.01) (Fig. 1C). VitD and DEX synergistically inhibited LPS-induced TNFα mRNA production in human monocytes as well (supplemental Fig. 1).
At the same time, significant changes in the cellular MKP-1 mRNA expression were observed (Fig. 1D). LPS treatment resulted in the up-regulation of MKP-1 mRNA expression (p < 0.05). Cellular MKP-1 mRNA levels were further induced 4.5 ± 1.4-fold (mean ± S.D.) in response to LPS/DEX (p < 0.01 as compared with LPS-treated cells). MKP-1 mRNA expression in response to LPS was significantly higher in VitD-pretreated cells (p < 0.01 as compared with the cells cultured in media alone for 24 h, followed by 6 h of LPS treatment). MKP-1 mRNA expression was the highest in the cells pretreated with VitD and stimulated with DEX and LPS as compared with all other treatment conditions (Fig. 1D).
Enhancing Effects of VitD on GC-mediated Gene Transcription Are Observed in Human Monocytes and Require CD14− Cells
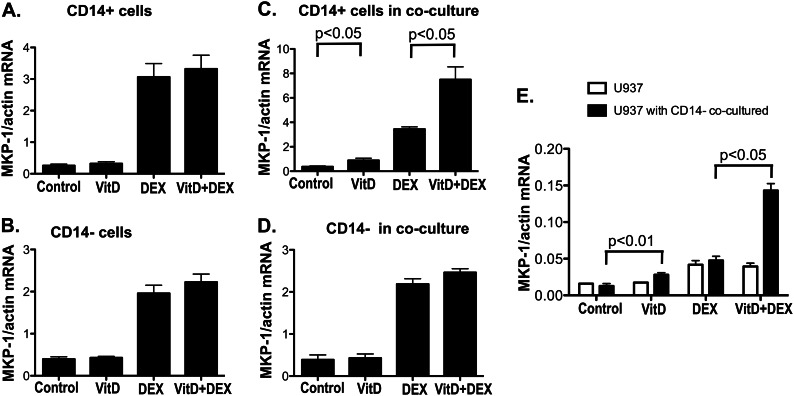
To further understand the effects of VitD on DEX-induced MKP-1, CD14− and CD14+ cells were cultured separately with VitD for 21 h, followed by 3 h of DEX treatment. MKP-1 induction was assessed by real-time PCR in CD14− and CD14+ cells. It was found that VitD lost its ability to enhance DEX induction of MKP-1 in purified CD14+ and CD14− cells separated from each other (Fig. 2, A and B). Co-culture of CD14+ and CD14− cells using a tissue culture insert, which prevented direct cell contact but allowed medium exchange between the two cell compartments, restored VitD steroid-enhancing action in CD14+ cells but not in CD14− cells (Fig. 2, C and D). Similarly, if the monocytic cell line, U937, was co-cultured with CD14− cells, MKP-1 mRNA induction by DEX in U937 cells was significantly greater in the presence of VitD as compared with DEX alone (Fig. 2E). These data suggested that an unknown soluble factor produced by CD14− cells in the presence of VitD was responsible for the steroid-enhancing effects of VitD in human CD14+ monocytes.
FIGURE 2.
VitD enhances DEX induction of MKP-1 in CD14+ cells and U937 cells monocytic cell line only in the presence of CD14− cells. A, purified CD14+ cells (n = 12); B, purified CD14− cells (n = 12); C, CD14+ cells with CD14− cells in tissue culture inserts (n = 4); D, CD14− cells in tissue culture inserts with CD14+ cells on the bottom of the cell culture wells (n = 4); E, U937 cells with CD14− cells in tissue culture inserts (n = 3) were preincubated with VitD for 21 h, followed by DEX (10 nm) treatment for 3 h. MKP-1 mRNA levels were detected by real-time PCR and were normalized to β-actin mRNA. Values represent mean ± S.E. (error bars).
GM-CSF Produced by CD14− Cells Significantly Increases DEX Induction of MKP-1 in U937 Cells in the Presence of VitD
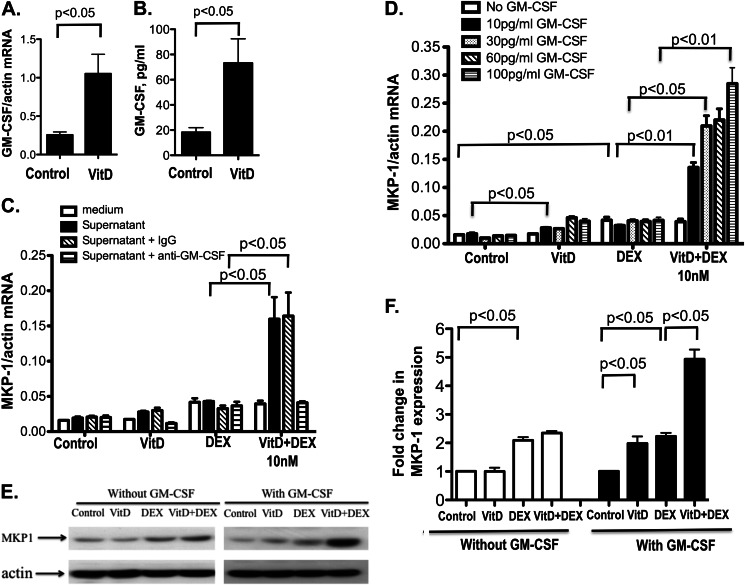
Soluble factors produced by CD14− were assessed in the supernatants of VitD-stimulated CD14− cells using a human cytokine array kit. GM-CSF was found in the supernatants from CD14− cells but not in the supernatants from CD14+ cells after 24 h of VitD stimulation (supplemental Fig. 2). To confirm this, GM-CSF mRNA and protein expression were examined in CD14+ and CD14− cells cultured with and without VitD for 24 h. CD14− cells cultured in media for 24 h produced GM-CSF mRNA and protein. The expression of GM-CSF mRNA and protein was significantly increased in CD14− cells after VitD treatment (4.0 ± 0.8-fold (p < 0.05) and 3.9 ± 0.6-fold (p < 0.05) for mRNA and protein, respectively (Fig. 3, A and B)). No detectable GM-CSF mRNA expression and GM-CSF protein in corresponding culture supernatants were observed in media- and VitD-cultured CD14+ cells (data not shown).
