Background: Endoplasmic reticulum (ER) stress is associated with fibrotic diseases, although the mechanisms are not completely understood.
Results: The ER stress protein calreticulin regulates TGF-β stimulated extracellular matrix through control of intracellular calcium and NFAT signaling.
Conclusion: Calreticulin is necessary for TGF-β stimulated extracellular matrix production.
Significance: These findings identify calreticulin as a mechanistic link between ER stress and fibrosis.
Keywords: Calcium, Collagen, Endoplasmic Reticulum Stress, Fibronectin, Fibrosis, NFAT Transcription Factor, Transforming Growth Factor β (TGFβ), Calreticulin
Abstract
Endoplasmic reticulum (ER) stress is an emerging factor in fibrotic disease, although precise mechanisms are not clear. Calreticulin (CRT) is an ER chaperone and regulator of Ca2+ signaling up-regulated by ER stress and in fibrotic tissues. Previously, we showed that ER CRT regulates type I collagen transcript, trafficking, secretion, and processing into the extracellular matrix (ECM). To determine the role of CRT in ECM regulation under fibrotic conditions, we asked whether CRT modified cellular responses to the pro-fibrotic cytokine, TGF-β. These studies show that CRT−/− mouse embryonic fibroblasts (MEFs) and rat and human idiopathic pulmonary fibrosis lung fibroblasts with siRNA CRT knockdown had impaired TGF-β stimulation of type I collagen and fibronectin. In contrast, fibroblasts with increased CRT expression had enhanced responses to TGF-β. The lack of CRT does not impact canonical TGF-β signaling as TGF-β was able to stimulate Smad reporter activity in CRT−/− MEFs. CRT regulation of TGF-β-stimulated Ca2+ signaling is important for induction of ECM. CRT−/− MEFs failed to increase intracellular Ca2+ levels in response to TGF-β. NFAT activity is required for ECM stimulation by TGF-β. In CRT−/− MEFs, TGF-β stimulation of NFAT nuclear translocation and reporter activity is impaired. Importantly, CRT is required for TGF-β stimulation of ECM under conditions of ER stress, as tunicamycin-induced ER stress was insufficient to induce ECM production in TGF-β stimulated CRT−/− MEFs. Together, these data identify CRT-regulated Ca2+-dependent pathways as a critical molecular link between ER stress and TGF-β fibrotic signaling.
Introduction
Endoplasmic reticulum (ER)3 stress is emerging as a factor in fibroproliferative diseases including pulmonary fibrosis, diabetic nephropathy, hypertension-associated cardiac fibrosis, and atherosclerosis (1–8). ER stress can be induced by glucose, glucosamine, and oxidative stress, factors known to induce fibroproliferative remodeling in multiple tissues (5, 9, 10). Chemical chaperones that reduce ER stress, such as valproate and 4-phenylbutyrate, reduce atherosclerosis and fibrosis (3, 11–13) and also inhibit TGF-β-induced type I collagen production independent of Smad reporter activity (14). In addition, induction of ER stress by tunicamycin exacerbates lung fibrosis in the bleomycin model of pulmonary fibrosis, although tunicamycin alone did not induce fibrosis (1). Enhanced ER stress in alveolar epithelial cells facilitates epithelial to mesenchymal transition, a process that occurs in some forms of fibrosis (15). Despite growing evidence for ER stress as factor in fibrosis, the mechanisms by which ER stress predisposes to or exacerbates fibrosis are not clear. In the lung, ER stress-induced alveolar epithelial cell apoptosis is thought to be a significant factor in the development of fibrosis (1, 6, 15). However, ER stress is also associated with fibroproliferative remodeling in tissues such as the diabetic vasculature where apoptosis is not a significant initiating factor (3, 4). This suggests that ER stress can drive pathways that promote fibrosis through additional mechanisms.
Calreticulin (CRT) is a 46-kDa ER protein that regulates cellular responses to stress through its roles in the unfolded protein response and its chaperone activity (16, 17). In addition, CRT also is important in ER Ca2+ buffering and regulation of downstream Ca2+-dependent signaling pathways such as calcineurin and NFAT (nuclear factor of activated T cells) (17). Calreticulin−/− mouse embryonic fibroblasts (MEFs) have decreased ER Ca2+ stores and impaired agonist-induced Ca2+ release from the ER, whereas cells overexpressing CRT have enhanced Ca2+ binding depots within thapsigargin-sensitive ER stores (18, 19). Impaired ER Ca2+ release in the absence of CRT leads to defects in downstream Ca2+/calcineurin signaling with reduced NFAT and MEF2C (myocyte enhancer factor 2c) nuclear translocation (20, 21). CRT−/− mice can be rescued from embryonic lethality by constitutively active calcineurin, which induces myocyte enhancer factor 2C and NFAT translocation to the nucleus providing evidence that CRT is an upstream modulator of calcineurin signaling (20, 22, 23).
Our laboratory recently demonstrated that CRT regulates transcription of multiple extracellular matrix (ECM) proteins in a Ca2+-dependent manner and that it has post-transcriptional effects on collagen trafficking and matrix assembly (24). CRT regulation of fibronectin is also thought to involve ER Ca2+ (25, 26). Interestingly, CRT expression is increased in multiple models of fibrosis including bleomycin-induced pulmonary fibrosis, the UUO (unilateral ureteral obstruction) model of renal fibrosis, and in chronic diseases of fibroproliferative remodeling, such as atherosclerosis (2–4). Cardiac-specific overexpression of CRT during development results in interstitial fibrosis, although the mechanisms have not been defined (27). CRT expression is up-regulated by factors that are known to induce both ER stress and fibrosis, including glucose, oxidative stress, cigarette smoke, hypoxia, and TGF-β (2, 3, 28–30).
TGF-β is a major stimulus of ECM production in fibroproliferative diseases (31). TGF-β stimulation of fibrotic pathways occurs primarily through Smad 2/3-dependent pathways, although the importance of other TGF-β stimulated pathways, including PI3K, ERK, and p38 MAPK, is now recognized (32–36). Ca2+-dependent pathways also regulate TGF-β stimulation of ECM (37, 38). TGF-β treatment leads to increased cytosolic Ca2+, which induces calcineurin-mediated NFAT dephosphorylation and enhanced expression of fibronectin (37, 38). Furthermore, constitutively active calcineurin or NFAT increases fibronectin promoter activity in mesangial cells, suggesting a role for Ca2+-regulated NFAT in control of TGF-β-driven matrix production (38). TGF-β can increase inositol 3-phosphate levels, thereby causing release of ER Ca2+ (39). In addition, TGF-β can increase cytoplasmic Ca2+ through translocation of type III inositol 3-phosphate receptors to the cell surface, through stimulation of H2O2-mediated Ca2+ release, or through a c-Jun-dependent mechanism (40–43).
Given our previous findings that CRT regulates fibronectin and type I collagen transcription and the known role of CRT in modulating calcineurin-NFAT activity, we asked whether CRT might play a role in regulating TGF-β stimulation of ECM proteins (20, 23, 24). These studies show that CRT is required for cellular responsiveness to TGF-β. CRT mediates TGF-β responsiveness through regulation of TGF-β-stimulated Ca2+ release and NFAT activity. Furthermore, CRT is required for TGF-β stimulation of ECM in tunicamycin-treated cells. Together, these data provide evidence that CRT is a critical regulator of TGF-β-mediated ECM production and establish a new mechanism by which ER stress contributes to fibrosis.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/liter glucose was purchased from Invitrogen. LY364947, SB203580, tunicamycin, l-ascorbic acid, protease inhibitor mixture, phosphatase inhibitor, and ionomycin were purchased from Sigma. DMEM and Dulbecco's phosphate buffered saline (D-PBS) were purchased from Cellgro (Manassas, VA). 11R-VIVIT and Hoechst (catalog #382061) were purchased from Calbiochem. A285222 was a gift of Abbott Laboratories. Fluo-4 AM was purchased from Invitrogen. NFAT (GGAGGAAAAACTGTTTCATACAGAAGGCGT) and Smad (AGCCAGACA) Cignal reporter assay kits were purchased from SA Biosciences (Valencia, CA). TGF-β was purchased from R&D Systems (Minneapolis, MN). Goat anti-fibronectin (catalog #6952), rabbit anti-β-tubulin (catalog #9104), mouse anti-NFATc3 (catalog #8405), and rabbit anti-GRP78 (catalog #13968) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-phospho-Smad3 (catalog #9520S) and rabbit anti-phospho-Smad2 (catalog #3101) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-β-actin IgG (catalog #IMG-5142A) was purchased from IMGENEX (San Diego, CA). Rabbit anti-collagen type I IgG (catalog #203002) was purchased from mdbioproducts (St. Paul, MN). Rabbit anti-collagen Iα2 (catalog #ab96723) was purchased from Abcam (Cambridge, MA). Mouse anti-Smad 2/3 (catalog #610842) was purchased from BD Transduction Laboratories. AlexaFluor 488 goat anti-rabbit IgG, and AlexaFluor 488 goat anti-mouse IgG were purchased from Invitrogen. Peroxidase-conjugated AffiniPure rabbit anti-goat IgG (catalog #305-035-003), goat anti-rabbit IgG (111-035-003), and goat anti-mouse IgG (115-035-146) secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Western Lightning Chemiluminescence Reagent Plus was purchased from PerkinElmer Life Sciences, and Re-Blot strong stripping solution was purchased from Chemicon (Temecula, CA).
Cells
Wild type MEFs, CRT−/− MEFs, calreticulin−/− MEFs stably transfected with the pcDNA3 expression vector to express rabbit HA-tagged CRT were gifts from Dr. Marek Michalak (University of Alberta, Edmonton, Alberta, Canada). Calreticulin−/− MEFs stably transfected with HA-tagged CRT lacking the TSP1 binding domain (amino acids 19–36) were generated as described previously (44). Mouse L fibroblasts (parental cells and CRT overexpressors) were provided by Dr. Michal Opas (University of Toronto). These cell lines were engineered to overexpress (CRT overexpressors) CRT by 1.6-fold compared with parental cells as described previously (45). Rat lung fibroblasts (RFL6) stably expressing an empty vector (pcDNA3.1, Invitrogen) were generated as previously described (gift of Dr. James Hagood) (46). Human IPF lung fibroblasts were provided by Dr. Victor Thannickal (University of Alabama at Birmingham). L fibroblasts were maintained in high (4.5 g/liter) glucose DMEM with 10% FBS in the presence of 100 μg/ml G418 sulfate (Cellgro).
Quantitative Real Time PCR
Cells were grown overnight in complete media containing 10% FBS, starved in low (0.5%) serum medium overnight, and treated with TGF-β or other compounds. After treatment, RNA was harvested with TRIzol reagent and isolated according to the manufacturer's specifications. Quantitative real time PCR was performed using standard protocols with an Opticon instrument (MJ Research, model CFD-3200). Primers for mouse type I collagen (Col1A1) (catalog #PPM03845F-200), fibronectin (catalog #QT00135758), CRT (catalog #QT00101206), and S9 (catalog #PPM03695A) were obtained from Qiagen and verified by melting curve analysis. Transcript levels were assayed using SYBR Green from Qiagen. Results were calculated using the ΔΔCT method and are expressed as the mean ± S.D. of three samples each assayed in triplicate as indicated in Fig. 1, 3, 4, 8, and 9 legends. Results are representative of at least two-three separate experiments.
