Abstract
Antagonism of group I metabotropic glutamate receptors (mGluR1 and mGluR5) reduces behavioral effects of drugs of abuse, including cocaine. However, the underlying mechanisms remain poorly understood. Activation of mGluR5 increases protein synthesis at synapses. Although mGluR5-induced excessive protein synthesis has been implicated in the pathology of fragile X syndrome, it remains unknown whether group I mGluR-mediated protein synthesis is involved in any behavioral effects of drugs of abuse. We report that group I mGluR agonist DHPG induced more pronounced initial depression of inhibitory postsynaptic currents (IPSCs) followed by modest long-term depression (I-LTD) in dopamine neurons of rat ventral tegmental area (VTA) through the activation of mGluR1. The early component of DHPG-induced depression of IPSCs was mediated by the cannabinoid CB1 receptors, while DHPG-induced I-LTD was dependent on protein synthesis. Western blotting analysis indicates that mGluR1 was coupled to extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling pathways to increase translation. We also show that cocaine conditioning activated translation machinery in the VTA via an mGluR1-dependent mechanism. Furthermore, intra-VTA microinjections of mGluR1 antagonist JNJ16259685 and protein synthesis inhibitor cycloheximide significantly attenuated or blocked the acquisition of cocaine-induced conditioned place preference (CPP) and activation of translation elongation factors. Taken together, these results suggest that mGluR1 antagonism inhibits de novo protein synthesis; this effect may block the formation of cocaine–cue associations and thus provide a mechanism for the reduction in CPP to cocaine.
Keywords: group I mGluRs, DHPG, long-term depression (LTD), conditioned place preference (CPP), protein synthesis
INTRODUCTION
Glutamatergic transmission plays a critical role in learning, memory, and drug addiction (Bird and Lawrence, 2009; Bliss and Collingridge, 1993; Kalivas, 2004). Glutamate signals through both ionotropic and metabotropic glutamate receptors (mGluRs) (Conn and Pin, 1997). Group I mGluRs (mGluR1 and mGluR5) are expressed in the mesolimbic dopamine system (Hubert et al, 2001; Shigemoto et al, 1992, 1993), a major component of the reward circuit of the brain (Leshner and Koob, 1999). Since the discovery that mGluR5 knockout mice do not self-administer cocaine and do not show hyperlocomotive responses to cocaine (Chiamulera et al, 2001), mGluR5 has received considerable attention as a therapeutic target for cocaine addiction (Herzig and Schmidt, 2004; Kenny et al, 2005; Kumaresan et al, 2009; Lee et al, 2005; McGeehan and Olive, 2003; Olive, 2009). However, fewer studies have investigated the role of mGluR1 in cocaine addiction. Selective mGluR1 antagonists reduce cocaine self-administration in monkeys (Achat-Mendes et al, 2012) and psychomotor sensitization in rodents (Dravolina et al, 2006; Xie et al, 2010). These studies suggest that mGluR1 is critically involved in behavioral effects of cocaine. However, the molecular and circuit mechanisms for group I mGluR antagonism-induced impairment of addictive behavior remain poorly understood.
Group I mGluRs are coupled to Gq/11, which activates the phospholipase C (PLC) pathway and induces Ca2+ release from internal stores (Conn and Pin, 1997). In addition, they are also coupled to extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling pathways (Gallagher et al, 2004; Grueter et al, 2006; Hou and Klann, 2004; Luscher and Huber, 2010), the activation of which leads to translation initiation and protein synthesis (Banko et al, 2005; Hoeffer and Klann, 2010; Huber et al, 2000; Ronesi et al, 2012). The activation of group I mGluRs, mGluR5 in particular, increases protein synthesis at synapses (Weiler and Greenough, 1993), and mGluR5-induced excessive protein synthesis has been implicated in the pathology of fragile X syndrome (Krueger and Bear, 2011). The formation and consolidation of memories require de novo protein synthesis (Nader et al, 2000a, 2000b; Schafe et al, 2001). The development of drug-associated memories and addictive behavior also requires new protein synthesis (Kuo et al, 2007; Mierzejewski et al, 2006; Milekic et al, 2006). However, the upstream signaling mechanisms by which learning and drug exposure lead to protein synthesis remain poorly understood. One possibility is that learning and cue–drug pairing cause the release of glutamate, which activates group I mGluRs to increase protein synthesis. By coupling to ERK and mTOR signaling pathways (Gallagher et al, 2004; Grueter et al, 2006; Hou and Klann, 2004; Luscher and Huber, 2010), group I mGluRs are well positioned to initiate or increase protein synthesis. However, to our knowledge, no previous studies have demonstrated a role of group I mGluR-dependent protein synthesis in mediating behavioral effects of any drugs of abuse.
The ventral tegmental area (VTA) of the midbrain and its dopaminergic projection play a critical role in reward processing and addictive behavior (Kauer, 2004). Both mGluR1 and mGluR5 are expressed in midbrain dopamine neurons, although mGluR1 is expressed at much higher density (Hubert et al, 2001). We hypothesized that mGluR1-dependent protein synthesis in the VTA is required for cocaine-induced conditioned place preference (CPP). In the present study, we tested this hypothesis using a combination of electrophysiological, biochemical, and behavioral approaches. We report here that cocaine conditioning activated protein synthesis machinery in the VTA through mGluR1 activation, while intra-VTA microinjections of an mGluR1 antagonist or a protein synthesis inhibitor blocked the acquisition of cocaine CPP. These results provide evidence that mGluR1-dependent protein synthesis is critically involved in behavioral effects of cocaine.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) were used for slice electrophysiology (P18–30 of age, 50–100 g), behavior (10–11 weeks old, 300–350 g) and western blotting (VTA slices, P18–30; in vivo VTA samples, 10–11 weeks old). Animal maintenance and use and all experimental procedures were approved by the Institution's Animal Care and Use Committees of the Medical College of Wisconsin, USA, and Shandong University, China.
Brain Slice Preparation
Midbrain slices (250 μm) from male Sprague Dawley rats (P18–30) were prepared as described in our previous studies (Pan et al, 2008a, 2011b; Zhong et al, 2012). Briefly, slices were cut in an ice-cold solution (4–6 °C) containing (in mM): 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgSO4, 26 NaHCO3, 25 glucose, 11.6 sodium ascorbate, and 3.1 sodium pyruvate. After cutting, slices were immediately transferred and incubated in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 3 KCl, 2.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose and were allowed to recover at room temperature for ⩾2.5 h.