FIGURE 3.
GM-CSF produced by CD14− cells stimulates MKP-1 induction by VitD and enhances MKP-1 induction by DEX in the presence of VitD. Purified CD14− cells were treated with VitD for 24 h. GM-CSF mRNA levels in the CD14− cells (A) and protein levels in the culture supernatants (B) were detected by real-time PCR and ELISA, respectively (n = 4). C, cell culture supernatants from VitD-stimulated CD14− cells allow VitD enhancement of DEX-induced MKP-1 mRNA production by U937 cells, and this effect is abolished if GM-CSF is depleted from cell culture supernatants. Cell culture supernatants from VitD-treated CD14− cells were depleted of GM-CSF using 0.5 μg of anti-GM-CSF-specific antibody (or isotype control) at 37 °C for 1 h, followed by precipitation with 60 μl of Protein A/G PLUS-agarose beads at room temperature for 2 h. Supernatants cleared of antibody·protein A/G complexes by centrifugation were added to the U937 monocytic cell line. The cells were treated with VitD for 21 h, followed by DEX (10 nm) treatment for 3 h. MKP-1 mRNA levels were detected by real-time PCR (n = 3). Recombinant GM-CSF dose-dependently stimulated VitD-mediated enhancement of DEX-induced MKP-1 mRNA (D) and protein (E) production in U937 cells. D, U937 cells were incubated with human recombinant GM-CSF at the indicated doses together with VitD for 21 h, followed by DEX (10 nm) treatment for 3 h. MKP-1 mRNA expression was detected as above (n = 3). E, U937 cells were incubated with 60 pg/ml human recombinant GM-CSF together with VitD for 21 h, followed by DEX (10 nm) treatment for 3 h, and MKP-1 expression was determined in cell protein extracts by Western blot. β-Actin expression in cell extracts was used as a loading control. Images are representative of three independent experiments. F, -fold changes in the densitometry readings of MKP-1 expression normalized to β-actin expression for the cells treated with and without GM-CSF are shown. -Fold changes were calculated in comparison with vehicle control set as 1. Values represent mean ± S.E. (error bars).
The effect of GM-CSF neutralization in cell culture supernatants from CD14− cells on steroid-enhancing actions of VitD was tested. After the depletion of GM-CSF from CD14− cell culture supernatants, the potentiation of DEX-induced MKP-1 mRNA production by VitD in CD14+ cells by these cell culture supernatants was completely abolished (Fig. 3C). VitD enhanced DEX-induced MKP-1 mRNA production by U937 cells in the presence of recombinant GM-CSF (Fig. 3D). Potentiation of VitD effects on DEX induction of MKP-1 was observed with as little as 10 pg/ml GM-CSF. MKP-1 induction by VitD alone was significantly increased in the presence of all of the tested doses of GM-CSF. These changes in MKP-1 mRNA expression reflected the changes in MKP-1 protein expression for the experimental conditions tested. As shown by Western blot, DEX treatment significantly induced MKP-1 protein expression in U937 cells (p < 0.05), and no VitD enhancement of MKP-1 protein production was observed in the absence of GM-CSF (Fig. 3, E and F). However, in the presence of GM-CSF, VitD significantly enhanced DEX-induced MKP-1 protein expression in these cells (p < 0.05) (Fig. 3, E and F).
GM-CSF-induced MED14 in U937 Cells
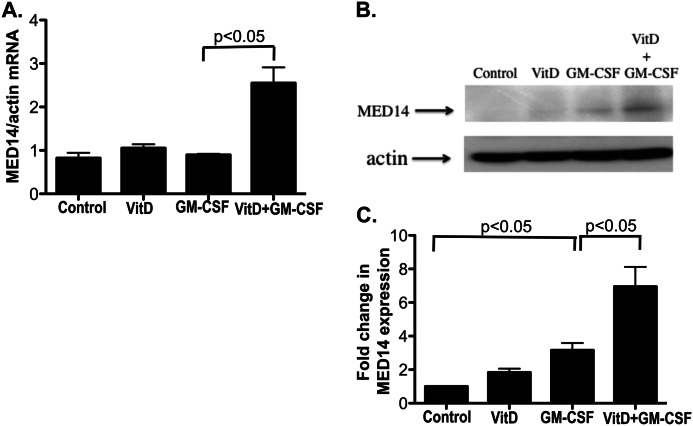
The expression of co-activators, co-repressors, and histone acetyltransferases known to interact with VDR and GR (including NCoR1, NCoR2, SMARCA2, SMARCA4, PCAF, NCOA1, NCOA2, NCOA3, CBP, P300, KAT5, ATF2, MED1, and MED14) was evaluated by real-time PCR in the U937 cells treated with the combination of GM-CSF and VitD as compared with VitD alone. The combination of GM-CSF and VitD significantly increased MED14 mRNA expression by U937 cells as compared with GM-CSF alone (Fig. 4A), and this resulted in up-regulation of the MED14 protein expression by U937 cells (Fig. 4, B and C).
