Abstract
We studied the repair of UV- and aflatoxin B1 (AFB1)-induced damage in the human metallothionein (hMT) gene family. After exposure to either UV or AFB1, DNA damage was initially repaired faster in the DNA fragments containing the transcribed hMT-IA, hMT-IE, and hMT-IIA genes than in the genome overall. By 6 h posttreatment, there was at least twice as much repair in these genes as in the rest of the genome. Repair of UV damage in the hMT-IB gene, which shows cell-type specific expression, and in the hMT-IIB gene, which is a nontranscribed processed pseudogene, was about the same as in the rest of the genome, whereas repair of AFB1-induced damage was deficient in these two genes. Inducing transcription of the three expressed hMT genes with CdCl2 or of only the hMT-IIA gene with dexamethasone increased the initial rate of repair in the induced genes another twofold over the rate observed when they were transcribed at a basal level. The rates of repair in the hMT-IB and hMT-IIB genes were not altered by these inducing treatments. Transcription of the hMT genes was transiently inhibited after UV irradiation. Inducing transcription of the genes did not shorten this UV-induced delay. Thus, the efficiency of repair of damage in a DNA sequence is dependent on the level of transcriptional activity associated with that sequence. However, an increased efficiency in repair of a gene itself is not necessarily coupled to recovery of its transcription after DNA damage.
Full text
PDF


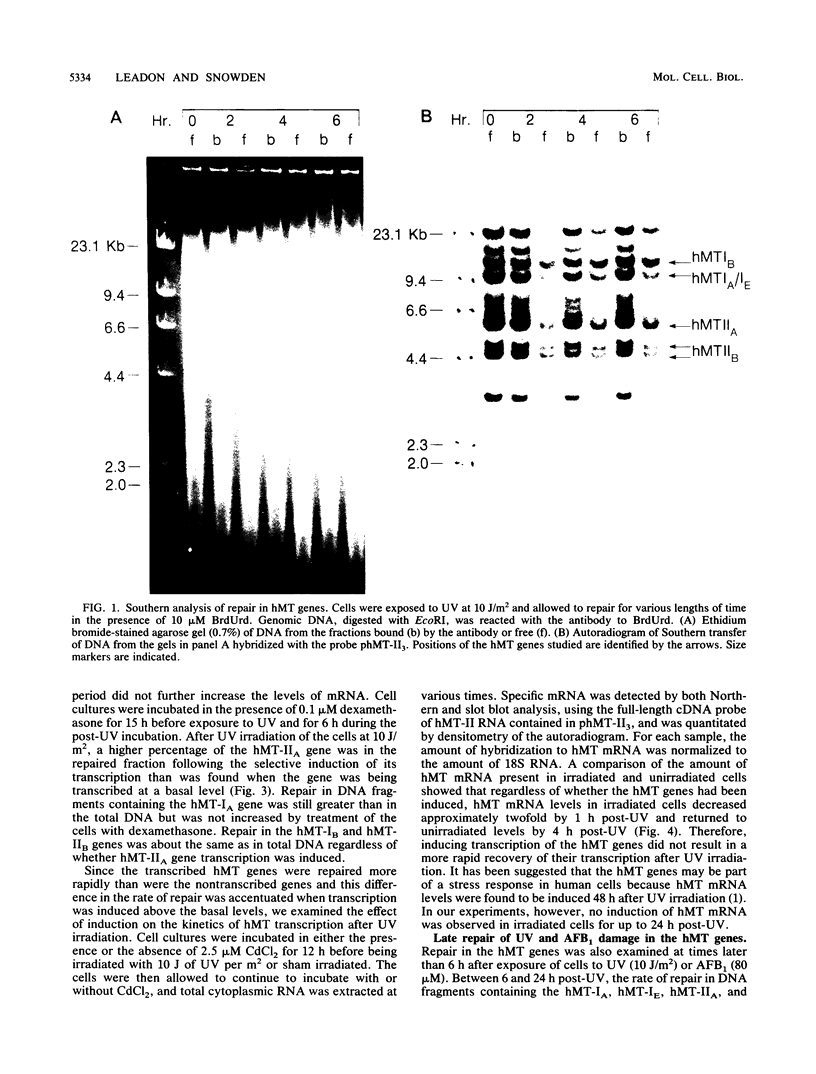
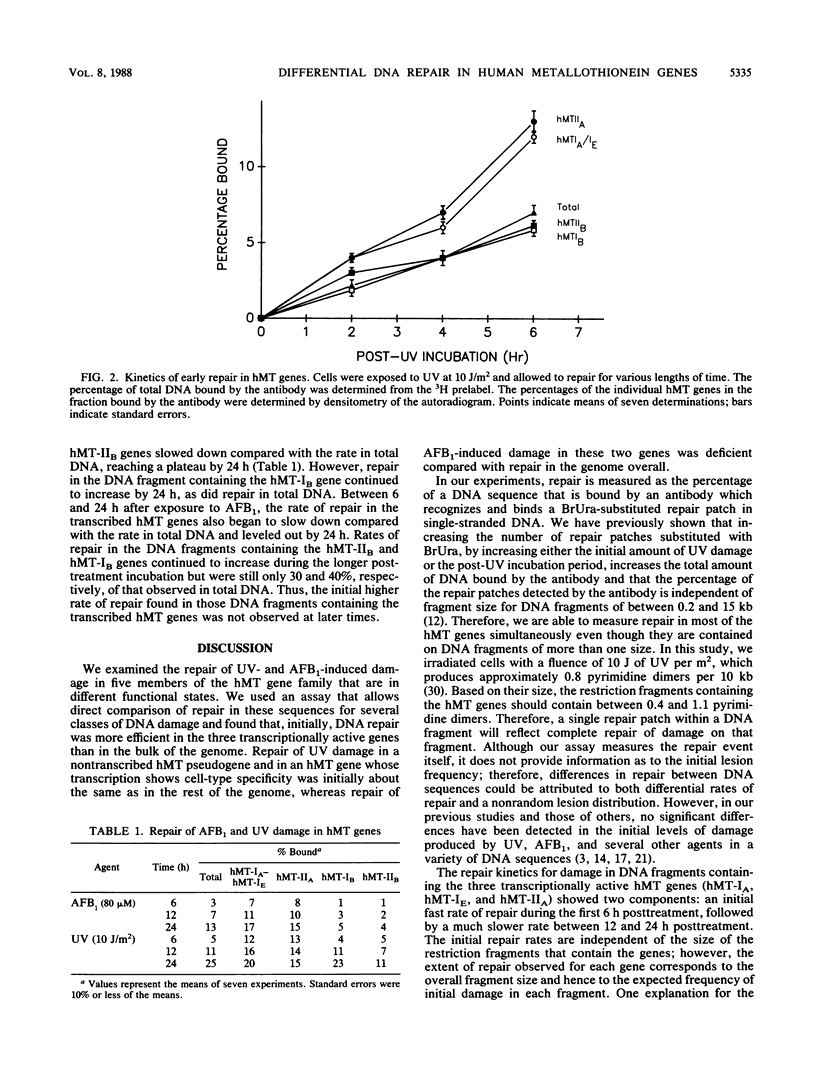
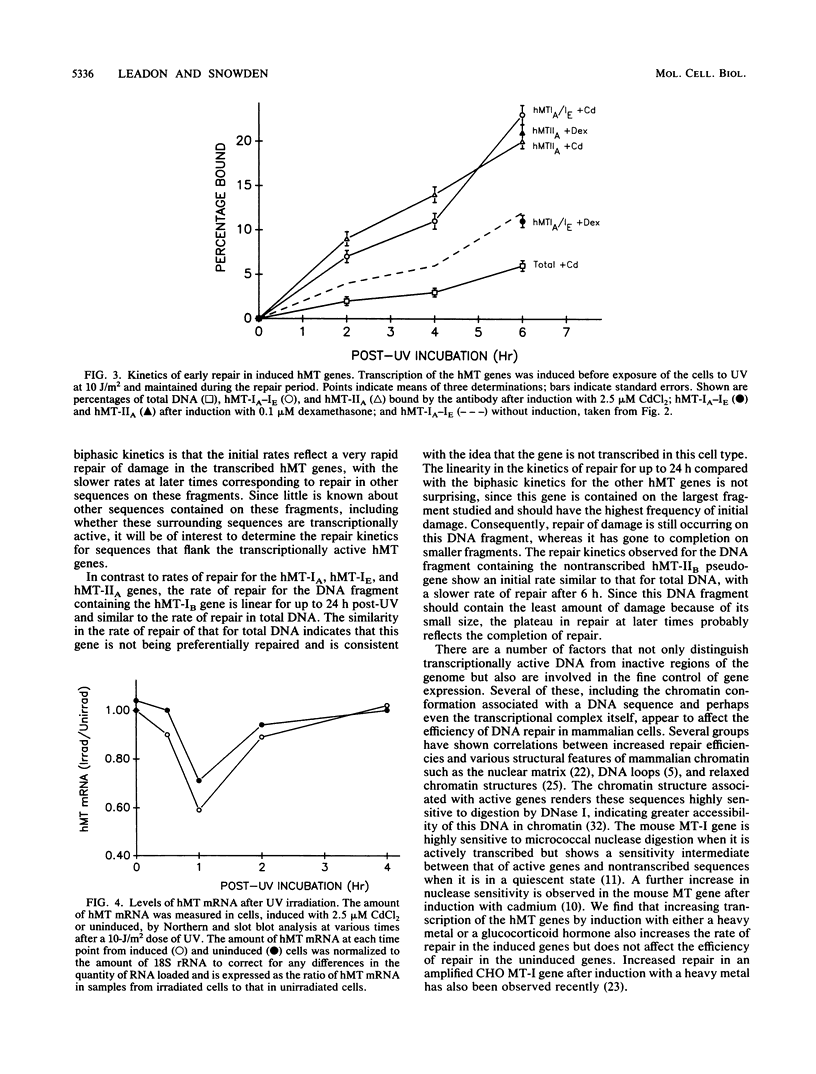