Electrophysiology
Whole-cell recordings were made using patch clamp amplifiers (Multiclamp 700B) under infrared-differential interference contrast microscopy. Data acquisition and analysis were performed using DigiData 1440A digitizer and analysis software pClamp 10 (Molecular Devices, Sunnyvale, CA). Dopamine neurons were identified by the width of action potential in cell-attached configuration (>1.2 ms) (Chieng et al, 2011) and the presence of large Ih currents, rhythmic firing at low frequency and prominent afterhyperpolarization (Johnson and North, 1992; Jones and Kauer, 1999; Liu et al, 2005). Glass pipettes were filled with a solution containing (in mM): 100 K-gluconate, 50 KCl, 10 HEPES, 0.2 EGTA, 2 MgCl2, 4 Mg-ATP, 0.3 Na2GTP, and 10 Na2-phosphocreatine at pH 7.2 (with KOH). Series resistance (15–25 MΩ) was monitored throughout the recordings and data were discarded if the resistance changed by >20%. All recordings were performed at 32±1 °C by using an automatic temperature controller (Warner Instrument).
Western Blotting
In experiments shown in Figure 5, VTA slices were maintained in a static incubation chamber in oxygenated ACSF at 32±1 °C. Slices were treated with vehicle or various drugs (see Results section), the VTA was dissected out and then homogenized in 0.2 ml lysis buffer (pH 7.6) containing 50 mM Tris-acetate, 50 mM NaF, 10 mM EDTA, 10 mM EGTA, 0.01% Triton-X, protease inhibitors (Research Product International, Mount Prospect, IL) and protein phosphatase inhibitors I and II (Sigma-Aldrich, St Louis, MO). In experiments shown in Figure 7 and Supplementary Figure S1, rats were anesthetized with isoflurane and rapidly decapitated ∼1 h after the CPP tests. The brains were immediately removed and placed in oxygenated ACSF at 4 °C. Midbrain slices were prepared, and the VTA was dissected out and homogenized in lysis buffer. Western blotting was performed as we have described (Pan et al, 2011b). Blots were blocked in solution containing 5% (w/v) milk and 0.1% (v/v) Tween-20 in tris-buffered saline (TBS-T) for 2 h at room temperature, and incubated overnight at 4 °C with anti-phospho-ERK (1 : 1000), anti-ERK (1 : 1000), anti-phospho-mTOR (1 : 1000), anti-mTOR (1 : 1000), anti-phospho-p70S6K antibody (1 : 500), anti-p70S6K antibody (1 : 500, Abcam, Cambridge, MA), anti-phospho-S6 antibody (1 : 1000), anti-S6 antibody (1 : 1000), anti-phospho-eIF4E (1 : 500), anti-eIF4E (1 : 500), anti-eEF1A (1 : 10000, Millipore, Billerica, MA), or anti-GAPDH (1 : 1000) antibodies. The above antibodies were purchased from Cell Signaling (Danvers, MA) except stated otherwise. Blots were then washed three times with TBS-T and probed with horseradish peroxidase-conjugated secondary antibody (1 : 3000, Bio-Rad Laboratories, Hercules, CA) for 2 h at room temperature before being developed using ECL immunoblotting detection system (Thermo Scientific, Rockford, IL). The intensity of western blots was quantified by densitometry using the ImageJ software (NIH, Bethesda, MD).
Animal Surgery, Intracranial Microinjections and CPP
Male Sprague-Dawley rats (300–350 g) were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed in a stereotaxic device (David Kopf Instruments, Tujunga, CA). Guide cannulae (26 gauge) (Plastics One, Roanoke, VA) were bilaterally implanted 2.8 mm above the VTA or substantia nigra (SN) using aseptic techniques. The stereotaxic coordinates for VTA were: anteroposterior, −5.6 mm; mediolateral, ±2.4 mm (10° angle); dorsoventral, −7.8 mm. The stereotaxic coordinates for the SN were: anteroposterior, −5.6 mm; mediolateral, ±2.4 mm (0° angle); dorsoventral, −7.8 mm (Paxinos and Watson, 1986). The cannulae were anchored to the skull with three stainless-steel screws and dental cement. After the surgery, rats received subcutaneous injections of analgesic (buprenorphine, 0.05 mg/kg) three times daily for 2 days. Rats were allowed to recover for about 1 week. CPP experiments were performed using three-chamber CPP apparatus (Med Associates, St Albans, Vermont) (Pan et al, 2011b). The apparatus has two conditioning chambers (28 cm × 21 cm × 21 cm) and a center chamber (12 cm × 21 cm × 21 cm). The left chamber has stainless-steel mesh floor with white walls, while the right chamber has stainless-steel grid floor with black walls. The three chambers were separated by manual guillotine doors. The CPP protocol consisted of the following sessions:
Pre-test (day 1): animals were allowed to explore both chambers for 20 min and time spent in each side was recorded. Rats showing unconditioned side preference (⩾180 s disparity) were excluded (n=4).
Conditioning (day 2–9): Rats received bilateral intra-VTA infusions of vehicle, JNJ16259685 or cycloheximide via the pre-implanted cannulae. Injector cannulae (33-gauge) were inserted into the guide cannulae. The intra-VTA infusions were made via C313C connectors to 2 μl-Hamilton microsyringes. Vehicle (1 μl per side), JNJ16259685 (0.1 ng, 1 μl per side) or cycloheximide (100 ng, 1 μl per side) was manually injected at a rate of 1 μl over 2 min, and the injectors were kept in place for an additional 2 min to ensure adequate drug diffusion from the injector tip. Thirty minutes after the microinjections, rats received cocaine or saline conditioning.
Cocaine conditioning
Rats received saline injection (0.9% NaCl, 1 ml/kg, i.p.) on days 2, 4, 6, and 8 and were immediately confined to one chamber for 20 min. On days 3, 5, 7, and 9, rats received cocaine injection (15 mg/kg, i.p.) and were immediately confined to the opposite chamber for 20 min.
Saline conditioning
Rats received daily saline injection and were immediately confined to one chamber for 20 min on days 2, 4, 6, and 8 and were confined to the opposite chamber for 20 min on days 3, 5, 7, and 9.
CPP test (day 10): all of the animals were allowed to explore freely for 20 min among the three chambers and time spent on each side is recorded.