FIGURE 4.
GM-CSF induces MED14 expression in U937 cells. MED14 mRNA (A) and protein expression (B) in U937 cells treated with VitD, GM-CSF (60 pg/ml), or both. MED14 mRNA levels in the cells were tested 6 h after stimulation by real-time PCR and were normalized to β-actin mRNA. MED14 protein expression by the cells was measured 24 h after stimulation. Whole cell extracts were blotted with antibodies against MED14 and β-actin. Images are representative of three independent experiments. C, -fold changes in the densitometry readings of MED14 normalized to β-actin were calculated in comparison with vehicle control set as 1. Values represent mean ± S.E. (error bars).
Recruitment of VDR and MED14 to Human MKP-1 Promoter Enhances GR Binding and Histone H4 Acetylation at the GRE Adjacent to the VDRE in Human MKP-1 Gene Promoter
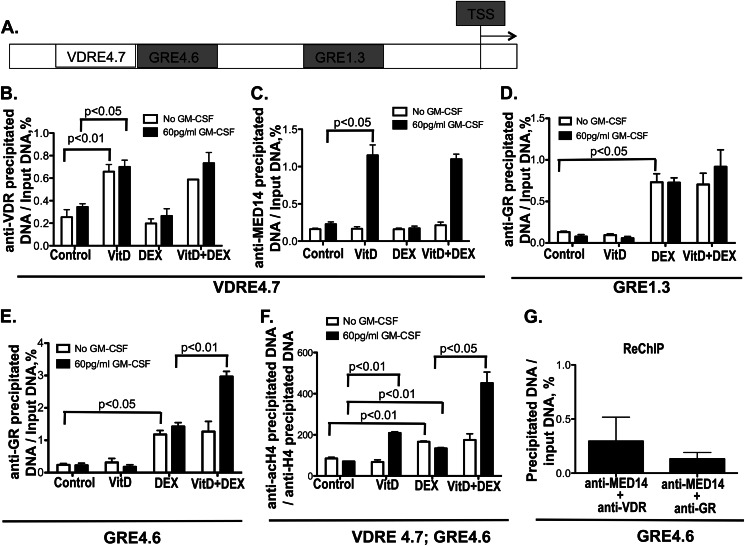
We examined VDR and GR interaction with the human MKP-1 gene promoter in U937 cells under various treatment conditions. VDR interaction with a potential VDRE (VDRE4.7) (23) and GR interaction with two reported GREs (GRE4.6 and GRE1.3) (12) in the MKP-1 gene promoter were evaluated by a ChIP assay. A schematic drawing of the human MKP-1 gene promoter and location of VDRE and GREs tested is shown in Fig. 5A.
FIGURE 5.
Recruitment of VDR, GR, and MED14 to human MKP-1 gene promoter. U937 cells were cultured in hormone-free medium with or without GM-CSF (60 pg/ml) and treated with VitD or vehicle control for 21 h, followed by DEX (10 nm) treatment for 3 h. VDR, GR, and MED14 binding to the VDRE and GRE in the human MKP-1 gene promoter and histone H4 acetylation in these regions were examined using a ChIP assay. The quantity of anti-VDR, anti-GR, and anti-MED14 antibody-precipitated DNA was normalized to input DNA, and anti-acetylated histone H4 antibody-precipitated DNA was normalized to anti-histone H4 antibody-precipitated DNA. A, schematic representation of the VDRE and GREs in the human MKP-1 promoter. TSS, transcriptional start site. The numbers represent kbp upstream of the TSS. B and C, recruitment of VDR and MED14 to VDRE4.7 site. D, recruitment of GR to the GRE1.3 site. E, recruitment of GR to the GRE4.6 site. F, histone H4 acetylation at the VDRE4.7/GRE4.6 site. G, recruitment of MED14·VDR and MED14·GR complexes to the GRE4.6 site as detected by re-ChIP experiments following GM-CSF, VitD, and DEX treatment (no specific precipitation of GRE4.6 was detected under control conditions; therefore, the data are not presented in this figure). Values represent mean ± S.E. (error bars) (n = 3).
The potential VDRE sequence AGTTCAAATCATTCA is located at −4708 to −4694 from the transcriptional start site (VDRE4.7) (23) in the human MKP-1 gene promoter. After treatment with VitD, a significant increase in VDR binding to this region of the MKP-1 gene promoter was observed (p < 0.01). No MED14 binding to this VDRE was found if the cells were treated only with VitD. If cells were stimulated by VitD and GM-CSF, VDR binding to VDRE was unaltered; however, a significant recruitment of MED14 to this VDRE in the MKP-1 gene promoter was observed (p < 0.05). DEX treatment did not influence VDR and MED14 recruitment to VDRE4.7 in the MKP-1 gene promoter (Fig. 5, B and C).