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Ho L., Hanawalt P. C. Characterization of a DNA repair domain containing the dihydrofolate reductase gene in Chinese hamster ovary cells. J Biol Chem. 1986 Dec 15;261(35):16666–16672. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Dighe N. R., Randerath K., Smith H. C. Distribution of initial and persistent 2-acetylaminofluorene-induced DNA adducts within DNA loops. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6605–6608. doi: 10.1073/pnas.82.19.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein IB gene. Mol Cell Biol. 1986 Jun;6(6):2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Eddy R. L., Henry W. M., Haley L. L., Byers M. G., Shows T. B. Human metallothionein genes are clustered on chromosome 16. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5494–5498. doi: 10.1073/pnas.81.17.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Koropatnick J., Andrews G., Duerksen J. D., Varshney U., Gedamu L. Mouse hepatic metallothionein-I gene cleavage by micrococcal nuclease is enhanced after induction by cadmium. Nucleic Acids Res. 1983 May 25;11(10):3255–3267. doi: 10.1093/nar/11.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatnick J., Duerksen J. D. Nuclease sensitivity of alpha-fetoprotein, metallothionein-1, and immunoglobulin gene sequences in mouse during development. Dev Biol. 1987 Jul;122(1):1–10. doi: 10.1016/0012-1606(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Leadon S. A. Differential repair of DNA damage in specific nucleotide sequences in monkey cells. Nucleic Acids Res. 1986 Nov 25;14(22):8979–8995. doi: 10.1093/nar/14.22.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Tyrrell R. M., Cerutti P. A. Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 1981 Dec;41(12 Pt 1):5125–5129. [PubMed] [Google Scholar]
