Review on tissue repair and M1-like to M2a-like macrophages, exhibiting important differences from in vitro phenotypes.
Keywords: wound healing, inflammation, cell therapy, fibrosis, M2a, regeneration
Abstract
Mp are crucial for tissue repair and regeneration but can also contribute to tissue damage and fibrosis. Mp can adopt a variety of functional phenotypes in response to different stimuli; two of the best-characterized in vitro phenotypes are a proinflammatory “M1” phenotype, produced by exposure to IFN-γ and TNF-α, and an anti-inflammatory “M2a” phenotype, produced by IL-4 or IL-13. M2a Mp are frequently termed “wound healing” Mp, as they express factors that are important for tissue repair. This review will summarize current knowledge of Mp phenotypes during tissue repair and will argue that these in vivo Mp populations are heterogeneous and temporally regulated and do not conform to existing, in vitro-defined M1 or M2 phenotypes. Mp during the early stages of tissue repair exhibit a more proinflammatory phenotype than their later counterparts, which in turn may exhibit some M2a-associated characteristics. However, phenotypic markers that appear to be coregulated in cultured Mp can be expressed independently of each other in vivo. Additionally, M1- and M2-associated markers may be expressed simultaneously by actual tissue-repair Mp. Improved understanding of Mp phenotypes and their regulation may assist in generation of novel therapies based on manipulating Mp function to improve healing.
Introduction
Across diverse tissues, repair after injury occurs in overlapping phases of inflammation, proliferation, and remodeling. Within hours after injury, circulating leukocytes invade the injury site, where they participate in host defense, phagocytosis of necrotic tissue, and secretion of paracrine factors. Mp, in particular, are known to be essential to regeneration, repair, and remodeling in numerous tissues [1–5]. However, chronic inflammation and persistent Mp accumulation are often associated with tissue destruction and fibrosis [6–8].
These seemingly contradictory roles of Mp in tissue injury and repair are likely related to the ability of Mp to assume markedly different phenotypes in response to specific environmental cues. Mp have long been known to exhibit a proinflammatory phenotype, now termed “classically activated” or M1, in response to bacterial components or IFN-γ and TNF-α; however, Mp can also assume a variety of “alternatively activated” or M2 phenotypes [9]. As in vitro IL-4 treatment of Mp causes an up-regulation of TGF-β and arginase, these M2a Mp are widely assumed to participate in tissue-repair processes and have often been termed wound-healing Mp [6, 9–11]. However, damaged tissue contains myriad factors that could influence Mp behavior in unexpected ways, and the actual factors influencing Mp phenotype during in vivo tissue injury and repair remain largely undetermined.
This review will address recent advances in our understanding of Mp phenotypes during tissue repair and will argue that the Mp populations that participate in tissue repair are not analogous to in vitro M2a Mp. In numerous physiological and pathological states, Mp exhibit extensive heterogeneity and plasticity, and tissue repair is no exception. Mp present during tissue repair are not a single homogenous population but include a temporally regulated spectrum of activation states to orchestrate the various phases of healing.
EVOLVING Mp ACTIVATION PARADIGMS
Our understanding of Mp biology has improved immensely over the past few decades, and new discoveries continue to redefine paradigms of Mp activation. In the past, Mp activation was viewed as a matter of on/off switching: naïve Mp were thought to become activated upon exposure to bacterial components or IFN-γ and TNF-α, which increase Mp production of inflammatory cytokines and ROS and enhance their pathogen-killing capacity. In contrast, the primary effect of anti-inflammatory cytokines on Mp was thought to be deactivation and resolution of inflammation [12]. However, in 1992, Stein and colleagues [13] demonstrated that IL-4 is not merely an off signal for Mp immune activation but also increases expression of the mannose receptor CD206 and enhances endocytosis of mannosylated ligands by murine Mp. Therefore, IL-4-treated Mp were termed “alternatively” activated to contrast with “classical” activation by IFN-γ/TNF-α and the general deactivation that was previously thought to occur with IL-4 treatment.
Subsequently, additional stimuli that were previously considered to be Mp deactivators were also shown to produce distinct Mp activation phenotypes in vitro. The term “alternative” activation has thus expanded to include these new phenotypes, now frequently referred to as M2a/b/c, whereas classical activation is referred to as M1 [14]. The M2a phenotype is produced in vitro by exposure to IL-4 or IL-13, which act through the common receptor IL-4Rα to increase expression of CD206, arginase, and TGF-β [15–17]. The M2b phenotype is produced by exposure to a combination of IgG-immune complexes and LPS, which increases production of IL-10 and decreases production of IL-12, imparting potent anti-inflammatory properties [18, 19]. In vitro exposure to IL-10 or glucocorticoids produces the M2c phenotype, which is similarly characterized by high IL-10 and low IL-12 production [19], as well as increased cell-surface expression of the scavenger receptor CD163 [20, 21]. Mp phenotype can also be altered by phagocytosis of apoptotic or necrotic cells [22–24]. Together, these studies have demonstrated that far from being merely turned “on” or “off” by proinflammatory versus anti-inflammatory cytokines, Mp can adopt a variety of different phenotypes in response to different stimuli.
Whereas discrete M1/2a/b/c phenotypes can certainly be produced in vitro, Mp phenotype is by no means restricted to these four categories. Indeed, the actual phenotypes acquired by Mp, even in vitro, depend on their stage of differentiation, the total biochemical milieu, and the dose and duration of the activating stimuli [19, 25, 26]. Additionally, Mp remain responsive to further stimuli after their initial activation [19, 27]. Thus, available evidence indicates that M1 and M2a/b/c are not discrete Mp differentiation states but instead represent convenient but limited and arbitrary in vitro-defined reference points on a time-dependent and multidimensional continuum of possible Mp phenotypes and functions [9].