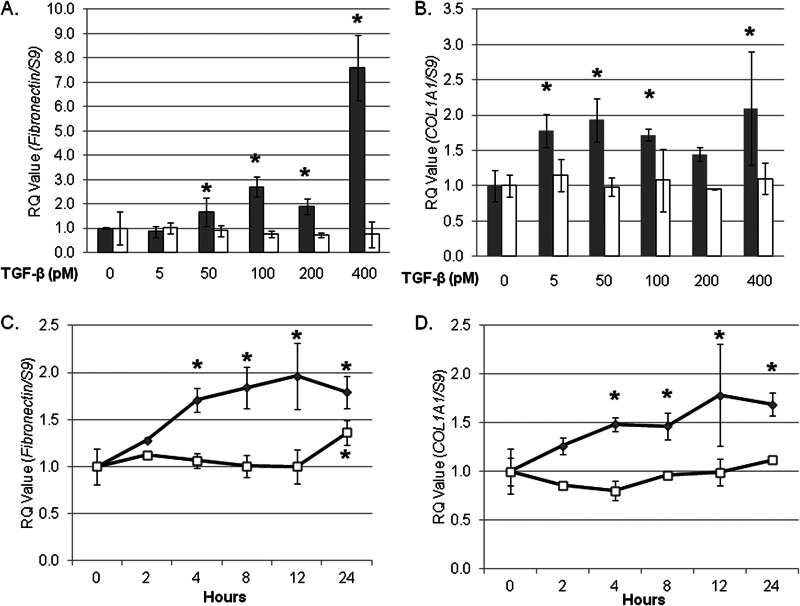
FIGURE 1.
TGF-β induces fibronectin and COL1A1 transcript in wild type but not CRT−/− MEFs. Wild type (gray bars, gray symbols) and CRT−/− MEFs (open bars, open symbols) were grown overnight in medium with 10% FBS, starved for 12 h in low serum (0.5% FBS) medium, and treated with increasing concentrations of TGF-β for 4 h (A and B). Cells were also treated with 100 pm TGF-β over a 24-h time period (C and D). RNA was harvested by TRIzol, and transcript levels of fibronectin (A and C) and COL1A1 (B and D) were determined by quantitative real time PCR (RQ). Values represent mean levels normalized to S9 ± S.D. of triplicate samples each performed in technical triplicates. Values for untreated wild type and CRT−/− cells were set to 1. Each assay shown is representative of three separate experiments with similar results. *, p < 0.05 versus non-treated or time zero cells.
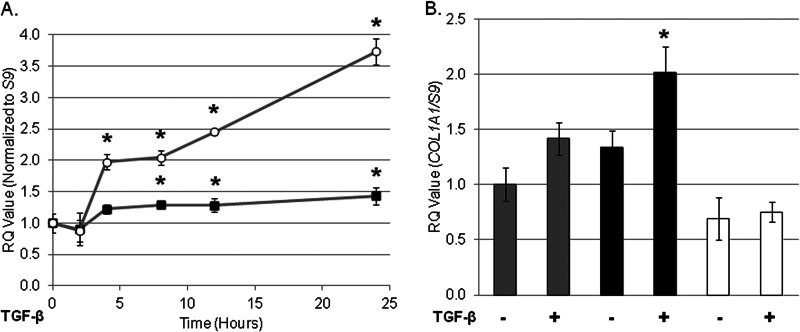
FIGURE 3.
Impaired responsiveness to TGF-β in the CRT−/− MEFs can be rescued by transfection with CRT plasmid or CRT plasmid lacking the TSP1 binding site. A, CRT−/− MEFs stably transfected with rabbit HA-tagged CRT were grown overnight in medium with 10% FBS, starved overnight in low (0.5%) serum medium, and treated with 100 pm TGF-β for 4 h. RNA was harvested by TRIzol, and transcript levels of fibronectin (open circles) and COL1A1 (closed squares) were determined by quantitative real time PCR. B, wild type MEFS (gray bars), CRT−/− MEFs stably expressing rabbit CRT lacking the TSP1 binding site (black bars), and CRT−/− MEFs (open bars) were treated as in A, RNA was harvested, and levels of COL1A1 were determined by RTQ-PCR (RQ). Results are the means normalized to S9 levels of triplicate samples ± S.D. from one representative experiment. Experiments were repeated on three (A) or two (B) separate occasions. The means of untreated cells were set to 1. *, p < 0.05 versus untreated cells.
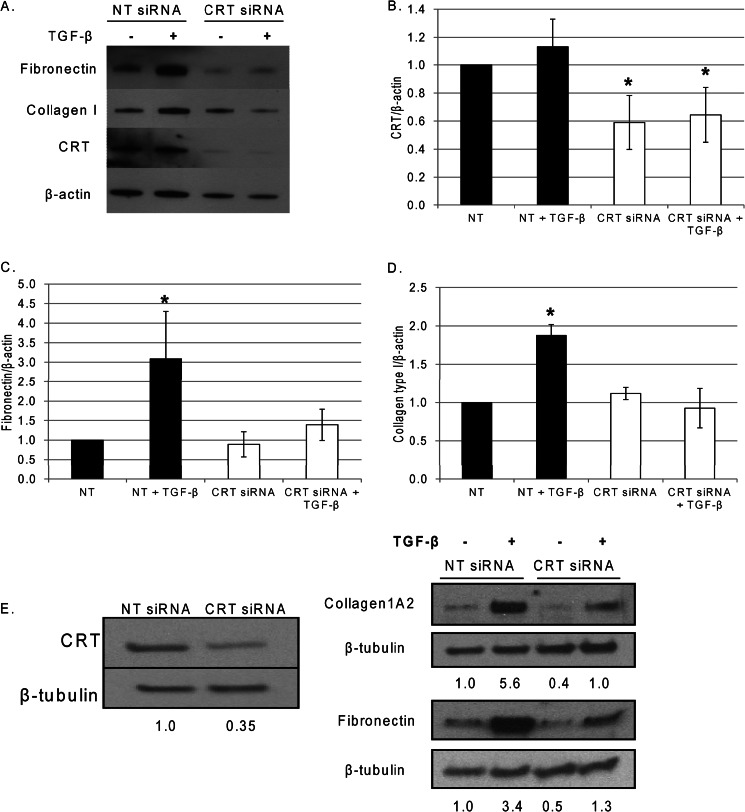
FIGURE 4.
Knockdown of CRT in Thy1−/− rat lung fibroblasts and human lung fibroblasts significantly inhibits TGF-β-stimulated matrix production. Thy1−/− rat lung fibroblasts were transfected with 100 nm non-targeting (NT) or CRT siRNA in medium with 10% FBS and maintained for 24 h. Cells were switched to low (0.5%) serum medium and stimulated with 100 pm TGF-β for 24 h. Laemmli cell lysates were immunoblotted for CRT (B), fibronectin (C), and collagen type I (D). A representative blot is shown in A. Results are the mean density normalized to β-actin ±S.D. from three separate experiments. E, human IPF lung fibroblasts were transfected with 200 nm non-targeting or CRT siRNA and maintained in medium with 10% FBS for 48 h. Cells were switched to low FBS medium for 6 h and then treated with 100 pm TGF-β with 2 μm ascorbic acid for 24 h. Laemmli cell lysates were separated by SDS-PAGE and immunoblotted for CRT, fibronectin, collagen type Iα2, and β-tubulin. Densitometric analysis of bands normalized to β-tubulin are indicated below each band. Results are representative of three separate experiments. *, p < 0.05 versus untreated cells.
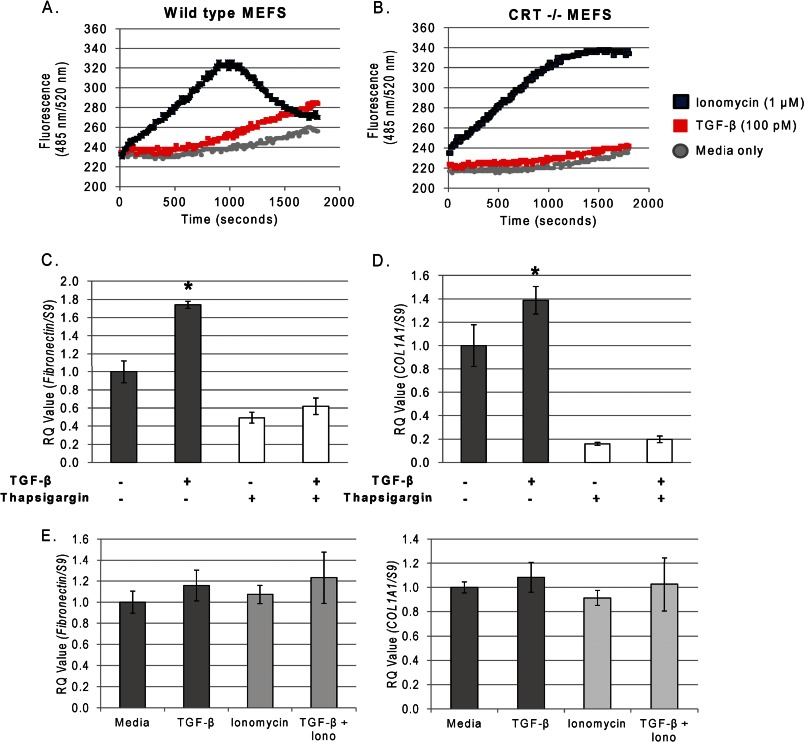
FIGURE 8.
TGF-β stimulates Ca2+ release, and Ca2+-dependent fibronectin and COL1A1 transcript are impaired in the CRT−/− MEFs. Wild type (A) and CRT−/− MEFs (B) were plated overnight in DMEM with 10% FBS, washed with low (0.5%) serum medium, and loaded with 5 μm Fluo-4 AM in low serum medium with 10 mm HEPES. Cells were loaded with dye for 20 min at 37 °C. After a 5-min equilibration, cells were stimulated with 100 pm TGF-β (red squares), 1 μm ionomycin (blue squares), or low serum medium (gray circle). Cells were excited at 485 nm, and emission was read at 520 nm. Results are representative of a typical experiment repeated in quadruplicate on at least four different occasions. C and D, wild type MEFs were plated overnight in medium with 10% FBS and starved overnight in low (0.5%) serum medium. Cells were pretreated with thapsigargin (0.5 μm) for 30 min and washed with low serum medium to remove the thapsigargin. Cells were treated with or without 100 pm TGF-β for 4 h. RNA was harvested with TRIzol and transcript levels of fibronectin (C) and COL1A1 (D) were determined by RTQ-PCR. Values represent the mean expression levels normalized to S9 ± S.D. of triplicate samples from a single representative experiment. Experiments were repeated three times with similar results. E, CRT−/− MEFs were plated overnight in medium with 10% FBS, starved overnight in low serum medium, and treated with TGF-β (100 pm), ionomycin (Iono, 1 μm), or both for 4 h. RNA was harvested with TRIzol, and transcript levels of fibronectin and COL1A1 were determined by RTQ-PCR (RQ). *, p < 0.05 versus untreated control.
FIGURE 9.
