Background: Decreased CaMKII activity after ischemia is correlated with the extent of neuronal damage.
Results: CaMKII inhibition within cortical astrocytes decreases glutamate uptake and leads to neurotoxic ATP release.
Conclusion: Astrocytic CaMKII inactivation leads to cellular dysfunction and compromised neuronal survival.
Significance: Pathophysiological inactivation of CaMKII contributes to ischemic damage via disrupting astrocyte-neuron communication.
Keywords: Calcium Signaling, CaMKII, Glia, Glutamate, Neurodegeneration, Neuronal-Glial Communication
Abstract
The extent of calcium/calmodulin-dependent protein kinase II (CaMKII) inactivation in the brain after ischemia correlates with the extent of damage. We have previously shown that a loss of CaMKII activity in neurons is detrimental to neuronal viability by inducing excitotoxic glutamate release. In the current study we extend these findings to show that the ability of astrocytes to buffer extracellular glutamate is reduced when CaMKII is inhibited. Furthermore, CaMKII inhibition in astrocytes is associated with the rapid onset of intracellular calcium oscillations. Surprisingly, this rapid calcium influx is blocked by the N-type calcium channel antagonist, ω-conotoxin. Although the function of N-type calcium channels within astrocytes is controversial, these voltage-gated calcium channels have been linked to calcium-dependent vesicular gliotransmitter release. When extracellular glutamate and ATP levels are measured after CaMKII inhibition within our enriched astrocyte cultures, no alterations in glutamate levels are observed, whereas ATP levels in the extracellular environment significantly increase. Extracellular ATP accumulation associated with CaMKII inhibition contributes both to calcium oscillations within astrocytes and ultimately cortical neuron toxicity. Thus, a loss of CaMKII signaling within astrocytes dysregulates glutamate uptake and supports ATP release, two processes that would compromise neuronal survival after ischemic/excitotoxic insults.
Introduction
Calcium/calmodulin (CaM)2-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine protein kinase that plays an essential role in synaptic plasticity, learning, and memory. All four CaMKII genes (α, β, δ, and γ) are expressed in the nervous system, with α and β isoforms predominating within neurons and the δ isoform predominating within astrocytes (1–6). The dodecameric structure of the CaMKII holoenzyme provides a platform for a complex intersubunit autophosphorylation reaction (7–10) that allows the kinase to uncouple from the calcium transient and extend its activity beyond Ca2+/CaM binding (10–13). Thus, CaMKII is believed to integrate temporal information inherent to the dynamic nature of calcium signaling to produce a level of “sustained” CaMKII activity that represents both past and present cellular activity. An interesting question that arises from this is: What happens to cellular activity/physiology in the absence of CaMKII signaling?
Insight into this question may be ascertained from previous studies examining the role of CaMKII after excitotoxic insults. CaMKII has been shown to rapidly inactivate and aggregate after excitotoxic insults such as ischemic stroke (14–18). The extent/pattern of this CaMKII inactivation radiating from the ischemic core into the surrounding penumbral region correlates to the extent/pattern of tissue damage induced by the ischemic insult (14). Interestingly, αCaMKII knock-out mice display greater damage after ischemic stroke than wild-type littermates (19). Furthermore, genetic as well as pharmacological inhibition of CaMKII within neurons has been associated with several pathological deficits including the disruption of calcium homeostasis, hyperexcitability, dysregulation of glutamate signaling, and neuronal death (20–22). Together, these studies show that a loss of CaMKII signaling is detrimental to neuronal function and survival.
There is an obvious knowledge gap regarding whether these detrimental effects associated with a loss of CaMKII activity are limited to excitable cells like neurons or whether a loss of CaMKII signaling compromises the function of other cell types, including astrocytes, which play a critical role in modulating neuronal physiology, as they not only buffer neurotoxic signals such as excitotoxic levels of glutamate but also release gliotransmitters (23–26). In support of the hypothesis that a loss of CaMKII in astrocytes may alter their activity, astrocyte dysfunction is observed during ischemic stroke. Not only is glutamate uptake reduced in astrocytes after ischemia, literature also suggests that the neurotoxic levels of extracellular ATP observed after insult are likely derived from astrocytes (27–32). To date, the mechanisms underlying astrocyte dysfunction have not been elucidated. We hypothesize that the astrocytic dysfunction observed after ischemia may, in part, be due to a loss of CaMKII signaling.
In the current study we used acute and chronic application of CaMKII inhibitors to investigate whether cultured astrocytes exhibited ischemia-related phenomena (altered calcium signaling, reduced glutamate uptake, and aberrant gliotransmitter release) when CaMKII was inactivated. Our data support a feed-forward model by which CaMKII inhibition in astrocytes compromises neuronal survival by decreasing glutamate uptake and dysregulating purinergic signaling. Together, our data has led to the development of a working model whereby loss of CaMKII signaling within both neurons and their supporting astrocytes sets the limit and severity of the neuronal death observed after ischemic insult.
EXPERIMENTAL PROCEDURES
Materials
Peptide inhibitors including tat-CN21 (YGRKKRRQRRKRPPKLGQIGRSKRVVIEDDR) and tat-CN21Ala (YGRKKRRQRRKAPAKAAQAAASKRVVIEDDR) as well as Fam-labeled versions of these peptides were synthesized by Biopeptide Co. Inc, San Diego, CA. KN-93 (422708) and KN-92 (422709) were purchased from EMD Millipore. Myristoylated AIP (64929) was purchased from Anaspec, Fremont, CA. MRS 2179 (0900), A 740003 (3701), and ARL 67156 (1283) were purchased from Tocris. MK-801 (M107), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (C239), nifedipine (N7634), ω-conotoxin (C9915), suramin (S2671), and apyrase (A6535) were purchased from Sigma.
Neuron and Astrocyte Cultures
Mixed co-cultures of neurons (both cortical and hippocampal) and astrocytes were derived from E18 to E19 Sprague-Dawley rat pups according to approved Institutional Animal Care and Use Committees guidelines as described previously (20). Pure astrocytes were derived from postnatal day 1–3 Sprague-Dawley rat pups following the methods established by McCarthy and de Vellis (33). In brief, after enzymatic digestion and trituration, cortical cells were resuspended in growth media (DMEM containing 2% NuSerum, 10% fetal bovine serum, penicillin (10 units/ml), streptomycin (10 μg/ml), and l-glutamine (29.2 μg/ml)) at a density of 2.5 million cells/ml and seeded on 50 μg/ml poly-d-lysine-coated 10-cm dishes. Cells were fed every 3–4 days, with half of the conditioned media being replaced with fresh growth media. When the cultures became confluent (7–8 DIV), the plates were shaken to remove oligodendrocytes and microglia. After a wash, trypsin was used to passage the astrocytes, and they were subsequently seeded on 12- or 15-mm coverslips or 30-mm dishes coated with poly-d-lysine. The cultures were then grown in neuronal growth media (Neurobasal containing 2% NuSerum, 2% NS21, penicillin (10 units/ml), streptomycin (10 μg/ml), and l-glutamine (29.2 μg/ml)) until treatment (after an additional 3–4 DIV). Pure cortical neurons were grown on 12- or 15-mm poly-d-lysine coverslips as previously described (20) for 8–10 DIV until treatment.
Imaging Intracellular Calcium and Mitochondrial Membrane Potential
Co-cultures of neurons and astrocytes (10–12 DIV) as well as cultures of pure astrocytes were loaded with Fluo-4AM or 2.6 μm Fura-2FF-AM/Rhodamine123 and subsequently imaged as described previously (20). The use of Fluo-4AM or Fura-2FF-AM is highlighted under “Results.” During imaging, the cultures were incubated in rat physiological saline (138 mm NaCl, 2.7 mm KCl, 1.8 mm CaCl2, 1.06 mm MgCl2, 12.4 mm HEPES, pH 7.4, 5.6 mm glucose; final pH adjusted to 7.3) as described (20). A Nikon Ti-E inverted fluorescent microscope was utilized to monitor fluorescence intensity once every 5–10 s. Base line was monitored for 2–5 min. To identify neurons in the co-culture experiments, a 20 mm KCl depolarization was employed at the start of the imaging. Neurons were identified as cells that immediately responded to KCl with a robust increase in calcium. In all experiments with CaMKII inhibitor application, the inhibitor was added 5 min after the start of imaging. For experiments requiring various receptor/channel antagonists, the drugs were applied at the 2-min mark to identify whether the drug itself had an impact on calcium levels before CaMKII inhibitor application. Analysis was performed using Nikon Elements 3.0 in which fluorescence intensity is measured in at least 10 cells per field. The fluorescence intensity of each cell was normalized to time 0 (or the 5 min mark) as the CaMKII inhibitor was applied.
Immunocytochemistry of Astrocyte Cultures
Glial cultures (3–4 DIV after split onto coverslips) were immunostained as previously described (20, 34). After fixation, permeabilization, and blocking, the cultures were incubated in polyclonal anti-GFAP (1:1000 Sigma), monoclonal anti-OX-42 (1:500 Abcam), polyclonal anti-pan-CaMKII (1:1000 Cell Signaling), or monoclonal anti-vimentin (1:500 Millipore) overnight at 4 °C. After three washes, secondary antibodies (anti-rabbit Alexa488 or anti-mouse Alexa594, 1:5000 (Molecular Probes) were applied for 1 h at room temperature. Coverslips were washed in PBS three times and were subsequently mounted in Prolong Gold Antifade with DAPI mounting media (Molecular Probes), and cells were imaged using a Zeiss Axio ObserverZ1 and processed with Axiovision 4.
CaMKII Activity Assay
Astrocyte cultures were lysed in lysis buffer (20 mm Tris, pH 7.4, 200 mm NaCl, 0.1 mm EDTA, and 2× protease inhibitor mixture (Millipore, 539137)) as described previously (20, 34), sonicated, and incubated with 0.1% Triton X-100 for 5 min. To measure total CaMKII activity, lysate was then incubated with 50 mm HEPES, pH 7.4, 100 mm NaCl, 10 mm MgCl2, 100 μm ATP, 2 mm CaCl2, 5 μm CaM, 50 μm AC-2 (KKALRRQETVDAL), and [γ-32P]ATP (3 μCi per reaction) for 3 min at 30 °C. To measure autonomous CaMKII activity, lysate was incubated in 50 mm HEPES, pH 7.4, 100 mm NaCl, 10 mm MgCl2, 100 μm ATP, 5 mm EGTA, 50 μm AC-2, and [γ-32P]ATP (3 μCi per reaction) for 3 min at 30 °C. The linear range of the phosphorylation reactions extended from 1 to 10 min. Protein levels were assessed using the DC protein assay kit (Bio-Rad), and activity was normalized to total protein.