The concepts of Mp heterogeneity and plasticity have now become widely acknowledged [9, 28, 29], and an appealing model of in vivo Mp activation classifies these cells based on function rather than expression of phenotypic “markers” [9]. Within this spectrum, wound-healing or tissue-repair functions are frequently ascribed to Mp of an M2a or M2a-like phenotype [6, 9–11]. However, tissue repair is a finely orchestrated, multistage process, and Mp perform critical but contrasting functions in each stage. Wound healing is thus not a single, well-defined function of Mp but comprises its own spectrum of overlapping functions, including phagocytosis, secretion of cytokines and growth factors, as well as matrix remodeling, all of which may be performed by Mp of different phenotypes. We argue below that nomination of M2a Mp as the primary Mp effectors of tissue repair is an oversimplification at best and likely not warranted.
EVIDENCE FOR M2a Mp AS TISSUE-REPAIR Mp
The notion that IL-4/IL-13-induced M2a Mp are tissue-repair Mp is based largely on their up-regulation of TGF-β and arginase, as well as their ability to produce certain ECM components [9, 17, 30]. IL-4-activated M2a Mp increase production of TGF-β [17], which is a powerful activator of fibroblast collagen production [31, 32]. It is likely that production of TGF-β by M2a Mp is at least partly responsible for the enhancement of fibroblast proliferation and collagen production observed in Mp/fibroblast cocultures [17]. Interestingly, however, Mp in a sponge-implantation model of mouse wound healing produced TGF-β, despite a lack of detection of the canonical M2a activators IL-4 or IL-13 in wound fluids or cells and an absence of Stat6 phosphorylation in wound Mp [33]. Therefore, M2a activation with IL-4 appears to be sufficient but not necessary for stimulation of Mp TGF-β production. It is possible that ingestion of apoptotic or necrotic cells, rather than IL-4/-13, may be a major trigger for production of healing-associated factors such as TGF-β [34].
Rodent M2a Mp also express arginase, which has been suggested to be important for tissue repair [9, 35]. Importantly, in contrast to rodent Mp, human M2a Mp or monocytes do not express arginase [36, 37]. Rodent Mp activated in vitro with IL-4 increase mRNA and protein expression of arginase, accompanied by an increase in enzymatic activity [16]. Arginine metabolism through the arginase pathway produces polyamines, which are important for cell proliferation [38], and proline, which is a major component of collagen. However, proline produced via the arginase pathway may not be a limiting factor for collagen synthesis [34], and the role of arginase in collagen production and fibrosis in vivo is complex and likely context-dependent [39–41]. Arginase is expressed during injury and repair in diverse models and tissues [40, 42–44]. However, arginase can be produced by cells other than Mp [34, 43], and as human Mp do not produce arginase [36, 37], human arginase appears to be derived entirely from non-Mp cell types [34]. Additionally, in rodent Mp, LPS appears to be a more powerful stimulus than IL-4 for arginase expression and activity [25, 43]. Thus, the presence of arginase or even of arginase-expressing Mp does not necessarily imply that IL-4/IL-13-induced M2a Mp are present in a tissue.
In addition to indirectly promoting fibroblast collagen synthesis via TGF-β and possibly arginase, M2a Mp are capable of directly synthesizing certain ECM components, including collagen type VI, fibronectin, and βIG-H3 [45, 46]. The ECM-associated protein βIG-H3 promotes adhesion and migration of monocytes, keratinocytes, and fibroblasts [47–49] and increases fibroblast collagen production [50]. βIG-H3 is expressed in numerous tissues in situations of injury or disease and can be expressed by many different cell types, including Mp, fibroblasts, epithelial cells, and keratinocytes [47–52]. The relative importance of Mp versus other cell types, particularly fibroblasts, for production of ECM components during tissue repair remains to be elucidated. Whereas cultured Mp can secrete collagen type VI at levels comparable with fibroblasts [45], the in vivo relevance of ECM production by Mp during tissue repair has yet to be demonstrated.
Clearly, cultured M2a Mp produce factors that are known to be involved in tissue repair, and in vivo Mp in an actual wound can produce many of these same factors. Thus, it may seem natural to conclude that M2a Mp perform tissue-repair functions in vivo. However, the assumption implicit in this line of reasoning is that the presence of an M2a marker (i.e., something that is up-regulated in Mp culture by IL-4) indicates that the Mp was, in fact, activated by IL-4 and/or shares other characteristics of cultured M2a Mp. This assumption is often false. For instance, in a mouse wound-healing model, Mp expression of the M2a marker CD206 does not require IL-4 or IL-13 and does not correlate, negatively or otherwise, with TNF-α production [33]. Additionally, in freshly isolated human peritoneal Mp, cell-surface expression of CD206 or the M2c marker CD163 does not correlate with expression of the M2-associated genes TGF-β, CCL18, or matrix metalloproteinase 9 [53]. Thus, whereas it is clear that treatment of cultured Mp with particular cytokines produces characteristic phenotypes, the presence of one or a few phenotypic markers in vivo does not demonstrate that the Mp was activated by the associated cytokine or that other aspects of the phenotype induced by that cytokine will also be present. The true in vivo phenotypes of tissue-repair Mp, as well as the factors that initiate and modulate these phenotypes, remain to be elucidated and are areas of active investigation.
Mp PHENOTYPES DURING TISSUE REPAIR AND FIBROSIS
Recent studies have sought to characterize Mp phenotype in vivo during tissue repair and to determine whether parallels can be drawn to in vitro-defined M1/M2 phenotypes. An emerging dogma in tissue-repair literature suggests that M1 Mp are the predominant population present during the first few days after injury, corresponding to the inflammatory and early proliferative phases, whereas M2a Mp are the primary effectors of later stages of repair or the later proliferative and remodeling phases [7, 8, 54]. If this were the case, then Mp would be expected to exhibit typical M1 markers during the early days after injury, whereas M2a markers would be expressed as repair progresses. For instance, Mp of the inflammatory and early proliferative phases would be expected to produce abundant inflammatory cytokines, whereas Mp at later stages would be expected to produce anti-inflammatory cytokines and express CD206 and other M2a surface markers. However, we argue here that whereas Mp of the inflammatory phase do, in fact, seem to present a more proinflammatory phenotype than their later-phase counterparts, in vivo tissue-repair Mp at any time-point do not share sufficient similarities with in vitro-defined phenotypes to justify labeling them as wholly M1 or M2.