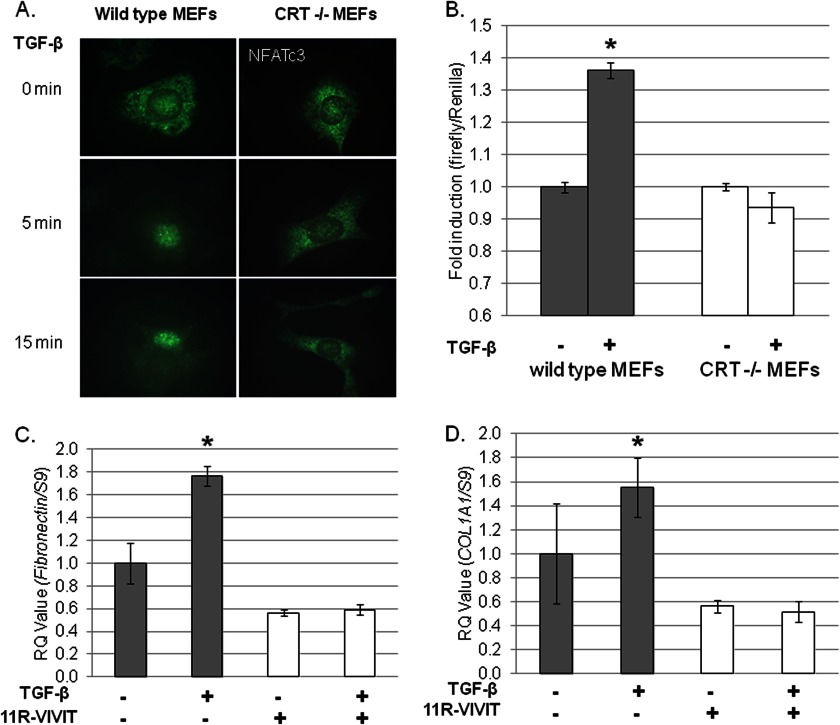
CRT−/− MEFs do not stimulate NFAT activity in response to TGF-β. A, wild type and CRT−/− MEFs were grown overnight on glass coverslips in medium with 10% FBS, starved overnight in low (0. 5%) serum medium, and stimulated with 400 pm TGF-β for 5 or 15 min. After treatment, cells were fixed, permeabilized, and incubated with anti-NFATc3 antibody. Cells were washed with PBS and incubated with a fluorescein-labeled secondary antibody. Results are representative of one experiment performed on at least three separate occasions. Original magnification = 1000×. B, wild type (gray bars) and CRT−/− MEFs (open bars) were transfected with an inducible NFAT reporter firefly luciferase reporter construct and a renilla luciferase control construct overnight in medium with 10% FBS. Cells were starved for 2 h in low serum medium and then treated every 2 h with 100 pm TGF-β over an 8-h span. After 8 h, cells were lysed, and triplicate samples were combined. Luciferase reporter activity was normalized to the renilla luciferase control. Data represent the mean normalized luciferase activity ± S.D. of one representative experiment performed in triplicate. The experiment was performed on three separate occasions with similar results. *, p < 0.05 versus untreated control. C and D, wild type MEFs were grown overnight in medium with 10% FBS, starved overnight in low serum medium, and pretreated with 2 μm 11R-VIVIT for 30 min (open bars). Cells were treated with or without 100 pm TGF-β ± 11R-VIVIT peptide for 4 h, and RNA was harvested with TRIzol. Transcript levels of fibronectin (C) or COL1A1 (D) were determined by RTQ-PCR and normalized to S9 levels ±S.D. Results are from one experiment of triplicate samples. Similar results were obtained in three separate experiments. *, p < 0.05 versus untreated control.
Immunoblotting for ECM Proteins
After treatment, cells were harvested using 1× Laemmli lysis buffer (Bio-Rad) containing 1× protease inhibitor mixture (Sigma catalog #p8340). After lysis, cells were sonicated for 7 s, 5% final β-mercaptoethanol was added, and samples were boiled at 100 °C for 7 min. Samples were centrifuged, and equal volumes were loaded onto 4–15 or 10% SDS-polyacrylamide gels. After separation by SDS-PAGE, samples were transferred onto a PVDF membrane at 100 V for 100 min. After transfer, membranes were blocked with 1% casein followed by application of the primary antibody. Membranes were washed in TBS-Tween, and secondary antibody was applied for 1 h at room temperature. Membranes were washed with TBS-Tween and developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences). Membranes were stripped and reprobed with rabbit anti-β-tubulin or rabbit anti-β-actin IgG to normalize for cell protein. Densitometric analysis of immunoblots was performed using the NIH Image J program. Data are expressed as the mean band density normalized for cell protein from at least three separate experiments.
Deoxycholate Extraction of the Extracellular Matrix Fraction
Deoxycholate (DOC) extractions of detergent-soluble and -insoluble fractions were performed similarly to previous reports (47). Briefly, wild type and CRT−/− MEFs were plated in full serum (10%) DMEM for 24 h, switched to low serum (0.5%) DMEM with 20 μm ascorbic acid, and treated with 100 pm TGF-β for 24 h. After 24 h, wells were rinsed with PBS and harvested by scraping with 300 μl of 4% DOC (4% DOC in 20 mm Tris-HCL, pH 8.8, with 1× protease inhibitor). Lysates were homogenized with a 27½-gauge needle and tumbled overnight at 4 °C. Precipitates containing the DOC-insoluble portion were pelleted at 13,500 × g and washed 3 times with 4% DOC solution. The supernatant containing the DOC soluble fraction was removed, placed into a new tube, and centrifuged twice as above. The DOC-insoluble pellet was resuspended in 30 μl of Laemmli buffer.
Soluble Collagen Assays
Wild type and CRT−/− MEFs were cultured for 48 h in DMEM with 10% FBS, switched to DMEM with 0.5% FBS and 20 μm ascorbic acid, and treated with or without 10 pm TGF-β for 72 h. Cells were dosed daily with TGF-β and ascorbic acid in 0.5% FBS. Conditioned medium was collected in the presence of protease inhibitor mixture (Sigma) and centrifuged at 15,000 × g for 5 min to remove cellular debris. Soluble collagen in the medium was measured using the Sircol assay (Biocolor) as described by the manufacturer.
siRNA Transfection of Thy-1 (−) Rat Lung Fibroblasts and Human IPF Lung Fibroblasts
Non-targeting siRNA (SI03650325) or rat CRT siRNA (SI04449004) were purchased from Qiagen and resuspended in RNase-free water. One million Thy-1 (−) rat lung fibroblasts were transfected via nucleofection using the MEF1 Nucleofector kit from Amaxa Biosystems (Amaxa GmbH, Lonza) in an Amaxa Nucleofector II using program A-023. Transfected cells were cultured in DMEM with 10% FBS for 24 h. Medium was switched to low serum (0.5% FBS) medium for 2 h followed by treatment with 100 pm TGF-β for 24 h. Cells were washed with D-PBS (Cellgro) and lysed with 1× Laemmli buffer containing 1× protease inhibitor.
Human IPF lung fibroblasts were transfected with non-targeting siRNA or human CRT siRNA (SI02654589) resuspended in RNase-free water. One million human IPF lung fibroblasts were transfected via nucleofection using the primary fibroblasts nucleofector kit from Amaxa Biosystems (Amaxa) in an Amaxa Nucleofector II using program A-023. Transfected cells were cultured in DMEM with 10% FBS for 48 h. Medium was switched to low serum (0.5% FBS) DMEM for 6 h followed by treatment with 2 μm ascorbic acid and 100 pm TGF-β for 24 h. Cells were washed with D-PBS and lysed with 1× Laemmli buffer containing 1× protease inhibitor.
Tunicamycin Experiment
Wild type and CRT−/− MEFs were plated with or without 0.01 μg/ml tunicamycin in DMEM with 10% FBS containing 20 μm ascorbic acid for 24 h as previously described (24). Cells were treated with or without 100 pm TGF-β for 24 h. Cells were washed with D-PBS and lysed with 1× Laemmli lysis buffer containing 1× protease inhibitor.
Immunofluorescence
Wild type and CRT−/− MEFs were plated on glass coverslips in a 24-well plate in DMEM with 10% FBS for 24 h and then switched to low serum (0.5% FBS) medium overnight. The next day cells were treated with TGF-β, fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X for 3 min. Cells were washed with D-PBS and blocked for 1 h with filtered, sterile 1% casein. Primary antibody was added as follows: rabbit anti-phospho-Smad 2 or 3 at a 1:150 dilution; mouse anti-NFATc3 at a 1:100 dilution in 1% casein solution overnight at 4 °C. Cells were washed with PBS, and the appropriate secondary AlexaFluor488 antibody (1:500 dilution) was added for 1 h at room temperature. After washing, cells were incubated with 4 μg/ml Hoechst for 5 min. Images were obtained using a Nikon Eclipse TE2000-U inverted microscope equipped for epifluorescence with a Nikon camera or a Zeiss LSM 710 confocal microscope. Non-immune IgG and secondary antibody alone were used as negative controls. Images in a particular experiment were obtained using a uniform exposure time, and images were adjusted uniformly.
Ca2+ Release Assay
Cells were plated in 24-well plates in complete (10% FBS) medium overnight. The next day low serum (0.5%) FBS medium containing 5 μm Fluo-4 AM was added to the cells. Dye was loaded into the cells for 20 min at 37 °C. The plate was allowed to equilibrate at 37 °C for 5 min. After equilibration, cells were treated with either TGF-β (100 pm) or ionomycin (1 μm) as indicated. Cells were excited at 485 nm, and emission was measured at 520 nm every 10 s for 30 min.
Reporter Assays
Wild type and CRT−/− MEFs were transfected with 2 μg of NFAT or Smad firefly luciferase reporter constructs with control renilla luciferase purchased from SABiosciences. Cells were transfected using the MEF 1 Nucleofector kit from Amaxa Biosystems in Amaxa Nucleofector II using program A-023. Transfected cells were cultured in complete (10% FBS) medium overnight, and GFP expression was confirmed the next morning. Cells were starved in low serum (0.5% FBS) medium and treated as indicated. After treatment, cells were lysed with 1× lysis buffer (Promega), and firefly and renilla luciferase activity were measured using a Dual Glo Luciferase kit from Promega according to the manufacturer's instructions. Luciferase reporter activity is normalized to the renilla luciferase control. Luciferase reporter construct data are representative of at least three individual experiments each performed in triplicate.
Statistics
Data were analyzed for statistical significance using one-way analysis of variance with Holm-Sidak post hoc analyses (Sigma Stat). p < 0.05 was considered significant.
RESULTS
Calreticulin Is Required for TGF-β-mediated Stimulation of Collagen and Fibronectin Transcript and Protein
Wild type and CRT−/− MEFs were treated with TGF-β, and levels of transcript were compared by RTQ-PCR. TGF-β stimulated a significant increase in fibronectin and COL1A1 transcript in wild type MEFs (Fig. 1, A and B). In contrast, TGF-β failed to stimulate an increase in either COL1A1 or fibronectin transcript in MEFs lacking CRT (Fig. 1, A and B). Levels of COL1A1 and fibronectin transcript were significantly increased 4 h post stimulation and were maintained for up to 24 h in wild type cells (Fig. 1, C and D). The lack of response in CRT−/− MEFs is not due to a delay in response to TGF-β, as no increase was observed over a 24-h period with COL1A1 or 12 h for fibronectin (Fig. 1, C and D).