In a subset of experiments in which we examined the target specificity of intra-VTA infusions (Figure 9), cocaine conditioning was performed, while the same amount of JNJ16259685 or cycloheximide as described above was microinjected into the SN bilaterally 30 min before each place conditioning, and CPP test was performed on day 10. In another subset of experiments in which we examined the environmental cues on the expression of p-ERK1/2 and p-mTOR levels (Supplementary Figure S1), the place conditioning was conducted without saline or cocaine injections.
Histological Verification of VTA Cannula Placements
Intra-VTA cannula placement was histologically verified by Cresyl Violet staining (Figure 8a) except the animals used in the experiment of western blotting (Figure 7), in which cannula placement was verified by visualizing VTA sections under dissecting microscope. After completion of the CPP experiments, animals were anesthetized with pentobarbital sodium (100 mg/kg, i.p.) and then perfused with phosphate-buffered saline following by 4% paraformaldehyde. The brains were cut into 40 μm sections, stained with Cresyl Violet and then examined with light microscopy. Based on the stereotaxic atlas of Paxinos and Watson (1986), 7 of 72 rats with misplaced cannulae were excluded from behavioral analysis.
Chemicals
Cocaine hydrochloride, CNQX-Na2, and AP-5 were obtained from Sigma-Aldrich (St Louis, MO). Anisomycin, cycloheximide, JNJ16259685, U0126, U0124, MTEP, and rapamycin were obtained from Tocris Bioscience (Ellisville, MO).
Statistics
Data are presented as the mean±SEM. The initial depression of evoked inhibitory postsynaptic currents (IPSCs) (%) was calculated as follows: 100 × (mean amplitude of IPSCs at last 5 min of drug application/mean amplitude of baseline IPSCs). Long-term depression (I-LTD) (%) was calculated as follows: 100 × (mean amplitude of IPSCs during the final 10 min of recording/mean amplitude of baseline IPSCs). The amplitude of IPSCs (evoked at 10–20 s intervals) was first averaged for every minute for each cell and then averaged from a group of cells that received the same treatment. The overall mean of the last 5 min of drug application (initial depression) or the final 10 min of recording (I-LTD) for each group was compared. The CPP score was calculated by the time spent in the cocaine-paired chamber minus the time spent in the saline-paired chamber. Data sets were compared with either Student's t-test (electrophysiology), one-way or two-way analyses of variance (ANOVA) followed by Tukey post hoc analysis (western blotting and CPP). For statistical analysis of locomotor activity, a mixed-design ANOVA, with the between-subjects factors of place conditioning (saline vs cocaine conditioning) and intra-VTA infusions (vehicle, JNJ16259685, or cycloheximide) and repeated measures on the conditioning days, was used. Results were considered to be significant at p<0.05.
RESULTS
The Early Component of DHPG-Induced Depression of IPSCs is CB1 Receptor-Dependent
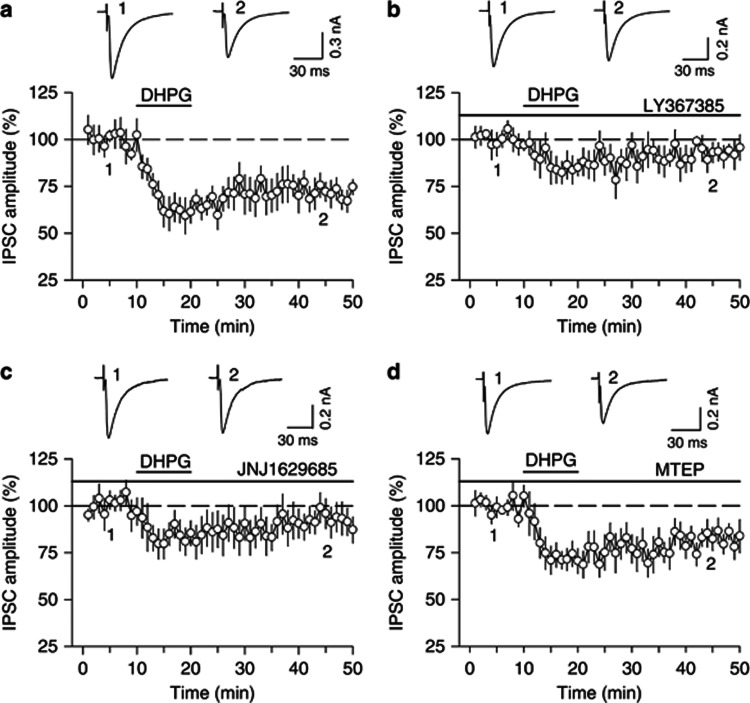
Whole-cell voltage-clamp recordings (holding potential −70 mV) were made from VTA dopamine neurons in midbrain slices prepared from 18- to 30-day-old rats. IPSCs were evoked by stimulating inhibitory synaptic afferents at 0.05–0.1 Hz. Glutamate receptor antagonists CNQX (20 μM) and AP-5 (50 μM) were present in the ACSF throughout the experiments to block excitatory synaptic transmission. Bath application of group I mGluR agonist DHPG (100 μM) for 10 min induced more pronounced initial depression of IPSCs (61.4±7.9% of baseline, 5–10 min of DHPG application, n=7, p<0.01) followed by modest I-LTD (71.8±6.1% of baseline, 40–50 min of the recording, n=7, p<0.01; Figure 1a). Group I mGluRs consist of mGluR1 and mGluR5, and both subtypes of receptors are expressed in the VTA (Hubert et al, 2001). Previous studies have shown that mGluR1-selective antagonists LY367385 (100 μM) and JNJ16259685 (100 nM) completed blocked DHPG-induced mGluR1 responses in brain slices (Mameli et al, 2007; Shin et al, 2009). LY367385 (100 μM) significantly attenuated the early component of DHPG-induced depression of IPSCs (84.3±6.8% of baseline, n=7, p<0.05 vs control) and I-LTD (93.1±5.6% of baseline, n=7, p<0.05 vs control; Figure 1b). Similarly, JNJ16259685 (100 nM) blocked DHPG-induced depression of IPSCs as well (early component: 85.4±7.2% of baseline, p<0.05 vs control; I-LTD: 93.6±7.5% of baseline, n=6, p<0.05 vs control; Figure1c). In contrast, the mGluR5-selective antagonist MTEP (10 μM) did not significantly affect DHPG-induced depression of IPSCs (early component: 71.9±6.3% of baseline, p>0.05 vs control; I-LTD: 82.2±6.1% of baseline, n=8, p>0.05 vs control; Figure1d). MTEP (1 μM) fully blocked DHPG-induced increase in neuronal excitability in amygdala slices (Li et al, 2011). The inability to block DHPG-induced I-LTD in the VTA by MTEP cannot be attributed to insufficient concentration. These results indicate that DHPG-induced depression of IPSCs in the VTA is mediated mainly by mGluR1 activation. Although both mGluR1 and mGluR5 are expressed in the VTA and SN, mGluR1 is expressed at much higher density than mGluR5 (Hubert et al, 2001), which may explain why DHPG-induced depression of IPSCs in the VTA is mediated mainly by mGluR1.