The pattern of proinflammatory cytokine production by tissue-repair Mp is perhaps the strongest evidence for the early-M1/late-M2 paradigm or at least for switching from a pro- to an anti-inflammatory phenotype. The M1 activator IFN-γ is up-regulated rapidly after injury to skeletal muscle and skin and is required for proper healing of these tissues [55–58]. Potential sources of IFN-γ in injured skeletal muscle include but are not limited to myoblasts, T cells, NK cells, and Mp themselves [57], although the most functionally important cellular source remains to be determined. Similar to its effects on cultured Mp, IFN-γ may promote an M1-like phenotype in Mp in the mdx mouse model of muscular dystrophy [59]. The M1-associated cytokines IL-1β, TNF-α, IL-6, and IL-12 are expressed by Mp during the first few hours to days after acute injury in numerous tissues, including skin [33, 60, 61], liver [62], kidney [63], and skeletal muscle [57, 64]. Mp expression of mRNA and protein for these cytokines exhibits an early peak and subsequent decline after skin wounding [33, 60] or toxin-induced muscle injury in mice [65], consistent with an early M1-like phenotype. However, further studies will be needed to determine whether proinflammatory cytokine production by early tissue-repair Mp is quantitatively similar to that of bona fide M1 Mp, as direct comparisons are currently unavailable. Additionally, anti-inflammatory and proinflammatory cytokine expression may be induced simultaneously at early time-points during tissue repair. For instance, at 48 h after acute liver injury in mice, Mp express IL-10 simultaneously with TNF-α and IL-1β mRNA [62]. Similarly, after mouse skeletal muscle laceration, Mp expression of TGF-β mRNA and IL-10 mRNA appears to peak early, ∼3 days postinjury (unpublished results). Further studies will be necessary to determine whether even the early-invading Mp population is not purely M1 and/or whether early anti-inflammatory cytokine expression is a negative-feedback mechanism initiating a phenotypic switch.
In addition to proinflammatory cytokine production, robust expression of iNOS is a hallmark of M1 activation in rodent Mp [14], although iNOS activity in human Mp appears to be much lower or absent entirely (∼20 nmol nitrite/106 cells/24 h in rodent M1 Mp; often undetectable in human Mp) [66]. In contrast to the fairly consistent up-regulation of proinflammatory cytokines by early tissue-repair Mp, expression of iNOS in rodent injury models appears to be highly context-dependent. In mouse kidney ischemia-reperfusion injury, Mp expression of iNOS mRNA is highest at 1-day postinjury and subsequently declines, consistent with an early M1 phenotype [63]. However, Mp in incisional mouse skin wounds appear to express iNOS only in response to invading pathogens and not as an inherent feature of the tissue-repair response [33, 67]. After excisional skin wounding in rats [56] or photochemical liver injury in mice [68], only a fraction of the wound Mp expresses iNOS (10–15% positive at peak in skin), and the heterogeneity of the Mp population may be dependent on proximity to the injured site [68]. Studies from our own laboratory have produced contradictory data in two different mouse muscle injury models: skeletal muscle Mp express iNOS mRNA at 5 days after chemically induced injury [57] but do not express iNOS at any time-point after laceration injury (unpublished results). The reasons for this discrepancy remain to be determined but may be related to different damage-associated molecular patterns produced by different modes of injury. The varying expression of iNOS by Mp during tissue repair highlights the complexity of in vivo phenotypic regulation. Taken together, the pattern of inflammatory cytokine production and iNOS expression suggests a proinflammatory but not entirely M1 Mp phenotype during the early stages of tissue repair. Further studies will be needed to determine the factors that regulate this phenotype.
If Mp transition from an M1- to an M2a-like phenotype as repair progresses, then anti-inflammatory cytokine expression by Mp would be expected to be low or absent during early stages of repair and elevated during the later stages. Indeed, during mouse skin wound healing, Mp expression of TGF-β mRNA and protein increases over the first 7–10 days postinjury, whereas proinflammatory cytokine production decreases [33, 60], consistent with the expression of an M2a-like phenotype during the later stages of repair. In murine skeletal muscle, however, Mp expression of TGF-β and IL-10 mRNA increases over the first few days after toxin-induced injury, peaks during the intermediate stages of healing, and then declines sharply, despite continued presence of Mp within the healing muscle [65]. Interestingly, during impaired muscle healing caused by loss of MAPK-phosphatase-1 [65], peak Mp expression of TGF-β and IL-10 is shifted to earlier time-points and occurs simultaneously with peak proinflammatory cytokine expression, contrary to expectations of an early-M1-to-late-M2 transition. Furthermore, levels of IL-10 and TGF-β expression are not necessarily coregulated during tissue repair; Mp display increased mRNA expression of IL-10 but not TGF-β during chronic liver fibrosis in mice, and deficiency of the chemokine receptor CX3CR1 results in reduced IL-10 but increased TGF-β expression by Mp [69]. Thus, even if particular M2a-associated cytokines are expressed by Mp during tissue repair, this does not guarantee that other aspects of the M2a phenotype are present.