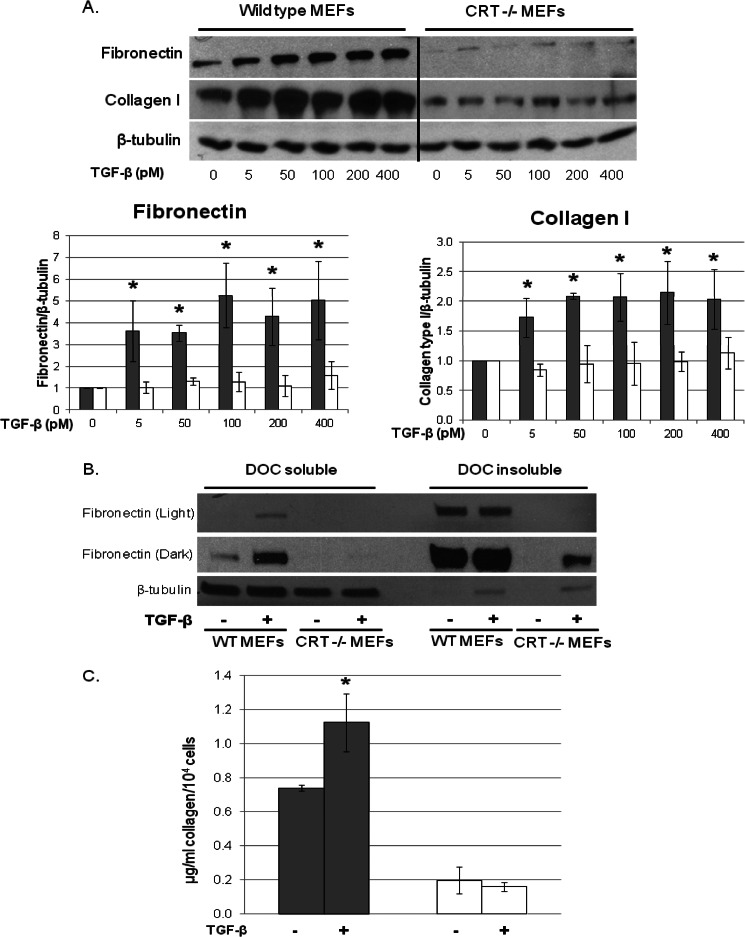
The failure of TGF-β to stimulate type I collagen and fibronectin transcription in CRT−/− MEFs correlates with a lack of protein stimulation as measured in cell lysates 24 h after TGF-β treatment (Fig. 2A). In addition, the increased ECM expression in TGF-β-treated wild type cells is due to increased protein synthesis and not incorporation of serum fibronectin into the extracellular matrix, as differential extraction of the DOC cell soluble from DOC insoluble extracellular matrix (48) shows increased fibronectin in the cellular fraction after TGF-β treatment (Fig. 2B). Similarly, TGF-β failed to stimulate an increase in secreted soluble collagen in the conditioned medium of CRT−/− MEFs (Fig. 2C). In these studies both wild type and CRT−/− MEFs were stimulated with TGF-β in the presence of 20 μm ascorbic acid. We showed previously that CRT is a collagen chaperone and that CRT−/− MEFs have reduced ER to Golgi trafficking and secretion of collagen, which is corrected by ascorbic acid (24). Ascorbic acid increases collagen transcript stability and is a cofactor for proline hydroxylation of procollagen, which enhances translation and secretion efficiency (49, 50).
FIGURE 2.
CRT is required for TGF-β stimulation of fibronectin and collagen I protein. A and B, wild type (gray bars) and CRT−/− MEFs (open bars) were grown overnight in medium with 10% FBS, starved for 2 h in low serum (0.5% FBS) medium, and then treated with 100 pm TGF-β with 20 μm ascorbic acid for 24 h. A, Laemmli cell lysates were immunoblotted for fibronectin and collagen. Results of a representative blot are shown. Results are the mean density of bands normalized to β-tubulin ± S.D. (n = 4 separate experiments). B, the 4% DOC soluble (cell fraction) and insoluble (extracellular matrix) fractions of cells treated as in A were separated by SDS-PAGE and immunoblotted for fibronectin. Membranes were re-probed with antibody to β-tubulin to determine loading and efficacy of fractionation of the cellular and extracellular matrix fractions. C, wild type (gray bars) and CRT−/− MEFs (open bars) were plated in medium with 10% FBS overnight, switched to low serum medium with 20 μm ascorbic acid, and treated with 10 pm TGF-β for 72 h, re-dosing TGF-β and ascorbic acid every 24 h. After 72 h, conditioned medium from triplicate samples were pooled, and levels of secreted soluble collagen were measured by Sircol assay according to the manufacturer's specifications. Results are the means ± S.D. from three separate experiments. *, p < 0.05 versus non-treated cells.
To test whether re-expression of CRT in the CRT−/− cells can rescue responsiveness to TGF-β, CRT−/− MEFs stably transfected with HA-tagged CRT were stimulated with TGF-β and transcript measured 4 h post stimulation (44). Similar to wild type cells, TGF-β induced a significant increase in COL1A1 and fibronectin transcript production in CRT−/− MEFs stably transfected with HA-tagged CRT (Fig. 3A).
Because cell surface CRT can act as a receptor for the matricellular protein thrombospondin 1 (TSP1) and stimulate collagen production, we examined whether TSP1 binding to cell surface CRT might be involved in regulating cellular responsiveness to TGF-β. TSP1 binding to cell surface CRT does not appear to be important for cellular responsiveness to TGF-β, as CRT−/− MEFs stably expressing CRT lacking the TSP1 binding site were able to respond to TGF-β (Fig. 3B) (44, 47).
Knockdown of CRT in Fibrogenic Lung Fibroblasts Inhibits the Ability of TGF-β to Stimulate ECM
We next determined whether CRT expression is important for TGF-β-induced ECM production in fibroblasts known to be highly responsive to TGF-β. Thy-1 (−) lung fibroblasts predominate in the fibrotic foci of lungs with idiopathic pulmonary fibrosis, and rat thy-1 (−) fibroblasts have robust production of ECM in response to TGF-β (46, 51, 52). SiRNA knockdown of CRT to 60% of control levels in the Thy-1 (−) rat lung fibroblasts blocked the ability of TGF-β to stimulate type I collagen and fibronectin protein (Fig. 4, A–D). Similar results were obtained by siRNA knockdown of human CRT in lung fibroblasts isolated from IPF patients (Fig. 4E). In these studies, knockdown of CRT to 35% of control levels reduced base-line ECM levels and attenuated TGF-β stimulated collagen I and fibronectin protein as compared to cells transfected with non-targeting siRNA.
TGF-β Induces a Greater Stimulation of ECM in Cells Overexpressing CRT
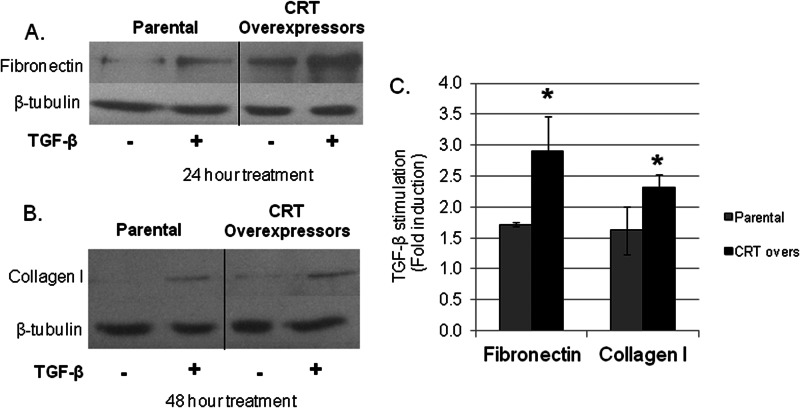
CRT and other ER stress response proteins are increased in several models of fibrosis, including bleomycin-induced lung fibrosis and unilateral ureteral obstruction renal fibrosis (2). Therefore, we asked whether overexpression of CRT correlates with enhanced ECM production in response to TGF-β. L-fibroblasts overexpressing CRT (∼1.6-fold increase in CRT) have increased collagen I and fibronectin transcript as compared with parental l-fibroblasts at base line (24). TGF-β treatment induced a greater increase in fibronectin and collagen I protein as compared with TGF-β-stimulated parental cells (Fig. 5, A–C).
FIGURE 5.
Overexpression of CRT increases TGF-β stimulation of ECM. Parental and CRT overexpressing L fibroblasts were grown overnight in medium with 10% FBS, starved for 2 h in medium with low (0.5%) serum, and treated with 100 pm TGF-β for 24 h for fibronectin determinations (A) or 48 h for collagen I determinations (B). Cells were harvested with Laemmli buffer, separated by SDS-PAGE, and immunoblotted for fibronectin, collagen I, and β-tubulin. C, bands were analyzed by densitometry (n = 3 separate experiments) and normalized to β-tubulin ±S.D. Untreated cells were set to 1.0. *, p < 0.05 versus parental cells. CRT overs, CRT overexpressors.
ER Stress in the Absence of CRT Is Not Sufficient to Stimulate ECM Production in Response to TGF-β
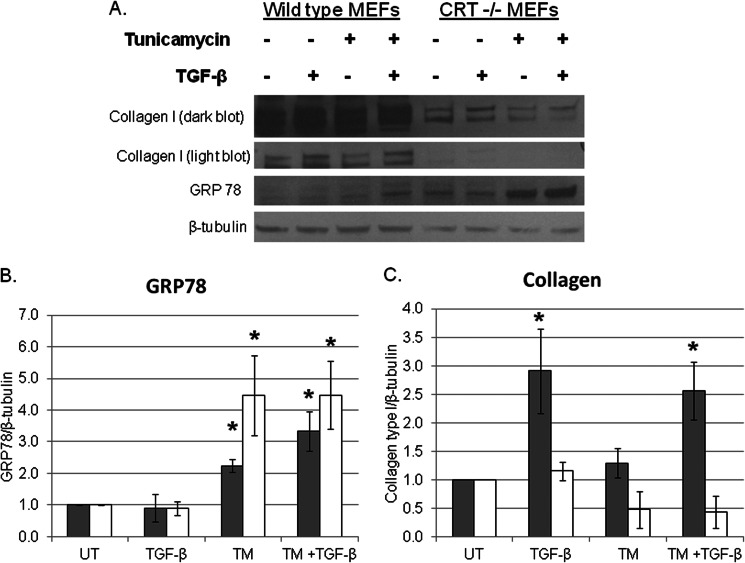
Increased ER stress exacerbates response to fibrogenic stimuli in vivo and in vitro (1, 14, 53). Because our data indicate that CRT is required for TGF-β-stimulated ECM production, we asked whether increased ER stress in the absence of CRT is sufficient to stimulate ECM production or whether CRT is a critical component of ER stress-induced ECM production. Wild type and CRT−/− MEFs were treated with TGF-β in the presence or absence of the ER stress inducer tunicamycin. In the presence of tunicamycin, both wild type and CRT−/− MEFs showed increased levels of the ER stress response protein GRP78, although tunicamycin increased GRP78 to a greater extent in CRT−/− MEFs as compared with wild type cells (Fig. 6, A and B). In the presence and absence of tunicamycin, wild type MEFs increased collagen I levels when treated with TGF-β, although TGF-β stimulation of collagen I was not enhanced in tunicamycin-treated cells as compared with cells treated with TGF-β alone (Fig. 6, A and C). However, enhanced ER stress due to tunicamycin treatment was not able to overcome the failure of CRT−/− MEFs to stimulate ECM in response to TGF-β (Fig. 6, A and C). Interestingly, tunicamycin did not increase CRT expression in wild type cells (data not shown). These data show that tunicamycin-induced ER stress is not sufficient for induction of ECM by TGF-β in the absence of CRT.
FIGURE 6.