As for GR recruitment to the published GRE4.6 and GRE1.3 sites in the human MKP-1 gene promoter (12), a significant increase in GR binding to both GRE regions was observed after the cells were treated with DEX. VitD did not affect the GR binding to these two sites, but in the presence of GM-CSF, VitD enhanced the binding of GR to GRE4.6 (p < 0.01) and did not affect the GR binding to GRE1.3 (Fig. 5, D and E).
Histone H4 acetylation in the vicinity of VDR and GR binding sites VDRE4.7 and GRE4.6 was examined by a ChIP assay using primers to amplify VDRE4.7. The results showed that in the absence of GM-CSF, DEX induced histone H4 acetylation (p < 0.01) in this region, and VitD treatment had no effect on histone H4 acetylation in this region. However, in the presence of GM-CSF, VitD alone induced histone H4 acetylation in the VDRE4.7 region (p < 0.01) and further enhanced histone H4 acetylation induced by DEX (p < 0.05) (Fig. 5F).
To further investigate whether MED14, VDR, and GR form a complex around the site of GRE4.6, re-ChIP experiments were done using chromatin precipitation with anti-MED14 antibody followed by the re-ChIP with anti-GR or anti-VDR. The results showed that MED14 formed a complex with VDR and GR at the site of GRE4.6 under the treatment of GM-CSF, VitD, and DEX, indicating simultaneous interaction of MED14·GR·VDR at this site of the human MKP-1 promoter (Fig. 5G).
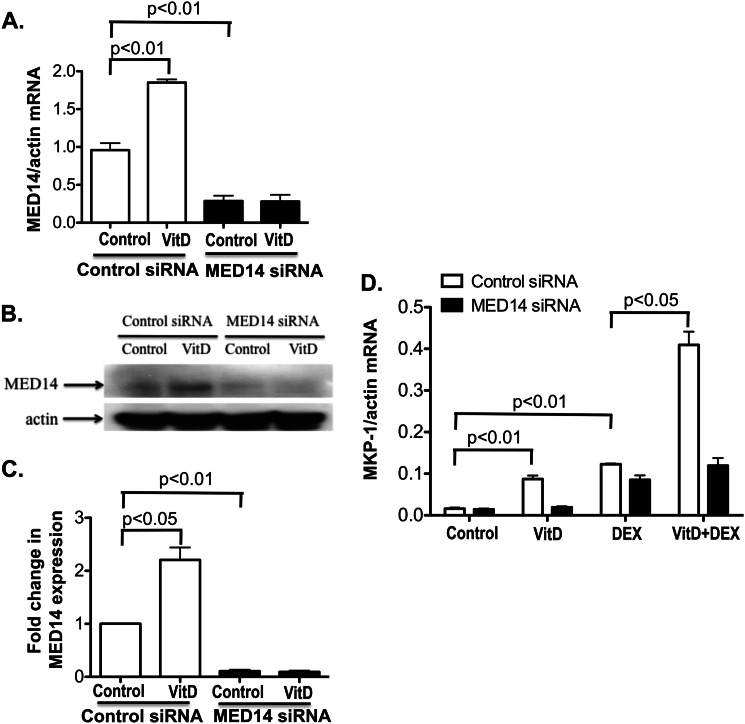
Knockdown of MED14 Expression in U937 Cells Abolishes the Enhancement of DEX Induction of MKP-1 by VitD
To determine whether VitD enhancement of DEX-induced MKP-1 production by U937 cells in the presence of GM-CSF is mediated by MED14, we specifically knocked down MED14 expression by RNA interference and assessed DEX induction of MKP-1. MED14 siRNA significantly inhibited the base-line and VitD-induced MED14 mRNA and protein expression in the presence of GM-CSF (Fig. 6, A–C). As before, VitD enhanced the DEX induction of MKP-1 with the presence of GM-CSF if the cells were transfected with control siRNA. In contrast, when cells were transfected with MED14 siRNA, VitD-mediated enhancement of DEX-induced MKP-1 mRNA production was abrogated (Fig. 6D). These data confirm that VitD enhances GR action through MED14 in the presence of GM-CSF.
FIGURE 6.
MED14 knockdown in U937 cells abolishes GC-sparing effects of VitD on MKP-1 induction by DEX. Base-line and VitD-induced MED14 mRNA (A) and protein (B and C) expression are inhibited by MED14 siRNA but not control siRNA in U937 cells. Following the transfection of siRNA, U937 cells were cultured in RPMI 1640 medium for 24 h. Cells were treated with or without VitD in the presence of 60 pg/ml GM-CSF to examine MED14 mRNA (A) and MED14 protein (B) expression 6 and 24 h post-stimulation, respectively. C, -fold changes in the densitometry readings of MED14 protein expression normalized to β-actin were calculated in comparison with vehicle control set as 1. D, MED14 siRNA inhibits VitD induction of MKP-1 mRNA and VitD enhancement of DEX-induced MKP-1 mRNA in the U937 cell line. Cells were treated with 60 pg/ml GM-CSF and VitD or vehicle control for 21 h, followed by DEX (10 nm) treatment for 3 h. MKP-1 mRNA levels were tested by real-time PCR and were normalized to β-actin mRNA. Values represent mean ± S.E. (error bars).