Inhibitor Uptake Analysis
Fluorescently conjugated peptides (tat-CN21-Fam and tat-CN21Ala-Fam) were diluted in fresh media and applied to astrocyte cultures at a final concentration of 10 μm for varying lengths of time (0–20 min). After treatment, coverslips were washed 3 times in PBS before blotting and were then mounted in Prolong Gold Antifade with DAPI mounting media. Coverslips were then imaged in three different fields with a Zeiss Axio Observer Z1 and processed with Axiovision 4. The total cell number (DAPI staining) and the total fluorescent cell number (FITC detection) were then quantified. No fluorescence was detected in cultures that were not treated with the fluorescent peptides.
Glutamate Uptake Assays
Astrocyte cultures were pretreated with various pharmacological antagonists for 20 min at 37 °C. Rat physiological saline (above) containing 0.1 μg/ml [3H]glutamate (American Radiolabeled Chemicals) and 100 μm unlabeled glutamate was then applied to astrocytes for 20 min at 37 °C. The cultures were then washed in cold PBS, lysed in lysis buffer (above) containing 0.1%Triton X-100, vortexed, incubated for 5 min on ice, and diluted in 8 ml RadSafe (Beckman Coulter). [3H]Glutamate within the lysates was quantified using a Beckman liquid scintillation counter. For normalization, protein levels within the lysates were assessed using a DC protein assay (Bio-Rad).
ATP Measurements
ATP concentrations in the media were assessed using the Enlighten ATP Assay System per the manufacturer's protocol (Promega). The luciferase was detected and quantified using a Victor V3 plate reader. The ATP detection assay standard curve was linear from 0.001 nm to 0.1 μm.
Glutamate Measurements
Concentrations of extracellular glutamate were quantified using a glutamate oxidase assay (Invitrogen) as described previously (20).
Cell Death Measurements
Neuronal coverslips were stained using the Live/Dead Viability/Cytotoxicity kit (Molecular Probes) as previously described (20, 34). The cells were imaged using a Zeiss Axio Observer Z1 and processed with Axiovision 4 (×100 magnification). Each coverslip was imaged in three different fields. The images were exported, and automated cell counting software (Nikon Elements v3.0) was used to quantify cytotoxic cells (Texas red filter), viable cells (FITC filter), or total cell number (DAPI filter). Viability analysis indicates toxicity in 5–10% of cells under control conditions (after media exchanges and washing during control treatments).
Data Analysis
Statistical analysis was performed using SigmaPlot 11 software. One-way ANOVA with a subsequent Dunnett's test was used to compare differences between the means of each group in the in situ calcium imaging experiments, in vitro catalytic assays, and in situ cell death assays. When appropriate, an unpaired Student's t test was performed. Significance was set at a p value of <0.05.
RESULTS
CaMKII Inhibition in Astrocytes
We hypothesized that a loss of CaMKII signaling in neuronal support cells, such as astrocytes, may contribute to ischemia-induced damage. To identify if astrocyte function is altered after CaMKII inactivation, we elected to pharmacologically inactivate CaMKII within cultured cortical astrocytes rather than use genetic approaches, as this mimics the rapid loss of CaMKII activity observed in brain tissue during an ischemic insult (14, 15). Importantly, this model allows for the exploration of functional changes in astrocytes produced by CaMKII inactivation in the absence of excitotoxic levels of glutamate. CaMKII was inhibited using the specific CaMKII inhibitor, CN21, conjugated to the tat cell-penetrant motif for intracellular delivery, as described previously (20, 34, 35). The inactive control, tat-CN21Ala, was utilized to characterize specificity of the tat-CN21-induced effects (34).
Although we employed a well characterized methodology for astrocyte cultures (33) (see “Experimental Procedures”), we used immunohistochemistry to further characterize these cultures. Fluorescent immunostaining indicated that 93.9 ± 10.8% (n = 6) of these cells were GFAP-positive, and 78.9 ± 21.2% (n = 6) were vimentin-positive (Fig. 1, A–C), whereas only 2.3 ± 3.9% (n = 6) were OX42-positive, suggesting these cultures were predominantly reactive astrocytes with little microglial contamination. The absence of MAP-2 immunostaining in these astrocyte cultures suggests no neuronal contamination.
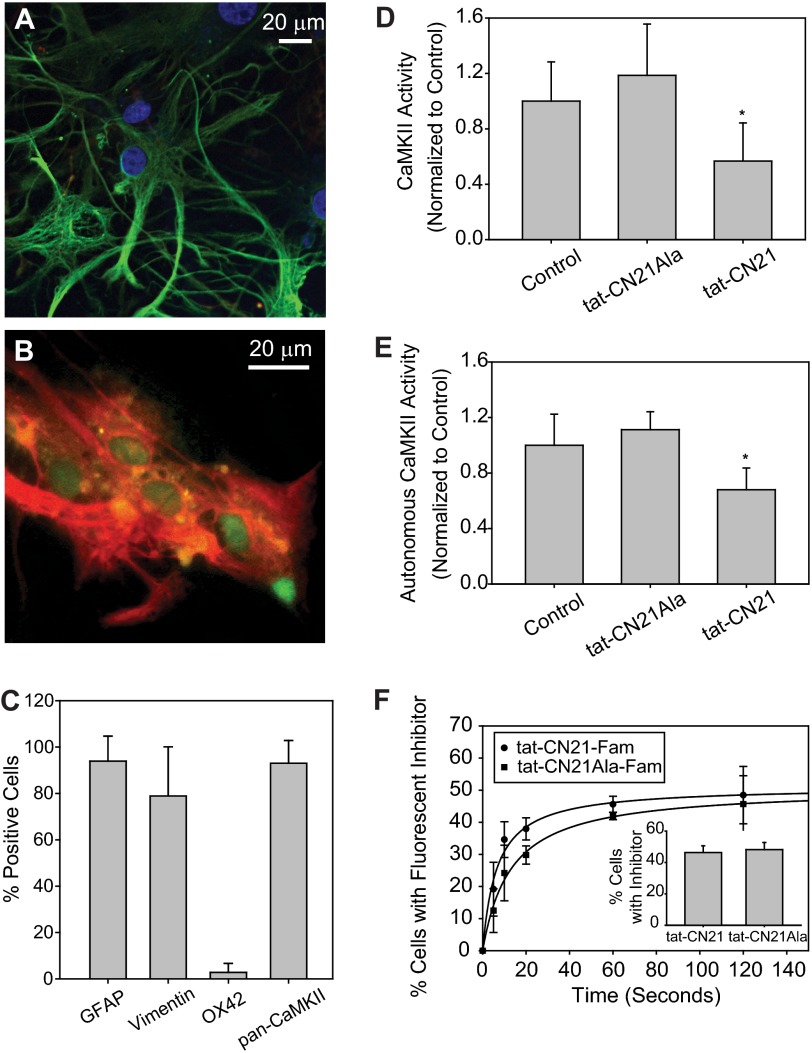
FIGURE 1.
CaMKII Expression and activity in cultured cortical astrocytes. A, shown is a representative image of a field of astrocytes immunostained with GFAP (green), OX42 (red), and Hoechst (blue). B, shown is a representative image of field of astrocytes immunostained with vimentin (red) and pan-CaMKII (green). C, shown is the average number of cells (n = 3, ±S.E.) positively stained with GFAP, vimentin, OX42, and CaMKII. D, shown is the average Ca2+/CaM-stimulated (total) CaMKII activity within astrocyte lysates treated with 10 μm tat-CN21 or tat-CN21Ala. Inhibitors were added to the cultures 10 min before lysis, and activity was measured in vitro via 32P incorporation into the CaMKII peptide substrate AC-2. The asterisk indicates significant difference compared with control (*, p < 0.05, one-way ANOVA, post-hoc Dunnett's test). E, shown is the average CaM-independent (autonomous) CaMKII activity within astrocyte lysates treated with 10 μm tat-CN21 or tat-CN21Ala as described in D. The asterisk indicates significant difference compared with control (*, p < 0.05, one-way ANOVA, post-hoc Dunnett's test). F, astrocyte uptake of fluorescent-conjugated tat-CN21 and tat-CN21Ala (10 μm) was examined using fluorescent microscopy. Total cell number was determined by Hoechst staining. Inset, shown is the average number of cells with fluorescently conjugated tat-CN21 and tat-CN21Ala 20 min after application.
We next examined CaMKII expression and activity within our astrocyte cultures. Immunocytochemical analysis indicated that 93.0 ± 9.8% of our astrocytes in culture exhibited CaMKII staining using a pan-CaMKII primary antibody (Fig. 1, B and C). The catalytic activity of CaMKII within our astrocyte cultures was assessed to determine the extent of activated (autonomous) CaMKII within our cultures. We measured the Ca2+/CaM-dependent and Ca2+/CaM-independent CaMKII activity using the highly specific CaMKII peptide, AC-2 (20, 36). The CaMKII activity measured in the presence of Ca2+/CaM is defined as the total pool of CaMKII activity, whereas the activity measured in the absence of Ca2+/CaM is defined as the pool of autonomous or Ca2+/CaM-independent activity (10, 11, 37). Thus, the fraction of autonomous CaMKII is believed to represent the pool of CaMKII activated in situ. We observed that under basal conditions, 14.0 ± 2.6% (n = 4) of the total CaMKII activity within our cultured astrocytes was autonomous.
To confirm that the high affinity CaMKII inhibitor, tat-CN21 (34, 38), in fact reduced CaMKII activity in the astrocyte cultures, we measured Ca2+/CaM-stimulated and autonomous CaMKII activity in the astrocytes after 10 min of exposure to the active and control tat-CN21 inhibitor. This relatively short exposure to tat-CN21 significantly reduced autonomous CaMKII activity (40.8 ± 19.9 and 38.7 ± 13.5% decrease in total and autonomous CaMKII activity) in tat-CN21-treated cultures compared with cultures treated with DMSO or control tat-CN21Ala respectively (Fig. 1, D and E).
In an effort to correlate the reduction in CaMKII activity with the uptake of the CaMKII inhibitors, we applied the carboxyfluorescein-tagged tat-CN21 to astrocytes and measured cellular uptake using fluorescent microscopy as described previously for cortical neurons (20). Remarkably, fluorescent peptide uptake was observed within seconds, with tat-CN21-Fam rapidly taken up by 46.3 ± 7.4% of astrocytes (tat-CN21Ala taken up in 44.8 ± 3.1% of cells) (Fig. 1F). The uptake of the peptide inhibitors in cultured astrocytes is considerably faster than we previously observed in cortical neurons (34). Although we cannot rule out that subpopulations of the cells may differentially respond to the inhibitor, it is interesting that the percentage of astrocytes that take up the peptide inhibitor (45%) is similar to the extent of CaMKII inhibition (40%).