ER stress is insufficient to drive TGF-β stimulation of ECM in CRT−/− MEFs. A, wild type (gray bars) and CRT−/− MEFs (open bars) were grown overnight in DMEM with 10% FBS and 20 μm ascorbic acid with or without 0.01 μg/ml tunicamycin. Cells were treated with 100 pm TGF-β in low (0.5%) serum medium containing 20 μm ascorbic acid for 24 h. Laemmli cell lysates were immunoblotted for collagen I, GRP78, or β-tubulin. A–C, bands were analyzed by densitometry and normalized to β-tubulin. Results are the mean densities normalized to β-tubulin ± S.D. from three separate experiments. *, p < 0.05 versus untreated cells. UT, untreated; TM, tunicamycin.
TGF-β-dependent Smad Signaling Is Active in Wild Type and CRT−/− MEFs
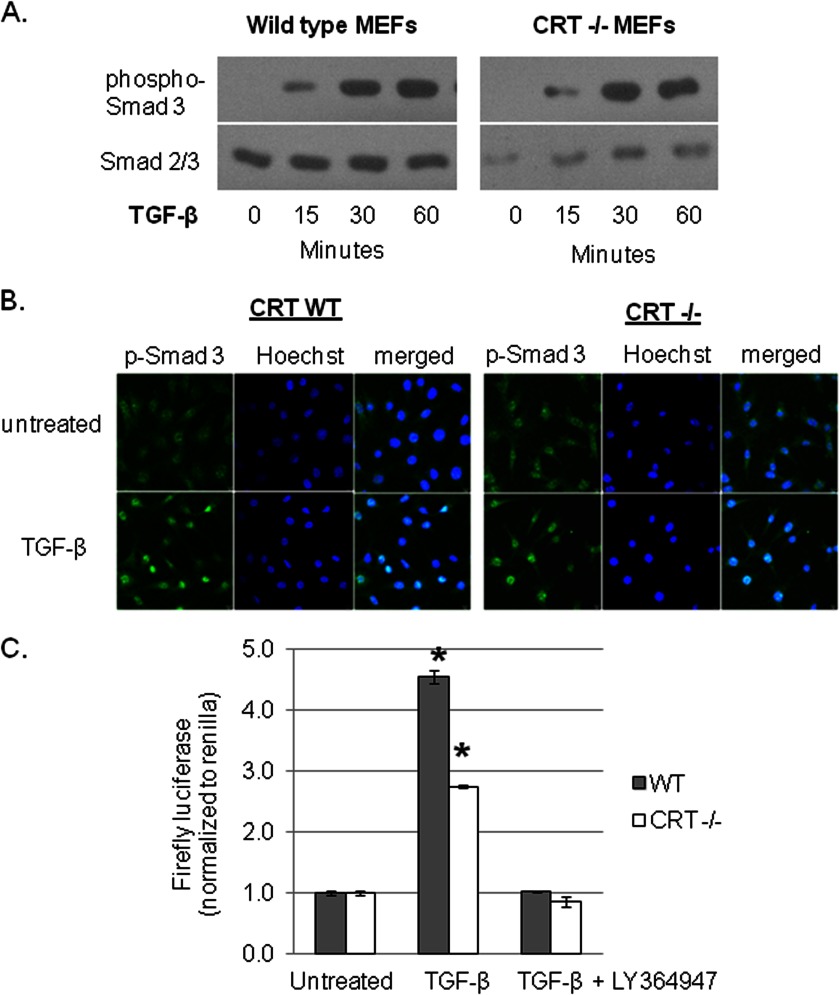
TGF-β signals ECM production primarily through Smad-dependent pathways, although other pathways can mediate TGF-β signaling (33, 36, 54). To determine if Smad signaling is impaired in the CRT−/− MEFs, we examined wild type and CRT−/− MEFS for Smad 3 phosphorylation after TGF-β stimulation. Phosphorylated Smad 3 was detected in both TGF-β treated wild type and CRT−/− MEFs, suggesting that TGF-β engagement of its signaling receptors and receptor Smad phosphorylation are not defective in CRT−/− MEFs (Fig. 7, A). Because CRT−/− MEFs are defective in their ability to induce MEFC2 nuclear translocation (21), we asked whether phosphorylated Smad 2/3 can be imported into the nucleus in CRT−/− MEFs treated with TGF-β. Immunofluorescence staining of TGF-β stimulated wild type and CRT−/− cells showed similar nuclear translocation of phosphorylated Smad 3 (Fig. 7B). Smad 2 phosphorylation and nuclear translocation were also similarly stimulated by TGF-β in CRT−/− MEFs (data not shown). Furthermore, Smad binding to Smad binding DNA elements is not deficient in the absence of CRT as TGF-β is able to induce Smad 2/3/4 reporter activity in wild type and CRT−/− MEFs (Fig. 7C). LY364947, an ALK5 (activin like kinase 5) TGF-βR1 inhibitor, blocked TGF-β stimulation of Smad 2/3/4 reporter activity by both wild type and CRT−/− MEFs in this assay, suggesting that TGF-β receptor signaling is not deficient in CRT−/− MEFs (Fig. 7C). We also confirmed that Smad signaling is important for TGF-β stimulation of ECM in wild type MEFs, as treatment of wild type MEFs with LY364947 significantly impaired TGF-β-induced collagen and -fibronectin transcript (data not shown). Finally, levels of active and total TGF-β were not decreased in the conditioned medium of CRT−/− MEFs (data not shown).
FIGURE 7.
TGF-β stimulates Smad activity in wild type and CRT−/− MEFs. A, wild type and CRT−/− MEFs were grown overnight in medium with 10% FBS, starved overnight in low (0.5%) serum medium, and treated with 100 pm TGF-β for 15, 30, or 60 min. Laemmli cell lysates containing phosphatase inhibitor were immunoblotted for phospho-Smad 3. Membranes were reprobed with antibody to Smad 2/3 or β-tubulin (data not shown) to normalize cell protein. The blot is representative of four separate experiments using 10–400 pm TGF-β. B, wild type and CRT−/− MEFs were grown overnight on glass coverslips in medium with 10% FBS, starved in low serum medium overnight, and treated with 100 pm TGF-β for 30 min. Cells were fixed, permeabilized, and incubated with antibody to phospho-Smad 3 followed by fluorescein-conjugated secondary antibody. Nuclei were stained with Hoechst. Results are representative of three separate experiments. Confocal images were obtained at an original magnification of 600×. C, wild type and CRT−/− MEFs were transfected with the Smad 2/3/4 firefly luciferase reporter construct and the control renilla luciferase construct and kept in medium with 10% FBS overnight, switched to low serum DMEM, and treated with 100 pm TGF-β for 8 h. Some cells were treated with 100 pm TGF-β in the presence of 3 μm LY364947. Cells were lysed with 1× lysis buffer (Promega), and triplicate samples were combined. Luciferase reporter activity is normalized to the renilla luciferase control. Data represent the means of samples from three separate experiments ± S.D. *, p < 0.05 versus untreated control.
CRT Is Required for TGF-β Induction of Cytosolic Ca2+
TGF-β can stimulate the slow release of intracellular Ca2+, which activates the calcineurin/NFAT pathway to increase fibronectin expression in mesangial cells (37, 41, 55–57). Because CRT regulates both ER Ca2+ and calcineurin/NFAT activity (17, 21, 22), we asked whether TGF-β stimulation of Ca2+ release was altered in CRT−/− MEFs. Wild type and CRT−/− MEFs were treated with TGF-β or the Ca2+ ionophore, ionomycin, as a positive control (58). Ca2+ release was measured over time using the Ca2+ binding fluorescent dye Fluo-4 AM. With ionomycin, both wild type and CRT−/− MEFS induced a large increase in cytosolic Ca2+, although Ca2+ re-uptake occurred more slowly in CRT−/− MEFs (Fig. 8, A and B). In contrast, TGF-β increased cytoplasmic Ca2+ levels in wild type but not in CRT−/− MEFs (Fig. 8, A and B), suggesting that CRT is required for TGF-β-dependent Ca2+ signaling.
To determine whether TGF-β stimulation of cytosolic Ca2+ is important for stimulation of ECM, wild type MEFs were treated with TGF-β in the presence or absence of thapsigargin, a SERCA2b inhibitor that blocks ER Ca2+ re-uptake to effectively deplete ER-releasable Ca2+ (59). Thapsigargin blocked the ability of TGF-β to stimulate collagen I and fibronectin transcript (Fig. 8, C and D), suggesting that TGF-β stimulation of cytosolic Ca2+ release is required for induction of ECM proteins. Cyclopiazonic acid, a SERCA2b inhibitor that acts in a manner similar to thapsigargin (60), also inhibited TGF-β-stimulated ECM production (data not shown). To determine if increased cytoplasmic Ca2+ is sufficient to support TGF-β-driven ECM expression in the absence of CRT, CRT−/− MEFs were treated with TGF-β in the presence of ionomycin. Despite an increase in cytoplasmic calcium with ionomycin, TGF-β was still unable to increase ECM transcript in CRT-deficient cells (Fig. 8E), suggesting that although CRT-mediated calcium regulation is critical for TGF-β-driven ECM stimulation, calcium alone is not sufficient, and other CRT-dependent factors are likely important.
CRT Is Required for TGF-β Stimulation of NFAT Activity
The activity of the transcription factor NFAT is regulated by Ca2+-activated calcineurin, and NFAT activity can regulate TGF-β-stimulated ECM production in the presence of sustained increases in cytoplasmic Ca2+ (37, 38). Given the importance of CRT for TGF-β stimulation of cytoplasmic Ca2+ levels and the deficient NFAT activation in CRT−/− mouse embryos (20, 23), we asked whether TGF-β stimulation of NFAT activity is defective in CRT−/− MEFs. TGF-β induces NFATc3 isoform nuclear translocation in wild type cells, whereas NFATc3 remained in the cytoplasm after TGF-β treatment of CRT−/− MEFs (Fig. 9A) (61). Furthermore, TGF-β stimulation NFAT reporter activity is also absent in CRT−/− MEFs (Fig. 9B).
NFAT Activity Is Required for TGF-β-stimulated ECM Production
To determine if TGF-β stimulation of NFAT activity is important for production of type I collagen and fibronectin, we asked whether inhibition of NFAT activity blocked TGF-β stimulation of fibronectin and collagen transcription. We used a cell-permeable peptide, 11R-VIVIT, which specifically blocks calcineurin binding to the NFAT PXIXIT sequence and prevents calcineurin-dependent NFAT dephosphorylation and nuclear translocation (61, 62). 11R-VIVIT blocked TGF-β stimulation of ECM in wild type MEFs (Fig. 9, C and D) and in human lung fibroblasts (data not shown). As expected, 11R-VIVIT had no effect on CRT−/− MEF ECM production by TGF-β (data not shown). Similarly, another NFAT inhibitor, A285222, which maintains NFAT in the cytoplasm regardless of calcineurin activity (63), showed a dose-dependent inhibition of TGF-β stimulated collagen and fibronectin transcript (data not shown). These results show that NFAT activity is necessary for TGF-β stimulation of ECM transcription in wild type MEFs and that CRT is required for TGF-β stimulation of NFAT activity.