DISCUSSION
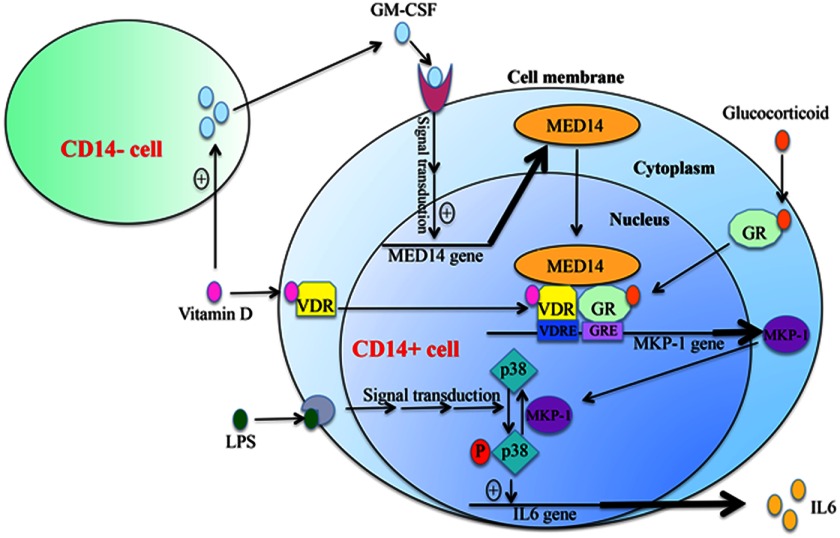
In this study, we demonstrate for the first time a mechanism for the steroid-enhancing effects of VitD in human monocytes. Further experiments demonstrated that GM-CSF, secreted by CD14− cells, induced MED14 expression in monocytes and subsequently contributed to the enhancement of GC-induced MKP-1 expression by VitD in monocytes. ChIP assays, in our study, demonstrated that GM-CSF and VitD treatment increased VDR and MED14 binding to the potential VDRE in the human MKP-1 gene promoter and subsequently enhanced GR binding to a GRE in the MKP-1 gene promoter located near the VDRE site following DEX treatment. Furthermore, histone H4 acetylation around the VDRE area in the MKP-1 gene promoter was significantly enhanced following the VDR and GR binding in the presence of GM-CSF. Our current study therefore demonstrates that steroid-enhancing effects of VitD, in human monocytes, require GM-CSF produced by CD14− cells and are mediated by the MED14 transcriptional co-activator, which enhances GR interaction with GRE in the vicinity of the VDRE site in the human MKP-1 gene promoter. This results in increased production of the anti-inflammatory gene, MKP-1, in human monocytes as summarized in Fig. 7.
FIGURE 7.
Proposed mechanism for the synergistic actions of VitD and GC on MKP-1 induction in human monocytes. VitD stimulates GM-CSF production by CD14− cells, which induces MED14 expression in CD14+ cells. MED14 acts as a bridge between ligand-bound VDR and GR at the adjacent VDRE and GRE of the human MKP-1 gene promoter and activates MKP-1 mRNA expression. MKP-1 dephosphorylates LPS-induced p38 MAPK activation and inhibits LPS-induced proinflammatory cytokine production.
The GR has two major anti-inflammation mechanisms. Transrepression involves interaction of GR with proinflammatory transcription factors, such as activator protein 1 (AP-1) and nuclear factor κB (NFκB), that inhibits their activity and accounts for the major anti-inflammatory and immunosuppressive effects of GC (24). GC-bound GR interacts with NFκB, recruits transcriptional repressors, and interferes with the NFκB transcriptional activity (25–27). In addition, the anti-inflammatory effects of GR can involve a second mechanism called transactivation involving gene induction of MKP-1 phosphatase that subsequently dampens the activation of p38 MAPK, an upstream activator of NFκB (28, 29) known to be involved in transcriptional and posttranscriptional regulation of proinflammatory cytokine production (15, 30). Despite the excellent efficacy of GC to control inflammation, side effects frequently limit long term GC application. The side effects of GCs have been shown to be strictly dose-dependent (31). Therefore, it is desirable to lower the dose of GC treatment while keeping the same level of production of GC-regulated genes that exhibit anti-inflammatory effects, such as MKP-1.
In recent years, VitD has been shown to have potent anti-inflammatory effects and is now considered as adjunctive therapy for numerous chronic diseases, including asthma, arthritis, and prostate cancer (19, 32, 33). It has been demonstrated that VitD directly induces the production of antimicrobial peptides, such as cathelicidin and human β-defensin 4, by human monocytes/macrophages and epithelial cells (34–36). VitD can also interfere with the nuclear translocation of NFκB by increasing the expression of IkBα (37). We recently identified the up-regulation of MKP-1 by VitD as a novel pathway by which vitamin D inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages. VitD inhibition of the LPS-induced cytokine production was significantly reduced in bone marrow-derived macrophages of MKP-1 KO mice as compared with the cells of wild type mice (23). In our study, we observed synergistic effects of vitamin D and DEX on suppression of LPS-induced cytokine production by human PBMC. Consistent with previous reports (38), MKP-1 mRNA expression was increased in the cells in response to LPS. MKP-1 mRNA expression, however, was the highest in the cells pretreated with VitD and stimulated with DEX and LPS as compared with all other treatment conditions. The current study demonstrates a novel function for vitamin D (i.e. enhancement of cellular responses to GCs) and provides a mechanism for VitD-mediated potentiation of GC responses in human monocytes, thus broadening the list of potential anti-inflammatory actions of VitD.
