Review on the ability of different TLR agonists to orchestrate antitumor immune responses, or promote tumor growth, underscoring the impact of choosing among TLR agonists when applying these therapies in the clinic.
Keywords: pathogen-associated molecular pattern, tumor therapy, tumor immunity
Abstract
Various TLR agonists are currently under investigation in clinical trials for their ability to orchestrate antitumor immunity. The antitumor responses are largely attributed to their aptitude to stimulate APCs such as DCs which in turn, activate tumor-specific T cell responses. However, there is a potential for TLR signaling to occur on cells other than professional APCs that could negate antitumor responses or even worse, promote tumor growth. The impetus for this review is twofold. First, there is accumulating data demonstrating that the engagement of TLRs on different T cell subsets and different cancer types could promote tumor growth or conversely, contribute to antitumor responses. Second, the efficacy of TLR agonists as monotherapies to treat cancer patients has been limited. In this review, we discuss how TLR signaling within different T cell subsets and cancer cells can potentially impact the generation of antitumor responses. Based on evidence from preclinical models and clinical trials, we draw attention to several criteria that we believe must be considered when selecting TLR agonists for developing effective immunotherapeutic strategies against cancer.
Introduction
In the late 1800s, Dr. William Coley reported that injection of killed bacteria (Streptocococcus pyogenes) into inoperable tumors reduced tumor growth in some patients [1]. The identification of the receptor that contributes to the immunostimulatory capacity of Coley's vaccine, however, was not discovered until almost one century later, when Beutler and colleagues [2] identified TLR4 as the receptor for LPS. It is now widely accepted that the proinflammatory activity of Coley's toxin, which contains various bacterial components, including highly immunostimulatory LPS, is, in part, mediated by the engagement of TLRs on immune cells [3–5].
TLRs play a vital role in activating immune responses. TLRs recognize conserved pathogen-associated molecular patterns (PAMPs) expressed on a wide array of microbes, as well as endogenous DAMPs released from stressed or dying cells [6–8]. Table 1 provides an overview of TLRs, cellular localization, agonists, and use in cancer therapeutics. TLR1, -2, -4, -5, -6, and -10 are expressed on the cell surface, whereas TLR3, -7, -8, and -9 are situated on endosomal membranes within the cell [5, 9, 10]. TLR1 and TLR2 can heterodimerize to recognize a variety of bacterial lipid structures and cell wall components, such as triacylated lipoproteins, lipoteichoic acid, and β-glucans [11]. TLR2 also heterodimerizes with TLR6 to bind diacylated lipopeptides [11]. Additionally, TLR2 can bind various endogenous DAMPs, such as HSPs, HMGB1, uric acid, fibronectin, and other extracellular matrix proteins [5]. It has also been suggested that TLR1 and TLR6 can heterodimerize with TLR10; however, the TLR agonist recognized by this dimer remains to be identified [12, 13]. TLR3 recognizes viral dsRNA, as well as synthetic analogs of dsRNA, such as ligand Poly I:C [11, 14]. TLR4 binds LPS in complex with lipid A binding protein, CD14, and myeloid differentiation protein 2, MD2 as well as recognizing various DAMPs [5]. Endogenous TLR4 ligands, which have been described, include β-defensin 2, fibronectin extra domain A EDA, HMGB1, Snapin, and tenascin C [15–19]. TLR5 recognizes bacterial flagellin, TLR7 and TLR8 bind viral ssRNA, whereas TLR9 interacts with unmethylated CpG DNA from bacteria and some viruses [5, 9]. Additional TLRs have been identified more recently in mice based on sequence homology of the highly conserved TIR domain [20]. TLR10 is a surface receptor whose natural ligand remains unknown [12, 13]. TLR11, -12, and -13 are present in mice but not in humans. TLR11 was shown to bind a T. gondii profilin and uropathogenic Escherichia coli. The ligand for TLR12 has not yet been identified, whereas TLR13 is an endosomal receptor that recognizes VSV [9, 20, 21].
Table 1. Overview of TLRs, Agonists, and Use in Cancer Therapeutics.
| TLR | Cellular localization | Adaptor molecule | Ligand/agonist | Source of ligand | Clinical |
|---|---|---|---|---|---|
| TLR1-TLR2 | Surface | MyD88 | Triacylated lipoproteins, lipoteichoic acid, peptidoglycans | Bacteria | BCGa |
| Zymosan | Fungi | – | |||
| Pam3CSK4 | Synthetic | – | |||
| TLR2-TLR6 | Surface | MyD88 | Diacylated lipopeptides | Bacteria | BCGa |
| HSPs, HMGB1, uric acid, fibronectin, ECM proteins | Endogenous | – | |||
| Pam3CSK4 | Synthetic | – | |||
| TLR3 | Endosome | TRIF | dsRNA | Virus | – |
| Poly I:C | Synthetic | Poly A:U | |||
| TLR4 | Surface (or endosome) | MyD88 or TRIF | LPS, lipoteichoic acid | Bacteria | BCGa |
| β-defensin 2, fibronectin EDA, HMGB1, snapin, tenascin C | Endogenous | ||||
| Synthetic | MPLa | ||||
| TLR5 | Surface | MyD88 | Flagellin | Bacteria | – |
| TLR7-TLR8 | Endosome | MyD88 | ssRNA | Virus | – |
| CpG-A, Poly G10, Poly G3 | Synthetic | Imiquimod (Aldara)a 852A (Phase II) | |||
| TLR9 | Endosome | MyD88 | Unmethylated CpG DNA | Bacteria and virus | – |
| Bacteria | BCGa | ||||
| Synthetic | EMD 120108 (Phase I) | ||||
| IMO-2055 (Phase II) | |||||
| TLR10 | Surface | MyD88 | Unknown natural ligand | Synthetic | – |
| Pam3CSK4, PamCysPamSK4 | |||||
| TLR11b | – | – | Toxoplasma gondii profilin | Protozoa | – |
| TLR12b | – | – | Unknown | – | – |
| TLR13b | Endosome | MyD88 | VSV | Virus | – |
TLRs are characterized by their cellular localization, adaptor proteins, and the PAMPs and DAMPs that they recognize. Agonists come from a variety of sources—natural and synthetic. Several TLR agonists are approved for clinical use, whereas others are being tested in clinical trials for their potential as anticancer therapies.
FDA-approved treatment for cancer;
expression detected in mouse cells but not human cells.
The engagement of all TLRs except TLR3 results in the recruitment of the adaptor protein MyD88 to the TIR domain on TLRs and is also required for IL-1R, IL-18R, and IL-33 signaling [22, 23]. IRAK-4 binds to MyD88, resulting in phosphorylation of IRAK-1, which through a series of steps involving TRAF-6, leads to the activation of the transcription factor NF-κB [5, 24]. NF-κB translocates into the nucleus and regulates the expression of a variety of genes involved in cell survival, proliferation, and proinflammatory cytokines [25]. Depending on the cell type and the TLR that is activated, TLR signaling can also activate MAPKs JNK, p38, and ERK [5, 8], resulting in the activation of numerous transcription factors, including, AP-1, Elk-1, and CREB [26–28].
TLR3 exclusively uses the TRIF adapter molecule, whereas TLR4 recruits TRIF through a bridging adapter called TRIF-related adaptor molecule (TRAM). Thus, TLR4 is the only TLR that can signal through MyD88 and TRIF [9]. TRIF signaling can also result in NF-κB, ERK, JNK, and p38 activation; however, the major transcription factor activated by TRIF is IFN regulatory factor 3, which is responsible for type I IFN production [29]. The engagement of TLR3 and TLR4 leads to the production of various cytokines, such as TNF-α, IL-6, pro-IL-1β, and IL-12, which help shape the proinflammatory response [5]. The precise pro- or antitumor outcome of TLR signaling depends on the cell type in which the TLR signaling occurs, the particular TLR that is stimulated, and the downstream signaling cascade that becomes activated in those cells. Table 1 summarizes the different TLRs and their cellular localization and ligands.
TLR agonists are currently under investigation as vaccine adjuvants in anticancer therapies for their ability to activate immune cells and promote inflammation. In humans, although TLRs have been detected on many cell types, most TLRs are expressed primarily on monocytes, mature macrophages, and DCs [11]. TLR stimulation on these cell types induces the expression of various membrane-bound costimulatory molecules, including B7.1 (CD80), B7.2 (CD86), and CD40, as well as the cytokine IL-12 that is necessary for the optimal activation of T cells [30, 31]. Beyond the impact of TLR stimulation on APCs, recent studies have revealed that TLR signaling within other cell types can also play a very important role in tumor growth. A growing body of evidence indicates that TLRs are expressed or can be induced on various cell types, including T cells and tumor cells [9, 32]. This review summarizes the current knowledge about TLR signaling in various T cell subsets and tumor cells and briefly comments on the results of several recent clinical trials using TLR agonists in tumor immunotherapy. In addition, we offer several criteria that we believe should be considered when selecting TLR agonists for treatments in cancer immunotherapy.
TLR SIGNALING IN T CELL SUBSETS
TLR agonists play a fundamental role in activating innate and adaptive immune responses. In mouse models, treatment with TLR agonists has been shown to reduce tumor growth and in some cases, destroy established tumors when used in combination with other therapeutic agents, such chemotherapy drugs, mAb, and various tumor antigen vaccines in the form of proteins, peptides, or plasmid DNA [16, 33–37]. The selection of TLR agonists has been premised on their ability to activate professional APCs, namely DCs. However, the engagement of TLRs on various T cell subsets has more recently been demonstrated to augment their responses and thus represents a novel and promising strategy to enhance the efficacy of cancer immunotherapies. In the next section, we review the effects that stimulating distinct TLRs have on different T cell subsets and comment on the implications these effects might have on generating effective immunotherapies.
T cell activation is characterized by three well-defined phases. During the initial phase, naïve T cells receive signals from the priming APC, which induces T cells to enter a highly proliferative state and also stimulates them to up-regulate the expression of various effector/cytolytic molecules, including IFN-γ, perforin, and granzyme. However, activation also results in the induction of activation-induced cell death within days of activation [38], resulting in a sharp contraction phase. The surviving T cells enter the third phase in which they become long-lived memory T cells with the capacity of self-renewal and ability to respond to antigen stimulation within hours of exposure. Memory T cells are dependent on specific cytokines, such as IL-7 and IL-15, for their survival [39, 40]. A greater understanding of the signals that can enhance the magnitude, duration, and survival of tumor-specific T cells would offer the opportunity for improving the T cell-based immunotherapies. We postulate that the engagement of specific TLRs on effector T cells contributes to antitumor activity and T cell survival and that TLR signaling in memory T cells might contribute to their homeostatic maintenance.
In general, the TLR expression profile on T cells varies according to their state of activation, as well as the T cell subset. Naïve T cells express low levels of TLR mRNA transcripts and protein. Upon activation through the TCR or using the stimulant PMA/ionomycin [41], TLR mRNA and protein expression levels are increased dramatically. Furthermore, it is important to note that the costimulatory effects of TLR on T cells are dependent on concomitant TCR stimulation, as TLR ligands alone have little effects on naïve or resting T cells [42, 43]. TLR expression is transient and is down-regulated gradually over the course of several days [41, 42, 44]. Interestingly, the expression of certain TLRs is maintained on mouse and human memory T cells, albeit at lower levels than on activated T cells, and is functional even in the absence of TCR triggering [45, 46].
TLR1-TLR2 and TLR2-TLR6
The engagement of TLR1-TLR2 on CD8+ CTLs dramatically increases the production of IFN-γ [42, 43, 47–50], TNF-α [48, 49], and IL-2 production [48–51]. TLR2 engagement can also enhance the production of granzyme B and perforin, which are two of the major cytolytic molecules secreted by cytotoxic CD8+ T cells [52]. The physiological significance of TLR signaling in T cells is highlighted in experiments demonstrating that the adoptive transfer of TCR transgenic CD8+ pmel T cells into tumor-bearing MyD88 knockout mice, followed by peritumoral injections of the TLR2 agonist Pam3CSK4, delayed or reversed B16 melanoma tumor growth [53]. The use of MyD88 knockout mice ensured that the costimulatory effects of the TLR1-TLR2 ligand arose from TLR signaling in CD8+ T cells and not on endogenous host cells. In addition to the synthetic TLR1-TLR2 agonists, bacterial lipoprotein has been reported to increase antigen-specific tumor killing by CD8+T cells, in in vitro and in vivo models [54, 55].
Certain DAMPs, such as HSPs, are also capable of directly modulating T cell responses. For example, Hsp60 has been found to stimulate TLR2 on human CD45RO+ memory and CD45RA+ naive T cells, resulting in increased β1-integrin-dependent adhesion and reduced chemotaxis by decreasing the expression of chemokine receptors CXCR4 and CCR7 [56]. Integrins on T cells play an important role in sustaining interactions between T cells and APCs or tumor cells and serve to potentiate T cell activation [57], and are also useful markers to distinguish memory and effector T cell subsets [58].
Immune suppression and T cell tolerance represent major obstacles for achieving effective and durable antitumor responses. Among the various cellular mechanisms that hinder a productive antitumor response are those mediated by TRegs. The immunosuppressive activity of these cells is, in part, mediated by the production of IL-10 and TGF-β, which severely limit the cytolytic activity of tumor-specific CD8+ T cells. Intriguingly, TLR2 stimulation directly on TRegs has been shown to reduce their suppressive function, as demonstrated by the proliferation of CD8+ T cells grown in coculture with bacterial lipoprotein-treated TRegs [50]. Similarly, in murine TRegs, the reversal of suppression following TLR2 engagement required TCR activation and IL-2 in vitro and in vivo. Liu and others [59–62] surmised that this inhibition could have been a result of the strong costimulatory signal induced by TLR2 activation, resulting in temporal reversal of TReg function.