DISCUSSION
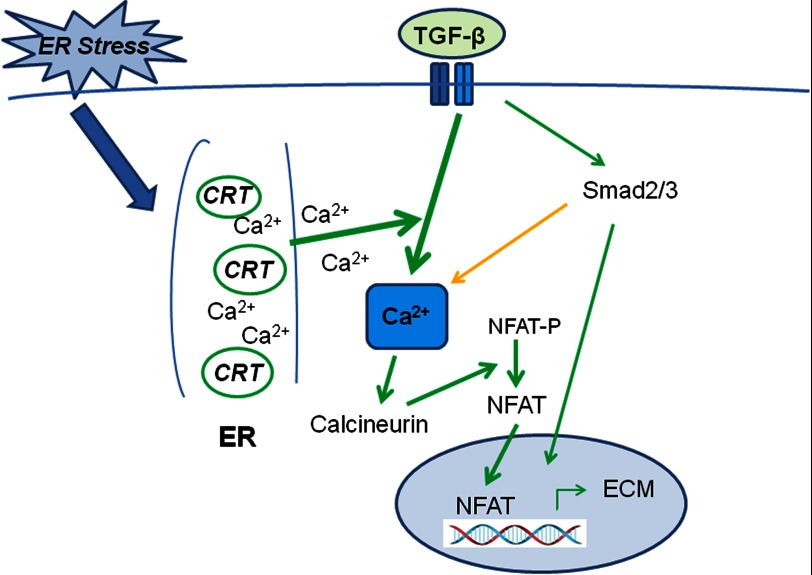
ER stress is emerging as a significant factor in fibrotic disorders (1, 2). Although ER stress-induced apoptosis is a contributing factor in fibrosis, a complete understanding of the mechanistic roles of ER stress in fibrosis remains to be defined. In our present studies we have shown that the ER stress-induced protein, CRT, is a critical regulator of fibrogenic responses to TGF-β. We show that CRT regulation of cytosolic Ca2+ and NFAT activity is required for TGF-β stimulation of collagen type I and fibronectin transcript (Fig. 10). Knock-out or knockdown of CRT abrogates cellular responsiveness to TGF-β even in the presence of active Smad 2/3 signaling. Consistent with these observations, cells with increased CRT expression exhibit a relative increase in responsiveness to TGF-β. Importantly, these studies show that tunicamycin-induced ER stress is not sufficient to support TGF-β stimulation of ECM in the absence of CRT. These studies identify a critical mechanistic link between ER stress and fibrosis.
FIGURE 10.
Model of CRT regulation of TGF-β ECM transcription. TGF-β binds to the heterotetrameric receptor complex (blue bars) to activate receptor type I kinase activity, which phosphorylates Smad2 and Smad 3. TGF-β also increases cytosolic Ca2+. The specific mechanism by which TGF-β regulates Ca2+ release and the involvement of Smad signaling in Ca2+ release are not yet clear (orange arrow). TGF-β stimulated Ca2+ release is dependent on CRT regulation of ER Ca2+ and possibly CRT-regulated store-operated Ca2+ entry (not depicted). Increased cytosolic Ca2+ leads to activation of calcineurin, which dephosphorylates cytoplasmic NFAT, resulting in NFAT activation and nuclear translocation. NFAT can directly stimulate transcription of ECM proteins or partner with other known matrix-inducing transcription factors such as AP-1 and SP-1. In the absence of CRT, there is a failure of TGF-β to increase cytoplasmic Ca2+, activate NFAT, and up-regulate ECM transcription. In contrast, under pathological ER stress, CRT expression can be up-regulated, resulting in increased TGF-β stimulated ECM.
CRT expression is increased in a number of different fibrotic tissues, including lung, kidney, and diabetic atherosclerotic vasculature (2, 4),4 suggesting an involvement in fibrotic processes. CRT acts as a collagen chaperone to mediate collagen ER-Golgi trafficking, processing, and incorporation into the ECM (24). In addition, CRT regulation of Src-dependent fibronectin expression and matrix deposition impacts collagen matrix assembly (24, 25). Our current studies now show that CRT regulation of Ca2+-dependent NFAT activity is required for the ability of TGF-β to stimulate ECM transcription.
TGF-β is a pro-fibrotic cytokine that drives expression of ECM proteins and integrin ECM receptors (64–67). TGF-β signals through both the canonical Smad pathway and other non-Smad pathways (20–24). Although not well studied, there is evidence that TGF-β can increase cytoplasmic Ca2+ and activate calcineurin, which dephosphorylates NFAT, resulting in NFAT nuclear translocation (37, 38). Despite these data, the mechanism by which TGF-β increases cytosolic Ca2+ remains unclear. Our data suggest that CRT is essential for TGF-β to increase cytoplasmic Ca2+ levels as cells lacking CRT were unable to increase cytoplasmic Ca2+ when treated with TGF-β. There are several mechanisms by which TGF-β increases intracellular Ca2+ levels, including increasing inositol 3-phosphate levels to cause release of Ca2+ from thapsigargin-sensitive stores in the ER, mediating translocation of type III inositol 3-phosphate receptors to the cell surface, stimulating H2O2-mediated Ca2+ release, and via a c-Jun-dependent mechanism (39–43). Rises in cytoplasmic Ca2+ levels are typically regulated by ER-mediated Ca2+ release or through store-operated Ca2+ entry through channels present on the plasma membrane (68, 69). CRT can regulate both ER-mediated Ca2+ release and store-operated Ca2+ entry (19, 20, 70–72). The ability of thapsigargin to block responses to TGF-β suggests that CRT regulation of ER Ca2+ stores might be involved, although TGF-β stimulation of wild type MEFs in medium lacking extracellular Ca2+ failed to stimulate a rise in cytoplasmic Ca2+, suggesting that CRT might also regulate cytoplasmic Ca2+ levels through regulation of store-operated Ca2+ entry (data not shown). In addition, treatment of CRT−/− MEFs with TGF-β in the presence of ionomycin failed to make CRT−/− cells responsive to TGF-β, showing that TGF-β stimulation of ECM is not simply a function of increased cytoplasmic calcium and suggesting that additional CRT-dependent functions are involved in regulating TGF-β stimulation of ECM.
TGF-β stimulation of NFAT activation and nuclear translocation were impaired in CRT−/− MEFs, suggesting that CRT-mediated calcineurin activity is likely impaired in CRT−/− MEFs. Our data are consistent with prior reports demonstrating that CRT is needed for efficient NFAT nuclear translocation (21, 23). Furthermore, studies by Gooch et al. (37) showed that TGF-β activates calcineurin in a time- and dose-dependent manner and that calcineurin is required for TGF-β-stimulated ECM in glomerular mesangial cells. These studies showed that TGF-β stimulation of fibronectin is dependent on NFAT binding to the fibronectin promoter, as cells expressing a dominant negative NFATc mutant are unable to induce ECM in response to TGF-β (38). NFAT signaling has also been shown to be important for myofibroblast induction and expression of collagens I and III by cardiac fibroblasts in response to mechanical stress and osteopontin by vascular smooth muscle cells in high glucose (73, 74).
CRT can regulate cell behavior, ECM production, and wound healing from multiple cellular compartments, including at the cell surface and as an extracellular ligand (75, 76). However, cell surface CRT, which can act as a co-receptor for TSP1 to stimulate collagen production, does not appear to be involved in mediating these TGF-β responses, as CRT−/− MEFs stably expressing CRT lacking the TSP1 binding sequence had normal responses to TGF-β (47).
The absence of CRT does not appear to negatively impact Smad 2/3 signaling as Smad 2/3 phosphorylation and nuclear translocation are unaffected. Because nuclear CRT can affect DNA binding of some nuclear hormones such as vitamin D, a nuclear receptor known to antagonize TGF-β signaling (45, 77), we also examined the ability of TGF-β to stimulate Smad reporter activity in CRT-deficient cells. Although reporter activity in CRT−/− MEFs was reduced as compared with wild type cells, TGF-β was still able to stimulate Smad reporter activity 2.5-fold in CRT−/− MEFs, levels that have been shown to be sufficient for Smad-induced gene transcription in other systems (78, 79). We also investigated whether the lack of CRT impacted non-Smad pathways regulated by TGF-β (32–36). In contrast to the negative effects of CRT deficiency on the calcium-NFAT pathway, the CRT−/− MEFs did not show alterations in levels of p-AKT, p-JNK, and p-ERK induced by TGF-β (data not shown). There was a trend toward increased phosphorylated p-38 MAPK in CRT−/− cells treated with TGF-β, although these data did not reach statistical significance, and the importance remains unclear.
Recent literature suggests that ER stress is associated with fibrotic disorders such as diabetic atherosclerosis, pulmonary fibrosis, and diabetic nephropathy (1–7). Chronic stimuli such as high glucose, glucosamine, or oxidative stress can up-regulate ER stress proteins and are associated with enhanced matrix production and fibrotic disease (3–5, 80, 81). Chemical chaperones such as 4-phenylbutyric reduce the expression of ER stress proteins such as CRT and GRP78 and also reduce fibrotic remodeling due to high glucose in animal models of atherosclerosis and nephropathy (3–5). Despite the growing appreciation for a role for ER stress in fibrotic disease, knowledge regarding the mechanisms by which ER stress regulates fibrosis is limited (8, 15). Although there is a clear role for ER stress-induced apoptosis in some models of fibrosis (6), there is also evidence for ER stress involvement in processes important for fibroproliferative remodeling that are independent of apoptosis (82, 83). Knockdown of GRP78, another ER stress response protein, also reduces TGF-β or tunicamycin-stimulated collagen and α-smooth muscle actin production in human lung fibroblasts (82). 150-kDa oxygen-regulated protein (ORP150) mediates TGF-β myofibroblast induction and collagen production in human lung fibroblasts (83). The mechanisms by which GRP78 and ORP150 mediate responsiveness to TGF-β were not addressed in these reports. Our data now suggest that CRT is an important ER stress factor in fibrotic remodeling through regulation TGF-β-stimulated ECM production. It is interesting that CRT mediates TGF-β responsiveness through its role as a regulator of calcium signaling rather than through its chaperone function, suggesting a unique role for CRT in the ER stress response. In contrast to these reports, Vonk et al. (84) showed that enhanced ER stress due to glucose and nutrient deprivation reduces collagen production in chondrocyte and dermal fibroblasts. Nonetheless, the majority of evidence suggests that enhanced ER stress is associated with exacerbated fibrotic outcomes (1–7, 10, 14, 15, 85).
TGF-β has been shown to increase expression of ER stress response proteins, including CRT in the human IPF lung fibroblasts and rat vascular smooth muscle cells (data not shown) (82, 83). However, we did not observe TGF-β stimulation of either GRP78 or CRT in MEFs or Thy-1 −/− rat lung fibroblasts, suggesting that basal levels of CRT expression are sufficient to mediate responses to TGF-β. Furthermore, knockdown of CRT in Thy1−/− rat lung fibroblasts to 60% that of control levels was able to attenuate TGF-β-stimulated matrix production, although base-line levels of matrix proteins were unaffected. The lack of effect on base-line matrix production might reflect a lower threshold of CRT required to maintain homeostatic levels of ECM expression in these cells. This would be consistent with our observations that knockdown of CRT to 35% that of control levels does reduce basal ECM expression in human lung fibroblasts. Interestingly, CRT−/− MEFs have increased basal levels of the ER stress proteins GRP78, GRP94 (glucose-regulated protein 94), calnexin, and protein disulfide isomerase (data not shown and Ref. 86) and reduced expression of multiple ECM proteins (24), suggesting that increased expression of other ER stress proteins cannot compensate for the lack of CRT in mediating ECM production in response to TGF-β (1). This conclusion is supported by our observation that TGF-β treatment of CRT−/− MEFs in the presence tunicamycin did not increase collagen I protein. These data suggest that enhanced levels of ER stress with TGF-β in the absence of CRT are not sufficient to drive fibrosis, implicating CRT as an important link between enhanced levels of ER stress and fibrotic disease. Furthermore, these data suggest that ER CRT might be a novel therapeutic target to attenuate fibrosis. In conclusion, these studies demonstrate that CRT is required for TGF-β-stimulated ECM production and provide a link between enhanced ER stress and TGF-β stimulation of ECM.