Additionally, the current study showed steroid-sparing effects of VitD, because the amount of MKP-1 induced by 10 nm DEX in the presence of VitD was similar to the MKP-1 levels induced by 100 nm DEX alone, reducing the required active dose of DEX by 90% while still maintaining the same level of MKP-1 induction. In the future, it will be important to evaluate whether VitD can enhance the capacity of GC to antagonize inflammatory response and lower GC doses in vivo in clinical treatments. As well, it would be critical to examine whether VitD can potentiate GC response under GC-insensitive conditions known to develop in subgroups of patients with chronic inflammatory diseases, such as asthma, rheumatoid arthritis, inflammatory bowel disease, etc. (39). Our preliminary data demonstrate in vitro potentiation of GC responses by VitD in cells not only from normal controls but also in subjects with asthma (19).
In our experiments, VitD enhancement of GC-induced MKP-1 was abolished both in purified CD14+ and CD14− cells, despite expression of VDR by these cells. VitD enhancement of GC responses was recovered in CD14+ cells co-cultured with CD14− cells separated by tissue culture inserts and was shown to be mediated by GM-CSF produced by CD14− cells in response to VitD. Depletion of GM-CSF from the CD14− cell supernatants abolished the supernatant-mediated VitD effect on MKP-1 induction. GM-CSF dose-dependently increased VitD induction of MKP-1 and its enhancing effect on DEX-induced MKP-1.
Previous studies reported GM-CSF gene repression by vitamin D and the VDR in mitogen-stimulated T-cells (40–42) by blocking NFAT-1·AP-1 complex activity. However, an increase in GM-CSF mRNA levels in Jurkat cells cultured with vitamin D only was also noted (41). Further investigation of VDR interaction with the GM-CSF promoter in CD14− and CD14+ cells is of interest to address GM-CSF regulation by vitamin D.
GM-CSF exerts its action by binding to the GM-CSF receptor and triggers cellular signal transduction via the JAK/STAT, MAPK, and PI3K pathways (43). GM-CSF is known to affect cell growth and differentiation (43, 44). Selective effects of VitD in monocytes can be explained by the fact that the GM-CSF receptor is expressed only by monocytes and not by other mononuclear cells in the peripheral blood (45). The receptor is composed of α and β chains. The α chain has specific affinity to GM-CSF, whereas the β chain is shared with IL-3 and IL-5 receptors (43). Because we did not observe detectable IL-3 and IL-5 expression in CD14− cells by real-time PCR (data not shown), the involvement of these cytokines in the GM-CSF/receptor-mediated signal transductions in monocytes was excluded.
In this study, we demonstrate GM-CSF-mediated induction of the MED14 transcriptional co-activator in human monocytes. MED14 was originally discovered as thyroid hormone receptor-associated protein, which enhanced transcriptional activation by thyroid hormone receptor (46). Later, MED14 was found to form a complex with VDR and mediate ligand-dependent enhancement of transcription by VDR (47). More recently, MED14 was reported to interact with GR and was shown to assist GR-mediated induction of interferon regulatory factor 8 (IRF8) and IGF-binding protein 1 (IGFBP1), but the expression of another GR-inducible gene, glucocorticoid-inducible leucine zipper (GILZ), did not require recruitment of MED14 (48, 49). Thus, MED14 recruitment by GR was found to be gene-specific (48).
We examined whether MED14 was required for VDR and GR interaction in the human MKP-1 gene promoter. We identified a potential VDRE site 4.7 kbp upstream of the transcriptional start site (close to GRE4.6) in the human MKP-1 gene promoter. We confirmed VDR and GR binding to the corresponding VDRE and two reported GREs (listed in Fig. 5A) after ligand stimulation. Under GM-CSF treatment, MED14 was recruited to VDRE4.7 following VitD but not DEX treatment, indicating that MED14 was recruited to the promoter via interaction with VDR. Furthermore, VitD enhanced the binding of GR to GRE4.6 in the presence of GM-CSF, indicating that the VDR·MED14 complex assisted GR binding to the GRE immediately downstream of VDRE. Re-ChIP data confirmed the presence of a MED14·VDR·GR complex at GRE4.6. The above data indicated a cross-talk between VitD and GC, mediated by MED14 in the human MKP-1 gene promoter. To address the role of MED14 in this process, we selectively knocked down MED14 expression in GM-CSF-treated U937 cells and found that the enhancing effect of VitD on DEX induction of MKP-1 was abolished. This novel discovery provides direct proof that MED14 bridges the cross-talk between VitD and GC.
For the described VDRE4.7 site in the human MKP-1 promoter (23), additional work should be done (i.e. promoter reporter constructs and gel shift assays, including site-directed mutagenesis) to confirm that this site is a bona fide VDRE.