Another obstacle for developing potent antitumor T cell responses resides in the fact that many tumor antigens are of low affinity and as such, provide insufficient stimulation to activate TCR signaling [63, 64]. Various studies, however, have demonstrated that TLR1-TLR2 activation on CD8+ T cells reduces the TCR activation threshold and facilitates the generation of memory cells in response to a weak TCR signal [43, 53, 65]. The costimulatory effects of TLR signaling are associated with increased PI3K and PKC signaling [40, 66]. TLR stimulation in CD8+ T cells was also found to increase the expression levels of the transcription factor T-bet and was associated with increased binding to the IFN-γ, granzyme B, and perforin promoter regions [52]. The physiological significance of lowering the TCR activation threshold to weakly immunogenic tumor antigens is highlighted in experiments demonstrating that tumor-bearing MyD88 knockout mice injected with the TLR2 ligand Pam3CSK4 and the TCR transgenic CD8+ pmel T cells reduced melanoma tumor growth compared with mice injected with pmel T cells alone. In contrast, mice injected with TLR2 knockout pmel or MyD88 knockout pmel T cells did not demonstrate enhanced antitumor responses when administered together with the TLR2 ligand [53]. Other studies have also alluded to the potential for lipopeptide-based vaccines to induce a broader antigen-specific T cell repertoire as a result of the activation of T cells with weak TCR antigen thresholds [67]. γδ T cells have also been found to express TLRs and upon concomitant activation through the TCR, enhance IFN-γ production and degranulation as marked by increased expression of CD107a [68].
TLR2 stimulation on human CD4+CD45RO+ memory cells also induces IFN-γ production, and these levels are increased when combined with IL-2 [43, 48]. Lipoproteins from Mycobacterium tuberculosis, a TLR2 agonist, can stimulate memory CD4+ T cells directly, resulting in enhanced proliferation, as well as IL-2 and IFN-γ production. Although resting CD4+ T cells responded to lipoproteins, as evidenced through NF-κB activation, such as CD8 T cells, CD4 T cells also required concomitant TCR signaling to induce proliferation and cytokine production [69]. In addition to enhancing T cell effector function, TLR2 agonists have been shown to promote T cell longevity and are associated with increased expression of antiapoptotic molecules A1 and Bcl-xL and down-regulation of the proapoptotic protein Bim [43, 53].
TLR3
Activated CD4+ T cells express TLR3, which upon stimulation with Poly I:C, increases NF-κB-dependent cell proliferation and survival [70]. Enhanced cell survival is associated with increased expression levels of the antiapoptotic molecule Bcl-xL [70]. Hervas-Stubbs demonstrated that Poly I:C also induced CD8+ T cell proliferation and enhanced their response in a manner that bypassed the need for CD4+T cell help or the expression of costimulatory molecules on APCs [54]. Additionally, TLR3 costimulation (along with TCR stimulation) promoted the generation of memory T cells, which was, in part, a result of the ability of TLR3 to prolong T cell survival. The aptitude for TLR3 signaling in T cells to sidestep the need for APC- or CD4-mediated costimulation and promote memory T cell generation is an important attribute when designing cancer vaccines, as the tumor environment lacks costimulatory signals [46].
In human CD8+ T cells, Poly I:C-mediated stimulation of PHA-activated effector or memory T cells enhanced IFN-γ production but did not appear to enhance their lytic function [71]. Likewise, pretreatment with Poly I:C followed by activation with antigen-pulsed splenocytes augmented mouse CD8+ T cell proliferation and IFN-γ secretion. TLR3-stimulated CD8+ T cells displayed increased levels of the activation marker CD69, as well as the high-affinity IL-2R α-chain (CD25) and upon adoptive transfer, demonstrated an increased expansion potential compared with untreated cells [72]. Freshly isolated γδ T cells also respond to TLR3 stimulation and show increased IFN-γ production and enhanced CD69 expression [73]. A study by Shojaei [74] showed that in vitro-expanded γδT cells pretreated with Poly I:C and TCR stimulation with bromohydrin pyrophosphate (a synthetic phosphoantigen recognized by γδT cells) increased the production of granzyme A and B, thus augmenting their cytolytic activity.
TLR4
The ability of LPS to activate and promote T cell proliferation has been largely attributed to its ability to stimulate APCs and induce the production of vast amounts of proinflammatory cytokines that promote nonspecific or bystander T cell expansion and activation [75]. However, CD4+ and CD8+ T cells can also respond to TLR4 stimulation with LPS. The first report of TLR4-stimulating T cells was demonstrated by Vogel et al. [76], who reported that a cloned murine IL-2-dependent cytotoxic T cell line, CT 6, proliferated in response to LPS. TLR4 engagement directly on human CD8+ T cells has also been shown to induce the production of IFN-γ, TNF-α, perforin, and granzyme B [77]. In sharp contrast, murine CD8+ T cells do not appear to express TLR4 or its accessory protein CD14 and do not respond to TLR4 stimulation [77]. In comparison with naïve murine T cells, which do not respond to most TLR agonists, the addition of LPS to naïve CD4+ T cell enhanced their proliferation and survival in vitro [78]. A more thorough analysis of the CD4+ T cell subset that expresses and responds to TLR4 signaling revealed that TLR4 mRNA was expressed primarily by murine Th17 CD4+ T cell subsets compared with Th1 and Th2 subsets [79]. Furthermore, in murine CD4+ T cells, LPS reduced IFN-γ levels but increased IL-17A production [80]. These effects on CD4+ Th cells were a result of decreased MAPK activation. TLR4 stimulation on CD4+ T cells has also been demonstrated to aggravate intestinal inflammation and play an important role in inducing colitis, highlighting the potent effects that TLR stimulation on T cells can provoke.
A role for TLR4 signaling in CD4+CD25+ TRegs is less clear. A study by Caramalho [81] demonstrated that LPS can activate CD4+CD25+ TRegs (from C3H/HeN mice), induce their proliferation, and enhance their immunosuppressive activity. This is in sharp contrast to the inhibitory effects that TLR1-TLR2 stimulation has on TRegs. On the other hand, Zhu et al. [66] found that TLR4 engagement on TRegs (from C57BL/6 mice) with HMGB1 reduced the expressions of CTLA4 and forkhead box p3 and diminished IL-10 production. Moreover, activating TLR4 on TRegs induced signals via TRIF (with less dependence on MyD88), whereas TLR4 stimulation on non-TReg T cells appears to occur primarily via p38 MAPK and MyD88 signaling [81]. These disparate results might have occurred from the use of different TLR4 agonists or from distinctions arising from different genetic backgrounds.
TLR5
Similar to the effects of other TLR agonists on T cells, the engagement of TLR5 on human CD4+ T cells with the bacterial component flagellin can induce IFN-γ, IL-8 and IL-10 but not IL-4 production. The costimulatory effects of the TLR5 ligand were more pronounced on effector memory CD4+CCR7− cells than CCR7+ central memory cells [82]. The production of IFN-γ (and lack of IL-4) can skew toward a Th1 response and is beneficial for generating efficient CD8+ T cell responses. Flagellin also induced proliferation and increased the expression of IFN-γ, TNF-α, and granzyme B of human cord blood CD8+ T cells [51]. Intriguingly, when used in combination with the synthetic TLR2 agonist Pam3CSK4, the responses generated in CD8+ T cells were stronger than either of these TLR agonists alone. These results highlight the potential additive or synergistic effects that combining different TLR ligands have on T cell responses and could serve to enhance the efficacy of antitumor T cell responses in vivo.
In contrast to the inhibitory effects that other TLR agonists (such as TLR1-TLR2 agonists) have on murine TRegs, TLR5 stimulation on human CD4+CD25+ TRegs has been reported to increase their expansion potential and augment their suppressive activity [83]. However, it is important to note that these effects were observed only when TRegs were stimulated in vitro. Whether TLR5 stimulation on TRegs occurs in vivo has yet to be determined. Furthermore, it is important to consider whether TLR5 stimulation on other cell types, such DCs or macrophages, might favor the generation of a cytokine milieu that suppresses TReg activity. These studies highlight the need to better characterize the effects that different TLR agonists might have on T cell subsets to avoid activating T cell subsets that could promote tumor growth. These reports also emphasize the importance of differentiating the effects that TLR agonists have on mouse and human T cells.
TLR7-TLR8
TLR8 is expressed on human TRegs but not on naïve CD4+ T cells. Peng et al. [84] demonstrated that CpG-A, which is a TLR8 ligand that induces high levels of IFN-α and IFN-β, reversed the suppressive activity of TRegs and thus, restored the proliferation of effector CD4+ T cells. Furthermore, TRegs pretreated with Poly-G10 (a modified TLR8 ligand) prior to adoptive transfer into a tumor-bearing mouse demonstrated a loss of suppressive activity and resulted in enhanced antitumor activity. This study also highlighted that in addition to TLR8, TLR9 (expressed on TRegs) can recognize CpG DNAs. Similarly, the stimulation of TLR8 using Poly-G3 or ssRNA40 on a unique population of human suppressor γδ T cells was reported to reverse their immunosuppressive function exerted on CD4+ T cells in in vivo and in vitro conditions [85]. In human CD4+ Th cells, the stimulation of TLR7/8 with the synthetic agonist resiquimod (R-848) increased IFN-γ, IL-2, and IL-10 production and enhanced proliferation in an APC-independent manner [82].
TLR9
Several studies have shown that TLR9 engagement on CD4+ T cells can enhance their survival and therefore, could potentiate antitumor responses by prolonging T cell activity [60]. Gelman et al. [70] reported that the enhanced longevity of TLR9-stimulated mouse T cells in vitro was dependent on NF-κB signaling and was associated with increased expression of the antiapoptotic protein Bcl-xL. Marsland et al. [86] reported that TLR9-mediated costimulation of T cells overcame T cell dependence for PKC-ϕ signaling. Furthermore, their studies indicated that TLR9 ligand reversed their anergic status and re-established proliferation and survival in vitro. Interestingly, the stimulation of TLR9 on rat CD4+ T cells with the agonist CpG-ODN made them (CD4+ T cells) moderately resistant against the suppressive effects mediated by TRegs. The inclusion of an immune adjuvant capable of abrogating the effects of TRegs is a worthy attribute in developing effective cancer immunotherapies considering the important role of TRegs in promoting tumor growth [87]. In addition to prolonging CD4+ T cell survival and suppressing TReg activity, TLR9 ligands increase CD4+ and CD8+ T cell numbers by augmenting IL-2 production and IL-2R expression. Of note, TLR9-mediated activation also functions in the absence of CD28, emphasizing the potential of TLR9 to serve as a costimulatory signal for CD4+ T cells, which is critical given the lack of costimulatory molecules within the tumor environment [88]. Finally, our group demonstrated that TLR9 engagement on murine CD4+ T cells reduces γ-radiation-induced apoptosis and was associated with increased DNA repair rates [89].
MyD88
Recent studies have also highlighted an obligatory role for MyD88 in murine CD4+ and CD8+ T cell survival [52, 90, 91]. For instance, two recent studies established that knocking out MyD88 in lymphocytic choriomeningitis virus-specific CD8+ T cells severely impaired their expansion in vivo [90, 91]. Interestingly, whereas the expression of MyD88 in CD8+ T cells was not necessary for activation, it was absolutely required for their survival. Likewise, Zhao et al. [92] verified that vv-specific, MyD88-deficient CD8+ T cells were significantly slower to expand in vivo compared with WT vv-specific T cells. We reported recently [53] that unlike WT pmel CD8+ T cells, MyD88-deficient pmel T cells demonstrated weak antitumor activity, which was, in part, a result of their inability to persist and generate adequate numbers of memory T cells. Furthermore, overexpressing TLR2 on CD8+ T cells enhanced their antitumor activity. Like CD8+ T cells, CD4+ T cells also required MyD88 expression to generate an effective response against a T. gondii infection in vivo [93]. It is important to note that whereas the absence of MyD88 impairs T cell survival, eliminating TRIF, TLR2, TLR4, TLR9, or IL-1R in T cells does not alter T cell survival, highlighting a critical and specific role for MyD88 signaling in T cells. The prosurvival effects of MyD88 appear to involve the activation of the PI3K–Akt pathway and to some degree, the mammalian target of rapamycin pathway [52, 94]. It is also important to note that in addition to transducing TLR signals, MyD88 is a key molecule for IL-1/IL-18/IL-33 signaling and could therefore have profound effects on T cell biology by transmitting signals via these other receptors.