Although IL-4 and IL-13 are often assumed to induce a wound-healing phenotype in Mp, the importance of these canonical M2a activators may vary across different situations of tissue repair. Genetic deletion of IL-4Rα greatly reduces mRNA expression of the M2a markers Ym1, FIZZ1, and arginase-1 in peritoneal exudate cells (which include Mp, neutrophils, eosinophils, and lymphocytes) after peritoneal incisional wounding in mice [70], indicating that IL-4/-13 are important phenotypic regulators in this model. In contrast, in a mouse sponge-implantation model of skin wounding, deletion of IL-4Rα has no effect on Mp phenotype, and IL-4 and IL-13 are not detected in wound cells or fluids at any time, indicating that despite their predominantly anti-inflammatory cytokine profile, these cells are not bona fide M2a Mp [33]. Both of these studies, however, are limited in that Mp obtained by peritoneal lavage or sponge compression may potentially differ from Mp present within the actual healing skin wound. In mouse skin wounds, IL-4 mRNA and protein are not detectable at early or late time-points [60, 71]. However, IL-4 gene expression is elevated from 1 to 7 days in rat skin wounds [56], and protein expression is elevated between 12 h and 10 days in human skin wounds (peak ∼12 pg/mg protein in injured skin vs. nearly undetectable in uninjured) [58]; IL-4 protein is present at similar levels in infarcted rat myocardium [72]. In mouse skeletal muscle injury by chemical toxin or unloading/reloading, IL-4 mRNA, although detectable, does not rise above uninjured levels over the course of healing [73, 74]. The reasons for and consequences of this differential expression of IL-4 remain unclear. Further studies will be needed to determine whether IL-4 and IL-13 are involved in Mp activation and healing of different tissues and to categorize the time course of anti-inflammatory cytokine expression by Mp during tissue repair. Taken together, existing data on anti-inflammatory cytokine production suggest that Mp may become more M2a-like as repair progresses, although this transition may not happen in all situations. Additionally, as IL-4/-13 activation is not always required for up-regulation of IL-10 and TGF-β [33], the presence of other aspects of the M2a phenotype should not be inferred from cytokine expression alone.
In addition to anti-inflammatory cytokine production, mouse M2a Mp are characterized by expression of phenotypic markers such as CD206, Ym1, dectin-1, and FIZZ1 [25]. If Mp undergo a switch from an M1-like to an M2a-like phenotype during tissue repair, Mp expression of these M2a markers would be expected to increase as repair progresses. Indeed, in mouse and human skin wounds and in injured mouse kidney, Mp expression of CD206 increases during the later stages of repair [33, 60, 61, 63]. However, although CD206, Ym1, and dectin-1 are expressed by murine subcutaneous wound Mp at 1, 3, and 7 days postinjury [33], these three M2a-associated proteins exhibit entirely different temporal patterns: CD206 increases somewhat with time, whereas Ym1 is expressed most highly at 1-day postinjury and then decreases, and dectin-1 expression remains constant [33]. Additionally, CD206 expression is similar among TNF-α-high, -low, or -negative Mp [33], which would not be expected if distinct M1 and M2a populations were present. Similarly, in mouse skin-wound Mp, CD206 protein expression does not appear to correlate, negatively or otherwise, with protein expression of Ly6C [60], which is sometimes associated with a proinflammatory phenotype [24, 64, 75]. Collectively, data from these studies indicate that the M2a markers CD206, Ym1, and dectin-1 can be regulated independently of each other in skin-wound Mp and can be coexpressed with M1-associated or proinflammatory genes and proteins. Thus, Mp during skin wound healing exhibit a heterogeneous and temporally regulated phenotype that does not conform strictly to M1/M2 categorization [33].
Numerous studies in other tissues have reported that M2a markers are present during tissue repair, but many of these studies have not determined whether these markers are expressed by Mp or by other cell types, and any conclusions drawn regarding Mp phenotype are therefore dubious. Seven days after photochemical injury to the liver, CD206+ cells are observed near the border of the injured site [68], although these cells were not verified specifically as Mp, and no other time-points were examined. Similarly, after peritoneal incisional wounding, peritoneal exudate cells transiently express elevated Ym1 and FIZZ1 mRNA, although this cell population contains lymphocytes and neutrophils in addition to Mp [70], and each of these cell types could express one or both genes [76, 77].
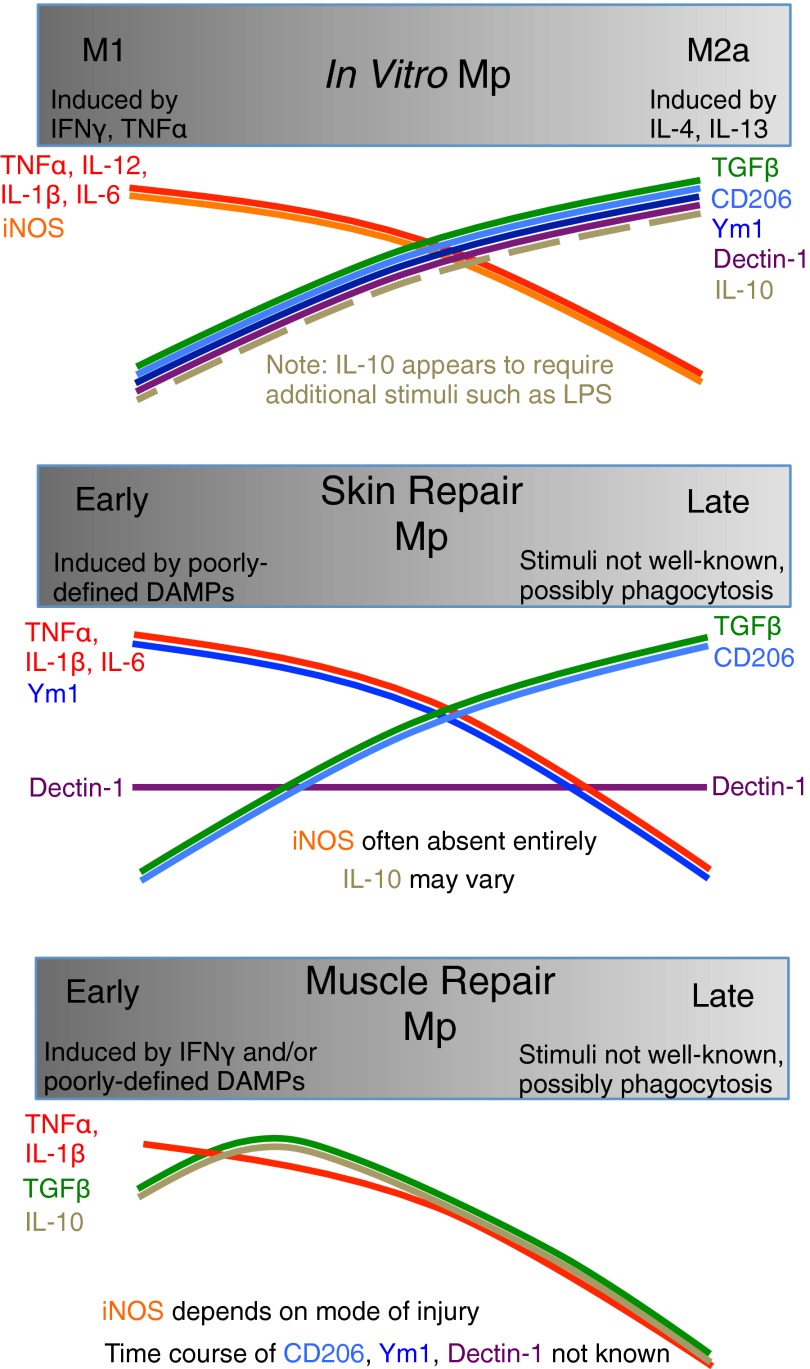