Compromised Glutamate Uptake with CaMKII Inhibition
The extent of neuronal damage after ischemic insult has been shown to be influenced by the increasing accumulation of extracellular glutamate within the infarcted tissue (for review, see Ref. 39). We previously identified that CaMKII inactivation within neurons enhances excitability via glutamate accumulation in the media (20). Moreover, a loss of CaMKII activity induced neurotoxicity via enhanced sensitivity to extracellular glutamate. Because astrocytes are known to greatly affect the tolerance of cytotoxic levels of glutamate in co-cultures (23), it was surprising that neurons were not spared in co-cultures with astrocytes (20). One potential explanation for these findings is that CaMKII inhibition in the co-cultures compromised astrocyte uptake of extracellular glutamate. To test this idea, [3H]glutamate was applied to astrocytes for 20 min in the presence and absence of CaMKII inhibitors, and [3H]glutamate uptake was quantified using liquid scintillation of cell lysates as described previously (40). Consistent with previous work showing that cultured cortical astrocytes express the GLT-1 and GLAST (EAAT-1 and EAAT-2, respectively) glutamate transporters (41–43), DL-threo-β-Benzyloxyaspartic acid (TBOA) (44) pretreatment within our cultures resulted in a significant decrease in glutamate uptake (Fig. 2). Thus, consistent with earlier studies, nearly ∼70% of glutamate uptake in cultured cortical astrocytes is active uptake via glutamate transporters (45, 46).
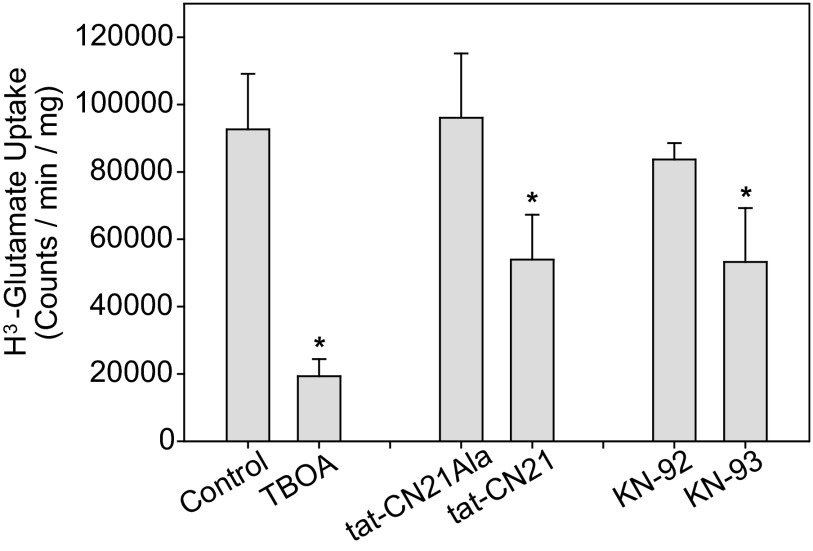
FIGURE 2.
Decreased glutamate uptake in astrocytes with CaMKII inhibition. Average [3H]glutamate uptake (n = 3, ±S.D.) in astrocytes pretreated with either 0.2% DMSO, 100 μm DL-threo-β-Benzyloxyaspartic acid (TBOA), 10 μm tat-CN21, 10 μm tat-CN21Ala, 1 μm KN-93, or 1 μm KN-92 for 20 min is shown. The asterisk indicates significant difference compared with DMSO control (*, p < 0.05, one-way ANOVA, post-hoc Dunnett's test).
Pretreatment with CaMKII inhibitors, both the peptide inhibitor tat-CN21 and the small molecule inhibitor KN-93 resulted in a significant reduction in [3H]glutamate uptake (Fig. 2). However, no change in uptake was observed when astrocytes were pretreated with the respective inactive controls, tat-CN21Ala or KN-92 (Fig. 2). Similarly, both a tat-tagged and a myristoylated form of a peptide inhibitor derived from the autoregulatory domain of CaMKII, termed AIP, reduced [3H]glutamate uptake, with a 43.8 ± 1.9% (n = 3) reduction associated with tat-AIP and a 43.5 ± 24.8% (n = 3) reduction associated with myr-AIP. These data suggest that small molecule and peptide inhibitors of CaMKII conjugated to various cell-penetrant motifs were able to diminish glutamate uptake within astrocytes. This ∼40% reduction in glutamate uptake is similar to uptake of the inhibitor in ∼45% of cells (Fig. 1F). Together, these data highlight that a loss of CaMKII signaling compromises glutamate uptake, a finding that could contribute to glutamate accumulation in penumbral regions where decreased CaMKII activity is observed after ischemia (14).
Calcium Oscillations Induced by CaMKII Inhibition
Application of tat-CN21 rapidly induced calcium oscillations in cortical astrocytes. Calcium transients in a single imaged astrocyte are shown in Fig. 3A, whereas time traces for multiple astrocytes are shown in Fig. 3B. The intracellular calcium increase observed in pure astrocyte cultures peaks (∼30 s after application) and is slowly reduced over a period of several minutes. The area under the curve was quantified to determine total changes in calcium concentration throughout the experiment. Only the active CaMKII inhibitor tat-CN21 induced oscillations in intracellular calcium levels within the astrocytes (Fig. 3, C and D), as no change was observed with inactive tat-CN21Ala. The small molecule, membrane-permeable inhibitor of CaMKII, KN-93, also produced a rapid increase in intracellular calcium concentrations (Fig. 3D), whereas KN-92 (inactive control) had no effect. Thus, both peptide and small molecule inhibitors of CaMKII generate oscillations in astrocytic calcium levels.
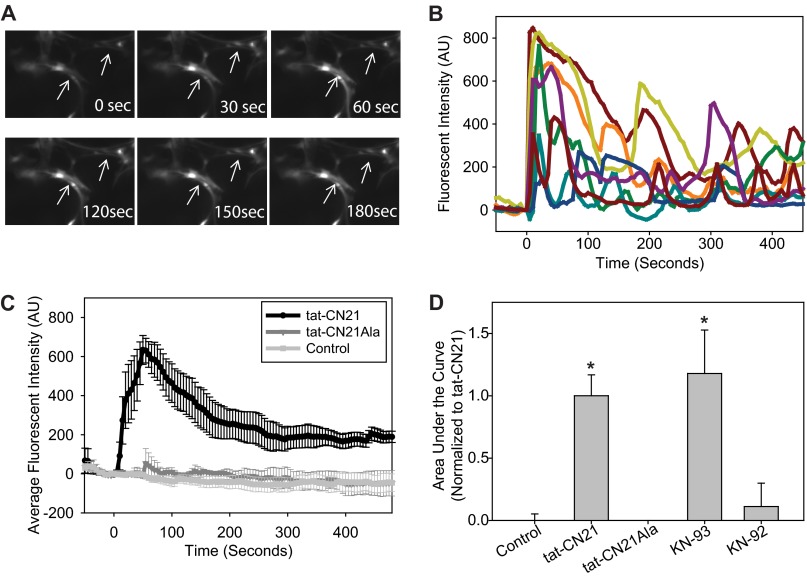
FIGURE 3.
CaMKII inhibition induces calcium oscillations in astrocytes. A, shown are astrocytes loaded with Fluo-4AM exhibit calcium transients after exposure to the CaMKII inhibitor tat-CN21 (10 μm). B, shown are representative Fluo-4AM traces of a field of astrocytes in response to tat-CN21 application at time 0. AU, arbitrary units. C, shown is the average trace (±S.E., n = 3) of calcium response in astrocytes treated with DMSO control, tat-CN21, or tat-CN21Ala (10 μm). D, shown is the average area under the curve from time 0 to 500 s (± S.E., n = 3–5) after treatment with tat-CN21 (10 μm), KN-93 (1 μm), or inactive controls-tat-CN21-Ala (10 μm) and KN-92 (1 μm), as indicated.
Although not all cells exhibited these oscillations in intracellular calcium with tat-CN21 application (Fig. 3B), the 40% of cells that displayed these oscillations is consistent with the percentage of cells (∼45%) with fluorescent inhibitor (Fam-tat-CN21) uptake (Fig. 1F). In addition, the rapid onset of calcium oscillations induced by CaMKII inhibition is consistent with the rapid rate of peptide inhibitor uptake we observed using fluorescently labeled peptides (Fig. 1F).
It is worth noting that the calcium oscillations induced by CaMKII inhibition within our astrocyte cultures appeared to be much faster than the calcium dysregulation we previously reported in cortical neurons (20). To verify that these kinetic differences in the disruption of calcium homeostasis observed in enriched cultures of neurons (20) and astrocytes is indeed temporally distinct and not simply due to different culture/experimental conditions, we repeated the intracellular calcium measurements after CaMKII inhibition in co-cultures of neurons and astrocytes. These neuronal-astrocyte co-cultures are ∼60% GFAP-positive astrocytes and ∼40% MAP2-positive neurons (20). Interestingly, application of tat-CN21 within the co-cultures resulted in a complex pattern of increased intracellular calcium levels that differed from cultures of highly pure astrocytes or neurons (Fig. 4A versus Fig. 3C and Ref. 20). As before, the inactive control tat-CN21Ala did not alter intracellular calcium homeostasis (Fig. 4A).
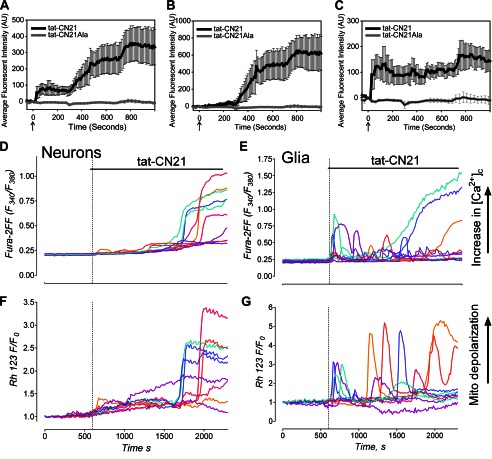
FIGURE 4.
Alterations in intracellular calcium and mitochondrial membrane potential in neuronal/astrocyte co-cultures after CaMKII inhibition. A, shown is the average trace (±S.E., n = 3) of calcium response in mixed cultures of cortical neurons and astrocytes treated with 10 μm tat-CN21 or tat-CN21Ala as measured by Fluo-4AM. AU, arbitrary units. B, shown is an average calcium response (± S.E., n = 3) after application of 10 μm tat-CN21 or tat-CN21Ala in cells responding to a depolarizing 20 mm KCl pulse at time −300 s (considered neurons). C, shown is an average trace (± S.E., n = 3) of calcium response with 10 μm tat-CN21 or tat-CN21Ala application in cells that did not respond to the KCl pulse at time −300 s (considered astrocytes). D, shown are representative traces of Fura-2FF calcium response in hippocampal neurons (identified by KCl pulse at time 0) after 10 μm tat-CN21 application. E, shown are representative traces of Fura-2FF calcium response in astrocytes (which did not respond to KCl at time 0) after tat-CN21 application. F and G, shown are representative traces of Rhodamine123 mitochondrial membrane potential responses in the same hippocampal neurons (F) and astrocytes (G) as in D and E.