Figure 1.
DHPG-induced depression of IPSCs in VTA dopamine neurons is mediated mainly by mGluR1. (a) Bath application of group I mGluR agonist DHPG (100 μM, 10 min) induced long-term depression (LTD) of evoked IPSCs (n=7, p<0.01 vs baseline). (b) The mGluR1-selective antagonist LY367385 (100 μM) blocked DHPG-induced depression of IPSCs (n=7, p<0.05 vs control). (c) Another mGluR1-selective antagonist, JNJ16259685 (100 nM) blocked DHPG-induced depression of IPSCs (n=6, p<0.05 vs control). (d) The mGluR5-selective antagonist MTEP (10 μM) did not affect DHPG-induced depression of IPSCs (n=8, p<0.05 vs control). Error bars indicate SEM.
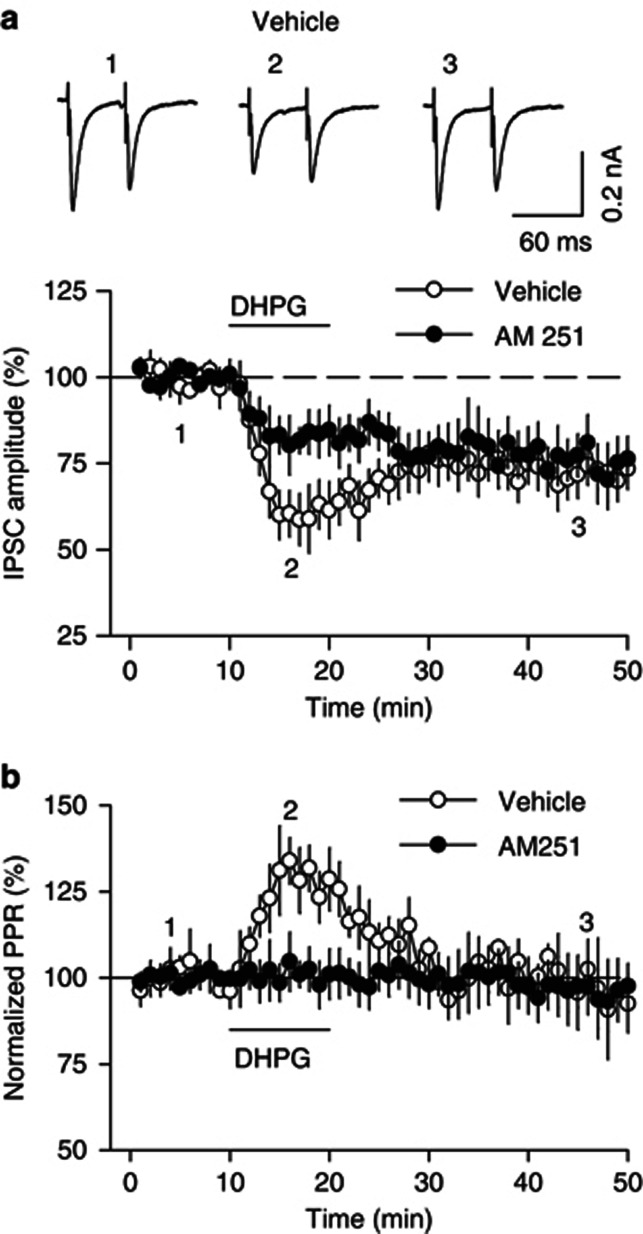
The activation of postsynaptic mGluRs induces release of endocannabinoids (eCBs), which activate presynaptic CB1 receptors to produce retrograde synaptic depression (Gerdeman et al, 2002; Maejima et al, 2001; Robbe et al, 2002; Varma et al, 2001). We examined whether the CB1 receptor antagonist AM251 affected DHPG-induced depression of IPSCs. To determine whether the depression of IPSCs was presynaptic or postsynaptic, we measured the paired-pulse ratio (PPR) by evoking IPSCs with paired-pulse stimuli at an inter-pulse interval of 50 ms. In the continuous presence of AM251 (2 μM), the initial component of DHPG-induced depression of IPSCs was significantly attenuated (82.8±6.4% of baseline, n=6, p<0.05 vs control; Figure 2a), while DHPG-induced I-LTD was not changed. There was a significant increase in the PPR during the initial depression of IPSCs (129.4±8.7%, n=6, p<0.05), and the increase in PPR was blocked by AM251 (Figure 2b). In contrast, AM251 had no significant effect on DHPG-induced I-LTD (75.7±7.4% of baseline, n=6, p>0.05 vs control; Figure 2b). Thus, the increase in the PPR during DHPG-induced initial depression is caused by CB1 receptor activation. Taken together, these results suggest that the CB1 receptor activation contributes to DHPG-induced initial depression of IPSCs, but not I-LTD.
Figure 2.
The early component of DHPG-induced depression of IPSCs is CB1 receptor-dependent. (a) The CB1 receptor antagonist AM251 (2 μM) attenuated the early component of DHPG-induced depression of IPSCs (n=6, p<0.05 vs control, n=6) but did not affect DHPG I-LTD (p>0.05). The amplitude of the first IPSCs was normalized. (b) DHPG increased the PPR during the early component of DHPG-induced depression of IPSCs (p<0.05 vs control) but did not affect the PPR during I-LTD (p>0.05). Error bars indicate SEM.