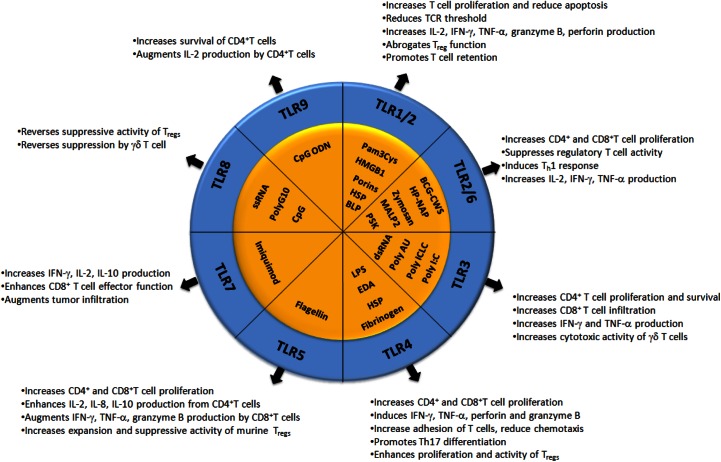
Collectively, these studies indicate that any future treatments intended to activate the immune system against cancer could benefit from the inclusion of TLR agonists that can: 1) stimulate CD4+ and CD8+ T cells to promote proliferation; 2) promote T cell longevity and memory T cell development; 3) augment effector function; 4) boost TCR signals to weakly immunogenic tumor antigens; 5) render T cells resistant to the suppressive effects of TReg; and 6) lessen CD4+ TReg-suppressive ability. It is also important to highlight that further studies elucidating the effects that these compounds have on different T cell subsets and delineating the effects that they have on mouse and human T cells will be essential to take full advantage of their immunostimulatory capacity. The effects of TLR engagement on different T cell subsets is provided in Fig. 1.
Figure 1. Effects of TLR engagement on different T cell subsets.
BLP, Bacterial lipoprotein; CWS, cell-wall skeleton; HP-NAP, Helicobacter pylori neutrophil-activating protein; MALP2, mycoplasma diacylated lipoprotein 2; PSK, polysaccharide krestin; Poly ICLC, polyriboinosinic-polyribocytidylic acid.
TLR SIGNALING IN TUMOR CELLS
Antitumor effects of TLRs
The engagement of specific TLRs on cancer cells can impact tumor growth by various mechanisms, including inducing apoptosis and potentiating the effects of chemotherapy [95]. The following sections outline examples of current studies that illustrate the antitumor effects of TLR signaling on tumor growth and development.
TLR1-TLR2
The expression of TLR2 on urothelium- and nonmuscle-invasive bladder tumors has been reported to be induced following incubation with Mycobacterium BCG in vitro [96–98]. BCG is a live-attenuated Mycobacterium bovis that is enriched in peptidoglycans and unmethylated CG-containing DNA, which primarily stimulates TLR2, TLR4, and TLR9. The engagement of TLR2 on bladder cancer cells leads to the nuclear translocation of NF-κB, activation of JNK, and production of IL-1β, IL-6, and IL-8 [99]. Interestingly, treatment with BCG results in the expression of MHC class II and costimulatory molecules, including CD86 and ICAM-1, respectively, on urothelial carcinoma cells [100]. The stimulation of urothelial cell carcinomas with BCG induced cell death and reduced proliferation and motility. The anti-cancer effects of BCG have been associated with increased production of cytotoxic NO in cell lines, as well as in patients treated with BCG [101]. These studies also emphasize the advantage of developing vaccination strategies that incorporate TLR ligands that can stimulate both immune responses and make tumor cells better targets for immune-mediated destruction.
TLR3
TLR3 has been implicated in promoting tumor cell death in various types of cancers. Breast cancer cells express TLR3, and signaling through this receptor induces autocrine type I IFN signaling that results in the apoptosis of human and mouse cancer cells [9, 102, 103]. In human colon cancer cells, for example, TLR3 stimulation with Poly I:C induced apoptosis and worked in synergy when combined with 5-fluorouracil or IFN-α [104]. TLR3 stimulation by BCG on bladder cancer cells also results in the production of IL-1β, IL-6, and IL-8, all of which correlate with favorable outcomes in the BCG treatment of bladder cancer patients [99]. Head and neck carcinoma cells stimulated with Poly I:C showed an increase in ICAM-I, IL-6, and IL-8 secretion. TLR3 stimulation also increased apoptotic and necrotic cell death in human pharynx carcinoma cells [105]. Similar effects were observed following stimulation of TLR2 and TLR5. In another study, endosomal stimulation, but not cell-surface engagement of TLR3 on human hepatocellular carcinoma cells, resulted in caspase-dependent apoptotic cell death [14, 102]. Primary nonsmall cell lung cancer cells were reported to express higher levels of TLR3 compared with cells from precancer patients [106]. The engagement of the TLR3 ligand in human lung cancer cell lines resulted in caspase-dependent apoptosis [107]. Interestingly, the cell death machinery appears to form a complex with the TLR3 itself, activating an extrinsic apoptotic cascade involving caspase-8 [107]. TLR3 stimulation in human prostate cancer cell lines triggers inflammation and antiproliferative signaling and, depending on the TLR3 ligand concentration, can lead to apoptosis [9, 102]. In human melanoma cell lines TLR3 stimulation can reduce cell division or induce cell death [102, 108]. Poly I:C stimulation of human myeloma cell lines also exhibited increased apoptosis, type I IFN secretion, and reduced cell growth in what appeared to be an NF-κB-dependent manner [102]. Type I IFNs can induce the Th1 effector phenotype and further potentiate antitumor immune response [109]. However, not all myeloma cells lines expressed TLR3 [110]. TLR3 polymorphisms have also been linked to an increased risk of nasopharyngeal carcinoma, breast cancer, cervical cancer, and Hodgkin's disease [111].
In addition to inducing cell death, TLR3 signaling can impact cancer cell migration. Rydberg et al. [105] reported that TLR3 engagement decreased the migration of cancer cells when added during the cell-attachment phase; however, if migration had already begun, Poly I:C treatment enhanced migration. These data suggest that TLR3 signaling can impact tumor cell metastases differrently depending on the stage of the tumor and requires further investigation [14, 102].
TLR4
Published reports regarding an antitumor effect of TLR4 signaling in cancer cells are scarce. However, Bauer et al. [112] showed that the expression of TLR4 on lung epithelium can have a protective effect against lung cancer development. In these studies, the authors sought to determine the role of TLR4 in chronic lung inflammation by examining lung permeability, leukocyte infiltration, and NF-κB activation in TLR4-responsive and -unresponsive mice. Butylated hydroxytoluene treatment was used to induce lung cancer. Mice with functional TLR4 signaling exhibited diminished lung permeability, leukocyte infiltration, and tumor formation [112]. It is worth noting that this study is an exception in the current literature, the majority of which portrays TLR4 to play a potent protumor role.
TLR5
TLR5 expression on cancer cells has been shown to abrogate tumor cell growth in some cancers. TLR5 is overexpressed in breast cancer, and activation of TLR5 signaling was demonstrated to block tumor cell proliferation and mechanistically linked to a down-regulation of cyclin B1, cyclin D1, and cyclin E2 expression in a mouse model [113]. The biological significance of TLR5 stimulation on breast cancer cells in mice was also highlighted by increased tumor necrosis and enhanced infiltration of neutrophils [113]. In tissue culture, head and neck cancer cells expressing TLR5 also demonstrated decreased viability and increased apoptosis in response to stimulation with flagellin [105]. Similarly, in a xenograft model of human colon cancer, TLR5 signaling in tumor cells suppressed tumor growth and was associated with tumor necrosis [114].
TLR9
TLR9 signaling on a human glioma cell line contributed to decreased proliferation by inducing cell-cycle arrest when stimulated in conjunction with irradiation [115]. These effects were mediated by NF-κB and NO. Therefore, one therapeutic advantage to the use of TLR9 agonists in this tumor model could be to sensitize tumors to the toxic effects of radiation treatment [115]. However, consideration has to be given to the fact that TLR9 has also been demonstrated to increase the invasive capacity of glioma [116]. Furthermore, TLR9 expression has been shown to exhibit antiproliferative and proapoptotic effects [117]. CpG-ODN stimulation of TLR9 on neuroblastoma cell lines has also been shown to decrease cell proliferation and increase caspase-dependent apoptosis, resulting in increased survival of tumor-bearing mice.
Protumor effects of TLRs
The engagement of TLRs on tumor cells can promote tumor growth by contributing to the maintenance of a chronically inflamed environment, inducing cancer cell proliferation, and promoting cell survival [24, 44, 118–124]. The following section discusses these protumor TLR-mediated mechanisms in more detail as they relate to specific neoplastic diseases.
TLR1-TLR2
TLR2 expression and tumor-promoting signaling have been described in many human cancers including gastric, liver, and lung carcinomas [32, 125–127]. For example, it is generally accepted that H. pylori is a causative entity for developing gastric carcinoma [128]. There is compelling evidence indicating that H. pylori can bind to and signal through various TLRs on gastric epithelial cells, which contributes to inflammation and disease progression [125, 129–132]. TLR2 signaling in human gastric cancer cell lines has also been shown to promote tumor growth by enhancing vascularization and cell invasion through the induction of COX-2, PGE2, and IL-8 [32, 125]. COX-2 and PGE2 contribute to tumor growth, in part, by regulating the expression of various other angiogenic factors [125, 133, 134]. Moreover, PGE2 can down-regulate T cell activation and proliferation by reducing IL-2 levels and lowering expression of the transferrin receptor on T cells [135]. In addition to the ability of IL-8 to induce cancer cell proliferation and survival, it exacerbates inflammation and promotes recruitment of tumor-associated macrophages, which support tumor growth [120, 121, 136]. Listeria monocytogenes was also shown to activate TLR2 signaling in a mouse hepatoma model, resulting in the secretion of NO and IL-6, which have been shown to suppress T cell function by inducing cell-cycle arrest and apoptosis and decreasing the expression of leukocyte adhesion molecules [127, 132, 137, 138]. To put some of these outcomes in perspective, NO and PGE2 are key players in the mechanism by which mesenchymal stem cells suppress immune-cell function [138], and inhibition of the PGE2 enzyme COX-2 has been shown to reduce inflammation and show potential as a cancer treatments. COX-2 inhibitors may potentiate the efficacy of certain chemotherapies [139, 140]. IL-6 has also been demonstrated to promote CD4+ T cell differentiation to TReg or Th17 subsets [141, 142]. Furthermore, it has been shown that autocrine IL-6 signaling by human ovarian cancer cell lines increases chemotherapy drug resistance by up-regulating multidrug resistance-related genes and GSTpi, in addition to the antiapoptotic proteins Bcl-2, Bcl-xL, and X-linked inhibitor of apoptosis [143].
TLR2 polymorphisms have been associated with a higher incidence of breast, colorectal, and prostate cancers in patients [111, 144]. Polymorphisms in TLR2 and TLR5 have also been suggested to have a significant role in gastric carcinogenesis [145]. TLR1 and TLR6 are located within the same gene cluster, and polymorphisms in these genes have also been identified with the increased risk of prostate cancer, nasopharyngeal carcinoma, and breast cancer [111].
TLR4
TLR4 signaling in tumor cells has more frequently been reported to exhibit protumor properties rather than antitumor responses. For example, mice deficient in TLR4 or MyD88 exhibit a significant reduction in the frequency, size, and overall number of chemically induced liver cancer [146]. Moreover, there is evidence supporting a role for long-term alcohol consumption and hepatitis C-mediated tumorigenesis, and hepatitis C virus NS5A transgenic mice exposed to long-term alcohol generate tumors with TLR4 overexpression [55]. These data emphasize a strong association between TLR signaling and hepatocarcinogenesis. In melanoma, Mittal et al. [147] reported that TLR4 engagement with the endogenous protein HMGB1, released from necrotic keratinocytes, induced the recruitment of inflammatory cells that perpetuated chronic inflammation, which they postulated was involved for tumor progression. The LPS from H. pylori has also been shown to activate TLR4 on human gastric cancer cell lines to increase tumor cell proliferation [129]. In addition to stimulation by bacterial LPS and HMGB1, TLR4 can be activated by an assortment of endogenous DAMPs, including HSPs and peptides with abnormal glycosylation patterns expressed by tumors [119, 148]. For instance, HSP90 has been demonstrated to contribute to human glioma cell migration in a TLR4-dependent manner through the association with and transactivation of EGFR, resulting in increased cytosolic Ca2+ levels, ATP release, and actin rearrangement [149]. Finally, individuals with TLR4 polymorphism have also been linked to having a higher susceptibility of developing prostate cancer [150, 151].
In addition to inducing tumor cell proliferation and enhancing cell survival, TLR4 engagement on human breast cancer cell lines has been shown to promote tumor progression by producing factors that promote immune evasion, such as VEGF, NO, IL-6, IL-12, and MMPs [152–154]. NO and IL-6 have been implicated in immune suppression by contributing to the immune evasion of cytotoxic CD8+ T cells and inhibition of NK activity [119, 153]. Furthermore, in mouse colon cancer models, TLR4 stimulation is linked to prolonging tumor-cell survival by up-regulating the expression of the programmed cell death ligand 1 (B7-H1) and ICOS ligand (B7-H2) and by down-regulating the expression of the death receptor Fas [119]. He et al. [155] reported that TLR4 stimulation on human lung cancer cells can also promote immune escape by inducing immunosuppressive cytokines, such as TGF-β, VEGF, and IL-8, while supporting an apoptosis-resistant phenotype.
TLR5
TLR5 is highly expressed on human gastric cancer cells, as well as precursor lesions [131, 156]. TLR5 recognizes ligands on H. pylori, such as flagellin, which upon stimulation in cancer cells, results in increased IL-8 production and tumor cell proliferation [130, 131]. IL-8 has been implicated to be involved in the advancement of human gastric carcinoma by influencing cell adhesion, metastasis, and drug resistance, in addition to its role as a chemoattractant for immune cells [122, 136]. TLR5 stimulation by H. pylori also enhanced the expression levels of TNF-α and promoted cell survival [124, 157, 158]. It is important to note that TNF-α has also been implicated in promoting the suppressive function of TRegs [159].