To tease apart the contribution of the neurons/astrocytes toward the changes in Fluo-4AM fluorescence within the mixed culture system, a KCl depolarizing stimulation was used to readily identify the neurons. Because nearly all of the cells that were not MAP-2-positive in these co-cultures were GFAP-positive (20), the cells that did not immediately respond to KCl were considered astrocytes. Using this method to deconvolute the calcium responses of neurons versus glial cells, we found that neurons undergo a characteristic delayed calcium dysregulation in the presence of astrocytes (Fig. 4B); this is kinetically similar to our previous findings in highly enriched cortical cultures (20). Similar to our findings within enriched astrocyte cultures (Fig. 3C), astrocytes within the co-culture exhibit a rapid increase in intracellular calcium concentration (Fig. 4, B and C). There was a delayed burst in intracellular calcium observed (∼800 s) in both the astrocytes and neurons of the co-culture (Fig. 4, A–C), which may have been generated by alterations in neuron-glia communication (i.e. released glutamate from neurons or released gliotransmitters from astrocytes).
Alterations in calcium homeostasis within both neurons and astrocytes was not limited to mixed cortical astrocyte/neuron cultures, as tat-CN21 also increased calcium concentration within hippocampal co-cultures loaded with Fura-2FF (Fig. 4, D and E). Examination of individual cellular calcium responses within these cultures again indicates that whereas neurons undergo delayed calcium dysregulation (Fig. 4D), intracellular calcium within astrocytes appears to oscillate when CaMKII is inhibited (Fig. 4E).
In addition to monitoring intracellular calcium, the mitochondrial membrane potential of the hippocampal neurons and astrocytes was measured before and after CaMKII inhibition. Interestingly, the mitochondria of neurons appeared to terminally depolarize at the time of the calcium dysregulation (Fig. 4F). This concept is consistent with our previous findings that CaMKII inhibition in neurons produces neurotoxicity (20). In contrast, the mitochondrial membrane potential of astrocytes appeared to track the calcium oscillations with very few cells displaying terminal membrane depolarization (Fig. 4G). This is consistent with the fact that we see very little astrocyte toxicity (10.71 ± 5.68%, n = 4) under these conditions. Thus, unlike neurons that undergo delayed calcium dysregulation and terminal mitochondrial depolarization associated with neuronal death after CaMKII inhibition, astrocytes display oscillations in calcium and mitochondrial membrane depolarization with minimal toxicity.
Mechanisms Underlying Calcium Influx in Astrocytes after CaMKII Inhibition
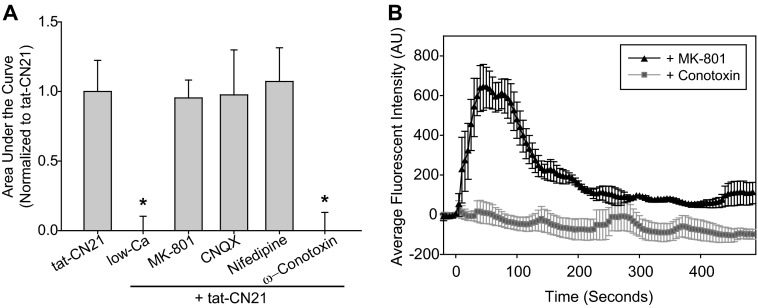
To determine if extracellular calcium contributed to the rapid calcium oscillations observed within the astrocytes after CaMKII inhibition, we reduced extracellular calcium and again monitored intracellular calcium using Fluo-4AM before and after CaMKII inhibition. Removal of extracellular calcium prevented tat-CN21-induced calcium increases within the astrocytes, indicating that extracellular calcium is indeed important for the rapid calcium increases associated with CaMKII inhibition (Fig. 5A). To further dissect mechanisms contributing to calcium entry after CaMKII inhibition, we pharmacologically probed the role of multiple CaMKII substrates within the plasma membrane that are known modulators of calcium signaling. Although the functional role of many of the voltage- and ligand-gated channels in the astrocyte plasma membrane is not fully appreciated, astrocytes express several of these proteins, including L-type (CaV1.2) and N-type (CaV2.2) calcium channels as well as the NMDA and AMPA receptors (47–50). Pretreatment with 20 μm MK-801, the NMDA-receptor antagonist, 10 μm CNQX, the AMPA-receptor antagonist, or 10 μm nifedipine, the L-type calcium channel blocker, did not reduce the calcium influx associated with the CaMKII inhibition (Fig. 5A). Surprisingly, the N-type calcium channel blocker, ω-conotoxin (1 μm), completely prevented the oscillations in intracellular calcium produced by CaMKII inhibition (Fig. 5, A and B). How a loss of CaMKII signaling contributes to aberrant N-type calcium channel activity is unknown, as little is known regarding mechanisms underlying CaMKII regulation of N-type calcium channels (51). However, it is intriguing that N-type calcium channels have been implicated in gliotransmitter release (ATP and glutamate) due to the dependence of this process on calcium/SNARE-dependent exocytosis (52–56).
FIGURE 5.
Mechanisms underlying astrocytic calcium influx induced by CaMKII inhibition. A, shown is the average area under the curve (±S.E., n = 3–5) after treatment with 10 μm tat-CN21 in combination with various pharmacological inhibitors as indicated. Pharmacological inhibitors were added at −120 s; none of the inhibitors altered the base line. The asterisk indicates significant difference compared with control, whereas the pound sign indicates a significant difference compared with tat-CN21 (*, p < 0.05, One-way ANOVA, post-hoc Dunnett's test). B, shown are representative traces of a field of astrocytes in response to pharmacological inhibitor application at −120 s and tat-CN21 application at time 0. AU, arbitrary units.
CaMKII Inhibition Induces ATP Release
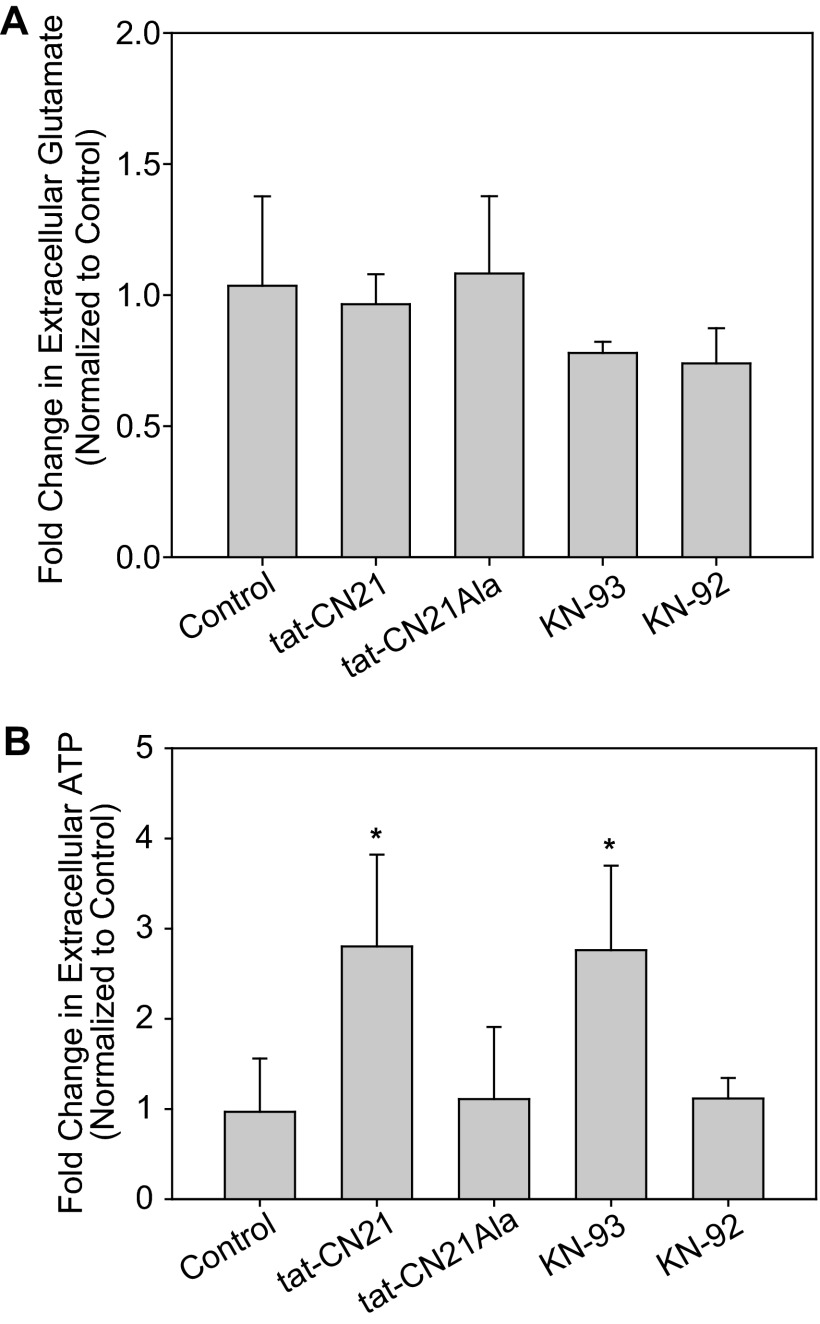
Because gliotransmitters are powerful modulators of calcium signaling in neurons and induce calcium oscillations in astrocytes (24, 57–62), we explored the hypothesis that CaMKII inhibition leads to aberrant gliotransmitter release in astrocytes. Because a loss of CaMKII in neurons has been shown to lead to aberrant glutamate release (20), we first elected to measure extracellular glutamate within the astrocyte cultures after application of the CaMKII inhibitors. Surprisingly, neither the peptide inhibitor, tat-CN21 (10 μm), nor the small molecule inhibitor, KN-93 (1 μm), altered the levels of extracellular glutamate within the astrocyte cultures even after 24 h of treatment (Fig. 6A).
FIGURE 6.
CaMKII inhibition induces ATP release from astrocytes. A, shown is the average change in extracellular glutamate concentration (n = 6, ±S.D.) in astrocyte cultures after 24 h application of various CaMKII inhibitors and controls (p > 0.05, one-way ANOVA). B, shown is the average change in extracellular ATP concentration (n = 6, ±S.D.) in astrocyte cultures after 24 h application of various CaMKII inhibitors and control. The asterisk indicates significant difference compared with control (*, p < 0.05, one-way ANOVA, post-hoc Dunnett's test).
Next, we measured changes in ATP accumulation in the media as ATP has been shown previously to be one of the most abundant gliotransmitters within cortical astrocytes (24, 26, 63) where its release modulates a number of different purinergic receptors in both neurons and astrocytes (64, 65). In contrast to extracellular glutamate, we observed that both tat-CN21 (10 μm) and KN-93 (1 μm) significantly increased extracellular ATP concentration compared with their matched controls (i.e. tat-CN21Ala and KN-92) (Fig. 6B). Furthermore, myr-AIP (10 μm) also significantly increased extracellular ATP with a 1.88 ± 0.50-fold (n = 3) increase over control. Thus, once again, multiple pharmacological inhibitors of CaMKII all lead to a similar phenotype in astrocytes; that is, accumulation of extracellular ATP.