DHPG-Induced I-LTD is Dependent on Protein Synthesis
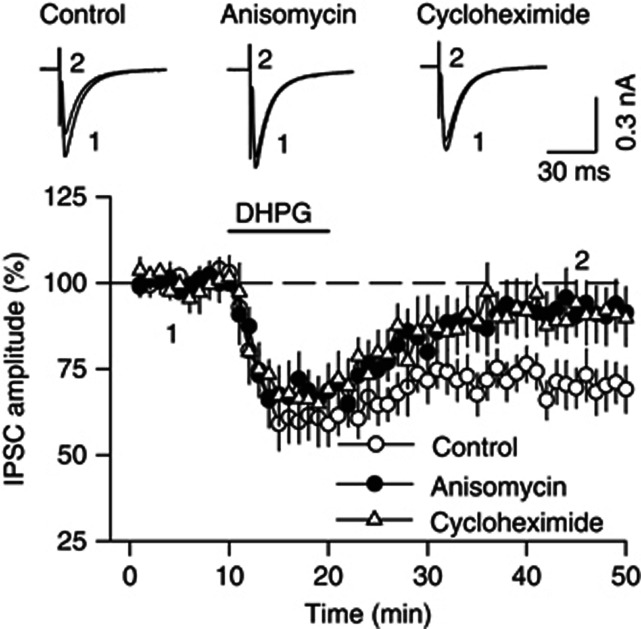
Group I mGluRs are known to be expressed on postsynaptic neurons (Hubert et al, 2001; Shigemoto et al, 1992, 1993). Group I mGluRs-mediated LTD, but not the initial depression of excitatory synaptic transmission, requires protein synthesis (Huber et al, 2000; Mameli et al, 2007; Mockett et al, 2011; Yin et al, 2006). However, it is unknown whether mGluR-mediated I-LTD also requires protein synthesis. We examined the effects of protein synthesis inhibitors on DHPG-induced I-LTD in the VTA. The protein synthesis inhibitor anisomycin (30 μM) or cycloheximide (80 μM) was included in the ACSF throughout the experiments while IPSCs were recorded. We found that either anisomycin (92.0±7.1% of baseline, n=8, p<0.05 vs control) or cycloheximide (91.0±6.2% of baseline, n=6, p<0.05 vs control) blocked the mGluR1-LTD but did not significantly affect the initial component of DHPG-induced depression of IPSCs (anisomycin: 68.1±6.9% of baseline, n=8, p>0.05 vs control; cycloheximide: 66.8±7.9% of baseline, n=6, p>0.05 vs control; Figure 3). These results indicate that mGluR1-mediated I-LTD in the VTA requires protein synthesis.
Figure 3.
Protein synthesis is required for DHPG-induced I-LTD in the VTA. The protein synthesis inhibitor anisomycin (30 μM, n=8, p<0.05 vs control) or cycloheximide (80 μM, n=6, p<0.05 vs control) blocked DHPG-induced I-LTD but did not significantly affect the early component of DHPG-induced depression of IPSCs (p<0.05 vs control). Error bars indicate SEM.
DHPG Activated ERK1/2 and mTOR Signaling Pathways in the VTA
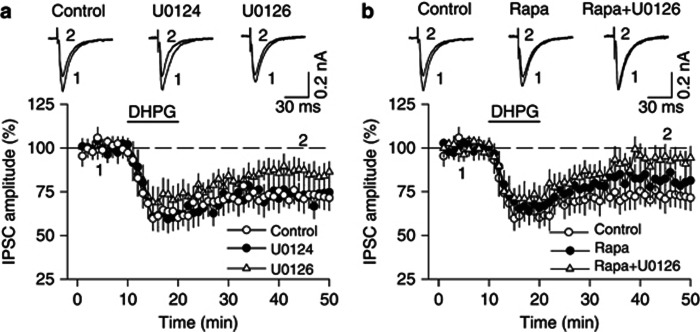
Group I mGluRs are coupled to the mTOR and ERK signaling pathways (Banko et al, 2006; Gallagher et al, 2004; Mao et al, 2005), which are known to be key regulators of protein synthesis (Cammalleri et al, 2003; Huo et al, 2011; Kelleher et al, 2004). We examined the effects of inhibition of ERK1/2 and mTOR signaling pathways on DHPG-induced depression of IPSCs in the VTA. ERK1/2 is phosphorylated and activated by MAPK/ERK kinase (MEK) (Nakielny et al, 1992). The MEK inhibitor U0126 (20 μM) partially attenuated DHPG-induced I-LTD but the attenuation did not reach statistical significance (control: 72.4±6.7% of baseline, n=6; U0126: 86.1±6.4% of baseline, n=8, p>0.05 vs control; Figure 4a). U0124 (20 μM), an inactive analog of U0126, did not affect DHPG-induced I-LTD (73.1±7.5% of baseline, n=7, p>0.05 vs control; Figure 4a). The mTOR inhibitor rapamycin (100 nM) showed a trend toward attenuation of DHPG-induced I-LTD (81.3±6.4% of baseline, n=7, p>0.05 vs control; Figure 4b). The combination of U0126 (20 μM) and rapamycin (100 nM) blocked DHPG-induced I-LTD (93.1±6.0% of baseline, n=8, p<0.05 vs control) without significantly affecting the early component of DHPG-induced depression of IPSCs (71.1±7.2% of baseline, n=8, p>0.05 vs control; Figure 4b). U0126, U0124, and rapamycin were present in the ACSF throughout the experiments. These results indicate that activation of both ERK1/2 and mTOR signaling is required for DHPG-induced I-LTD in VTA dopamine neurons.
Figure 4.
DHPG-induced I-LTD requires the activation of ERK1/2 and mTOR signaling pathways. (a) The MEK inhibitor U0126 (20 μM) exerted only a modest effect on DHPG-induced I-LTD that did not reach statistical significance (n=8, p>0.05 vs control). U0124 (20 μM), an inactive analog of U0126, had no effect on DHPG I-LTD (n=7, p>0.05 vs control). (b) The mTOR inhibitor rapamycin (Rapa, 100 nM) also had no significant effect on DHPG-induced I-LTD (n=7, p>0.05 vs control), whereas co-application of rapamycin (100 nM) and U0126 (20 μM) blocked DHPG-induced I-LTD (n=8, p<0.05 vs control). Error bars indicate SEM.