In summary, existing evidence is limited but does not support the notion that Mp present during tissue repair are bona fide M2a Mp. A proinflammatory-to-anti-inflammatory transition may indeed occur in many situations, although this appears to be context-dependent, and the coexpression of M1- and M2a-associated markers precludes classification of early or late Mp as purely M1 or M2a (Table 1 and Fig. 1). Given the growing belief that M2a Mp participate in tissue repair, surprisingly few studies have actually examined Mp-specific expression of M2a-associated genes or proteins in vivo or performed quantitative comparisons of cytokine production by cultured M1/M2a Mp versus in vivo tissue-repair Mp. Whole-tissue content of cytokines or other markers of M2a activation is insufficient to determine the phenotype of Mp during in vivo tissue repair, as whole-tissue levels could be affected by expression in non-Mp cells or by changes in the number of Mp in the tissue. Additionally, a comprehensive in vivo Mp phenotype cannot be deduced solely from a limited set of phenotypic markers, as different M2a-associated genes or proteins can exhibit entirely different temporal regulation and can be coexpressed with M1-associated markers [19, 25, 33, 60, 78]. Further studies of Mp phenotype during tissue repair are essential, but we suggest that investigation of specific functions (such as phagocytosis or stimulation of stem-cell proliferation and differentiation) of Mp isolated from tissues undergoing repair may be more fruitful than attempts to sort tissue-repair Mp into discrete, in vitro -defined subsets that may not exist in vivo and are based on markers that may have no direct role in tissue injury and repair.
Table 1. Summary of In Vitro and In Vivo Mp Phenotypes.
| Mp phenotype | Cultured Mp |
Tissue repair Mp in vivo |
||
|---|---|---|---|---|
| M1 | M2a | Early | Late | |
| Activator | IFN-γ, TNF-α, LPS | IL-4, IL-13 | Poorly defined DAMPs; IFN-γ may promote M1-like polarization (skeletal muscle). IL-4 may or may not be present. | Not well-known, possibly phagocytosis; IL-4 may or may not be present.IL-4/-13 do not appear to be required for activation (skin). |
| Proinflammatory cytokines | IL-1β, TNF-α, IL-6, IL-12 | Low | IL-1β (skin, liver, skeletal muscle), TNF-α (skin, liver, skeletal muscle), IL-6 (skin), IL-12 (kidney) | Decrease with time (skin, skeletal muscle); IL-12 also decreases with time in kidney. |
| iNOS | High (only in rodent) | Low | Context-dependent; may be related to host defense, not tissue repair. | Decreases with time (kidney); if present, time course not known in other tissues. |
| Anti-inflammatory cytokines | Low | TGF-β high, IL-10 low | TGF-β (skeletal muscle, skin) and IL-10 (liver and skeletal muscle; may not be present in skin) | TGF-β increases with time (skin); IL-10 time course not known. |
| CD206 | Low | High | Present (skin) | Increase with time (skin) |
| Dectin-1 | Low | High | Present (skin) | No change with time (skin) |
| Ym1 | Low | High | High (skin) | Decrease with time (skin) |
Figure 1. Similarities and differences among phenotypes of Mp in vitro and after acute injury to skin or skeletal muscle in vivo.
CONCLUDING REMARKS
Mp are critical orchestrators of repair and regeneration in numerous tissues, but may also contribute to chronic tissue damage and fibrosis. Different stimuli can produce a broad spectrum of functional Mp phenotypes, and this plasticity likely contributes to the seemingly contradictory roles of Mp in tissue injury, regeneration, and fibrosis. The plethora of factors that regulate Mp activation also complicate identification of Mp phenotypes in vivo; tissue-repair Mp are exposed to a vastly more complex microenvironment than Mp treated with one or a few cytokines in a cell-culture dish. Therefore, it is not surprising that in vivo tissue-repair Mp do not conform to existing, in vitro-defined phenotypic categories. Whereas Mp during the early stages of tissue repair seem to present a more generally proinflammatory profile than their later counterparts, early Mp may lack iNOS expression or may express CD206, Ym1, and IL-10 in addition to proinflammatory cytokines, indicating that these early-repair Mp do not exhibit an entirely M1 phenotype. Similarly, whereas later Mp populations may exhibit a more anti-inflammatory profile with increased expression of some M2a-associated markers, such as CD206, others, such as Ym1, may be oppositely regulated, and the canonical M2a activators, IL-4 and IL-13, may or may not be present, depending on the tissue and mode of injury. These data indicate that Mp during the later stages of tissue repair are not entirely of the M2a phenotype. In short, tissue-repair Mp exhibit complex and heterogeneous phenotypes that change throughout the repair process and do not correspond to existing, in vitro -defined categories.