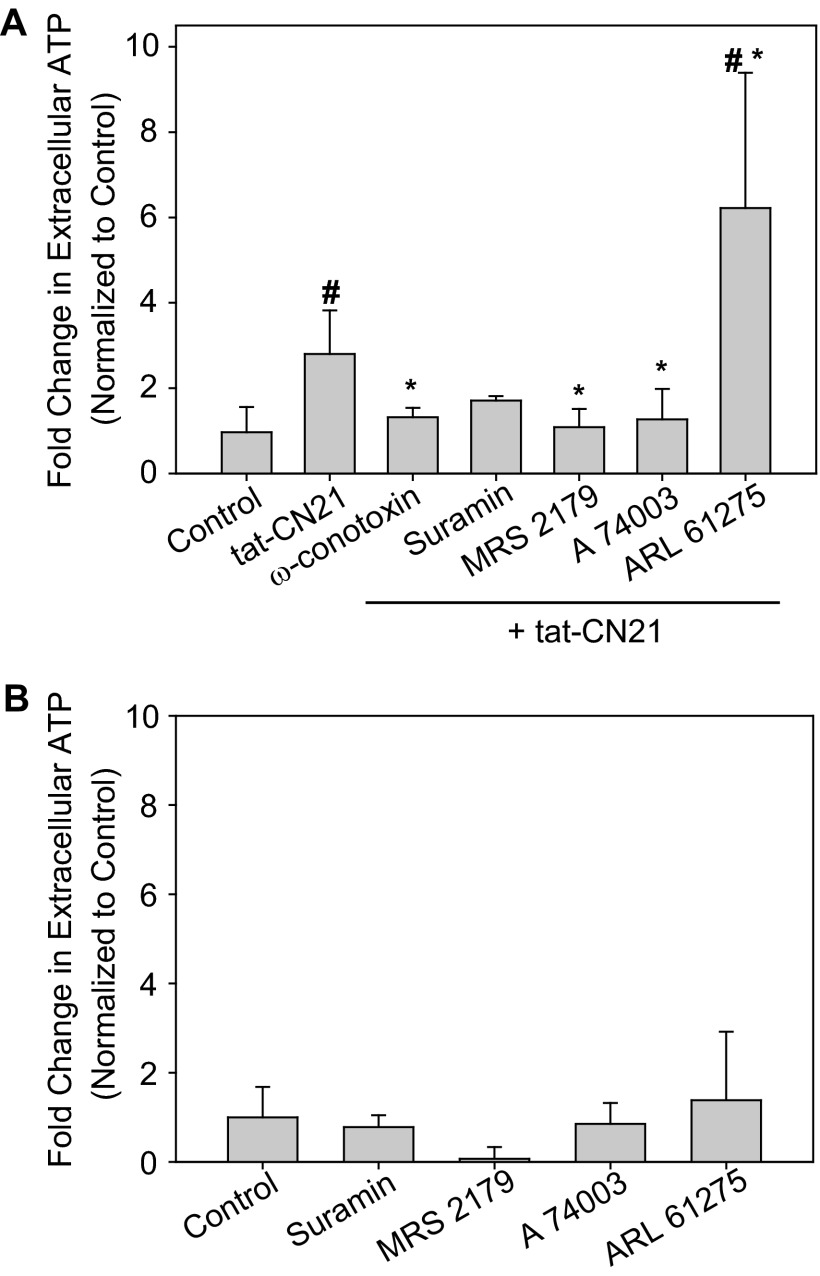
Because N-type calcium channel blockade inhibited increases in intracellular calcium, we pretreated astrocytes with 1 μm ω-conotoxin and again measured extracellular ATP content after CaMKII inhibition. Application of ω-conotoxin prevented the accumulation of extracellular ATP when CaMKII was inhibited (Fig. 7A). These data support a model whereby a loss of CaMKII signaling leads to N-type voltage-gated calcium channel activation (Fig. 5, E and F), calcium influx, and ATP release (Fig. 7A). As a control, 10 μm nifedipine, the L-type calcium channel antagonist, which had no effect on the calcium homeostasis when CaMKII was inhibited, was unable to reduce ATP accumulation (2.02 ± 0.32-fold increase over control n = 3).
FIGURE 7.
Purinergic signaling modulates ATP release in response to CaMKII inhibition. A, shown is the average change in extracellular ATP concentration when tat-CN21 was applied alone or in combination with various pharmacological modulators of purinergic signaling and the N-type calcium channel blocker, ω-conotoxin. The asterisk indicates significant difference compared with tat-CN21, whereas the pound sign (#) indicates significant difference compared with control (* and #: p < 0.05, one-way ANOVA, post-hoc Dunnett's test). B, shown is the average fold change in extracellular ATP levels (n = 3–4, ±S.D.) when astrocytes were treated with various pharmacological modulators of purinergic signaling in the absence of CaMKII inhibition compared with control (p > 0.05, one-way ANOVA).
Purinergic signaling within astrocytes has been shown to induce a feed-forward release of ATP (66). Thus, we measured extracellular ATP levels when tat-CN21 was applied to astrocytes in combination with various purinergic receptor antagonists. Suramin, the non-selective purinergic receptor antagonist, decreased extracellular ATP levels compared with cultures treated with tat-CN21 alone (Fig. 7A); however, suramin was unable to reduce extracellular ATP levels back to base line (Fig. 7A). It is difficult to interpret whether the inability to completely abrogate ATP release is a drug potency/specificity issue or whether the initial phase of CaMKII-induced ATP release is produced primarily by N-type calcium channel-activity, as ω-conotoxin ablated both the alterations in calcium homeostasis and ATP accumulation associated with CaMKII inhibition (Figs. 5 and 7A).
Although astrocytes express several purinergic receptors, CaMKII signaling has been previously linked to P2Y1 and P2X7 receptors (67). Thus, we next examined whether pharmacological antagonists of these subtypes of purinergic receptors reduced the aberrant increase in extracellular ATP concentration induced by CaMKII inhibition. Interestingly, co-application of tat-CN21 with either MRS 2179, the P2Y1 antagonist, or A 740003, the P2X7 antagonist, led to a significant reduction in extracellular ATP concentrations compared with tat-CN21 alone (Fig. 7A). Importantly, none of the purinergic receptor antagonists had an effect on basal extracellular ATP concentration (i.e. without tat-CN21) (Fig. 7B). Because ATP can be broken down within the extracellular environment by ectoATPases, we next measured the extracellular ATP concentration when the ectoATPase inhibitor ARL 6127 was applied in combination with tat-CN21. This resulted in a robust increase in extracellular ATP levels (Fig. 7A), suggesting that although CaMKII inhibition led to the accumulation of extracellular ATP, this is an underestimate due to ATP degradation by ectoATPases.
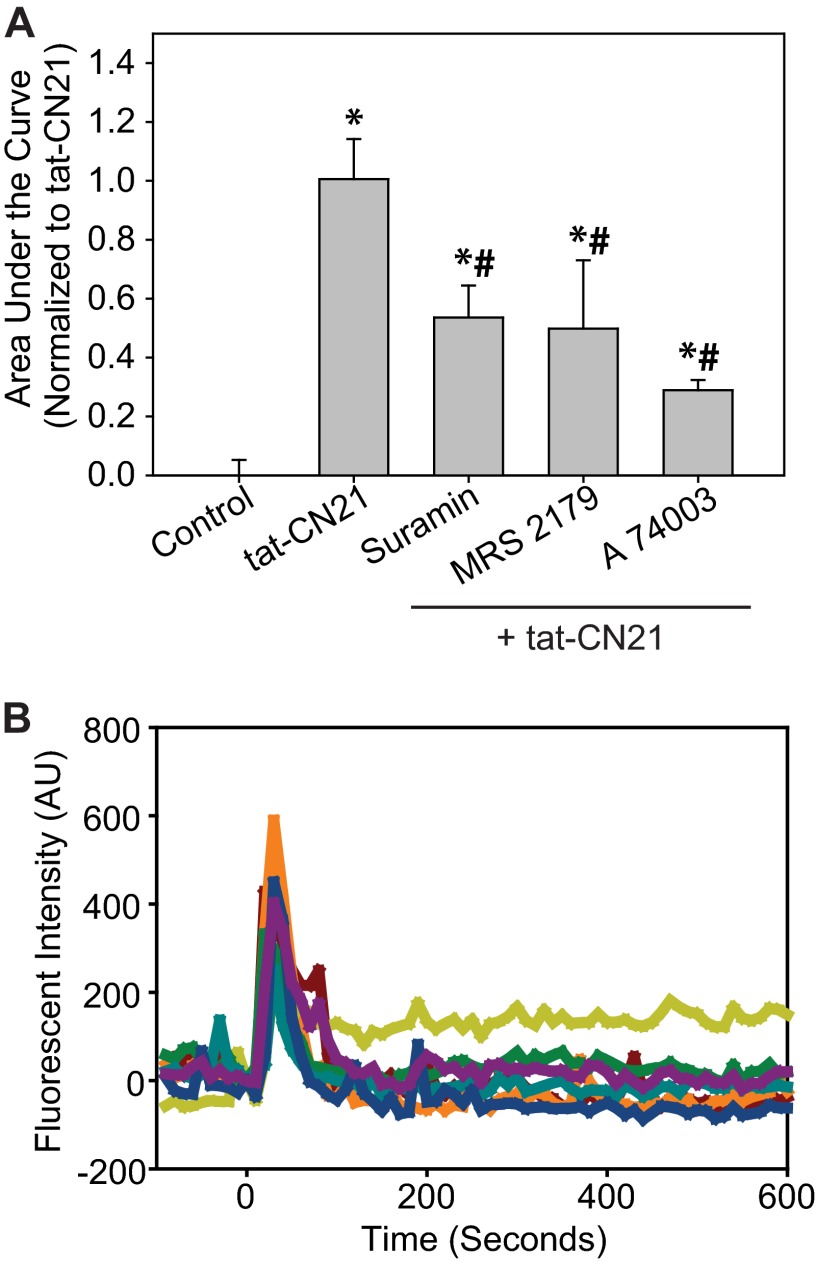
To further connect the immediate increase in intracellular calcium concentration observed with CaMKII inhibition (Fig. 5) to the long term accumulation of extracellular ATP (Figs. 6 and 7), intracellular calcium levels were monitored when purinergic receptors were antagonized before tat-CN21 application. Interestingly, suramin, MRS 2179, and A 740003 all significantly reduced the total calcium influx induced by CaMKII inhibition (Fig. 8). The inability of these inhibitors to reduce calcium influx back to base line is not surprising, as the N-type calcium channels appear to be responsible for the initial phase of increased calcium concentration and release of ATP (Figs. 5F and 7A). Interestingly, when multiple calcium wave forms for the individual astrocytes were plotted rather than simply integrating the area under the curve as shown in Fig. 8A, it became quite apparent that the oscillations of calcium observed with tat-CN21 (Fig. 5, A and B) are absent in the presence of purinergic antagonists (Fig. 8B). This is consistent with previous studies indicating that purinergic signaling underlies intracellular calcium waves within astrocytes (68). The potential for aberrant ATP release induced by a loss of CaMKII signaling is particularly intriguing, as antagonism of purinergic receptors has been shown to decrease infarct size after stroke, suggesting that ATP release may potentiate glutamate-induced excitotoxicity (69, 70). Together, these data support the model that CaMKII inhibition in astrocytes leads to aberrant N-type calcium channel activity, which in turn leads to ATP release and a feed-forward loop of subsequent calcium oscillations supported by further purinergic signaling.