Based on MKP-1 regulation by VitD and GC in the presence of GM-CSF, our studies suggest that the combination of VitD and GC in anti-inflammatory treatment may potentiate the actions of GC and lower the dose of GC required to reduce inflammation, thereby supporting a reduction in GC-mediated side effects. They further suggest that any factor that inhibits this pathway (e.g. GM-CSF receptor or MED14 mutations) may influence the cross-talk between VitD and GC; discovering factors that stimulate this pathway sets the basis for future development of drugs that benefits the combination usage of VitD and GC. Our current study provides a mechanistic basis for VitD enhancement of GC anti-inflammatory effects and potential biomarkers that can be used in clinical trials assessing the beneficial effects of vitamin D in patients on chronic anti-inflammatory treatment with GCs. In the current study, we exclusively used 1,25(OH)2-VitD, which is not the form that is commonly used for VitD supplementation. This work is not in a position to advise VitD supplementation to enhance glucocorticoid responses in a clinical practice setting, because more work in vivo needs to be done to address relevance of this work to clinical practice.
Acknowledgment
We thank Shih-Yun Lyman for help with preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health, NIAID and NHLBI, Grants AI070140, 2R56AI070140, and HL37260. This work was also supported by the Edelstein Family Foundation.

This article contains supplemental Figs. 1 and 2.
- VitD
- vitamin D
- VDR
- VitD receptor
- VDRE
- VitD response element
- GR
- glucocorticoid receptor
- GC
- glucocorticoid
- GRE
- GC-responsive element
- PBMC
- peripheral blood mononuclear cell
- DEX
- dexamethasone
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Holick M. F. (2007) Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 [DOI] [PubMed] [Google Scholar]
- 2. Adams J. S., Hewison M. (2008) Unexpected actions of vitamin D. New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 4, 80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. (1995) The nuclear receptor superfamily. The second decade. Cell 83, 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlberg C. (1996) The vitamin D3 receptor in the context of the nuclear receptor superfamily. The central role of the retinoid X receptor. Endocrine 4, 91–105 [DOI] [PubMed] [Google Scholar]
- 5. Quack M., Carlberg C. (2000) Ligand-triggered stabilization of vitamin D receptor/retinoid X receptor heterodimer conformations on DR4-type response elements. J. Mol. Biol. 296, 743–756 [DOI] [PubMed] [Google Scholar]
- 6. Kim S., Shevde N. K., Pike J. W. (2005) 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J. Bone Miner. Res. 20, 305–317 [DOI] [PubMed] [Google Scholar]
- 7. Barnes P. J. (1998) Anti-inflammatory actions of glucocorticoids. Molecular mechanisms. Clin. Sci. 94, 557–572 [DOI] [PubMed] [Google Scholar]
- 8. Rhen T., Cidlowski J. A. (2005) Antiinflammatory action of glucocorticoids. New mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 9. Newton R. (2000) Molecular mechanisms of glucocorticoid action. What is important? Thorax 55, 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beato M., Herrlich P., Schütz G. (1995) Steroid hormone receptors. Many actors in search of a plot. Cell 83, 851–857 [DOI] [PubMed] [Google Scholar]
- 11. Kassel O., Sancono A., Krätzschmar J., Kreft B., Stassen M., Cato A. C. (2001) Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20, 7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tchen C. R., Martins J. R., Paktiawal N., Perelli R., Saklatvala J., Clark A. R. (2010) Glucocorticoid regulation of mouse and human dual specificity phosphatase 1 (DUSP1) genes. Unusual cis-acting elements and unexpected evolutionary divergence. J. Biol. Chem. 285, 2642–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owens D. M., Keyse S. M. (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 [DOI] [PubMed] [Google Scholar]
- 14. Keyse S. M. (2000) Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12, 186–192 [DOI] [PubMed] [Google Scholar]
- 15. Clark A. R. (2003) MAP kinase phosphatase 1. A novel mediator of biological effects of glucocorticoids? J. Endocrinol. 178, 5–12 [DOI] [PubMed] [Google Scholar]
- 16. Clark A. R., Lasa M. (2003) Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr. Opin. Pharmacol. 3, 404–411 [DOI] [PubMed] [Google Scholar]
- 17. Skversky A. L., Kumar J., Abramowitz M. K., Kaskel F. J., Melamed M. L. (2011) Association of glucocorticoid use and low 25-hydroxyvitamin D levels. Results from the National Health and Nutrition Examination Survey (NHANES). 2001–2006. J. Clin. Endocrinol. Metab. 96, 3838–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutherland E. R., Goleva E., Jackson L. P., Stevens A. D., Leung D. Y. (2010) Vitamin D levels, lung function, and steroid response in adult asthma. Am. J. Respir. Crit. Care Med. 181, 699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Searing D. A., Zhang Y., Murphy J. R., Hauk P. J., Goleva E., Leung D. Y. (2010) Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J. Allergy Clin. Immunol. 125, 995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brehm J. M., Schuemann B., Fuhlbrigge A. L., Hollis B. W., Strunk R. C., Zeiger R. S., Weiss S. T., Litonjua A. A. (2010) Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J. Allergy Clin. Immunol. 126, 52–58.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bikle D. D. (2010) Vitamin D. Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 21, 375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y., Leung D. Y., Nordeen S. K., Goleva E. (2009) Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J. Biol. Chem. 284, 24542–24552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Leung D. Y., Richers B. N., Liu Y., Remigio L. K., Riches D. W., Goleva E. (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 188, 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Bosscher K., Vanden Berghe W., Haegeman G. (2003) The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1. Molecular mechanisms for gene repression. Endocr. Rev. 24, 488–522 [DOI] [PubMed] [Google Scholar]