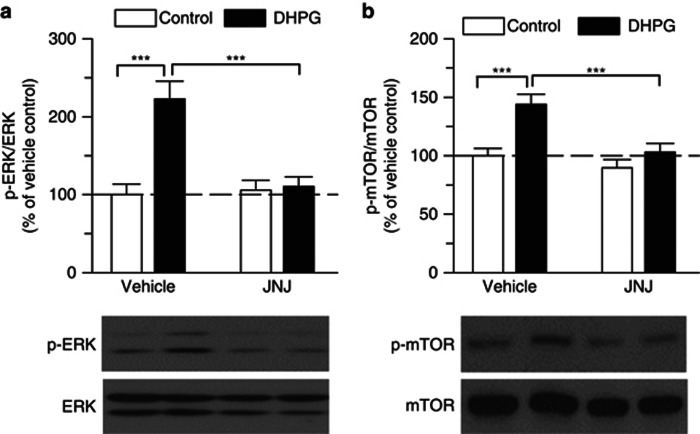
To further investigate whether DHPG activates ERK1/2 in the VTA, we examined the effect of DHPG on the levels of phosphorylated ERK1/2 in isolated VTA slices, using phospho-specific antibodies recognizing the dually phosphorylated (Thr202/Tyr204) and active ERK (Dalby et al, 1998). VTA slices were incubated with DHPG (100 μM) for 10 min in the continuous presence of vehicle or mGluR1 antagonist JNJ16259685 (100 nM). Western blots of VTA homogenates were performed. Two-way ANOVA revealed significant effects of DHPG (F(1,20)=16.06, p<0.001) and JNJ16259685 treatment (F(1,20)=11.15, p<0.01) on p-ERK1/2 levels, as well as a significant interaction between DHPG and JNJ16259685 treatment (F(1,20)=13.48, p<0.01). Tukey's post hoc analysis showed that DHPG significantly increased p-ERK1/2 levels (p<0.001), which was blocked by JNJ16259685 (p<0.001, n=6 each group; Figure 5a). Similarly, DHPG (F(1,24)=14.99, p<0.001) and JNJ16259685 treatment (F(1,24)=12.14, p<0.01) had significant effects on p-mTOR levels, and there was a significant interaction between DHPG and JNJ16259685 treatment (F(1,24)=4.39, p<0.05). Tukey's post hoc analysis showed that DHPG significantly increased p-mTOR levels (p<0.001), and JNJ16259685 blocked DHPG-induced increase in p-mTOR (p<0.001, n=7 each group; Figure 5b). These results indicate that DHPG induced ERK1/2 and mTOR phosphorylation and activation in the VTA in an mGluR1-dependent manner.
Figure 5.
DHPG induced ERK and mTOR phosphorylation and activation in the VTA via mGluR1. (a) DHPG (100 μM, 10 min) increased p-ERK1/2 levels in VTA homogenates compared, this effect was blocked by the JNJ16259685 (JNJ, 100 nM, ***p<0.001, Tukey post hoc test). (b) DHPG also increased p-mTOR levels in VTA homogenates compared with control, this effect was blocked by JNJ16259685 (100 nM, ***p<0.001). Each group of data was obtained from 6 to 7 VTA slices prepared from 3 to 4 rats. Error bars indicate SEM.
Intra-VTA Infusions of JNJ16259685 and Cycloheximide Blocked Cocaine CPP
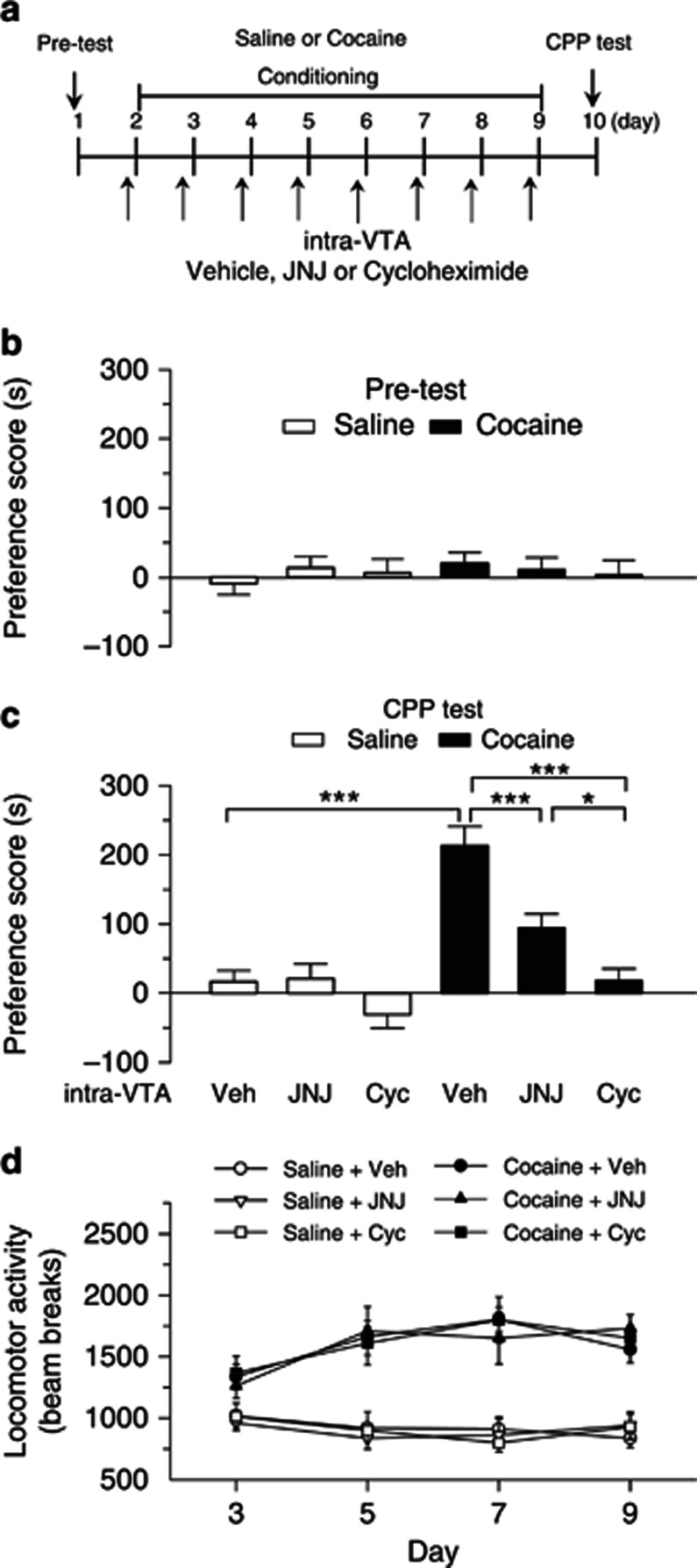
The above results suggest that mGluR1 activation with DHPG induced protein synthesis-dependent I-LTD in the VTA. To test whether this finding is relevant to behavioral effects of cocaine, we examined the effects of intra-VTA microinjections of selective mGluR1 antagonist JNJ16259685 or protein synthesis inhibitor cycloheximide on cocaine CPP. The timeline for baseline preference test (pre-test), cocaine or saline conditioning, intra-VTA microinjections and CPP test is described in Figure 6a. During pre-test, four rats showing unconditioned side preference (⩾180 s disparity) were excluded. The remaining rats did not exhibit significant difference in the time spent in each chamber (p>0.05; Figure 6b). Then, cocaine or saline place conditioning was conducted once daily for 8 days (see Materials and methods). Vehicle (0.01% DMSO in saline, 1 μl per side), JNJ16259685 (0.1 ng, 1 μl per side), or cycloheximide (100 ng, 1 μl per side) was bilaterally microinjected into the VTA via the pre-implanted cannulae 30 min prior to each cocaine- or saline-pairing with a particular chamber. Twenty-four hours after the last pairing, CPP was tested without any drug or vehicle administration. Two-way ANOVA revealed that cocaine treatment (F(1,45)=40.29, p<0.001) and intra-VTA microinjections (F(2,45)=17.10, p<0.001) had significant main effects on CPP scores and there was a significant interaction between cocaine and intra-VTA infusions (F(2,45)=7.06, p<0.01). Tukey's post hoc test showed that intra-VTA microinjections of JNJ 16259685 produced a significant decrease in the CPP score in cocaine-conditioned rats (p<0.001) but did not affect the CPP score in saline-conditioned rats (p>0.05). In addition, intra-VTA microinjection of cycloheximide blocked the acquisition of CPP to cocaine (p<0.001) without affecting CPP score in saline-conditioned rats (p>0.05; Figure 6c). Thus, JNJ16259685 and cycloheximide attenuated CPP to cocaine without inducing CPP or conditioned place aversion in saline-conditioned rats.