FIGURE 8.
ATP signaling is required for calcium oscillations in astrocytes induced by CaMKII inhibition. A, shown is the average area under the curve (± S.E., n = 3–5) for Fluo-4AM-loaded cells (calcium influx) after treatment with 10 μm tat-CN21 and/or co-treatment with various other pharmacological inhibitors, as indicated. Pharmacological inhibitors were added at −120 s; none of the inhibitors altered base line. The asterisk indicates significant difference compared with control, whereas the pound sign (#) indicates a significant difference compared with tat-CN21 (one-way ANOVA, post-hoc Dunnett's test, * and #, p < 0.05). B, shown are representative traces of astrocytic calcium response after tat-CN21 application at time 0 with suramin pretreatment at time −120 s. AU, arbitrary units.
CaMKII Inhibition in Astrocytes Is Detrimental for Neuronal Viability
Astrocytes play a critical role in maintaining neuronal function and viability; therefore, alterations in their calcium signaling as well as gliotransmitter uptake and release could have dire consequences on neuronal survival. Previous studies have identified that extracellular ATP contributes to the expansion of neuronal death after ischemia (69, 71, 72), supporting our hypothesis that the increased extracellular ATP resulting from CaMKII inhibition in astrocytes may induce neurotoxicity. Thus, we examined cellular viability when naïve neurons were treated with astrocyte-conditioned media after astrocyte exposure to CaMKII inhibitors. Compared with control media and conditioned media from astrocytes treated with inactive tat-CN21Ala, conditioned media from astrocytes treated with tat-CN21 significantly increased levels of neurotoxicity (Fig. 9, A and B).
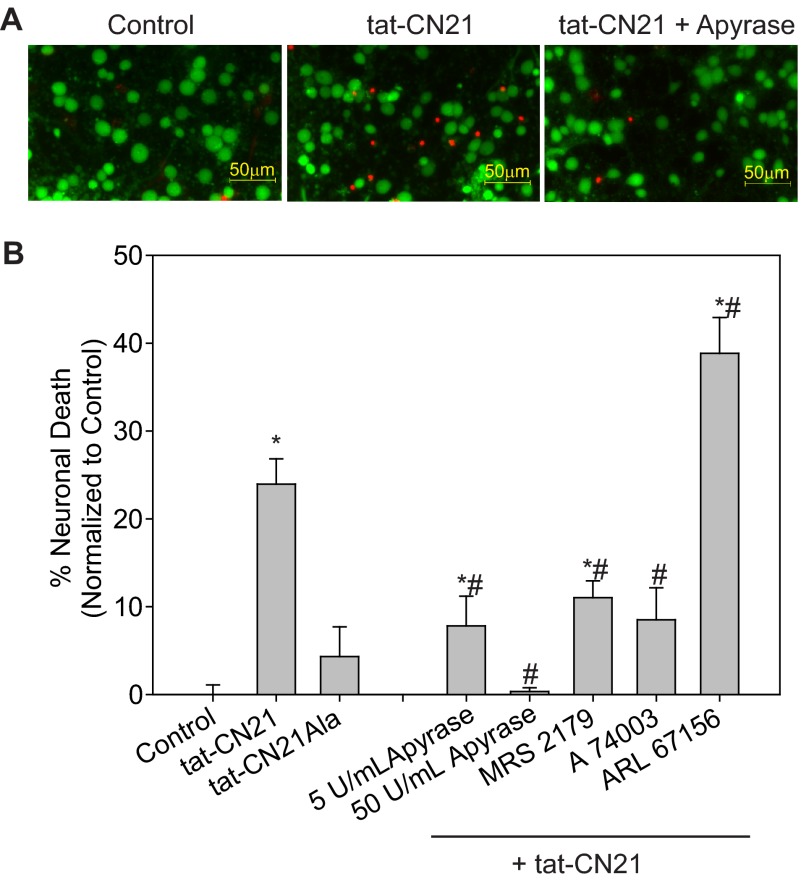
FIGURE 9.
Neuronal death induced by extracellular ATP released by astrocytes after CaMKII inhibition. Average neuronal death (n = 5–8, ±S.E.) in neurons treated (24 h) with conditioned media from astrocytes subjected to 10 μm tat-CN21Ala or tat-CN21 alone or in combination with MRS 2179 (1 μm), A 740003 (1 μm), or ARL 67156 (10 μm) for 24 h is shown. The asterisk indicates significant difference compared with control, whereas the pound sign indicates significant difference compared with tat-CN21 alone (* and #, p < 0.05, one-way ANOVA, post-hoc Dunnett's test).
Reducing the extracellular ATP levels with antagonists of the P2Y1 or P2X7 receptors (MRS 2179 and A 740003, which significantly reduced extracellular ATP concentrations compared with tat-CN21 alone in Fig. 7), led to a significant reduction in neuronal death (Fig. 9). Furthermore, in Fig. 7A, we show that ectoATPase (ARL 6127) inhibition significantly increased extracellular ATP concentrations compared with tat-CN21 alone. In parallel, we see that this medium with the highest ATP concentration results in the highest level of neuronal death (Fig. 9).
To show that the increased extracellular ATP associated with CaMKII inhibition is indeed the factor released by astrocytes leading to neuronal death, we applied apyrase to degrade ATP in the astrocytes-conditioned media before testing neuronal toxicity. When increasing amounts of apyrase were added to the conditioned media of astrocytes subjected to CaMKII inhibitors, the levels of neurotoxicity significantly decreased (Fig. 9). Together, these data indicate that CaMKII inhibition in astrocytes leads to N-type channel activity, alterations in calcium homeostasis, and ultimately ATP release; a process that exacerbates calcium oscillations in astrocytes and induces neuronal death.
DISCUSSION
Astrocytes play critical roles in regulating neuronal function. In addition to regulating the metabolic coupling of neurons and surrounding blood vessels, astrocytes can also modulate neuronal activity and plasticity by regulating ion homeostasis, transmitter release, and uptake (for review, see Refs. (23 and 73–76). After an ischemic stroke, astrocytes are activated and accumulate within the ischemic core and penumbral regions (77–80). This infiltration and activation has been shown to have both beneficial and harmful effects. Neuroprotection can be seen when astrocytes are properly functioning and are able to take up glutamate, buffer K+, and scavenge reactive oxygen species (for review, see Refs. 74 and 81). However, astrocytes within this region can also contribute to neuronal death when these neuroprotective effects are reversed and glutamate, reactive oxygen species, and ATP are released from the astrocytes (27, 28, 31). Thus, the dysregulation of astrocyte function can have a profound impact on neuronal survival after excitotoxic insult.
Previous studies have indicated that the loss of CaMKII activity after ischemic stroke correlates with the extent of damage (14). Whether CaMKII is inactivated preferentially in neurons or astrocytes in vivo is not yet known. Our current in vitro data indicate that a loss of CaMKII signaling in astrocytes could have dire consequences on neuronal survival, especially if specific subpopulations of neurons also experience deregulated calcium-glutamate signaling due to decreased CaMKII activity. One major finding of our work is that CaMKII activity is essential for normal glutamate buffering by astrocytes. Thus, the expanding loss of CaMKII activity within the penumbral tissue leads to both increased glutamate release from neurons and decreased glutamate uptake in astrocytes. This in combination with the classical model of glutamate invasion into the penumbra from the damaged cells in the ischemic core may underlie the expansion of neuronal death following insult.
Inhibition of CaMKII in astrocytes altered astrocytic calcium homeostasis and induced oscillations in both intracellular calcium concentration and mitochondrial membrane potential. Calcium oscillations and wave propagation from one astrocyte to a nearby astrocyte are dependent on purinergic receptor activation (63, 68, 82). Consistent with this, we saw that blockade of purinergic receptors resulted in a decrease in the calcium influx and ablation of the calcium oscillations induced by CaMKII inhibition. Furthermore, an increase in extracellular ATP concentration was observed. Interestingly, there was a direct association with the aberrant calcium influx and the accumulation of extracellular ATP; antagonists that reduced calcium influx also reduced extracellular ATP. These findings are consistent with the cyclic relationship between intracellular calcium and extracellular ATP observed in astrocytes. Increases in cytosolic calcium underlie vesicular ATP release (26, 83), which then activates purinergic receptors to induce further influx of calcium (24, 57–59). Our data highlight how CaMKII inhibition can initiate the cycle of increased ATP release presumably by directly or indirectly leading to N-type calcium channel activation, a process leading to a vicious cycle between calcium influx and increased purinergic signaling. N-type calcium channel dysregulation also contributed to neuronal death when CaMKII was inhibited in cultured cortical neurons (20). Together, these studies indicate that CaMKII may be a key regulator of N-type calcium channel function; however, further work is necessary to delineate the mechanisms underlying this regulation.
Increased purinergic signaling has long-been implicated in neurodegeneration (for review, see Ref. 84). Up-regulation of purinergic receptors and increased extracellular ATP have been associated with ischemia (85–87), traumatic brain and spinal cord injury (88, 89), epilepsy, Parkinson disease, and Alzheimer disease (for review, see Ref. 84). Antagonism of purinergic receptors has been shown to decrease infarct size after stroke (69–72) and improve functional recovery after stroke and spinal cord injury (70, 71, 88). Although a decrease in overall ATP availability is seen within the ischemic core and penumbral regions, there is a significant increase in extracellular ATP concentration, suggesting that either ATP release is increased or ATP breakdown mechanisms are impaired after ischemia (90). Our data provide a potential mechanism underlying the accumulation of ATP after ischemia; the inactivation of CaMKII induces astrocytic release of ATP.
The purinergic signaling initiated by CaMKII inhibition negatively impacts both astrocytes and neurons. Within astrocytes, it sustains the calcium oscillations initiated by N-type calcium channels. The increased ATP in the astrocyte-conditioned media instigates neurotoxicity when applied to cultured cortical neurons. Although neurons and astrocytes express a variety of purinergic receptors, we chose to focus on two receptors, P2Y1 and P2X7, both of which have been linked to CaMKII signaling and are known to play a role in maintaining astrocyte function and regulating neuronal viability (67). P2X7 receptor activation has been shown to produce gliotransmitter release (82, 91, 92) and is often associated with cellular death signaling (93, 94). P2Y1 signaling has also been implicated in gliotransmitter release (95), apoptosis (96, 97), and calcium dysregulation (98). Thus, it was not surprising when the highly selective antagonists of both P2Y1 and P2X7 were effective at reducing the oscillations observed in astrocyte cultures.