TLR7-TLR8
TLR7 and TLR8 are overexpressed in lung cancer patients and have been implicated in contributing to inflammation, tumor growth, cell survival, and metastasis [106, 123]. Additionally, TLR7 or TLR8 stimulation significantly increased the chemoresistance of human lung cancer cell lines following treatment with combinations of cycloheximide, cisplatine, carboplatine, doxorubicine, and Navelbine [123]. The TLR7/8-dependent activation of NF-κB in these cells also results in the up-regulation of proinflammatory cytokines (IL-6, IL-8, GM-CSF, IL-1α, IL-12), the antiapoptotic protein Bcl-2, VEGFR2 involved in angiogenesis, and chemokine receptors associated with increased cell migration [123].
TLR9
Human breast cancer and gastric carcinoma cells expressing TLR9 have enhanced invasive capability as a result of the increased secretion of MMP13 and COX-2 upon TLR9 stimulation in tissue culture [125, 154]. MMPs play an important role in tumor-cell invasion, as they cleave ECM proteins that maintain tissue structure and allow for tumor growth [121, 160]. TLR9 also plays a role in the increased production of IL-8, IL-1, and IL-6 in lung cancer cells, further promoting tumor growth [106]. TLR9 engagement on human ovarian cancer has also been shown to increase the invasion and migration of tumor cells [116, 158]. Furthermore, in glioma, tumor cell expression of TLR9 correlates with disease severity in patients [116]. It is interesting to note that in the case of glioma, despite its metastasis-inducing properties, TLR9 had no effect on cell proliferation, highlighting the diverse effects that TLR9 signaling can have on different tumor cell types. CpG DNA, recognized by TLR9 on human prostate epithelial cells, induces NF-κB activation, resulting in increased proliferation and reduced apoptosis, and has been suggested to play a role in early cancer development [161]. The majority of human hepatocellular cancer cells expresses TLR9, which like in many tumor types, aides in tumor cell proliferation, survival, and drug resistance [162]. Intriguingly, these protumor effects are not always mediated by NF-κB activation but in some cases, involve the induction of oncogenes and other regulatory proteins involved in carcinogenesis [162].
MyD88 and IRAK
Aside from the effects of TLR stimulation on cancer cells, it appears that constitutive activation of the MyD88 signaling pathway (and downstream molecules) might also play a role in tumor progression in a TLR agonist-independent pathway. Mizobe and colleagues [163] found a constitutive association between MyD88 and IRAK-1 in HTLV-I-transformed T cell lines but not in uninfected cells. Interestingly, HTLV-1 infection resulted in the expression of TLR1, -6, and -10 mRNA. NF-κB activation in HTLV-transformed cells was inhibited by the overexpression of dominant-negative forms of MyD88 and consequently reduced proliferation and increased apoptosis. We reported recently that various melanoma cell lines express the activated form of p-IRAK-1 and/or p-IRAK-4 in the absence of TLR stimulation [164]. Moreover, ∼45% of melanoma tumor biopsies (n=242) expressed p-IRAK-4 levels, as determined by immunohistochemical evaluation. The levels of p-IRAK-4 did not correlate with clinical stage, gender, or age, but inhibiting IRAK signaling with pharmacological inhibitors or small interfering RNA enhanced cell death in vitro and in vivo in combination with vinblastine. Ngo et al. [165] also demonstrated a critical role for oncogenically active MyD88 mutations in human lymphoma. Collectively, these data suggest that any changes that lead to constitutive TLR-MyD88 signaling can intensify tumor growth.
Summary of TLR Signaling in Tumor Cells
In summary, various TLRs have been reported to be expressed on a multitude of cancer types. TLR3 and TLR5 show the most promise for eliciting direct antitumor effects, whereas TLR4, -7, -8, and -9 display primarily protumor properties when stimulated on tumor cells. It is important to highlight that whereas the engagement of one TLR on a particular tumor type might induce cell death, the stimulation of the same TLR on a different tumor could promote survival, induce proliferation, or promote a chemoresistant phenotype. Therefore, identifying optimal TLR agonists in the framework of cancer immunotherapy requires special consideration with regard to the effects that TLR engagement on tumor cells will have on antitumor responses. We suggest that the selection of TLR agonists should be, in part, based on the TLR expression profile and functional consequence of TLR signaling that occurs within a specific type of cancer. Table 2 provides a summary of the effects of TLR signaling in tumor cells.
Table 2. Summary of the Effects of TLR Signaling in Tumor Cells.
| TLR | Type of cancer | Functional outcome |
|
|---|---|---|---|
| Protumor | Antitumor | ||
| TLR2 | Gastric cancer | Inflammation, vascularization, metastasis, ↑IL-8, COX-2, PGE2 | |
| Hepatocellular carcinoma | Immunosuppression, ↑IL-6, NO | ||
| TLR3 | Breast cancer | Apoptosis, ↑type I IFN | |
| Colon cancer | Apoptosis | ||
| Cervical cancer | Apoptosis | ||
| Head and neck cancer | Apoptosis, necrosis, ↑ICAM-I | ||
| Hepatocellular carcinoma | Apoptosis | ||
| Melanoma | Decreased proliferation, apoptosis | ||
| Myeloma | Apoptosis, ↑type I IFN | ||
| Lung cancer | Proliferation and survival | Apoptosis | |
| Prostate cancer | Inflammation, apoptosis | ||
| TLR4 | Breast cancer | Viability, immune evasion, ↑VEGF, NO, IL-6, IL-12, MMPs | |
| Colon cancer | Inflammation, tumor growth, immune evasion, ↑B7-H2, B7-H2, ↓Fas | ||
| Gastric cancer | Proliferation | ||
| Hepatocellular carcinoma | Carcinogenesis | ||
| Lung cancer | Immunosuppression, immune evasion, reduced apoptosis, ↑TGF-β, VEGF, IL-8 | Decreased lung permeability and inflammation | |
| Melanoma | Carcinogenesis | ||
| TLR5 | Breast cancer | Decreased proliferation, neutrophil infiltration, necrosis | |
| Head and neck cancer | Decreased proliferation, apoptosis | ||
| Colon cancer | Decreased proliferation, necrosis | ||
| Gastric cancer | Proliferation, ↑IL-8, TNF-α, IL-8 | ||
| TLR7/8 | Lung cancer | Proliferation, survival, drug resistance, metastasis, ↑IL-1α, IL-6, IL-8, IL-12, GM-CSF, VEGFR2, Bcl-2 | |
| TLR9 | Breast cancer | Metastasis, ↑MMP | |
| Gastric cancer | Metastasis, ↑COX-2 | ||
| Glioma | Metastasis | Cell-cycle arrest, ↑NO | |
| Hepatocellular carcinoma | Proliferation, survival, drug resistance | ||
| Lung cancer | Proliferation, survival, ↑IL-1, IL-6, IL-8 | ||
| Neuroblastoma | Decreased proliferation, apoptosis | ||
| Ovarian cancer | Metastasis | ||
| Prostate cancer | Proliferation, reduced apoptosis | ||
This table provides a brief overview of the outcome of TLR signaling on various cancer types organized by TLR.
We believe that it is important to identify rapid molecular diagnostics that could provide insights as to which TLR agonist to choose to optimize therapeutic strategies. Our group has examined the expression levels of various TLRs on several types of cancers derived from patients, including melanoma, pancreatic cancer, colon cancer, as well as T-lineage acute lymphoblastic leukemia and AML (ref. [164] and unpublished data). In general, we can detect various TLRs by PCR and Western blot. However, TLR expression on cells is not always sufficient to trigger TLR signaling, and thus, unfortunately, it appears that the expression levels of TLR (or TLR-related proteins) are not likely to be a reliable method to predict the potential effects of TLR stimulation on cancer cells (unpublished data). Another consideration is that the inability for TLR-expressing cells to respond to TLR agonists could be a result of the lack of downstream proteins involved in TLR signaling or the overexpression of negative regulators of TLR signaling [166]. However, these concepts have yet to be tested experimentally. Another potential screen might involve using patients' tumor cells to characterize (by RNA expression profile) their response to TLR ligands in vitro. We envision that the identification of specific gene patterns associated with cell survival, proliferation, metastases, cytokine expression, and chemotherapy and radiotherapy resistance might provide insight for better selection of TLR agonists for immunotherapy. We recognize that a limitation to these types of in vitro analyses does not take into consideration the ability for TLR agonists to localize to the tumor site in vivo. Furthermore, this testing does not take into consideration the effects that the presence of immune cells, which are likely to have a greater aptitude to take up TLR agonists, might have on tumor responses to TLR agonists. However, in general, these assays could provide useful insights as to the potential that certain TLR agonists might have in promoting tumor cell proliferation or enhancing chemotherapy or radiotherapy resistance.
A MULTI-TLR AGONIST APPROACH IN CANCER IMMUNOTHERAPY
The only TLR agonists approved by the FDA for use in cancer patients are BCG (which stimulates TLR2, TLR3, TLR4, and possibly TLR9), MPL (a TLR4 agonist), and imiquimod (TLR7 agonist). Arguably, the most impressive antitumor responses observed have been in patients with bladder cancer treated with BCG, which is composed of a “mixture” of TLR agonists. The potent immunostimulatory effects of BCG were indeed dependent on the simultaneous activation of TLR2 and TLR4 [98], highlighting the advantage to engaging multiple TLRs. BCG is the only FDA-approved agent therapy for bladder carcinomas and is in clinical trials to treat patients with various types of malignancies. In attempts to further enhance the antitumor efficacy of BCG therapy, efforts have also focused on developing approaches to refine tumor-specific immune responses. In particular, the development of autologous whole-cell tumor lysate vaccines administered together with BCG appears to augment antitumor responses and enhance survival in patients with colorectal cancer [167–169], melanoma [170], and renal cell cancer [171]. This vaccination approach appears to elicit strong T cell responses; however, a role for antibody and innate-immune cell responses has not been ruled out. It is important to note that in melanoma patients, DTH reactions associated with antitumor responses (following this type of therapy) decreased after successive vaccinations that lacked BCG. These data suggest that a continuous source of TLR agonists is required to maintain antitumor responses. The inclusion of tumor lysate in vaccine formulations might also have the advantage of stimulating additional TLRs and/or other PRRs via lysate-derived DAMPs [172]. Although the stimulation of TLRs on DCs is critical for the induction of tumor-specific T cell responses, whether the antitumor responses observed in patients were, in part, a result of the stimulation of TLRs on T cells or tumor cells is a topic that merits further investigation.
Recent advances in preclinical studies have also shown evidence of TLR synergy. Conforti et al. [173] reported that injection of the TLR3 agonist Poly A:U or TLR9 agonist CpG-ODN alone failed to control tumor growth unless the two TLR agonists were combined and administered with tumor-associated antigen in mice. Zhu et al. [174] found that engaging specific pairs of TLRs resulted in a synergistic response in their ability to activate DCs and promote antigen-specific T cell responses and proliferation compared with injecting a single TLR agonist. In another study, it was found that specific combinations of TLR2, -3, and -9 ligands, administered at suboptimal doses, synergized and that responses depended on cross-talk between the MyD88 and TRIF signaling pathways [175]. Ahonen et al. [176] also reported that combining the TLR7 agonist imiquimod with CD40 agonists synergized and augmented CD8+ T cell responses nearly 20-fold above the use of either agonist alone. These findings highlight the advantage of combining certain TLR agonists with one another or with other immunostimulatory agents (i.e., anti-CD40 antibody) and indicate that optimizing the anticancer effects of TLR agonists will require careful selection and appropriate sequential timing of TLR ligand administration.
The expression of costimulatory molecules on APCs (or tumor cells) and the cytokine milieu elicited by different TLR agonists will also play a determining role as to whether protumor or antitumor responses are produced. The TLR mixture would ideally consist of one that efficiently matures DCs and produces a cytokine mixture that potentiates T cell activation while having cytotoxic or cytostatic effects on tumor cells. TLR9 stimulation on DCs induces the production of IL-12 and IFN-γ, whereas TLR1-TLR2 stimulation on CD8+ and CD4+ T cells enhances their survival, proliferation, and cytotoxicity [42, 52, 53, 89]. The engagement of various TLRs on human and mouse DCs has also been demonstrated to skew immunity toward an antitumor Th1 response via increased production of the inflammatory mediator IL-12 [177, 178]; however, depending on the TLR agonist, the generation of a Th2-type response can also be elicited in response to MyD88-dependent TLR signaling [179–181]. Interestingly, whereas the engagement of TLR4 alone induced the generation of IL-4-producing DCs (Th2-type), the coengagement of TLR4 and TLR7/TLR8 tilted toward the generation of IFN-γ-producing (Th1-type) DCs [178]. Therefore, it is necessary to identify a TLR agonist combination that does not promote the production of cytokines (i.e., IL-4 and IL-10) capable of supporting a protumor environment through the induction of CD4+ TRegs or other immunosuppressive mechanisms. TLR-mediated DC maturation must also be able to induce the expression of costimulatory molecules required for optimal T cell activation. Warger et al. [182] also demonstrated that the synchronized activation of the MyD88 and TRIF pathways in DCs resulted in increased IL-6 and IL-12p70 production, as well as increased expression of CD40, CD70, and CD86. Noteworthy, T cells elicited under these conditions were reported to be resistant against the suppressive effects of CD4+ TRegs [182]. Mitchell et al. [177] reported that the simultaneous engagement of TLRs that signal through the same or different pathways on mouse bone marrow-derived DCs induced distinct patterns and levels of cytokines. Interestingly, despite the fact that both TLR agonists (TLR2 and TLR9 ligands) transduce signals via the MyD88 pathway, the synergistic effects were differentially dependent on MAPK signaling but relied on NF-κB signaling.