Acknowledgments
We thank Drs. Michal Opas (University of Toronto) and Dr. Marek Michalak (University of Alberta) for the generous gifts of L fibroblasts and CRT−/− MEFs and Dr. Victor Thannickal (University of Alabama at Birmingham) for the gift of human IPF lung fibroblasts. We thank Abbott Laboratories for the gift of A285222. We thank Dr. Majd Zayzafoon and Dr. John Chatham, Department of Pathology, University of Alabama at Birmingham for helpful discussions. We also thank Claire Gamlin for work on signaling studies on non-canonical TGF-β pathways. We also thank Shawn Williams of the University of Alabama at Birmingham High Resolution Imaging Facility for assistance in acquiring confocal images. This work was performed in facilities supported by National Institutes of Health Grant C06RR15490.
This work was supported, in whole or in part, by National Institutes of Health Grants T32 HL007918 (to K. A. Z.) and T32 GM008361 (to L. V. G.). This work was also supported by American Heart Association Innovation Grant 12IRG160008 (to J. M.-U.).
K. A. Zimmerman, M. A. Pallero, and J. E. Murphy-Ullrich, unpublished data.
- ER
- endoplasmic reticulum
- NFAT
- nuclear factor of activated T cell
- IPF
- idiopathic pulmonary fibrosis
- CRT
- calreticulin
- ECM
- extracellular matrix
- MEF
- mouse embryonic fibroblast
- Smad
- small mothers against decapentaplegic
- TSP1
- thrombospondin 1
- GRP78
- glucose regulated protein 78
- SERCA2b
- sarcoplasmic/endoplasmic reticulum calcium ATPase
- JNK
- c-Jun N-terminal kinase
- ORP150
- oxygen-regulated protein 150
- DOC
- deoxycholate.
REFERENCES
- 1. Lawson W. E., Cheng D. S., Degryse A. L., Tanjore H., Polosukhin V. V., Xu X. C., Newcomb D. C., Jones B. R., Roldan J., Lane K. B., Morrisey E. E., Beers M. F., Yull F. E., Blackwell T. S. (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. U. S. A. 108, 10562–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kypreou K. P., Kavvadas P., Karamessinis P., Peroulis M., Alberti A., Sideras P., Psarras S., Capetanaki Y., Politis P. K., Charonis A. S. (2008) Altered expression of calreticulin during the development of fibrosis. Proteomics 8, 2407–2419 [DOI] [PubMed] [Google Scholar]
- 3. Khan M. I., Pichna B. A., Shi Y., Bowes A. J., Werstuck G. H. (2009) Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid. Redox. Signal. 11, 2289–2298 [DOI] [PubMed] [Google Scholar]
- 4. Kurokawa M., Hideshima M., Ishii Y., Kyuwa S., Yoshikawa Y. (2009) Aortic ER stress in streptozotocin-induced diabetes mellitus in APA hamsters. Exp. Anim. 58, 113–121 [DOI] [PubMed] [Google Scholar]
- 5. Qi W., Mu J., Luo Z. F., Zeng W., Guo Y. H., Pang Q., Ye Z. L., Liu L., Yuan F. H., Feng B. (2011) Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metabolism 60, 594–603 [DOI] [PubMed] [Google Scholar]
- 6. Korfei M., Ruppert C., Mahavadi P., Henneke I., Markart P., Koch M., Lang G., Fink L., Bohle R. M., Seeger W., Weaver T. E., Guenther A. (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 178, 838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kassan M., Galán M., Partyka M., Saifudeen Z., Henrion D., Trebak M., Matrougui K. (2012) Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 32, 1652–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lenna S., Trojanowska M. (2012) The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol. 24, 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui Y., Robertson J., Maharaj S., Waldhauser L., Niu J., Wang J., Farkas L., Kolb M., Gauldie J. (2011) Oxidative stress contributes to the induction and persistence of TGF-β1 induced pulmonary fibrosis. Int. J. Biochem. Cell Biol. 43, 1122–1133 [DOI] [PubMed] [Google Scholar]
- 10. Beriault D. R., Sharma S., Shi Y., Khan M. I., Werstuck G. H. (2011) Glucosamine supplementation promotes endoplasmic reticulum stress, hepatic steatosis, and accelerated atherogenesis in apoE−/− mice. Atherosclerosis 219, 134–140 [DOI] [PubMed] [Google Scholar]
- 11. Ayala P., Montenegro J., Vivar R., Letelier A., Urroz P. A., Copaja M., Pivet D., Humeres C., Troncoso R., Vicencio J. M., Lavandero S., Díaz-Araya G. (2012) Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp. Mol. Pathol. 92, 97–104 [DOI] [PubMed] [Google Scholar]
- 12. Wang J. Q., Chen X., Zhang C., Tao L., Zhang Z. H., Liu X. Q., Xu Y. B., Wang H., Li J., Xu D. X. (2013) Phenylbutyric acid protects against carbon tetrachloride-induced hepatic fibrogenesis in mice. Toxicol. Appl. Pharmacol. 266, 307–316 [DOI] [PubMed] [Google Scholar]
- 13. Bowes A. J., Khan M. I., Shi Y., Robertson L., Werstuck G. H. (2009) Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice. Evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. Am. J. Pathol. 174, 330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rishikof D. C., Ricupero D. A., Liu H., Goldstein R. H. (2004) Phenylbutyrate decreases type I collagen production in human lung fibroblasts. J. Cell Biochem. 91, 740–748 [DOI] [PubMed] [Google Scholar]
- 15. Tanjore H., Cheng D. S., Degryse A. L., Zoz D. F., Abdolrasulnia R., Lawson W. E., Blackwell T. S. (2011) Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J. Biol. Chem. 286, 30972–30980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coe H., Michalak M. (2009) Calcium binding chaperones of the endoplasmic reticulum. Gen. Physiol. Biophys. 28, F96–F103 [PubMed] [Google Scholar]
- 17. Michalak M., Groenendyk J., Szabo E., Gold L. I., Opas M. (2009) Calreticulin, a multiprocess calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 417, 651–666 [DOI] [PubMed] [Google Scholar]
- 18. Groenendyk J., Lynch J., Michalak M. (2004) Calreticulin, Ca2+, and calcineurin signaling from the endoplasmic reticulum. Mol. Cells 17, 383–389 [PubMed] [Google Scholar]
- 19. Mery L., Mesaeli N., Michalak M., Opas M., Lew D. P., Krause K. H. (1996) Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 271, 9332–9339 [DOI] [PubMed] [Google Scholar]
- 20. Mesaeli N., Nakamura K., Zvaritch E., Dickie P., Dziak E., Krause K. H., Opas M., MacLennan D. H., Michalak M. (1999) Calreticulin is essential for cardiac development. J. Cell Biol. 144, 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch J., Guo L., Gelebart P., Chilibeck K., Xu J., Molkentin J. D., Agellon L. B., Michalak M. (2005) Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca2+-dependent signaling cascade. J. Cell Biol. 170, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lynch J., Michalak M. (2003) Calreticulin is an upstream regulator of calcineurin. Biochem. Biophys. Res. Commun. 311, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 23. Guo L., Nakamura K., Lynch J., Opas M., Olson E. N., Agellon L. B., Michalak M. (2002) Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J. Biol. Chem. 277, 50776–50779 [DOI] [PubMed] [Google Scholar]
- 24. Van Duyn Graham L., Sweetwyne M. T., Pallero M. A., Murphy-Ullrich J. E. (2010) Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix. J. Biol. Chem. 285, 7067–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papp S., Fadel M. P., Kim H., McCulloch C. A., Opas M. (2007) Calreticulin affects fibronectin-based cell-substratum adhesion via the regulation of c-Src activity. J. Biol. Chem. 282, 16585–16598 [DOI] [PubMed] [Google Scholar]
- 26. Papp S., Szabo E., Kim H., McCulloch C. A., Opas M. (2008) Kinase-dependent adhesion to fibronectin. Regulation by calreticulin. Exp. Cell Res. 314, 1313–1326 [DOI] [PubMed] [Google Scholar]
- 27. Hattori K., Nakamura K., Hisatomi Y., Matsumoto S., Suzuki M., Harvey R. P., Kurihara H., Hattori S., Yamamoto T., Michalak M., Endo F. (2007) Arrhythmia induced by spatiotemporal overexpression of calreticulin in the heart. Mol. Genet. Metab. 91, 285–293 [DOI] [PubMed] [Google Scholar]
- 28. Pan C., Giraldo G. S., Prentice H., Wu J. Y. (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J. Biomed. Sci. 17, S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelsen S. G., Duan X., Ji R., Perez O., Liu C., Merali S. (2008) Cigarette smoke induces an unfolded protein response in the human lung. A proteomic approach. Am. J. Respir. Cell Mol. Biol. 38, 541–550 [DOI] [PubMed] [Google Scholar]
- 30. Jia L., Xu M., Zhen W., Shen X., Zhu Y., Wang W., Wang X. (2008) Novel anti-oxidative role of calreticulin in protecting A549 human type II alveolar epithelial cells against hypoxic injury. Am. J. Physiol. Cell Physiol. 294, C47–C55 [DOI] [PubMed] [Google Scholar]
- 31. Roberts A. B. (1998) Molecular and cell biology of TGF-β. Miner. Electrolyte Metab. 24, 111–119 [DOI] [PubMed] [Google Scholar]
- 32. Jayaraman L., Massague J. (2000) Distinct oligomeric states of SMAD proteins in the transforming growth factor-β pathway. J. Biol. Chem. 275, 40710–40717 [DOI] [PubMed] [Google Scholar]
- 33. Wrana J. L. (2000) Regulation of Smad activity. Cell 100, 189–192 [DOI] [PubMed] [Google Scholar]
- 34. Hanafusa H., Ninomiya-Tsuji J., Masuyama N., Nishita M., Fujisawa J., Shibuya H., Matsumoto K., Nishida E. (1999) Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J. Biol. Chem. 274, 27161–27167 [DOI] [PubMed] [Google Scholar]
- 35. Hayashida T., Decaestecker M., Schnaper H. W. (2003) Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-β-dependent responses in human mesangial cells. FASEB J. 17, 1576–1578 [DOI] [PubMed] [Google Scholar]
- 36. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 37. Gooch J. L., Gorin Y., Zhang B. X., Abboud H. E. (2004) Involvement of calcineurin in transforming growth factor-β-mediated regulation of extracellular matrix accumulation. J. Biol. Chem. 279, 15561–15570 [DOI] [PubMed] [Google Scholar]
- 38. Cobbs S. L., Gooch J. L. (2007) NFATc is required for TGFβ-mediated transcriptional regulation of fibronectin. Biochem. Biophys. Res. Commun. 362, 288–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muldoon L. L., Rodland K. D., Magun B. E. (1988) Transforming growth factor β and epidermal growth factor alter calcium influx and phosphatidylinositol turnover in rat-1 fibroblasts. J. Biol. Chem. 263, 18834–18841 [PubMed] [Google Scholar]
- 40. McGowan T. A., Madesh M., Zhu Y., Wang L., Russo M., Deelman L., Henning R., Joseph S., Hajnoczky G., Sharma K. (2002) TGF-β-induced Ca2+ influx involves the type III IP3 receptor and regulates actin cytoskeleton. Am. J. Physiol. Renal Physiol. 282, F910–F920 [DOI] [PubMed] [Google Scholar]
- 41. Ishiyama N., Shibata H., Kanzaki M., Shiozaki S., Miyazaki J., Kobayashi I., Kojima I. (1996) Calcium as a second messenger of the action of transforming growth factor-β on insulin secretion. Mol. Cell. Endocrinol. 117, 1–6 [DOI] [PubMed] [Google Scholar]