We show for the first time that CaMKII inhibition in astrocytes compromises neuronal survival by increasing astrocytic ATP release. The decrease in glutamate uptake displayed by astrocytes that have lost CaMKII signaling may further contribute to neurotoxicity, as we recently have shown that a loss of CaMKII signaling in neurons exacerbates glutamate and ROS excitotoxicity in cortical neurons (20). Thus, our working model is that the loss of CaMKII activity within the core and penumbral regions of an ischemic stroke is detrimental for neuronal survival by altering neuronal-neuronal and glial-neuronal communications. Based on this, it is possible that CaMKII inactivation delimits the expansion of neuronal death from the ischemic core into the penumbra. Considering this, identification of avenues that restore CaMKII activity within these tissues may be imperative for affording neuroprotection and stopping the expansion of cellular death away from the core after the insult.
This work was supported, in whole or in part, by National Institutes of Health Grants NS078171 (to A. H.) and NS078008 (N. B.). This work was also supported by the Indiana State Department of Health, Spinal Cord Brain Injury (ISDH/A70-2-079607) research grants (A. H.).
- CaM
- calmodulin
- CaMKII
- CaM-dependent protein kinase II
- Fam
- 6-carboxyfluorescein
- DIV
- days in vitro
- ANOVA
- analysis of variance
- GFAP
- glial fibrillary acidic protein.
REFERENCES
- 1. Sakagami H., Watanabe M., Kondo H. (1992) Gene expression of Ca2+/calmodulin-dependent protein kinase of the cerebellar granule cell type or type IV in the mature and developing rat brain. Brain. Res. Mol. Brain. Res. 16, 20–28 [DOI] [PubMed] [Google Scholar]
- 2. Bayer K. U., Löhler J., Schulman H., Harbers K. (1999) Developmental expression of the CaM kinase II isoforms. Ubiquitous γ- and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain. Res. Mol. Brain. Res. 70, 147–154 [DOI] [PubMed] [Google Scholar]
- 3. Ouimet C. C., McGuinness T. L., Greengard P. (1984) Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc. Natl. Acad. Sci. U.S.A. 81, 5604–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erondu N. E., Kennedy M. B. (1985) Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 5, 3270–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGuinness T. L., Lai Y., Greengard P. (1985) Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J. Biol. Chem. 260, 1696–1704 [PubMed] [Google Scholar]
- 6. Takeuchi Y., Yamamoto H., Fukunaga K., Miyakawa T., Miyamoto E. (2000) Identification of the isoforms of Ca2+/calmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J. Neurochem. 74, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 7. Chao L. H., Pellicena P., Deindl S., Barclay L. A., Schulman H., Kuriyan J. (2010) Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat. Struct. Mol. Biol. 17, 264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanson P. I., Kapiloff M. S., Lou L. L., Rosenfeld M. G., Schulman H. (1989) Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 3, 59–70 [DOI] [PubMed] [Google Scholar]
- 9. Colbran R. J. (1993) Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J. Biol. Chem. 268, 7163–7170 [PubMed] [Google Scholar]
- 10. Lai Y., Nairn A. C., Greengard P. (1986) Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 83, 4253–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lou L. L., Lloyd S. J., Schulman H. (1986) Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation. ATP modulates production of an autonomous enzyme. Proc. Natl. Acad. Sci. U.S.A. 83, 9497–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller S. G., Kennedy M. B. (1986) Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation. A Ca2+-triggered molecular switch. Cell 44, 861–870 [DOI] [PubMed] [Google Scholar]
- 13. Schworer C. M., Colbran R. J., Soderling T. R. (1986) Reversible generation of a Ca2+-independent form of Ca2+(calmodulin)-dependent protein kinase II by an autophosphorylation mechanism. J. Biol. Chem. 261, 8581–8584 [PubMed] [Google Scholar]
- 14. Hanson S. K., Grotta J. C., Waxham M. N., Aronowski J., Ostrow P. (1994) Calcium/calmodulin-dependent protein kinase II activity in focal ischemia with reperfusion in rats. Stroke 25, 466–473 [DOI] [PubMed] [Google Scholar]
- 15. Aronowski J., Grotta J. C., Waxham M. N. (1992) Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II. Potential role in neuronal damage. J. Neurochem. 58, 1743–1753 [DOI] [PubMed] [Google Scholar]
- 16. Kolb S. J., Hudmon A., Waxham M. N. (1995) Ca2+/calmodulin kinase II translocates in a hippocampal slice model of ischemia. J. Neurochem. 64, 2147–2156 [DOI] [PubMed] [Google Scholar]
- 17. Dosemeci A., Reese T. S., Petersen J., Tao-Cheng J. H. (2000) A novel particulate form of Ca2+/calmodulin-dependent (correction of Ca2+/CaMKII-dependent) protein kinase II in neurons. J. Neurosci. 20, 3076–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudmon A., Lebel E., Roy H., Sik A., Schulman H., Waxham M. N., De Koninck P. (2005) A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J. Neurosci. 25, 6971–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waxham M. N., Grotta J. C., Silva A. J., Strong R., Aronowski J. (1996) Ischemia-induced neuronal damage. A role for calcium/calmodulin-dependent protein kinase II. J. Cereb. Blood Flow Metab. 16, 1–6 [DOI] [PubMed] [Google Scholar]
- 20. Ashpole N. M., Song W., Brustovetsky T., Engleman E. A., Brustovetsky N., Cummins T. R., Hudmon A. (2012) Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J. Biol. Chem. 287, 8495–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carter D. S., Haider S. N., Blair R. E., Deshpande L. S., Sombati S., DeLorenzo R. J. (2006) Altered calcium/calmodulin kinase II activity changes calcium homeostasis that underlies epileptiform activity in hippocampal neurons in culture. J. Pharmacol. Exp. Ther. 319, 1021–1031 [DOI] [PubMed] [Google Scholar]
- 22. Klug J. R., Mathur B. N., Kash T. L., Wang H. D., Matthews R. T., Robison A. J., Anderson M. E., Deutch A. Y., Lovinger D. M., Colbran R. J., Winder D. G. (2012) Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability. PloS One 7, e45323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenberg P. A., Aizenman E. (1989) Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett. 103, 162–168 [DOI] [PubMed] [Google Scholar]
- 24. Guthrie P. B., Knappenberger J., Segal M., Bennett M. V., Charles A. C., Kater S. B. (1999) ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cotrina M. L., González-Hoyuela M., Barbas J. A., Rodríguez-Tébar A. (2000) Programmed cell death in the developing somites is promoted by nerve growth factor via its p75(NTR) receptor. Dev. Biol. 228, 326–336 [DOI] [PubMed] [Google Scholar]
- 26. Coco S., Calegari F., Pravettoni E., Pozzi D., Taverna E., Rosa P., Matteoli M., Verderio C. (2003) Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi S., Shibata M., Fukuuchi Y. (1997) Effects of increased extracellular potassium on influx of sodium ions in cultured rat astroglia and neurons. Brain. Res. Dev. Brain. Res. 104, 111–117 [DOI] [PubMed] [Google Scholar]
- 28. Parpura V., Scemes E., Spray D. C. (2004) Mechanisms of glutamate release from astrocytes. Gap junction “hemichannels,” purinergic receptors, and exocytotic release. Neurochem. Int. 45, 259–264 [DOI] [PubMed] [Google Scholar]
- 29. Dallas M., Boycott H. E., Atkinson L., Miller A., Boyle J. P., Pearson H. A., Peers C. (2007) Hypoxia suppresses glutamate transport in astrocytes. J. Neurosci. 27, 3946–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillis J. W., Ren J., O'Regan M. H. (2000) Transporter reversal as a mechanism of glutamate release from the ischemic rat cerebral cortex. Studies with dl-threo-β-benzyloxyaspartate. Brain Res. 880, 224. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., Wang W., Gu X. S., Duan S. (2007) Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 [DOI] [PubMed] [Google Scholar]
- 32. Franke H., Verkhratsky A., Burnstock G., Illes P. (2012) Pathophysiology of astroglial purinergic signalling. Purinergic Signal 8, 629–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarthy K. D., de Vellis J. (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell. Biol. 85, 890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashpole N. M., Hudmon A. (2011) Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol. Cell. Neurosci. 46, 720–730 [DOI] [PubMed] [Google Scholar]
- 35. Vest R. S., O'Leary H., Coultrap S. J., Kindy M. S., Bayer K. U. (2010) Effective post-insult neuroprotection by a novel Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor. J. Biol. Chem. 285, 20675–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hudmon A., Aronowski J., Kolb S. J., Waxham M. N. (1996) Inactivation and self-association of Ca2+/calmodulin-dependent protein kinase II during autophosphorylation. J. Biol. Chem. 271, 8800–8808 [DOI] [PubMed] [Google Scholar]
- 37. Schworer C. M., Colbran R. J., Keefer J. R., Soderling T. R. (1988) Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J. Biol. Chem. 263, 13486–13489 [PubMed] [Google Scholar]
- 38. Vest R. S., Davies K. D., O'Leary H., Port J. D., Bayer K. U. (2007) Dual mechanism of a natural CaMKII inhibitor. Mol. Biol. Cell 18, 5024–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lipton S. A., Rosenberg P. A. (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 [DOI] [PubMed] [Google Scholar]
- 40. Zhou B. Y., Liu Y., Kim B. o., Xiao Y., He J. J. (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol. Cell. Neurosci. 27, 296–305 [DOI] [PubMed] [Google Scholar]
- 41. Rothstein J. D., Martin L., Levey A. I., Dykes-Hoberg M., Jin L., Wu D., Nash N., Kuncl R. W. (1994) Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 [DOI] [PubMed] [Google Scholar]
- 42. Perego C., Vanoni C., Bossi M., Massari S., Basudev H., Longhi R., Pietrini G. (2000) The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J. Neurochem. 75, 1076–1084 [DOI] [PubMed] [Google Scholar]
- 43. Anderson C. M., Swanson R. A. (2000) Astrocyte glutamate transport. Review of properties, regulation, and physiological functions. Glia 32, 1–14 [PubMed] [Google Scholar]
- 44. Shimamoto K., Lebrun B., Yasuda-Kamatani Y., Sakaitani M., Shigeri Y., Yumoto N., Nakajima T. (1998) dl-Threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 53, 195–201 [DOI] [PubMed] [Google Scholar]
- 45. Höltje M., Hofmann F., Lux R., Veh R. W., Just I., Ahnert-Hilger G. (2008) Glutamate uptake and release by astrocytes are enhanced by Clostridium botulinum C3 protein. J. Biol. Chem. 283, 9289–9299 [DOI] [PubMed] [Google Scholar]