- Leadon S. A., Zolan M. E., Hanawalt P. C. Restricted repair of aflatoxin B1 induced damage in alpha DNA of monkey cells. Nucleic Acids Res. 1983 Aug 25;11(16):5675–5689. doi: 10.1093/nar/11.16.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lieberman H. B., Rabin M., Barker P. E., Ruddle F. H., Varshney U., Gedamu L. Human metallothionein-II processed gene is located in region p11----q21 of chromosome 4. Cytogenet Cell Genet. 1985;39(2):109–115. doi: 10.1159/000132117. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Kesteren A. C., Bussmann C. J., van Zeeland A. A., Natarajan A. T. Preferential repair of nuclear matrix associated DNA in xeroderma pigmentosum complementation group C. Mutat Res. 1984 Oct;141(2):75–82. doi: 10.1016/0165-7992(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Okumoto D. S., Bohr V. A. DNA repair in the metallothionein gene increases with transcriptional activation. Nucleic Acids Res. 1987 Dec 10;15(23):10021–10030. doi: 10.1093/nar/15.23.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Ryan A. J., Billett M. A., O'Connor P. J. Selective repair of methylated purines in regions of chromatin DNA. Carcinogenesis. 1986 Sep;7(9):1497–1503. doi: 10.1093/carcin/7.9.1497. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H., McBride O. W. Chromosomal location of human metallothionein genes: implications for Menkes' disease. Science. 1984 Jun 8;224(4653):1104–1106. doi: 10.1126/science.6719135. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]