Figure 6.
Intra-VTA infusions of the mGluR1 antagonist JNJ16259685 or protein synthesis inhibitor cycloheximide during the conditioning phase attenuated the acquisition of CPP to cocaine. (a) Timeline of drug treatment and behavioral paradigm. Groups of rats received saline and cocaine place conditioning once daily for 8 days. Vehicle (Veh), JNJ 16259685 (JNJ) or cycloheximide (Cyc) was bilaterally infused into the VTA 30 min prior to each saline- or cocaine-pairing. (b) Pre-test indicates that rats did not exhibit baseline bias in place preference in all groups (n=7–10, p>0.05). (c) Intra-VTA infusions of JNJ16259685 or cycloheximide significantly attenuated CPP in cocaine-conditioned rats but did not affect CPP scores in saline-conditioned rats (n=7–10, *p<0.05, ***p<0.001, Tukey post hoc test). (d) Intra-VTA infusions of JNJ16259685 or cycloheximide did not significantly affect locomotor activity in cocaine- or saline-conditioned rats (p>0.05, n=7–10). Error bars indicate SEM.
During place conditioning, locomotor activities in the conditioning chambers were tracked by infrared photobeam breaks. We compared locomotor activities in saline- or cocaine-conditioned rats that received intra-VTA infusions of vehicle, JNJ16259685, and cycloheximide over the 4 days of cocaine conditioning or correspondent saline conditioning (i.e., day 3, 5, 7, 9). Locomotor activities were analyzed with a mixed ANOVA that included between-subjects factors of place conditioning and intra-VTA infusions and within-subject factor of conditioning days. Cocaine significantly increased locomotor activities over the 4 days of conditioning (F(1,180)=183.67, p<0.001), and there was a significant interaction between cocaine and conditioning days (F(3,180)=6.06, p<0.001). However, intra-VTA infusions of JNJ16259685 or cycloheximide did not significantly alter locomotor activities in saline- and cocaine-conditioned rats (F(2,180)=0.05, p>0.05) and there was no significant interaction between place conditioning and intra-VTA infusions (F(2,180)=0.08, p>0.05) (Figure 6d).
Cocaine Conditioning Activated Protein Synthesis Machinery in the VTA Through mGluR1
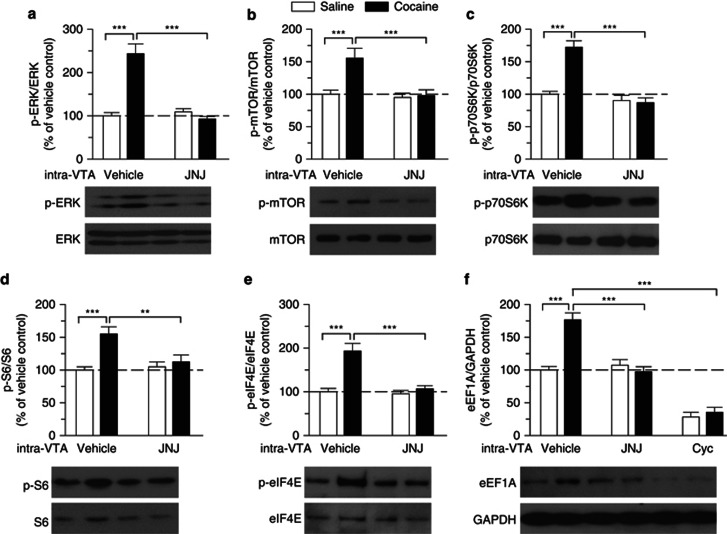
We determined whether the acquisition of CPP to cocaine was accompanied with mGluR1-dependent phosphorylation and activation of ERK1/2 and mTOR in the VTA. About 1 h after the CPP tests, some of saline- or cocaine-conditioned rats (shown in Figure 6) were euthanized and the VTA was dissected out bilaterally from midbrain slices. Western blotting was performed using antibodies against p-ERK1/2, ERK1/2, p-mTOR, and mTOR. Two-way ANOVA indicated that place conditioning and intra-VTA infusions had significant effects on p-ERK1/2 levels (place conditioning: F(1,20)=24.06, p<0.001; intra-VTA infusion: F(1,20)=30.14, p<0.001; place conditioning × intra-VTA infusion interaction: F(1,20)=38.34, p<0.001) and p-mTOR levels (place conditioning: F(1,20)=8.66, p<0.01; intra-VTA infusion: F(1,20)=9.95, p<0.01; place conditioning × intra-VTA infusion interaction: F(1,20)=6.95, p<0.05). Tukey's post hoc test showed that p-ERK1/2 and p-mTOR levels were significantly increased in cocaine-conditioned rats compared with those in saline-conditioned rats (p<0.001 for both p-ERK1/2 and p-mTOR), while intra-VTA infusions of JNJ16259685 significantly attenuated p-ERK1/2 and p-mTOR levels in cocaine-conditioned rats (p<0.001 for both p-ERK1/2 and p-mTOR; n=6 each group; Figure 7a and b). These results indicate that the acquisition of CPP to cocaine is accompanied by increases in ERK and mTOR phosphorylation and activation in the VTA, and mGluR1 activation contributes to cocaine-induced ERK and mTOR activation.