The use of TLR agonists as a monotherapy or in combination with tumor antigens, chemotherapy, or radiotherapy is still early in clinical development, and their antitumor potential against different malignancies remains to be firmly established. For example, the TLR3 agonist Poly A:U, albeit safe, has produced moderate success for treating patients with various types of cancers [183–187]. Interestingly, however, stratification of breast cancer patients, whose breast tumors were TLR3-positive, revealed an association with decreased risk of metastatic relapse compared with TLR3-negative tumors. For a detailed list of ongoing clinical trials using TLR agonists, see www.clinicaltrials.gov, and review article by Adams [6]. The TLR7 agonist imiquimod (Aldara) appears to be effective against warts and primary skin tumors such as basal cell carcinomas [188]. Although still early in clinical stages, recent results from a Phase II study of imiquimod in breast cancer patients with chest-wall recurrences or skin metastases revealed modest antitumor effects with two of 10 patients achieving a partial response [189]. Imiquimod treatment was reported to be well-tolerated. The antitumor activity of another TLR7 agonist 852A was also examined for its ability to treat metastatic breast, ovarian, endometrial, or cervical cancer in patients not responding to standard treatment. Most patients exhibited increased cytokine levels in response to 825A treatment; two of three ovarian cancer patients, who received all 24 doses of 825A, showed progressive or stable disease (Study NCT00319748). A detailed summary of these completed studies are still pending. In a randomized Phase II trial of a TLR9 agonist, patients receiving taxane plus platinum chemotherapy for first-line treatment of nonsmall-cell lung cancer were suggested to improve an objective response [190]. The objective response rate in patients who received TLR9 agonist plus chemotherapy was 30% compared with 19% who received chemotherapy alone. Brody et al. [191] also reported that in situ tumor vaccination with a TLR9 agonist induced clinically significant anti-B cell lymphoma responses. These studies highlight the efficacy and advantage to administering TLR agonists locally (vs. systemically) at the tumor site.
Despite the moderate antitumor responses observed using certain TLR agonists (and in specific cancer types), a clearly beneficial role for the mono-TLR agonist in cancer immunotherapy has yet to be established. It is important to note that a recent trial using the TLR9 agonist EMD 1201081 in metastatic squamous cell carcinoma was terminated as a result of potential safety concerns when administered in combination with platinum-based therapies (Study NCT01360827). This comes shortly after Pfizer's discontinuation of its TLR9 agonist CPG 7909 following the drug's inability to demonstrate efficacy (in combination with chemotherapy) in Phase III trials in nonsmall-cell lung cancer. In yet a more recent study conducted by Idera Pharmaceuticals, Phase II results of the TLR9 agonist IMO 2055 did not improve progression-free survival in recurrent or metastatic head and neck cancer. A more promising approach includes ongoing Phase III studies by GlaxoSmithKline, which are testing the antitumor activity of a multipronged tactic using melanoma-associated antigen 3 with the TLR9 and TLR4 agonist MPL in melanoma patients with the objective of decreasing tumor recurrence.
DOES THIS MEAN THE ENDGAME FOR TLR AGONISTS IN CANCER IMMUNOTHERAPY?
Despite the moderate antitumor effects observed in most clinical trials, we believe that the use of TLR agonists still holds great potential in cancer immunotherapy. However, we consider that the successful implementation of TLR agonists in cancer immunotherapy must include the following characteristics.
First, TLR agonists should be used in combination with other agents to synergistically enhance their immunostimulatory capacity. This can be accomplished by combining different TLR agonists that activate the MyD88-dependent and MyD88-independent pathways. Combining TLR agonists with other immunostimulatory agents, such as anti-CD40 (to activate DCs, B cells, and macrophages), anti-OX40, and anti-4-1BB (to potentiate T cell responses), can enhance TLR effects in a synergistic fashion [176, 192]. Moreover, combining TLR agonists with tumor antigens has the potential for enhancing the generation of tumor-reactive T cells. The mixture of TLR agonists must also take into account their capacity to mature DCs, as some TLR agonists are more effective than others at doing so [49], and to induce a phenotype that favors Th1-skewing, as evidenced by IL-12 and IFN-γ production and the up-regulation of MHCII, CD80, CD86, and CD40.
Secondly, TLR agonists must also be able to stimulate TLRs directly on CD8+ T cells and CD4+ Th cells as a means to induce a polyclonal T cell response [193], as well as to augment T cell survival, memory T cell generation, and cytotoxicity. Furthermore, as TLR expression and its costimulatory effects on T cells are dependent on TCR stimulation, it is important that the TLR agonists be present at the tumor site at the same time that T cells are present. Therefore, the timing of TLR ligand administration as well as the ligand's ability to make it to the tumor site or capacity to be effectively delivered to the site represent important factors to consider when formulating TLR agonist-based cancer vaccines. The ability to direct TLR ligands to specific organ sites is under investigation in several laboratories. Data from our group demonstrate that localized TLR1-TLR2 therapy intended to costimulate tumor-specific T cells in B16 melanoma tumor-bearing mice enhanced antitumor responses only when administered peritumorally but was ineffective when administered systemically via i.p. injection or via a s.c. injection at a distal site from the tumor (refs. [53, 194] and Geng et al., unpublished results). The ideas that systemic TLR agonist-based therapies are not effective in clinical trials and pose a hurdle in developing optimal cancer therapies are echoed by other experts in the field [195]. In more recent studies, we have observed that tumor-specific human T cells engineered to secrete the TLR5 ligand at the tumor site enhance antitumor responses and prolong T cell survival. Autocrine TLR5 signaling in T cells also results in the expression of various cytokines/chemokines at the tumor site that further promotes antitumor activity by enhancing T cell and DC infiltration (ref. [194] and Geng et al., unpublished results).
Taking into consideration that CD4+ TRegs also express functional TLRs, the inclusion of TLR agonists with the capacity to inhibit CD4+ TReg activity, or at least not able to stimulate their suppressive ability, can further potentiate antitumor responses.
Ideally, TLR agonists would be toxic or cytostatic to tumor cells and would not promote tumor cell proliferation. Moreover, the inclusion of TLR agonists that can induce the expression of costimulatory molecules and/or cytokines/chemokines by tumors cells that can promote immune cell infiltration could serve to enhance the efficacy of TLR agonist-based therapies.
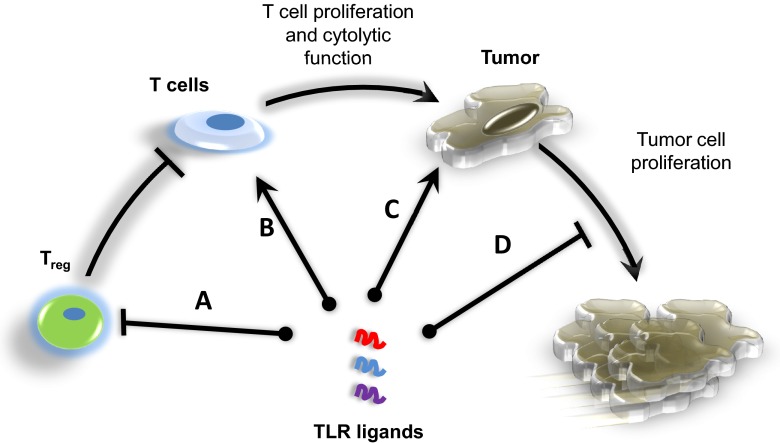
Finally, it also important to keep in mind that the TLR expression profiles, as well as the response to specific TLR agonists, differ between mouse and human cells. It is therefore essential that we delineate succinctly the effects that the stimulation of different TLRs has on human immune and cancerous cells when advancing studies into clinical trials. Figure 2 provides an overview of how the selection of distinct TLR agonists can enhance antitumor efficacy by stimulating TLRs on different T cell subsets and tumor cells.
Figure 2. The proper selection of TLR agonists that stimulate TLRs on different T cell subsets and tumor cells could enhance the efficacy of TLR agonist-based cancer therapies.
(A) In addition to activating DCs and skewing toward a Th1 cytokine profile, the optimal TLR agonist-based therapy would consist of a combination of TLR agonists to inhibit the immunosuppressive effects of TReg. (B) Furthermore, the inclusion of TLR ligands that can stimulate CD4+ and CD8+ T cells and prolong their survival, augment cytokine production, and increase cytotoxicity would further potentiate antitumor responses. (C and D) The TLR agonists should also be able to kill tumor cells directly and/or induce the production of chemokines or costimulatory molecules to facilitate antitumor T cell activity or prevent cells from proliferating. Furthermore, the timing at which distinct TLR ligands are administered, as well as the ligand's ability to make it to the tumor site, will impact efficacy and significant aspects to reflect on when formulating TLR agonist-based cancer vaccines.
CONCLUDING REMARKS
The use of multi-TLR agonists to induce potent antitumor responses remains a promising topic of research despite limited clinical evidence supporting the use of systemic TLR agonists as monotherapies. However, exploiting the potent immunostimulatory properties of TLR ligands is like wielding a two-sided light saber. Whereas their ability to activate TLRs on DCs, CD4+ Th, and CD8+ T cells is critical for generating antitumor responses, their capacity to support tumor progression by stimulating TLRs on tumor cells and TRegs in preclinical models cautions us to carefully consider the choice of TLR agonists. We remain hopeful that further studies elucidating the antitumor effects of combining different TLR agonists, identifying novel methods to direct the TLR agonists to the tumor site (and to specific cell subsets), and testing different timing schedules will yield important, new information that will enhance antitumor responses in patients.
ACKNOWLEDGMENTS
This study was supported by NCI 1R01CA140917-01, Leukemia and Lymphoma Society Translational Research Progam, U.S. National Institutes of Health Center for Biomedical Research Center Excellence grant 1P20 RR021970, and University of Maryland Marlene and Stewart Greenebaum NCI Cancer Center.
Footnotes
- BCG
- Bacillus Calmette-Guérin
- DAMP
- damage-associated molecular pattern
- EDA
- extra domain A
- FDA
- U.S. Food and Drug Administration
- HMGB1
- high-mobility group box 1
- HSP
- heat shock protein
- HTLV-1
- human T-lymphotropic virus type I
- IRAK
- IL-1R-associated kinase
- MMP
- matrix metalloproteinase
- MPL
- monophosphoryl lipid A
- NCI
- National Cancer Institute
- p-IRAK
- phosphorylated IL-1R-associated kinase
- Pam3CSK4
- palmitoyl-3-cysteine-serine-lysine-4
- pmel
- T cells specific for the gp100 antigen
- Poly A:U
- polyadenylic:polyuridylic acid
- Poly I:C
- polyinosinic:polycytidylic acid
- TIR
- Toll/IL-1R
- TReg
- regulatory CD4+ T cell
- TRIF
- Toll/IL-1R domain-containing adapter-inducing IFN-β
- vv
- vaccinia virus
AUTHORSHIP
All authors contributed equally.