- 25. Adcock I. M., Caramori G. (2001) Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol. 79, 376–384 [DOI] [PubMed] [Google Scholar]
- 26. Ogawa S., Lozach J., Benner C., Pascual G., Tangirala R. K., Westin S., Hoffmann A., Subramaniam S., David M., Rosenfeld M. G., Glass C. K. (2005) Molecular determinants of crosstalk between nuclear receptors and Toll-like receptors. Cell 122, 707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ito K., Barnes P. J., Adcock I. M. (2000) Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol. 20, 6891–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. King E. M., Holden N. S., Gong W., Rider C. F., Newton R. (2009) Inhibition of NF-κB-dependent transcription by MKP-1. Transcriptional repression by glucocorticoids occurring via p38 MAPK. J. Biol. Chem. 284, 26803–26815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghosh S., Hayden M. S. (2008) New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 8, 837–848 [DOI] [PubMed] [Google Scholar]
- 30. Clark A. R., Dean J. L., Saklatvala J. (2003) Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 546, 37–44 [DOI] [PubMed] [Google Scholar]
- 31. Rosen J., Miner J. N. (2005) The search for safer glucocorticoid receptor ligands. Endocr. Rev. 26, 452–464 [DOI] [PubMed] [Google Scholar]
- 32. Krishnan A. V., Feldman D. (2010) Molecular pathways mediating the anti-inflammatory effects of calcitriol. Implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer 17, R19–R38 [DOI] [PubMed] [Google Scholar]
- 33. Plum L. A., DeLuca H. F. (2010) Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 9, 941–955 [DOI] [PubMed] [Google Scholar]
- 34. Wang T. T., Nestel F. P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J. W., Mader S., White J. H. (2004) Cutting edge. 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 173, 2909–2912 [DOI] [PubMed] [Google Scholar]
- 35. Gombart A. F., Borregaard N., Koeffler H. P. (2005) Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 19, 1067–1077 [DOI] [PubMed] [Google Scholar]
- 36. Weber G., Heilborn J. D., Chamorro Jimenez C. I., Hammarsjo A., Törmä H., Stahle M. (2005) Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 124, 1080–1082 [DOI] [PubMed] [Google Scholar]
- 37. Stio M., Martinesi M., Bruni S., Treves C., Mathieu C., Verstuyf A., d'Albasio G., Bagnoli S., Bonanomi A. G. (2007) The vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn's disease. J. Steroid Biochem. Mol. Biol. 103, 51–60 [DOI] [PubMed] [Google Scholar]
- 38. Chen P., Li J., Barnes J., Kokkonen G. C., Lee J. C., Liu Y. (2002) Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 169, 6408–6416 [DOI] [PubMed] [Google Scholar]
- 39. Leung D. Y., Bloom J. W. (2003) Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 111, 3–22; quiz 23 [DOI] [PubMed] [Google Scholar]
- 40. Tobler A., Gasson J., Reichel H., Norman A. W., Koeffler H. P. (1987) Granulocyte-macrophage colony-stimulating factor. Sensitive and receptor-mediated regulation by 1,25-dihydroxyvitamin D3 in normal human peripheral blood lymphocytes. J. Clin. Invest. 79, 1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Towers T. L., Freedman L. P. (1998) Granulocyte-macrophage colony-stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. Implications for allosteric influences on nuclear receptor structure and function by a DNA element. J. Biol. Chem. 273, 10338–10348 [DOI] [PubMed] [Google Scholar]
- 42. Towers T. L., Staeva T. P., Freedman L. P. (1999) A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene. Vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol. Cell. Biol. 19, 4191–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinez-Moczygemba M., Huston D. P. (2003) Biology of common β receptor-signaling cytokines. IL-3, IL-5, and GM-CSF. J. Allergy Clin. Immunol. 112, 653–665; quiz 666 [DOI] [PubMed] [Google Scholar]
- 44. Kim Y. R., Abraham N. G., Lutton J. D. (1991) Mechanisms of differentiation of U937 leukemic cells induced by GM-CSF and 1,25(OH)2 vitamin D3. Leuk. Res. 15, 409–418 [DOI] [PubMed] [Google Scholar]
- 45. Lee J., Kim Y., Lim J., Kim M., Han K. (2008) G-CSF and GM-CSF concentrations and receptor expression in peripheral blood leukemic cells from patients with chronic myelogenous leukemia. Ann. Clin. Lab. Sci. 38, 331–337 [PubMed] [Google Scholar]
- 46. Fondell J. D., Ge H., Roeder R. G. (1996) Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc. Natl. Acad. Sci. U.S.A. 93, 8329–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rachez C., Lemon B. D., Suldan Z., Bromleigh V., Gamble M., Näär A. M., Erdjument-Bromage H., Tempst P., Freedman L. P. (1999) Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398, 824–828 [DOI] [PubMed] [Google Scholar]
- 48. Chen W., Rogatsky I., Garabedian M. J. (2006) MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor. Mol. Endocrinol. 20, 560–572 [DOI] [PubMed] [Google Scholar]
- 49. Hittelman A. B., Burakov D., Iñiguez-Lluhi J. A., Freedman L. P., Garabedian M. J. (1999) Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J. 18, 5380–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]