Many open questions remain regarding the role of Mp in tissue repair. Whereas Mp are known to promote tissue-repair functions such as wound debridement, cell proliferation, angiogenesis, and matrix remodeling, much remains to be learned about the mechanisms by which Mp accomplish these functions. Similarly, whereas many potential modulators of Mp activation have been identified in vitro, the actual factors that regulate Mp phenotype during tissue repair remain to be elucidated. Furthermore, whether Mp of different phenotypes during the inflammatory, proliferative, and remodeling phases are derived from sequential invasion of distinct blood-monocyte populations or from a change in phenotype of existing wound Mp is under debate [64, 79]. Finally and perhaps most importantly, rodent and human Mp do not exhibit entirely similar responses to in vitro activation [9, 34, 66, 80], and it remains to be determined whether there are also species-dependent differences in Mp function during in vivo tissue repair. As Mp are known to play important roles in normal and impaired healing, an improved understanding of the reciprocal regulation of Mp phenotype and the tissue-repair environment will provide insight into novel therapies based on manipulating Mp function to promote healing.
ACKNOWLEDGMENTS
Grant support was provided by Department of Defense #W81XWH-05-1-0159 to T.J.K., U.S. National Institutes of Health #R01GM092850 to T.J.K., American College of Sports Medicine Doctoral Student Research grant #2011-03604-00-00 to M.L.N., and University of Illinois at Chicago Graduate College Dean's Scholar Award to M.L.N.
The authors thank Luisa DiPietro and Giamila Fantuzzi for their insightful feedback.
Footnotes
- βIG
- TGFβ-inducible gene
- FIZZ1
- found in inflammatory zone
- Mp
- macrophage
AUTHORSHIP
M.L.N. and T.J.K. outlined the review and wrote the manuscript.
REFERENCES
- 1. Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Müller W., Roers A., Eming S. A. (2010) Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184, 3964–3977 [DOI] [PubMed] [Google Scholar]
- 2. Mirza R., DiPietro L. A., Koh T. J. (2009) Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175, 2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Summan M., Warren G. L., Mercer R. R., Chapman R., Hulderman T., Van Rooijen N., Simeonova P. P. (2006) Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. 290, R1488–R1495 [DOI] [PubMed] [Google Scholar]
- 4. Van Amerongen M. J., Harmsen M. C., van Rooijen N., Petersen A. H., van Luyn M. J. (2007) Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duffield J. S., Forbes S. J., Constandinou C. M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J. P. (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreira A. P., Hogaboam C. M. (2011) Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J. Interferon Cytokine Res. 31, 485–491 [DOI] [PubMed] [Google Scholar]
- 7. Moyer A. L., Wagner K. R. (2011) Regeneration versus fibrosis in skeletal muscle. Curr. Opin. Rheumatol. 23, 568–573 [DOI] [PubMed] [Google Scholar]
- 8. Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heymann F., Trautwein C., Tacke F. (2009) Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm. Allergy Drug Targets 8, 307–318 [DOI] [PubMed] [Google Scholar]
- 11. Ricardo S. D., van Goor H., Eddy A. A. (2008) Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathan C. (1991) Mechanisms and modulation of macrophage activation. Behring Inst. Mitt. February, 200–207 [PubMed] [Google Scholar]
- 13. Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 15. Gordon S. (2003) Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 16. Louis C. A., Mody V., Henry W. L., Reichner J. S., Albina J. E. (1999) Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am. J. Physiol. 276, R237–R242 [DOI] [PubMed] [Google Scholar]
- 17. Song E., Ouyang N., Hörbelt M., Antus B., Wang M., Exton M. S. (2000) Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 204, 19–28 [DOI] [PubMed] [Google Scholar]
- 18. Anderson C. F., Gerber J. S., Mosser D. M. (2002) Modulating macrophage function with IgG immune complexes. J. Endotoxin Res. 8, 477–481 [DOI] [PubMed] [Google Scholar]
- 19. Stout R. D., Jiang C., Matta B., Tietzel I., Watkins S. K., Suttles J. (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175, 342–349 [DOI] [PubMed] [Google Scholar]
- 20. Buechler C., Ritter M., Orsó E., Langmann T., Klucken J., Schmitz G. (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67, 97–103 [PubMed] [Google Scholar]
- 21. Ambarus C. A., Krausz S., van Eijk M., Hamann J., Radstake T. R., Reedquist K. A., Tak P. P., Baeten D. L. (2012) Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 375, 196–206 [DOI] [PubMed] [Google Scholar]
- 22. Ren Y., Savill J. (1998) Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563–568 [DOI] [PubMed] [Google Scholar]
- 23. Fadok V. A., McDonald P. P., Bratton D. L., Henson P. M. (1998) Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem. Soc. Trans. 26, 653–656 [DOI] [PubMed] [Google Scholar]
- 24. Bréchot N., Gomez E., Bignon M., Khallou-Laschet J., Dussiot M., Cazes A., Alanio-Bréchot C., Durand M., Philippe J., Silvestre J. S., Van Rooijen N., Corvol P., Nicoletti A., Chazaud B., Germain S. (2008) Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One 3, e3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menzies F. M., Henriquez F. L., Alexander J., Roberts C. W. (2010) Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin. Exp. Immunol. 160, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hart P. H., Jones C. A., Finlay-Jones J. J. (1995) Monocytes cultured in cytokine-defined environments differ from freshly isolated monocytes in their responses to IL-4 and IL-10. J. Leukoc. Biol. 57, 909–918 [DOI] [PubMed] [Google Scholar]