REFERENCES
- 1. Coley W. B. (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat Res. Jan., 3–11 [PubMed] [Google Scholar]
- 2. Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. (1985) Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 316, 552–554 [DOI] [PubMed] [Google Scholar]
- 3. Shear M. J., Turner F. C., Perrault A., Shovelton T. (1943) Chemical treatment of tumors. V. Isolation of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrate. J. Natl. Cancer Inst. 4, 81–97 [Google Scholar]
- 4. Rakoff-Nahoum S., Medzhitov R. (2008) Role of Toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 73, 555–561 [DOI] [PubMed] [Google Scholar]
- 5. Rakoff-Nahoum S., Medzhitov R. (2009) Toll-like receptors and cancer. Nat. Rev. Cancer 9, 57–63 [DOI] [PubMed] [Google Scholar]
- 6. Adams S. (2009) Toll-like receptor agonists in cancer therapy. Immunotherapy 1, 949–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matzinger P. (1994) Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- 8. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 9. So E. Y., Ouchi T. (2010) The application of Toll like receptors for cancer therapy. Int. J. Biol. Sci. 6, 675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim D., Kim Y. J., Koh H. S., Jang T. Y., Park H. E., Kim J. Y. (2010) Reactive oxygen species enhance TLR10 expression in the human monocytic cell line THP-1. Int. J. Mol. Sci. 11, 3769–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hennessy E. J., Parker A. E., O'Neill L. A. (2010) Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307 [DOI] [PubMed] [Google Scholar]
- 12. Govindaraj R. G., Manavalan B., Lee G., Choi S. (2010) Molecular modeling-based evaluation of hTLR10 and identification of potential ligands in Toll-like receptor signaling. PLoS ONE 5, e12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasan U., Chaffois C., Gaillard C., Saulnier V., Merck E., Tancredi S., Guiet C., Briere F., Vlach J., Lebecque S.., et al. (2005) Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J. Immunol. 174, 2942–2950 [DOI] [PubMed] [Google Scholar]
- 14. Yoneda K., Sugimoto K., Shiraki K., Tanaka J., Beppu T., Fuke H., Yamamoto N., Masuya M., Horie R., Uchida K.., et al. (2008) Dual topology of functional Toll-like receptor 3 expression in human hepatocellular carcinoma: differential signaling mechanisms of TLR3-induced NF-κB activation and apoptosis. Int. J. Oncol. 33, 929–936 [PubMed] [Google Scholar]
- 15. Biragyn A., Ruffini P. A., Leifer C. A., Klyushnenkova E., Shakhov A., Chertov O., Shirakawa A. K., Farber J. M., Segal D. M., Oppenheim J. J.., et al. (2002) Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 298, 1025–1029 [DOI] [PubMed] [Google Scholar]
- 16. Rudilla F., Fayolle C., Casares N., Durantez M., Arribillaga L., Lozano T., Villanueva L., Pio R., Sarobe P., Leclerc C.., et al. (2012) Combination of a TLR4 ligand and anaphylatoxin C5a for the induction of antigen-specific cytotoxic T cell responses. Vaccine 30, 2848–2858 [DOI] [PubMed] [Google Scholar]
- 17. Park J. S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J. Y., Strassheim D., Sohn J. W., Yamada S., Maruyama I., Banerjee A.., et al. (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell. Physiol. 290, C917–C924 [DOI] [PubMed] [Google Scholar]
- 18. Shi B., Huang Q., Tak P. P., Vervoordeldonk M. J., Huang C. C., Dorfleutner A., Stehlik C., Pope R.M. (2012) SNAPIN: an endogenous Toll-like receptor ligand in rheumatoid arthritis. Ann. Rheum. Dis. 71, 1411–1417 [DOI] [PubMed] [Google Scholar]
- 19. Midwood K., Sacre S., Piccinini A. M., Inglis J., Trebaul A., Chan E., Drexler S., Sofat N., Kashiwagi M., Orend G.., et al. (2009) Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 [DOI] [PubMed] [Google Scholar]
- 20. Shi Z., Cai Z., Sanchez A., Zhang T., Wen S., Wang J., Yang J., Fu S., Zhang D. (2011) A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 286, 4517–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kucera K., Koblansky A. A., Saunders L. P., Frederick K. B., De La Cruz E. M., Ghosh S., Modis Y. (2010) Structure-based analysis of Toxoplasma gondii profilin: a parasite-specific motif is required for recognition by Toll-like receptor 11. J. Mol. Biol. 403, 616–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith D. E. (2011) The biological paths of IL-1 family members IL-18 and IL-33. J. Leukoc. Biol. 89, 383–392 [DOI] [PubMed] [Google Scholar]
- 23. Thomas C., Bazan J. F., Garcia K. C. (2012) Structure of the activating IL-1 receptor signaling complex. Nat. Struct. Mol. Biol. 19, 455–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medzhitov R. (2001) Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 25. Karin M., Greten F. R. (2005) NF-κB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 26. Scharf S., Hippenstiel S., Flieger A., Suttorp N., N′Guessan P. D. (2010) Induction of human β-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-κB, and AP-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L687–L695 [DOI] [PubMed] [Google Scholar]
- 27. Thapa B., Kim Y. H., Kwon H. J., Kim D. S. (2012) Novel regulatory mechanism and functional implication of plasminogen activator inhibitor-1 (PAI-1) expression in CpG-ODN-stimulated macrophages. Mol. Immunol. 49, 572–581 [DOI] [PubMed] [Google Scholar]
- 28. Mellett M., Atzei P., Jackson R., O'Neill L. A., Moynagh P. N. (2011) Mal mediates TLR-induced activation of CREB and expression of IL-10. J. Immunol. 186, 4925–4935 [DOI] [PubMed] [Google Scholar]
- 29. Beutler B. (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 [DOI] [PubMed] [Google Scholar]
- 30. Iwasaki A., Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 [DOI] [PubMed] [Google Scholar]
- 31. Boudreau J. E., Bonehill A., Thielemans K., Wan Y. (2011) Engineering dendritic cells to enhance cancer immunotherapy. Mol. Ther. 19, 841–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukata M., Abreu M. T. (2008) Role of Toll-like receptors in gastrointestinal malignancies. Oncogene 27, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stone G. W., Barzee S., Snarsky V., Santucci C., Tran B., Langer R., Zugates G. T., Anderson D. G., Kornbluth R. S. (2009) Nanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-like receptor agonists as a treatment for melanoma. PLoS One 4, e7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider C., Schmidt T., Ziske C., Tiemann K., Lee K. M., Uhlinsky V., Behrens P., Sauerbruch T., Schmidt-Wolf I. G., Muhlradt P. F.., et al. (2004) Tumour suppression induced by the macrophage activating lipopeptide MALP-2 in an ultrasound guided pancreatic carcinoma mouse model. Gut 53, 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Triozzi P. L., Aldrich W., Ponnazhagan S. (2011) Inhibition and promotion of tumor growth with adeno-associated virus carcinoembryonic antigen vaccine and Toll-like receptor agonists. Cancer Gene Ther. 18, 850–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broomfield S. A., van der Most R. G., Prosser A. C., Mahendran S., Tovey M. G., Smyth M. J., Robinson B. W., Currie A. J. (2009) Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J. Immunol. 182, 5217–5224 [DOI] [PubMed] [Google Scholar]
- 37. Davis M. B., Vasquez-Dunddel D., Fu J., Albesiano E., Pardoll D., Kim Y. J. (2011) Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses. Clin. Cancer Res. 17, 3984–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badovinac V. P., Porter B. B., Harty J. T. (2002) Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 3, 619–626 [DOI] [PubMed] [Google Scholar]
- 39. Surh C. D., Sprent J. (2002) Regulation of naive and memory T-cell homeostasis. Microbes Infect. 4, 51–56 [DOI] [PubMed] [Google Scholar]
- 40. Kaech S. M., Tan J. T., Wherry E. J., Konieczny B. T., Surh C. D., Ahmed R. (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 41. Sobek V., Birkner N., Falk I., Wurch A., Kirschning C. J., Wagner H., Wallich R., Lamers M. C., Simon M. M. (2004) Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res. Ther. 6, R433–R446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asprodites N., Zheng L., Geng D., Velasco-Gonzalez C., Sanchez-Perez L., Davila E. (2008) Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 22, 3628–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cottalorda A., Verschelde C., Marcais A., Tomkowiak M., Musette P., Uematsu S., Akira S., Marvel J., Bonnefoy-Berard N. (2006) TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur. J. Immunol. 36, 1684–1693 [DOI] [PubMed] [Google Scholar]
- 44. Morrison C., Baer M. R., Zandberg D. P., Kimball A., Davila E. (2011) Effects of Toll-like receptor signals in T-cell neoplasms. Future Oncol. 7, 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Babu S., Blauvelt C. P., Kumaraswami V., Nutman T. B. (2006) Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J. Immunol. 176, 3885–3889 [DOI] [PubMed] [Google Scholar]
- 46. Hervas-Stubbs S., Olivier A., Boisgerault F., Thieblemont N., Leclerc C. (2007) TLR3 ligand stimulates fully functional memory CD8+ T cells in the absence of CD4+ T-cell help. Blood 109, 5318–5326 [DOI] [PubMed] [Google Scholar]
- 47. Imanishi T., Hara H., Suzuki S., Suzuki N., Akira S., Saito T. (2007) Cutting edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 178, 6715–6719 [DOI] [PubMed] [Google Scholar]
- 48. Komai-Koma M., Jones L., Ogg G. S., Xu D., Liew F. Y. (2004) TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 101, 3029–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu H., Yang Y., Gad E., Wenner C. A., Chang A., Larson E. R., Dang Y., Martzen M., Standish L. J., Disis M. L. (2011) Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 17, 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y., Luo F., Cai Y., Liu N., Wang L., Xu D., Chu Y. (2011) TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J. Immunol. 186, 1963–1969 [DOI] [PubMed] [Google Scholar]
- 51. McCarron M., Reen D. J. (2009) Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J. Immunol. 182, 55–62 [DOI] [PubMed] [Google Scholar]
- 52. Geng D., Zheng L., Srivastava R., Asprodites N., Velasco-Gonzalez C., Davila E. (2010) When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effectors function. Blood 116, 3494–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geng D., Zheng L., Srivastava R., Riker A. I., Velasco-Gonzales C., Markovic S. N., Davila E. (2010) Amplifying TLR-MyD88 signals within tumor-specific T-cells enhances antitumor activity to suboptimal levels of weakly-immunogenic tumor-antigens. Cancer Res. 70, 7442–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seki E., Tsutsui H., Tsuji N. M., Hayashi N., Adachi K., Nakano H., Futatsugi-Yumikura S., Takeuchi O., Hoshino K., Akira S.., et al. (2002) Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169, 3863–3868 [DOI] [PubMed] [Google Scholar]
- 55. Seki E., Brenner D. A. (2008) Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48, 322–335 [DOI] [PubMed] [Google Scholar]
- 56. Zanin-Zhorov A., Nussbaum G., Franitza S., Cohen I. R., Lider O. (2003) T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 17, 1567–1569 [DOI] [PubMed] [Google Scholar]
- 57. Mueller K. L., Daniels M. A., Felthauser A., Kao C., Jameson S. C., Shimizu Y. (2004) Cutting edge: LFA-1 integrin-dependent T cell adhesion is regulated by both ag specificity and sensitivity. J. Immunol. 173, 2222–2226 [DOI] [PubMed] [Google Scholar]
- 58. Kobayashi N., Takata H., Yokota S., Takiguchi M. (2004) Down-regulation of CXCR4 expression on human CD8+ T cells during peripheral differentiation. Eur. J. Immunol. 34, 3370–3378 [DOI] [PubMed] [Google Scholar]
- 59. Liu H., Komai-Koma M., Xu D., Liew F. Y. (2006) Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 103, 7048–7053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rahman A. H., Taylor D. K., Turka L. A. (2009) The contribution of direct TLR signaling to T cell responses. Immunol. Res. 45, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salem M. L. (2011) Triggering of Toll-like receptor signaling pathways in T cells contributes to the anti-tumor efficacy of T cell responses. Immunol. Lett. 137, 9–14 [DOI] [PubMed] [Google Scholar]
- 62. Sutmuller R. P., den Brok M. H., Kramer M., Bennink E. J., Toonen L. W., Kullberg B. J., Joosten L. A., Akira S., Netea M. G., Adema G. J. (2006) Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rosenberg S. A. (2001) Progress in human tumour immunology and immunotherapy. Nature 411, 380–384 [DOI] [PubMed] [Google Scholar]
- 64. Rosenberg S. A., Yang J. C., Restifo N. P. (2004) Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mercier B. C., Cottalorda A., Coupet C. A., Marvel J., Bonnefoy-Berard N. (2009) TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J. Immunol. 182, 1860–1867 [DOI] [PubMed] [Google Scholar]
- 66. Zhu X. M., Yao Y. M., Liang H. P., Xu C. T., Dong N., Yu Y., Sheng Z. Y. (2011) High mobility group box-1 protein regulate immunosuppression of regulatory T cells through Toll-like receptor 4. Cytokine 54, 296–304 [DOI] [PubMed] [Google Scholar]
- 67. Day E. B., Zeng W., Doherty P. C., Jackson D. C., Kedzierska K., Turner S. J. (2007) The context of epitope presentation can influence functional quality of recalled influenza A virus-specific memory CD8+ T cells. J. Immunol. 179, 2187–2194 [DOI] [PubMed] [Google Scholar]
- 68. Deetz C. O., Hebbeler A. M., Propp N. A., Cairo C., Tikhonov I., Pauza C. D. (2006) γ Interferon secretion by human Vγ2Vδ2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect. Immun. 74, 4505–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lancioni C. L., Li Q., Thomas J. J., Ding X., Thiel B., Drage M. G., Pecora N. D., Ziady A. G., Shank S., Harding C. V.., et al. (2011) Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect. Immun. 79, 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gelman A. E., Zhang J., Choi Y., Turka L. A. (2004) Toll-like receptor ligands directly promote activated CD4+ T cell survival. J. Immunol. 172, 6065–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tabiasco J., Devevre E., Rufer N., Salaun B., Cerottini J. C., Speiser D., Romero P. (2006) Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J. Immunol. 177, 8708–8713 [DOI] [PubMed] [Google Scholar]