- 42. Junn E., Lee K. N., Ju H. R., Han S. H., Im J. Y., Kang H. S., Lee T. H., Bae Y. S., Ha K. S., Lee Z. W., Rhee S. G., Choi I. (2000) Requirement of hydrogen peroxide generation in TGF-β 1 signal transduction in human lung fibroblast cells. Involvement of hydrogen peroxide and Ca2+ in TGF-β 1-induced IL-6 expression. J. Immunol. 165, 2190–2197 [DOI] [PubMed] [Google Scholar]
- 43. Janowski E., Jiao X., Katiyar S., Lisanti M. P., Liu M., Pestell R. G., Morad M. (2011) c-Jun is required for TGF-β-mediated cellular migration via nuclear Ca2+ signaling. Int. J. Biochem. Cell Biol. 43, 1104–1113 [DOI] [PubMed] [Google Scholar]
- 44. Pallero M. A., Elzie C. A., Chen J., Mosher D. F., Murphy-Ullrich J. E. (2008) Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J. 22, 3968–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burns K., Duggan B., Atkinson E. A., Famulski K. S., Nemer M., Bleackley R. C., Michalak M. (1994) Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature 367, 476–480 [DOI] [PubMed] [Google Scholar]
- 46. Barker T. H., Grenett H. E., MacEwen M. W., Tilden S. G., Fuller G. M., Settleman J., Woods A., Murphy-Ullrich J., Hagood J. S. (2004) Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization, and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp. Cell Res. 295, 488–496 [DOI] [PubMed] [Google Scholar]
- 47. Sweetwyne M. T., Pallero M. A., Lu A., Van Duyn Graham L., Murphy-Ullrich J. E. (2010) The calreticulin binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am. J. Pathol. 177, 1710–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Midwood K. S., Wierzbicka-Patynowski I., Schwarzbauer J. E. (2002) Preparation and analysis of synthetic multicomponent extracellular matrix. Methods Cell Biol. 69, 145–161 [DOI] [PubMed] [Google Scholar]
- 49. Prockop D. J., Kivirikko K. I. (1995) Collagens. Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 50. Davidson J. M., LuValle P. A., Zoia O., Quaglino D., Jr., Giro M. (1997) Ascorbate differentially regulates elastin and collagen biosynthesis in vascular smooth muscle cells and skin fibroblasts by pretranslational mechanisms. J. Biol. Chem. 272, 345–352 [DOI] [PubMed] [Google Scholar]
- 51. Zhou Y., Hagood J. S., Murphy-Ullrich J. E. (2004) Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-β in response to fibrogenic stimuli. Am. J. Pathol. 165, 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hagood J. S., Prabhakaran P., Kumbla P., Salazar L., MacEwen M. W., Barker T. H., Ortiz L. A., Schoeb T., Siegal G. P., Alexander C. B., Pardo A., Selman M. (2005) Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am. J. Pathol. 167, 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee G. H., Oh H. W., Lim H. D., Lee W., Chae H. J., Kim H. R. (2011) 4-Phenylbutyric acid regulates collagen synthesis and secretion induced by high concentrations of glucose in human gingival fibroblasts. Korean J. Physiol. Pharmacol. 15, 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piek E., Ju W. J., Heyer J., Escalante-Alcalde D., Stewart C. L., Weinstein M., Deng C., Kucherlapati R., Bottinger E. P., Roberts A. B. (2001) Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 276, 19945–19953 [DOI] [PubMed] [Google Scholar]
- 55. Yang K. L., Chang W. T., Chuang C. C., Hung K. C., Li E. I. (2008) Antagonizing TGF-β induced liver fibrosis by a retinoic acid derivative through regulation of ROS and calcium influx. Biochem. Biophys. Res. Commun. 365, 484–489 [DOI] [PubMed] [Google Scholar]
- 56. Dolmetsch R. E., Lewis R. S., Goodnow C. C., Healy J. I. (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 [DOI] [PubMed] [Google Scholar]
- 57. Timmerman L. A., Clipstone N. A., Ho S. N., Northrop J. P., Crabtree G. R. (1996) Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383, 837–840 [DOI] [PubMed] [Google Scholar]
- 58. Morgan A. J., Jacob R. (1994) Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 300, 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seidler N. W., Jona I., Vegh M., Martonosi A. (1989) Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 264, 17816–17823 [PubMed] [Google Scholar]
- 61. Crabtree G. R., Olson E. N. (2002) NFAT signaling. Choreographing the social lives of cells. Cell 109, S67–S79 [DOI] [PubMed] [Google Scholar]
- 62. Aramburu J., Yaffe M. B., López-Rodríguez C., Cantley L. C., Hogan P. G., Rao A. (1999) Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285, 2129–2133 [DOI] [PubMed] [Google Scholar]
- 63. Trevillyan J. M., Chiou X. G., Chen Y. W., Ballaron S. J., Sheets M. P., Smith M. L., Wiedeman P. E., Warrior U., Wilkins J., Gubbins E. J., Gagne G. D., Fagerland J., Carter G. W., Luly J. R., Mollison K. W., Djuric S. W. (2001) Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J. Biol. Chem. 276, 48118–48126 [DOI] [PubMed] [Google Scholar]
- 64. Inagaki Y., Truter S., Ramirez F. (1994) Transforming growth factor-β stimulates α2(I) collagen gene expression through a cis-acting element that contains an Sp1-binding site. J. Biol. Chem. 269, 14828–14834 [PubMed] [Google Scholar]
- 65. Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. (1987) Transforming growth factor-β increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J. Clin. Invest. 79, 1285–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Varga J., Rosenbloom J., Jimenez S. A. (1987) Transforming growth factor β (TGF β) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 247, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ignotz R. A., Massagué J. (1986) Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345 [PubMed] [Google Scholar]
- 68. Putney J. W., Jr. (1990) Capacitative calcium entry revisited. Cell Calcium 11, 611–624 [DOI] [PubMed] [Google Scholar]
- 69. Baksh S., Michalak M. (1991) Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 266, 21458–21465 [PubMed] [Google Scholar]
- 70. Nakamura K., Zuppini A., Arnaudeau S., Lynch J., Ahsan I., Krause R., Papp S., De Smedt H., Parys J. B., Muller-Esterl W., Lew D. P., Krause K. H., Demaurex N., Opas M., Michalak M. (2001) Functional specialization of calreticulin domains. J. Cell Biol. 154, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bastianutto C., Clementi E., Codazzi F., Podini P., De Giorgi F., Rizzuto R., Meldolesi J., Pozzan T. (1995) Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 130, 847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu W., Longo F. J., Wintermantel M. R., Jiang X., Clark R. A., DeLisle S. (2000) Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphosphate-induced Ca2+ store depletion. J. Biol. Chem. 275, 36676–36682 [DOI] [PubMed] [Google Scholar]
- 73. Herum K. M., Lunde I. G., Skrbic B., Florholmen G., Behmen D., Sjaastad I., Carlson C. R., Gomez M. F., Christensen G. (2013) Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J. Mol. Cell. Cardiol. 54, 73–81 [DOI] [PubMed] [Google Scholar]
- 74. Nilsson-Berglund L. M., Zetterqvist A. V., Nilsson-Ohman J., Sigvardsson M., González Bosc L. V., Smith M. L., Salehi A., Agardh E., Fredrikson G. N., Agardh C. D., Nilsson J., Wamhoff B. R., Hultgårdh-Nilsson A., Gomez M. F. (2010) Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler. Thromb. Vasc. Biol. 30, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nanney L. B., Woodrell C. D., Greives M. R., Cardwell N. L., Pollins A. C., Bancroft T. A., Chesser A., Michalak M., Rahman M., Siebert J. W., Gold L. I. (2008) Calreticulin enhances porcine wound repair by diverse biological effects. Am. J. Pathol. 173, 610–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gold L. I., Eggleton P., Sweetwyne M. T., Van Duyn L. B., Greives M. R., Naylor S. M., Michalak M., Murphy-Ullrich J. E. (2010) Calreticulin. Non-endoplasmic reticulum functions in physiology and disease. FASEB J. 24, 665–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dedhar S., Rennie P. S., Shago M., Hagesteijn C. Y., Yang H., Filmus J., Hawley R. G., Bruchovsky N., Cheng H., Matusik R. J. (1994) Inhibition of nuclear hormone receptor activity by calreticulin. Nature 367, 480–483 [DOI] [PubMed] [Google Scholar]
- 78. Tili E., Michaille J. J., Alder H., Volinia S., Delmas D., Latruffe N., Croce C. M. (2010) Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 80, 2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Isono M., Chen S., Hong S. W., Iglesias-de la Cruz M. C., Ziyadeh F. N. (2002) Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-β-induced fibronectin in mesangial cells. Biochem. Biophys. Res. Commun. 296, 1356–1365 [DOI] [PubMed] [Google Scholar]
- 80. Srinivasan V., Tatu U., Mohan V., Balasubramanyam M. (2009) Molecular convergence of hexosamine biosynthetic pathway and ER stress leading to insulin resistance in L6 skeletal muscle cells. Mol. Cell. Biochem. 328, 217–224 [DOI] [PubMed] [Google Scholar]
- 81. Sage A. T., Walter L. A., Shi Y., Khan M. I., Kaneto H., Capretta A., Werstuck G. H. (2010) Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am. J. Physiol. Endocrinol. Metab. 298, E499–E511 [DOI] [PubMed] [Google Scholar]
- 82. Baek H. A., Kim do S., Park H. S., Jang K. Y., Kang M. J., Lee D. G., Moon W. S., Chae H. J., Chung M. J. (2012) Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 46, 731–739 [DOI] [PubMed] [Google Scholar]
- 83. Tanaka K., Shirai A., Ito Y., Namba T., Tahara K., Yamakawa N., Mizushima T. (2012) Expression of 150-kDa oxygen-regulated protein (ORP150) stimulates bleomycin-induced pulmonary fibrosis and dysfunction in mice. Biochem. Biophys. Res. Commun. 425, 818–824 [DOI] [PubMed] [Google Scholar]
- 84. Vonk L. A., Doulabi B. Z., Huang C. L., Helder M. N., Everts V., Bank R. A. (2010) Endoplasmic reticulum stress inhibits collagen synthesis independent of collagen-modifying enzymes in different chondrocyte populations and dermal fibroblasts. Biochem. Cell Biol. 88, 539–552 [DOI] [PubMed] [Google Scholar]
- 85. Torres-González E., Bueno M., Tanaka A., Krug L. T., Cheng D. S., Polosukhin V. V., Sorescu D., Lawson W. E., Blackwell T. S., Rojas M., Mora A. L. (2012) Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am. J. Respir. Cell Mol. Biol. 46, 748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Knee R., Ahsan I., Mesaeli N., Kaufman R. J., Michalak M. (2003) Compromised calnexin function in calreticulin-deficient cells. Biochem. Biophys. Res. Commun. 304, 661–666 [DOI] [PubMed] [Google Scholar]