- 27. Heusinkveld M., de Vos van Steenwijk P. J., Goedemans R., Ramwadhdoebe T. H., Gorter A., Welters M. J., van Hall T., van der Burg S. H. (2011) M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J. Immunol. 187, 1157–1165 [DOI] [PubMed] [Google Scholar]
- 28. Murray P. J., Wynn T. A. (2011) Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 89, 557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 [DOI] [PubMed] [Google Scholar]
- 30. Mosser D. M. (2003) The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212 [DOI] [PubMed] [Google Scholar]
- 31. Vidal B., Serrano A. L., Tjwa M., Suelves M., Ardite E., De Mori R., Baeza-Raja B., Martínez de Lagrán M., Lafuste P., Ruiz-Bonilla V., Jardí M., Gherardi R., Christov C., Dierssen M., Carmeliet P., Degen J. L., Dewerchin M., Muñoz-Cánoves P. (2008) Fibrinogen drives dystrophic muscle fibrosis via a TGFβ/alternative macrophage activation pathway. Genes Dev. 22, 1747–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foster W., Li Y., Usas A., Somogyi G., Huard J. (2003) γ Interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 21, 798–804 [DOI] [PubMed] [Google Scholar]
- 33. Daley J. M., Brancato S. K., Thomay A. A., Reichner J. S., Albina J. E. (2010) The phenotype of murine wound macrophages. J. Leukoc. Biol. 87, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brancato S. K., Albina J. E. (2011) Wound macrophages as key regulators of repair: origin, phenotype, and function. Am. J. Pathol. 178, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shearer J. D., Richards J. R., Mills C. D., Caldwell M. D. (1997) Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am. J. Physiol. 272, E181–E190 [DOI] [PubMed] [Google Scholar]
- 36. Munder M., Mollinedo F., Calafat J., Canchado J., Gil-Lamaignere C., Fuentes J. M., Luckner C., Doschko G., Soler G., Eichmann K., Müller F. M., Ho A. D., Goerner M., Modolell M. (2005) Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105, 2549–2556 [DOI] [PubMed] [Google Scholar]
- 37. Raes G., Brys L., Dahal B. K., Brandt J., Grooten J., Brombacher F., Vanham G., Noël W., Bogaert P., Boonefaes T., Kindt A., Van den Bergh R., Leenen P. J., De Baetselier P., Ghassabeh G. H. (2005) Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J. Leukoc. Biol. 77, 321–327 [DOI] [PubMed] [Google Scholar]
- 38. Satriano J., Matsufuji S., Murakami Y., Lortie M. J., Schwartz D., Kelly C. J., Hayashi S., Blantz R. C. (1998) Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J. Biol. Chem. 273, 15313–15316 [DOI] [PubMed] [Google Scholar]
- 39. Wynn T. A., Barron L. (2010) Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wehling-Henricks M., Jordan M. C., Gotoh T., Grody W. W., Roos K. P., Tidball J. G. (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One 5, e10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villalta S. A., Nguyen H. X., Deng B., Gotoh T., Tidball J. G. (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hesse M., Modolell M., La Flamme A. C., Schito M., Fuentes J. M., Cheever A. W., Pearce E. J., Wynn T. A. (2001) Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 [DOI] [PubMed] [Google Scholar]
- 43. Jansen A., Lewis S., Cattell V., Cook H. T. (1992) Arginase is a major pathway of L-arginine metabolism in nephritic glomeruli. Kidney Int. 42, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 44. Jude E. B., Boulton A. J., Ferguson M. W., Appleton I. (1999) The role of nitric oxide synthase isoforms and arginase in the pathogenesis of diabetic foot ulcers: possible modulatory effects by transforming growth factor β 1. Diabetologia 42, 748–757 [DOI] [PubMed] [Google Scholar]
- 45. Schnoor M., Cullen P., Lorkowski J., Stolle K., Robenek H., Troyer D., Rauterberg J., Lorkowski S. (2008) Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J. Immunol. 180, 5707–5719 [DOI] [PubMed] [Google Scholar]
- 46. Gratchev A., Guillot P., Hakiy N., Politz O., Orfanos C. E., Schledzewski K., Goerdt S. (2001) Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand. J. Immunol. 53, 386–392 [DOI] [PubMed] [Google Scholar]
- 47. Kim H. J., Kim I. S. (2008) Transforming growth factor-β-induced gene product, as a novel ligand of integrin αMβ2, promotes monocytes adhesion, migration and chemotaxis. Int. J. Biochem. Cell Biol. 40, 991–1004 [DOI] [PubMed] [Google Scholar]
- 48. Bae J. S., Lee S. H., Kim J. E., Choi J. Y., Park R. W., Yong Park J., Park H. S., Sohn Y. S., Lee D. S., Bae Lee E., Kim I. S. (2002) βIg-H3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 294, 940–948 [DOI] [PubMed] [Google Scholar]
- 49. LeBaron R. G., Bezverkov K. I., Zimber M. P., Pavelec R., Skonier J., Purchio A. F. (1995) β IG-H3, a novel secretory protein inducible by transforming growth factor-β, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J. Invest. Dermatol. 104, 844–849 [DOI] [PubMed] [Google Scholar]
- 50. Nacu N., Luzina I. G., Highsmith K., Lockatell V., Pochetuhen K., Cooper Z. A., Gillmeister M. P., Todd N. W., Atamas S. P. (2008) Macrophages produce TGF-β-induced (β-Ig-H3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J. Immunol. 180, 5036–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thapa N., Lee B. H., Kim I. S. (2007) TGFBIp/βIg-H3 protein: a versatile matrix molecule induced by TGF-β. Int. J. Biochem. Cell Biol. 39, 2183–2194 [DOI] [PubMed] [Google Scholar]