- 72. Salem M. L., Diaz-Montero C. M., El-Naggar S. A., Chen Y., Moussa O., Cole D. J. (2009) The TLR3 agonist poly(I: C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naive recipient mice. Vaccine 27, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wesch D., Beetz S., Oberg H. H., Marget M., Krengel K., Kabelitz D. (2006) Direct costimulatory effect of TLR3 ligand poly(I: C) on human γ δ T lymphocytes. J. Immunol. 176, 1348–1354 [DOI] [PubMed] [Google Scholar]
- 74. Shojaei H., Oberg H. H., Juricke M., Marischen L., Kunz M., Mundhenke C., Gieseler F., Kabelitz D., Wesch D. (2009) Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human γδ T cells. Cancer Res. 69, 8710–8717 [DOI] [PubMed] [Google Scholar]
- 75. Yadav R., Zammit D. J., Lefrancois L., Vella A. T. (2006) Effects of LPS-mediated bystander activation in the innate immune system. J. Leukoc. Biol. 80, 1251–1261 [DOI] [PubMed] [Google Scholar]
- 76. Vogel S. N., Hilfiker M. L., Caulfield M. J. (1983) Endotoxin-induced T lymphocyte proliferation. J. Immunol. 130, 1774–1779 [PubMed] [Google Scholar]
- 77. Komai-Koma M., Gilchrist D. S., Xu D. (2009) Direct recognition of LPS by human but not murine CD8+ T cells via TLR4 complex. Eur. J. Immunol. 39, 1564–1572 [DOI] [PubMed] [Google Scholar]
- 78. Reynolds J. M., Martinez G. J., Chung Y., Dong C. (2012) Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc. Natl. Acad. Sci. USA 109, 13064–13069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Reynolds J. M., Pappu B. P., Peng J., Martinez G. J., Zhang Y., Chung Y., Ma L., Yang X. O., Nurieva R. I., Tian Q.., et al. (2010) Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32, 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gonzalez-Navajas J. M., Fine S., Law J., Datta S. K., Nguyen K. P., Yu M., Corr M., Katakura K., Eckman L., Lee J.., et al. (2010) TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J. Clin. Invest. 120, 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Caramalho I., Lopes-Carvalho T., Ostler D., Zelenay S., Haury M., Demengeot J. (2003) Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Caron G., Duluc D., Fremaux I., Jeannin P., David C., Gascan H., Delneste Y. (2005) Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-γ production by memory CD4+ T cells. J. Immunol. 175, 1551–1557 [DOI] [PubMed] [Google Scholar]
- 83. Crellin N. K., Garcia R. V., Hadisfar O., Allan S. E., Steiner T. S., Levings M. K. (2005) Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 175, 8051–8059 [DOI] [PubMed] [Google Scholar]
- 84. Peng G., Guo Z., Kiniwa Y., Voo K. S., Peng W., Fu T., Wang D. Y., Li Y., Wang H. Y., Wang R. F. (2005) Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309, 1380–1384 [DOI] [PubMed] [Google Scholar]
- 85. Peng G., Wang H. Y., Peng W., Kiniwa Y., Seo K. H., Wang R. F. (2007) Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity 27, 334–348 [DOI] [PubMed] [Google Scholar]
- 86. Marsland B. J., Nembrini C., Grun K., Reissmann R., Kurrer M., Leipner C., Kopf M. (2007) TLR ligands act directly upon T cells to restore proliferation in the absence of protein kinase C-θ signaling and promote autoimmune myocarditis. J. Immunol. 178, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 87. Chiffoleau E., Heslan J. M., Heslan M., Louvet C., Condamine T., Cuturi M. C. (2007) TLR9 ligand enhances proliferation of rat CD4+ T cell and modulates suppressive activity mediated by CD4+ CD25+ T cell. Int. Immunol. 19, 193–201 [DOI] [PubMed] [Google Scholar]
- 88. Bendigs S., Salzer U., Lipford G. B., Wagner H., Heeg K. (1999) CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur. J. Immunol. 29, 1209–1218 [DOI] [PubMed] [Google Scholar]
- 89. Zheng L., Asprodites N., Keene A. H., Rodriguez P., Brown K. D., Davila E. (2008) TLR9 engagement on CD4 T lymphocytes represses γ-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood 111, 2704–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bartholdy C., Christensen J. E., Grujic M., Christensen J. P., Thomsen A. R. (2009) T-cell intrinsic expression of MyD88 is required for sustained expansion of the virus-specific CD8+ T-cell population in LCMV-infected mice. J. Gen. Virol. 90, 423–431 [DOI] [PubMed] [Google Scholar]
- 91. Rahman A. H., Cui W., LaRosa D. F., Taylor D. K., Zhang J., Goldstein D. R., Wherry E. J., Kaech S. M., Turka L. A. (2008) MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J. Immunol. 181, 3804–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhao Y., De T. C., Flynn R., Ware C. F., Croft M., Salek-Ardakani S. (2009) The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J. Immunol. 182, 6278–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. LaRosa D. F., Stumhofer J. S., Gelman A. E., Rahman A. H., Taylor D. K., Hunter C. A., Turka L. A. (2008) T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 105, 3855–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Quigley M., Martinez J., Huang X., Yang Y. (2009) A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood 113, 2256–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Salaun B., Romero P., Lebecque S. (2007) Toll-like receptors' two-edged sword: when immunity meets apoptosis. Eur. J. Immunol. 37, 3311–3318 [DOI] [PubMed] [Google Scholar]
- 96. Means T. K., Wang S., Lien E., Yoshimura A., Golenbock D. T., Fenton M. J. (1999) Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163, 3920–3927 [PubMed] [Google Scholar]
- 97. Tsuji S., Matsumoto M., Takeuchi O., Akira S., Azuma I., Hayashi A., Toyoshima K., Seya T. (2000) Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of Toll-like receptors. Infect. Immun. 68, 6883–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Uehori J., Matsumoto M., Tsuji S., Akazawa T., Takeuchi O., Akira S., Kawata T., Azuma I., Toyoshima K., Seya T. (2003) Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guerin peptidoglycan. Infect. Immun. 71, 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ayari C., Bergeron A., LaRue H., Menard C., Fradet Y. (2011) Toll-like receptors in normal and malignant human bladders. J. Urol. 185, 1915–1921 [DOI] [PubMed] [Google Scholar]
- 100. Bevers R. F., Kurth K. H., Schamhart D. H. (2004) Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br. J. Cancer 91, 607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jansson O. T., Morcos E., Brundin L., Lundberg J. O., Adolfsson J., Soderhall M., Wiklund N. P. (1998) The role of nitric oxide in bacillus Calmette-Guerin mediated anti-tumour effects in human bladder cancer. Br. J. Cancer 78, 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cheng Y. S., Xu F. (2011) Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 10, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 103. Salaun B., Coste I., Rissoan M. C., Lebecque S. J., Renno T. (2006) TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 176, 4894–4901 [DOI] [PubMed] [Google Scholar]
- 104. Taura M., Fukuda R., Suico M. A., Eguma A., Koga T., Shuto T., Sato T., Morino-Koga S., Kai H. (2010) TLR3 induction by anticancer drugs potentiates poly I: C-induced tumor cell apoptosis. Cancer Sci. 101, 1610–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rydberg C., Mansson A., Uddman R., Riesbeck K., Cardell L. O. (2009) Toll-like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas. Immunology 128, e600–e611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Samara K. D., Antoniou K. M., Karagiannis K., Margaritopoulos G., Lasithiotaki I., Koutala E., Siafakas N. M. (2012) Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int. J. Oncol. 40, 1397–1404 [DOI] [PubMed] [Google Scholar]
- 107. Estornes Y., Toscano F., Virard F., Jacquemin G., Pierrot A., Vanbervliet B., Bonnin M., Lalaoui N., Mercier-Gouy P., Pacheco Y.., et al. (2012) dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 19, 1482–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Salaun B., Lebecque S., Matikainen S., Rimoldi D., Romero P. (2007) Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin. Cancer Res. 13, 4565–4574 [DOI] [PubMed] [Google Scholar]
- 109. Proietti E., Bracci L., Puzelli S., Di Pucchio T., Sestili P., De Vincenzi E., Venditti M., Capone I., Seif I., De Maeyer E.., et al. (2002) Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169, 375–383 [DOI] [PubMed] [Google Scholar]
- 110. Chiron D., Pellat-Deceunynck C., Amiot M., Bataille R., Jego G. (2009) TLR3 ligand induces NF-κB activation and various fates of multiple myeloma cells depending on IFN-α production. J. Immunol. 182, 4471–4478 [DOI] [PubMed] [Google Scholar]
- 111. Kutikhin A. G. (2011) Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum. Immunol. 72, 1095–1116 [DOI] [PubMed] [Google Scholar]
- 112. Bauer A. K., Dixon D., Degraff L. M., Cho H. Y., Walker C. R., Malkinson A. M., Kleeberger S. R. (2005) Toll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesis. J. Natl. Cancer Inst. 97, 1778–1781 [DOI] [PubMed] [Google Scholar]
- 113. Cai Z., Sanchez A., Shi Z., Zhang T., Liu M., Zhang D. (2011) Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 71, 2466–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rhee S. H., Im E., Pothoulakis C. (2008) Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li X., Liu D., Liu X., Jiang W., Zhou W., Yan W., Cen Y., Li B., Cao G., Ding G.., et al. (2012) CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-κB activation and NO production. Tumour Biol. 33, 1607–1618 [DOI] [PubMed] [Google Scholar]
- 116. Wang C., Cao S., Yan Y., Ying Q., Jiang T., Xu K., Wu A. (2010) TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer 10, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brignole C., Marimpietri D., Di P. D., Perri P., Morandi F., Pastorino F., Zorzoli A., Pagnan G., Loi M., Caffa I.., et al. (2010) Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res. 70, 9816–9826 [DOI] [PubMed] [Google Scholar]
- 118. Pikarsky E., Porat R. M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. (2004) NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 [DOI] [PubMed] [Google Scholar]
- 119. Huang B., Zhao J., Li H., He K. L., Chen Y., Chen S. H., Mayer L., Unkeless J. C., Xiong H. (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 65, 5009–5014 [DOI] [PubMed] [Google Scholar]
- 120. Li A., Dubey S., Varney M. L., Dave B. J., Singh R. K. (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 170, 3369–3376 [DOI] [PubMed] [Google Scholar]
- 121. Kitadai Y., Sasaki A., Ito M., Tanaka S., Oue N., Yasui W., Aihara M., Imagawa K., Haruma K., Chayama K. (2003) Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem. Biophys. Res. Commun. 311, 809–814 [DOI] [PubMed] [Google Scholar]
- 122. Kuai W. X., Wang Q., Yang X. Z., Zhao Y., Yu R., Tang X. J. (2012) Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J. Gastroenterol. 18, 979–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cherfils-Vicini J., Platonova S., Gillard M., Laurans L., Validire P., Caliandro R., Magdeleinat P., Mami-Chouaib F., Dieu-Nosjean M. C., Fridman W. H.., et al. (2010) Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J. Clin. Invest. 120, 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kumar P. S., Brandt S., Madassery J., Backert S. (2011) Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS One 6, e19614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chang Y. J., Wu M. S., Lin J. T., Chen C. C. (2005) Helicobacter pylori-induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J. Immunol. 175, 8242–8252 [DOI] [PubMed] [Google Scholar]
- 126. Huang Y., Cai B., Xu M., Qiu Z., Tao Y., Zhang Y., Wang J., Xu Y., Zhou Y., Yang J.., et al. (2012) Gene silencing of Toll-like receptor 2 inhibits proliferation of human liver cancer cells and secretion of inflammatory cytokines. PLoS One 7, e38890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Huang B., Zhao J., Shen S., Li H., He K. L., Shen G. X., Mayer L., Unkeless J., Li D., Yuan Y.., et al. (2007) Listeria monocytogenes promotes tumor growth via tumor cell Toll-like receptor 2 signaling. Cancer Res. 67, 4346–4352 [DOI] [PubMed] [Google Scholar]
- 128. Hardin F. J., Wright R. A. (2002) Helicobacter pylori: review and update. Hospital Physician, Turner White Communications, Wayne, PA, USA, 23–31 [Google Scholar]
- 129. Chochi K., Ichikura T., Kinoshita M., Majima T., Shinomiya N., Tsujimoto H., Kawabata T., Sugasawa H., Ono S., Seki S.., et al. (2008) Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-Toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin. Cancer Res. 14, 2909–2917 [DOI] [PubMed] [Google Scholar]
- 130. Song E. J., Kang M. J., Kim Y. S., Kim S. M., Lee S. E., Kim C. H., Kim D. J., Park J. H. (2011) Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5. Int. J. Mol. Med. 28, 115–119 [DOI] [PubMed] [Google Scholar]
- 131. Schmausser B., Andrulis M., Endrich S., Muller-Hermelink H. K., Eck M. (2005) Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int. J. Med. Microbiol. 295, 179–185 [DOI] [PubMed] [Google Scholar]
- 132. Yuan A., Chen J. J., Yao P. L., Yang P. C. (2005) The role of interleukin-8 in cancer cells and microenvironment interaction. Front. Biosci. 10, 853–865 [DOI] [PubMed] [Google Scholar]
- 133. Ristimaki A., Sivula A., Lundin J., Lundin M., Salminen T., Haglund C., Joensuu H., Isola J. (2002) Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 62, 632–635 [PubMed] [Google Scholar]
- 134. Tsujii M., Kawano S., Tsuji S., Sawaoka H., Hori M., DuBois R. N. (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705–716 [DOI] [PubMed] [Google Scholar]
- 135. Chouaib S., Welte K., Mertelsmann R., Dupont B. (1985) Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J. Immunol. 135, 1172–1179 [PubMed] [Google Scholar]
- 136. Bogdan C. (2001) Nitric oxide and the immune response. Nat. Immunol. 2, 907–916 [DOI] [PubMed] [Google Scholar]