- 46. Matos M., Augusto E., Oliveira C. R., Agostinho P. (2008) Amyloid-β peptide decreases glutamate uptake in cultured astrocytes. Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 156, 898–910 [DOI] [PubMed] [Google Scholar]
- 47. Zhou Y., Li H. L., Zhao R., Yang L. T., Dong Y., Yue X., Ma Y. Y., Wang Z., Chen J., Cui C. L., Yu A. C. (2010) Astrocytes express N-methyl-d-aspartate receptor subunits in development, ischemia, and post-ischemia. Neurochem. Res. 35, 2124–2134 [DOI] [PubMed] [Google Scholar]
- 48. D'Ascenzo M., Vairano M., Andreassi C., Navarra P., Azzena G. B., Grassi C. (2004) Electrophysiological and molecular evidence of L (Cav1)-, N (Cav2.2)-, and R (Cav2.3)-type Ca2+ channels in rat cortical astrocytes. Glia 45, 354–363 [DOI] [PubMed] [Google Scholar]
- 49. Latour I., Hamid J., Beedle A. M., Zamponi G. W., Macvicar B. A. (2003) Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41, 347–353 [DOI] [PubMed] [Google Scholar]
- 50. Fan D., Grooms S. Y., Araneda R. C., Johnson A. B., Dobrenis K., Kessler J. A., Zukin R. S. (1999) AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J. Neurosci. Res. 57, 557–571 [PubMed] [Google Scholar]
- 51. Tang Q., Bangaru M. L., Kostic S., Pan B., Wu H. E., Koopmeiners A. S., Yu H., Fischer G. J., McCallum J. B., Kwok W. M., Hudmon A., Hogan Q. H. (2012) Ca2+-dependent regulation of Ca2+ currents in rat primary afferent neurons. Role of CaMKII and the effect of injury. J. Neurosci. 32, 11737–11749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yasuda K., Itakura M., Aoyagi K., Sugaya T., Nagata E., Ihara H., Takahashi M. (2011) PKC-dependent inhibition of Ca2+-dependent exocytosis from astrocytes. Glia 59, 143–151 [DOI] [PubMed] [Google Scholar]
- 53. Liu T., Sun L., Xiong Y., Shang S., Guo N., Teng S., Wang Y., Liu B., Wang C., Wang L., Zheng L., Zhang C. X., Han W., Zhou Z. (2011) Calcium triggers exocytosis from two types of organelles in a single astrocyte. J. Neurosci. 31, 10593–10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Araque A., Li N., Doyle R. T., Haydon P. G. (2000) SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 20, 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yaguchi T., Nishizaki T. (2010) Extracellular high K+ stimulates vesicular glutamate release from astrocytes by activating voltage-dependent calcium channels. J. Cell. Physiol. 225, 512–518 [DOI] [PubMed] [Google Scholar]
- 56. Parpura V., Zorec R. (2010) Gliotransmission. Exocytotic release from astrocytes. Brain. Res. Rev. 63, 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCarthy K. D., Salm A. K. (1991) Pharmacologically distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience 41, 325–333 [DOI] [PubMed] [Google Scholar]
- 58. Salter M. W., Hicks J. L. (1994) ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J. Neurosci. 14, 1563–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Centemeri C., Bolego C., Abbracchio M. P., Cattabeni F., Puglisi L., Burnstock G., Nicosia S. (1997) Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br. J. Pharmacol. 121, 1700–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Angulo M. C., Kozlov A. S., Charpak S., Audinat E. (2004) Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Parpura V., Haydon P. G. (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. U.S.A. 97, 8629–8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parpura V., Basarsky T. A., Liu F., Jeftinija K., Jeftinija S., Haydon P. G. (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747 [DOI] [PubMed] [Google Scholar]
- 63. Cotrina M. L., Lin J. H., López-García J. C., Naus C. C., Nedergaard M. (2000) ATP-mediated glia signaling. J. Neurosci. 20, 2835–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Inoue K., Koizumi S., Tsuda M. (2007) The role of nucleotides in the neuron-glia communication responsible for the brain functions. J. Neurochem. 102, 1447–1458 [DOI] [PubMed] [Google Scholar]
- 65. Köles L., Leichsenring A., Rubini P., Illes P. (2011) P2 receptor signaling in neurons and glial cells of the central nervous system. Adv. Pharmacol. 61, 441–493 [DOI] [PubMed] [Google Scholar]
- 66. Anderson C. M., Bergher J. P., Swanson R. A. (2004) ATP-induced ATP release from astrocytes. J. Neurochem. 88, 246–256 [DOI] [PubMed] [Google Scholar]
- 67. León D., Hervás C., Miras-Portugal M. T. (2006) P2Y1 and P2X7 receptors induce calcium/calmodulin-dependent protein kinase II phosphorylation in cerebellar granule neurons. Eur. J. Neurosci. 23, 2999–3013 [DOI] [PubMed] [Google Scholar]
- 68. Bowser D. N., Khakh B. S. (2007) Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 129, 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lämmer A., Günther A., Beck A., Krügel U., Kittner H., Schneider D., Illes P., Franke H. (2006) Neuroprotective effects of the P2 receptor antagonist PPADS on focal cerebral ischaemia-induced injury in rats. Eur. J. Neurosci. 23, 2824–2828 [DOI] [PubMed] [Google Scholar]
- 70. Kuboyama K., Harada H., Tozaki-Saitoh H., Tsuda M., Ushijima K., Inoue K. (2011) Astrocytic P2Y1 receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J. Cereb. Blood Flow Metab. 31, 1930–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lämmer A. B., Beck A., Grummich B., Förschler A., Krügel T., Kahn T., Schneider D., Illes P., Franke H., Krügel U. (2011) The P2 receptor antagonist PPADS supports recovery from experimental stroke in vivo. PloS One 6, e19983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arbeloa J., Pérez-Samartín A., Gottlieb M., Matute C. (2012) P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol. Dis. 45, 954–961 [DOI] [PubMed] [Google Scholar]
- 73. Parpura V., Heneka M. T., Montana V., Oliet S. H., Schousboe A., Haydon P. G., Stout R. F., Jr., Spray D. C., Reichenbach A., Pannicke T., Pekny M., Pekna M., Zorec R., Verkhratsky A. (2012) Glial cells in (patho)physiology. J. Neurochem. 121, 4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barres B. A. (2008) The mystery and magic of glia. A perspective on their roles in health and disease. Neuron 60, 430–440 [DOI] [PubMed] [Google Scholar]
- 75. Banker G. A. (1980) Trophic interactions between astroglial cells and hippocampal neurons in culture. Science 209, 809–810 [DOI] [PubMed] [Google Scholar]
- 76. Pfrieger F. W., Barres B. A. (1997) Synaptic efficacy enhanced by glial cells in vitro. Science 277, 1684–1687 [DOI] [PubMed] [Google Scholar]
- 77. Kajihara H., Tsutsumi E., Kinoshita A., Nakano J., Takagi K., Takeo S. (2001) Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction. Immunohistochemical and electron microscopic studies. Brain Res. 909, 92–101 [DOI] [PubMed] [Google Scholar]
- 78. Zamanian J. L., Xu L., Foo L. C., Nouri N., Zhou L., Giffard R. G., Barres B. A. (2012) Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Petito C. K., Olarte J. P., Roberts B., Nowak T. S., Jr., Pulsinelli W. A. (1998) Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol. 57, 231–238 [DOI] [PubMed] [Google Scholar]
- 80. Schmidt-Kastner R., Aguirre-Chen C., Saul I., Yick L., Hamasaki D., Busto R., Ginsberg M. D. (2005) Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res. 1052, 28–39 [DOI] [PubMed] [Google Scholar]
- 81. Barres B. A. (1991) New roles for glia. J. Neurosci. 11, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Suadicani S. O., Brosnan C. F., Scemes E. (2006) P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pryazhnikov E., Khiroug L. (2008) Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56, 38–49 [DOI] [PubMed] [Google Scholar]
- 84. Franke H., Illes P. (2006) Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol. Ther. 109, 297–324 [DOI] [PubMed] [Google Scholar]
- 85. Phillis J. W., Smith-Barbour M., O'Regan M. H., Perkins L. M. (1994) Amino acid and purine release in rat brain following temporary middle cerebral artery occlusion. Neurochem. Res. 19, 1125–1130 [DOI] [PubMed] [Google Scholar]
- 86. Franke H., Günther A., Grosche J., Schmidt R., Rossner S., Reinhardt R., Faber-Zuschratter H., Schneider D., Illes P. (2004) P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 63, 686–699 [DOI] [PubMed] [Google Scholar]
- 87. Volonté C., Amadio S., Cavaliere F., D'Ambrosi N., Vacca F., Bernardi G. (2003) Extracellular ATP and neurodegeneration. Curr. Drug Targets CNS Neurol. Disord. 2, 403–412 [DOI] [PubMed] [Google Scholar]
- 88. Wang X., Arcuino G., Takano T., Lin J., Peng W. G., Wan P., Li P., Xu Q., Liu Q. S., Goldman S. A., Nedergaard M. (2004) P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827 [DOI] [PubMed] [Google Scholar]
- 89. Ryu J. K., Kim J., Choi S. H., Oh Y. J., Lee Y. B., Kim S. U., Jin B. K. (2002) ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. Neuroreport 13, 1611–1615 [DOI] [PubMed] [Google Scholar]
- 90. Melani A., Turchi D., Vannucchi M. G., Cipriani S., Gianfriddo M., Pedata F. (2005) ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem. Int. 47, 442–448 [DOI] [PubMed] [Google Scholar]
- 91. Sperlágh B., Köfalvi A., Deuchars J., Atkinson L., Milligan C. J., Buckley N. J., Vizi E. S. (2002) Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 81, 1196–1211 [DOI] [PubMed] [Google Scholar]
- 92. Duan S., Anderson C. M., Keung E. C., Chen Y., Chen Y., Swanson R. A. (2003) P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schulze-Lohoff E., Hugo C., Rost S., Arnold S., Gruber A., Brüne B., Sterzel R. B. (1998) Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 275, F962–F971 [DOI] [PubMed] [Google Scholar]
- 94. Ferrari D., Los M., Bauer M. K., Vandenabeele P., Wesselborg S., Schulze-Osthoff K. (1999) P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 447, 71–75 [DOI] [PubMed] [Google Scholar]
- 95. Jourdain P., Bergersen L. H., Bhaukaurally K., Bezzi P., Santello M., Domercq M., Matute C., Tonello F., Gundersen V., Volterra A. (2007) Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339 [DOI] [PubMed] [Google Scholar]
- 96. Sellers L. A., Simon J., Lundahl T. S., Cousens D. J., Humphrey P. P., Barnard E. A. (2001) Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J. Biol. Chem. 276, 16379–16390 [DOI] [PubMed] [Google Scholar]
- 97. Mamedova L. K., Gao Z. G., Jacobson K. A. (2006) Regulation of death and survival in astrocytes by ADP activating P2Y1 and P2Y12 receptors. Biochem. Pharmacol. 72, 1031–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gallagher C. J., Salter M. W. (2003) Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J. Neurosci. 23, 6728–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]