- 52. Yun S. J., Kim M. O., Kim S. O., Park J., Kwon Y. K., Kim I. S., Lee E. H. (2002) Induction of TGF-β-inducible gene-h3 (βIg-H3) by TGF-β1 in astrocytes: implications for astrocyte response to brain injury. Brain Res. Mol. Brain Res. 107, 57–64 [DOI] [PubMed] [Google Scholar]
- 53. Bellón T., Martínez V., Lucendo B., del Peso G., Castro M. J., Aroeira L. S., Rodríguez-Sanz A., Ossorio M., Sánchez-Villanueva R., Selgas R., Bajo M. A. (2011) Alternative activation of macrophages in human peritoneum: implications for peritoneal fibrosis. Nephrol. Dial. Transplant. 26, 2995–3005 [DOI] [PubMed] [Google Scholar]
- 54. Mahdavian Delavary B., van der Veer W. M., van Egmond M., Niessen F. B., Beelen R. H. (2011) Macrophages in skin injury and repair. Immunobiology 216, 753–762 [DOI] [PubMed] [Google Scholar]
- 55. Schäffer M., Bongartz M., Hoffmann W., Viebahn R. (2006) Regulation of nitric oxide synthesis in wounds by IFN-γ depends on TNF-α. J. Invest. Surg. 19, 371–379 [DOI] [PubMed] [Google Scholar]
- 56. Miao M., Niu Y., Xie T., Yuan B., Qing C., Lu S. (2012) Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 20, 203–213 [DOI] [PubMed] [Google Scholar]
- 57. Cheng M., Nguyen M. H., Fantuzzi G., Koh T. J. (2008) Endogenous interferon-γ is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell. Physiol. 294, C1183–C1191 [DOI] [PubMed] [Google Scholar]
- 58. Takamiya M., Fujita S., Saigusa K., Aoki Y. (2008) Simultaneous detection of eight cytokines in human dermal wounds with a multiplex bead-based immunoassay for wound age estimation. Int. J. Legal Med. 122, 143–148 [DOI] [PubMed] [Google Scholar]
- 59. Villalta S. A., Deng B., Rinaldi C., Wehling-Henricks M., Tidball J. G. (2011) IFN-γ promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J. Immunol. 187, 5419–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mirza R., Koh T. J. (2011) Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 56, 256–264 [DOI] [PubMed] [Google Scholar]
- 61. Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J. M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karlmark K. R., Zimmermann H. W., Roderburg C., Gassler N., Wasmuth H. E., Luedde T., Trautwein C., Tacke F. (2010) The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52, 1769–1782 [DOI] [PubMed] [Google Scholar]
- 63. Lee S., Huen S., Nishio H., Nishio S., Lee H. K., Choi B. S., Ruhrberg C., Cantley L. G. (2011) Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perdiguero E., Sousa-Victor P., Ruiz-Bonilla V., Jardí M., Caelles C., Serrano A. L., Muñoz-Cánoves P. (2011) p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. 195, 307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Albina J. E. (1995) On the expression of nitric oxide synthase by human macrophages. Why no NO? J. Leukoc. Biol. 58, 643–649 [DOI] [PubMed] [Google Scholar]
- 67. Mahoney E., Reichner J., Bostom L. R., Mastrofrancesco B., Henry W., Albina J. (2002) Bacterial colonization and the expression of inducible nitric oxide synthase in murine wounds. Am. J. Pathol. 161, 2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kawao N., Nagai N., Tamura Y., Okada K., Yano M., Suzuki Y., Umemura K., Ueshima S., Matsuo O. (2011) Urokinase-type plasminogen activator contributes to heterogeneity of macrophages at the border of damaged site during liver repair in mice. Thromb. Haemost. 105, 892–900 [DOI] [PubMed] [Google Scholar]
- 69. Aoyama T., Inokuchi S., Brenner D. A., Seki E. (2010) CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loke P., Gallagher I., Nair M. G., Zang X., Brombacher F., Mohrs M., Allison J. P., Allen J. E. (2007) Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179, 3926–3936 [DOI] [PubMed] [Google Scholar]
- 71. Bryan D., Walker K. B., Ferguson M., Thorpe R. (2005) Cytokine gene expression in a murine wound healing model. Cytokine 31, 429–438 [DOI] [PubMed] [Google Scholar]
- 72. Fraccarollo D., Galuppo P., Schraut S., Kneitz S., van Rooijen N., Ertl G., Bauersachs J. (2008) Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension 51, 905–914 [DOI] [PubMed] [Google Scholar]
- 73. Deng B., Wehling-Henricks M., Villalta S. A., Wang Y., Tidball J. G. (2012) IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 189, 3669–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Toschi A., Severi A., Coletti D., Catizone A., Musarò A., Molinaro M., Nervi C., Adamo S., Scicchitano B. M. (2011) Skeletal muscle regeneration in mice is stimulated by local overexpression of V1a-vasopressin receptor. Mol. Endocrinol. 25, 1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yona S., Jung S. (2010) Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 17, 53–59 [DOI] [PubMed] [Google Scholar]
- 76. Nair M. G., Gallagher I. J., Taylor M. D., Loke P., Coulson P. S., Wilson R. A., Maizels R. M., Allen J. E. (2005) Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 73, 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harbord M., Novelli M., Canas B., Power D., Davis C., Godovac-Zimmermann J., Roes J., Segal A. W. (2002) Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J. Biol. Chem. 277, 5468–5475 [DOI] [PubMed] [Google Scholar]
- 78. Spencer M., Yao-Borengasser A., Unal R., Rasouli N., Gurley C. M., Zhu B., Peterson C. A., Kern P. A. (2010) Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 299, E1016–E1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Raes G., Van den Bergh R., De Baetselier P., Ghassabeh G. H., Scotton C., Locati M., Mantovani A., Sozzani S. (2005) Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 174, 6561–6562 [DOI] [PubMed] [Google Scholar]