- 137. Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234 [DOI] [PubMed] [Google Scholar]
- 138. Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A. I., Zhao R. C., Shi Y. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 [DOI] [PubMed] [Google Scholar]
- 139. Khan O. A., Blann A. D., Payne M. J., Middleton M. R., Protheroe A. S., Talbot D. C., Taylor M., Kirichek O., Han C., Patil M.., et al. (2011) Continuous low-dose cyclophosphamide and methotrexate combined with celecoxib for patients with advanced cancer. Br. J. Cancer 104, 1822–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Midgley R. S., McConkey C. C., Johnstone E. C., Dunn J. A., Smith J. L., Grumett S. A., Julier P., Iveson C., Yanagisawa Y., Warren B.., et al. (2010) Phase III randomized trial assessing rofecoxib in the adjuvant setting of colorectal cancer: final results of the VICTOR trial. J. Clin. Oncol. 28, 4575–4580 [DOI] [PubMed] [Google Scholar]
- 141. Jones S. A. (2005) Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 [DOI] [PubMed] [Google Scholar]
- 142. Pellegrini P., Contasta I., Del B. T., Ciccone F., Berghella A. M. (2011) Gender-specific cytokine pathways, targets, and biomarkers for the switch from health to adenoma and colorectal cancer. Clin. Dev. Immunol. 2011, 819724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wang Y., Niu X. L., Qu Y., Wu J., Zhu Y. Q., Sun W. J., Li L. Z. (2010) Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 295, 110–123 [DOI] [PubMed] [Google Scholar]
- 144. Jelavic T. B., Barisic M. D., Boraska V., Vrdoljak E., Peruzovic M., Hozo I., Puljiz Z., Terzic J. (2006) Microsatelite GT polymorphism in the Toll-like receptor 2 is associated with colorectal cancer. Clin. Genet. 70, 156–160 [DOI] [PubMed] [Google Scholar]
- 145. Zeng H. M., Pan K. F., Zhang Y., Zhang L., Ma J. L., Zhou T., Su H. J., Li W. Q., Li J. Y., Gerhard M.., et al. (2011) Genetic variants of Toll-like receptor 2 and 5, Helicobacter pylori infection, and risk of gastric cancer and its precursors in a Chinese population. Cancer Epidemiol. Biomarkers Prev. 20, 2594–2602 [DOI] [PubMed] [Google Scholar]
- 146. Naugler W. E., Sakurai T., Kim S., Maeda S., Kim K., Elsharkawy A. M., Karin M. (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 [DOI] [PubMed] [Google Scholar]
- 147. Mittal D., Saccheri F., Venereau E., Pusterla T., Bianchi M. E., Rescigno M. (2010) TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 29, 2242–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vabulas R. M., Wagner H., Schild H. (2002) Heat shock proteins as ligands of Toll-like receptors. Curr. Top. Microbiol. Immunol. 270, 169–184 [DOI] [PubMed] [Google Scholar]
- 149. Thuringer D., Hammann A., Benikhlef N., Fourmaux E., Bouchot A., Wettstein G., Solary E., Garrido C. (2011) Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. J. Biol. Chem. 286, 3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zheng S. L., Augustsson-Balter K., Chang B., Hedelin M., Li L., Adami H. O., Bensen J., Li G., Johnasson J. E., Turner A. R.., et al. (2004) Sequence variants of Toll-like receptor 4 are associated with prostate cancer risk: results from the Cancer Prostate in Sweden Study. Cancer Res. 64, 2918–2922 [DOI] [PubMed] [Google Scholar]
- 151. Chen Y. C., Giovannucci E., Lazarus R., Kraft P., Ketkar S., Hunter D. J. (2005) Sequence variants of Toll-like receptor 4 and susceptibility to prostate cancer. Cancer Res. 65, 11771–11778 [DOI] [PubMed] [Google Scholar]
- 152. Harmey J. H., Bucana C. D., Lu W., Byrne A. M., McDonnell S., Lynch C., Bouchier-Hayes D., Dong Z. (2002) Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int. J. Cancer 101, 415–422 [DOI] [PubMed] [Google Scholar]
- 153. Yang H., Zhou H., Feng P., Zhou X., Wen H., Xie X., Shen H., Zhu X. (2010) Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. J. Exp. Clin. Cancer Res. 29, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bhattacharya D., Yusuf N. (2012) Expression of Toll-like receptors on breast tumors: taking a toll on tumor microenvironment. Int. J. Breast Cancer 2012, 716564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. He W., Liu Q., Wang L., Chen W., Li N., Cao X. (2007) TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol. Immunol. 44, 2850–2859 [DOI] [PubMed] [Google Scholar]
- 156. Pimentel-Nunes P., Afonso L., Lopes P., Roncon-Albuquerque R., Jr., Goncalves N., Henrique R., Moreira-Dias L., Leite-Moreira A. F., Dinis-Ribeiro M. (2011) Increased expression of Toll-like receptors (TLR) 2, 4 and 5 in gastric dysplasia. Pathol. Oncol. Res. 17, 677–683 [DOI] [PubMed] [Google Scholar]
- 157. Suganuma M., Watanabe T., Yamaguchi K., Takahashi A., Fujiki H. (2012) Human gastric cancer development with TNF-α-inducing protein secreted from Helicobacter pylori. Cancer Lett. 322, 133–138 [DOI] [PubMed] [Google Scholar]
- 158. Muccioli M., Sprague L., Nandigam H., Pate M., Benencia F. (2012) Toll-like receptors as novel therapeutic targets for ovarian cancer. ISRN Oncol. 2012, 642141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Grinberg-Bleyer Y., Saadoun D., Baeyens A., Billiard F., Goldstein J. D., Gregoire S., Martin G. H., Elhage R., Derian N., Carpentier W.., et al. (2010) Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J. Clin. Invest. 120, 4558–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Toriseva M., Laato M., Carpen O., Ruohonen S. T., Savontaus E., Inada M., Krane S. M., Kahari V. M. (2012) MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS One 7, e42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kundu S. D., Lee C., Billips B. K., Habermacher G. M., Zhang Q., Liu V., Wong L. Y., Klumpp D. J., Thumbikat P. (2008) The Toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate 68, 223–229 [DOI] [PubMed] [Google Scholar]
- 162. Tanaka J., Sugimoto K., Shiraki K., Tameda M., Kusagawa S., Nojiri K., Beppu T., Yoneda K., Yamamoto N., Uchida K.., et al. (2010) Functional cell surface expression of Toll-like receptor 9 promotes cell proliferation and survival in human hepatocellular carcinomas. Int. J. Oncol. 37, 805–814 [PubMed] [Google Scholar]
- 163. Mizobe T., Tsukada J., Higashi T., Mouri F., Matsuura A., Tanikawa R., Minami Y., Yoshida Y., Tanaka Y. (2007) Constitutive association of MyD88 to IRAK in HTLV-I-transformed T cells. Exp. Hematol. 35, 1812–1822 [DOI] [PubMed] [Google Scholar]
- 164. Srivastava R., Geng D., Liu Y., Zheng L., Li Z., Joseph M. A., McKenna C., Bansal N., Ochoa A. C., Davila E. (2012) Augmentation of therapeutic responses in melanoma by inhibition of IRAK-1, -4. Cancer Res.72, 6209–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ngo V. N., Young R. M., Schmitz R., Jhavar S., Xiao W., Lim K. H., Kohlhammer H., Xu W., Yang Y., Zhao H.., et al. (2011) Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 167. De Gruijl T. D., van den Eertwegh A. J., Pinedo H. M., Scheper R. J. (2008) Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol. Immunother. 57, 1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hanna M. G., Jr., Ransom J. H., Pomato N., Peters L., Bloemena E., Vermorken J. B., Hoover H. C., Jr., (1993) Active specific immunotherapy of human colorectal carcinoma with an autologous tumor cell/bacillus Calmette-Guerin vaccine. Ann. N. Y. Acad. Sci. 690, 135–146 [DOI] [PubMed] [Google Scholar]
- 169. Hoover H. C., Jr., Brandhorst J. S., Peters L. C., Surdyke M. G., Takeshita Y., Madariaga J., Muenz L. R., Hanna M. G., Jr., (1993) Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J. Clin. Oncol. 11, 390–399 [DOI] [PubMed] [Google Scholar]
- 170. Baars A., Claessen A. M., van den Eertwegh A. J., Gall H. E., Stam A. G., Meijer S., Giaccone G., Meijer C. J., Scheper R. J., Wagstaff J.., et al. (2000) Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann. Oncol. 11, 965–970 [DOI] [PubMed] [Google Scholar]
- 171. Jocham D., Richter A., Hoffmann L., Iwig K., Fahlenkamp D., Zakrzewski G., Schmitt E., Dannenberg T., Lehmacher W., von Wietersheim J.., et al. (2004) Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 363, 594–599 [DOI] [PubMed] [Google Scholar]
- 172. Favaro W. J., Nunes O. S., Seiva F. R., Nunes I. S., Woolhiser L. K., Duran N., Lenaerts A. J. (2012) Effects of P-MAPA immunomodulator on Toll-like receptors and p53: potential therapeutic strategies for infectious diseases and cancer. Infect. Agent Cancer 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Conforti R., Ma Y., Morel Y., Paturel C., Terme M., Viaud S., Ryffel B., Ferrantini M., Uppaluri R., Schreiber R.., et al. (2010) Opposing effects of Toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 70, 490–500 [DOI] [PubMed] [Google Scholar]
- 174. Zhu Q., Egelston C., Gagnon S., Sui Y., Belyakov I. M., Klinman D. M., Berzofsky J. A. (2010) Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Invest. 120, 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhu Q., Egelston C., Vivekanandhan A., Uematsu S., Akira S., Klinman D. M., Belyakov I. M., Berzofsky J. A. (2008) Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc. Natl. Acad. Sci. USA 105, 16260–16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ahonen C. L., Doxsee C. L., McGurran S. M., Riter T. R., Wade W. F., Barth R. J., Vasilakos J. P., Noelle R. J., Kedl R. M. (2004) Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199, 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mitchell D., Yong M., Schroder W., Black M., Tirrell M., Olive C. (2010) Dual stimulation of MyD88-dependent Toll-like receptors induces synergistically enhanced production of inflammatory cytokines in murine bone marrow-derived dendritic cells. J. Infect. Dis. 202, 318–329 [DOI] [PubMed] [Google Scholar]
- 178. Napolitani G., Rinaldi A., Bertoni F., Sallusto F., Lanzavecchia A. (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Didierlaurent A., Ferrero I., Otten L. A., Dubois B., Reinhardt M., Carlsen H., Blomhoff R., Akira S., Kraehenbuhl J. P., Sirard J. C. (2004) Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 172, 6922–6930 [DOI] [PubMed] [Google Scholar]
- 180. Dillon S., Agrawal A., Van Dyke T., Landreth G., McCauley L., Koh A., Maliszewski C., Akira S., Pulendran B. (2004) A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172, 4733–4743 [DOI] [PubMed] [Google Scholar]
- 181. Redecke V., Hacker H., Datta S. K., Fermin A., Pitha P. M., Broide D. H., Raz E. (2004) Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J. Immunol. 172, 2739–2743 [DOI] [PubMed] [Google Scholar]
- 182. Warger T., Osterloh P., Rechtsteiner G., Fassbender M., Heib V., Schmid B., Schmitt E., Schild H., Radsak M. P. (2006) Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 108, 544–550 [DOI] [PubMed] [Google Scholar]
- 183. Lacour F., Lacour J., Spira A., Michelson M., Petit J. Y., Delage G., Contesso G., Merlin-Nahon E., Sarrazin D., Viguier J. (1980) [Adjuvant immunotherapy using polyadenylic acid and polyuridylic acid (Poly A., Poly, U.) in operable carcinoma of the breast (author's transl)]. Chirurgie 106, 737–743 [PubMed] [Google Scholar]
- 184. Lacour J., Lacour F., Spira A., Michelson M., Petit J. Y., Delage G., Sarrazin D., Contesso G., Viguier J. (1980) Adjuvant treatment with polyadenylic-polyuridylic acid (Polya.Polyu) in operable breast cancer. Lancet 2, 161–164 [DOI] [PubMed] [Google Scholar]
- 185. Laplanche A., Alzieu L., Delozier T., Berlie J., Veyret C., Fargeot P., Luboinski M., Lacour J. (2000) Polyadenylic-polyuridylic acid plus locoregional radiotherapy versus chemotherapy with CMF in operable breast cancer: a 14 year follow-up analysis of a randomized trial of the Federation Nationale des Centres de Lutte contre le Cancer (FNCLCC). Breast Cancer Res. Treat. 64, 189–191 [DOI] [PubMed] [Google Scholar]
- 186. Hawkins M. J., Levin M., Borden E. C. (1985) An Eastern Cooperative Oncology Group phase I-II pilot study of polyriboinosinic-polyribocytidylic acid poly-L-lysine complex in patients with metastatic malignant melanoma. J. Biol. Response Mod. 4, 664–668 [PubMed] [Google Scholar]
- 187. Robinson R. A., DeVita V. T., Levy H. B., Baron S., Hubbard S. P., Levine A. S. (1976) A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patients with leukemia or solid tumors. J. Natl. Cancer Inst. 57, 599–602 [DOI] [PubMed] [Google Scholar]
- 188. Geisse J., Caro I., Lindholm J., Golitz L., Stampone P., Owens M. (2004) Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 50, 722–733 [DOI] [PubMed] [Google Scholar]
- 189. Adams S., Kozhaya L., Martiniuk F., Meng T. C., Chiriboga L., Liebes L., Hochman T., Shuman N., Axelrod D., Speyer J. L.., et al. (2012) Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. 18, 6748–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Manegold C., Gravenor D., Woytowitz D., Mezger J., Hirsh V., Albert G., Al-Adhami M., Readett D., Krieg A. M., Leichman C. G. (2008) Randomized phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 26, 3979–3986 [DOI] [PubMed] [Google Scholar]
- 191. Brody J. D., Ai W. Z., Czerwinski D. K., Torchia J. A., Levy M., Advani R. H., Kim Y. H., Hoppe R. T., Knox S. J., Shin L. K.., et al. (2010) In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 28, 4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Houot R., Goldstein M. J., Kohrt H. E., Myklebust J. H., Alizadeh A. A., Lin J. T., Irish J. M., Torchia J. A., Kolstad A., Chen L.., et al. (2009) Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 114, 3431–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Varghese B., Widman A., Do J., Taidi B., Czerwinski D. K., Timmerman J., Levy S., Levy R. (2009) Generation of CD8+ T cell-mediated immunity against idiotype-negative lymphoma escapees. Blood 114, 4477–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li J., Song W., Czerwinski D. K., Varghese B., Uematsu S., Akira S., Krieg A. M., Levy R. (2007) Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J. Immunol. 179, 2493–2500 [DOI] [PubMed] [Google Scholar]
- 195. Guha M. (2012) Anticancer TLR agonists on the ropes. Nat. Rev. Drug Discov. 11, 503–505 [DOI] [PubMed] [Google Scholar]