Figure 7.
Cocaine CPP activates ERK and mTOR signaling pathways and increases the synthesis of the 5′TOP-encoded protein eEF1A through mGluR1 activation. (a, b) p-ERK1/2 (a) and mTOR levels (b) in the VTA were significantly increased in cocaine-conditioned rats compared with those in saline-conditioned rats, and the increase was blocked by intra-VTA infusions of JNJ16259685 (JNJ) (***p<0.001, Tukey post hoc test). (c, d, e) p-p70S6K (c), p-S6 (d), and p-eIF4E (e) levels in the VTA were significantly increased in cocaine-conditioned rats compared with those in saline-conditioned rats, and these increases were blocked by intra-VTA infusions of JNJ16259685 during the conditioning phase (**p<0.01, ***p<0.001). (f) eEF1A was significantly increased in cocaine-conditioned rats compared with that in saline-conditioned rats (***p<0.001), and this increase was blocked by intra-VTA infusions of JNJ16259685 or cycloheximide (Cyc) during the conditioning phase (***p<0.001). Western blotting was performed on VTA samples collected ∼1 h after the CPP tests. n=6 rats each group. Error bars indicate SEM.
To test whether exposure to novel cues (the conditioning chamber) caused the increases in p-ERK1/2 and p-mTOR, we compared p-ERK1/2 and p-mTOR levels in the VTA between rats raised in home cages and rats that received place conditioning alone. The place conditioning was performed following the timeline of cocaine conditioning but the rats did not receive any saline or cocaine treatment. Place conditioning alone had no significant effect on p-ERK1/2 or p-mTOR levels in the VTA compared with control rats raised in home cages (p>0.05, n=3 rats each group; Supplementary Figure S1). These results suggest that place conditioning without cocaine injections does not affect p-ERK1/2 or p-mTOR levels in the VTA.
Both ERK1/2 and mTOR control protein synthesis at the level of translation (Cammalleri et al, 2003; Huo et al, 2011; Kelleher et al, 2004). We examined whether cocaine-induced activation of ERK1/2 and mTOR signaling pathways in turn activated the protein translation machinery in the VTA. mTOR phosphorylates the 70-kDa ribosomal protein S6 kinase (p70S6K) at T389 site (Jefferies et al, 1997). To determine whether cocaine CPP affected phosphorylation of p70S6K in the VTA, we performed western blotting to detect phosphorylated p70S6K (p-p70S6K) at T389 site and total p70S6K in VTA homogenates from saline- and cocaine-conditioned rats shown in Figure 6. Two-way ANOVA indicated that place conditioning (F(1,20)=21.20, p<0.001) and intra-VTA infusions (F(1,20)=40.38, p<0.001) had significant effects on p-p70S6K levels and there was a significant interaction between place conditioning and intra-VTA infusions (F(1,20)=25.26, p<0.001). Tukey's post hoc test showed that the levels of p-p70S6K at T389 site were significantly increased in cocaine-conditioned rats compared with those of saline-conditioned rats (p<0.001), while intra-VTA infusions of mGluR1 antagonist JNJ16259685 blocked the increase in p-p70S6K (p<0.001; Figure 7c).
The activated p70S6K phosphorylates its target substrate ribosomal protein S6 at Ser235/236. S6 phosphorylation initiates 5′ terminal oligopyrimidine (5′ TOP) mRNA translation (Proud, 2007). Two-way ANOVA indicated that place conditioning (F(1,20)=12.89, p<0.01) and intra-VTA infusions (F(1,20)=4.69, p<0.05) had significant effects on phosphorylated S6 (p-S6) levels and there was a significant interaction between place conditioning and intra-VTA infusions (F(1,20)=7.44, p<0.05). Tukey's post hoc test showed that p-S6 was significantly increased in cocaine-conditioned rats (p<0.001), and this increase was also blocked by mGluR1 antagonist JNJ16259685 (p<0.001; Figure 7d).
ERK1/2 indirectly phosphorylates eukaryotic translation initiation factor 4E (eIF4E) by activating the MAPK-interacting kinase (Proud, 2007). Two-way ANOVA indicated that place conditioning (F(1,20)=22.85, p<0.001) and intra-VTA infusions (F(1,20)=17.32, p<0.001) had significant effects on phosphorylated eIF4E (p-eIF4E at S209) levels and there was a significant interaction between place conditioning and intra-VTA infusions (F(1,20)=14.14, p<0.001). Tukey's post hoc test showed that cocaine conditioning increased p-eIF4E levels in the VTA (p<0.001), and this effect was attenuated by JNJ16259685 (p<0.001; Figure 7e). In addition, we found that place conditioning (F(1,30)=14.69, p<0.001) and intra-VTA infusions (F(2,30)=94.27, p<0.001) had significant effects on protein levels of eukaryotic elongation factor 1A (eEF1A), a 5′ TOP mRNA-encoded protein, and there was a significant interaction between place conditioning and intra-VTA infusions (F(2,30)=16.75, p<0.001). Tukey's post hoc test showed that cocaine conditioning significantly increased protein levels of eEF1A (p<0.001), and this increase was blocked by intra-VTA infusions of JNJ16259685 (p<0.001) or protein synthesis inhibitor cycloheximide (p<0.001; Figure 7f). These results suggest that cocaine conditioning activates mGluR1, which is coupled to ERK1/2 and mTOR signaling pathways to stimulate de novo protein synthesis.
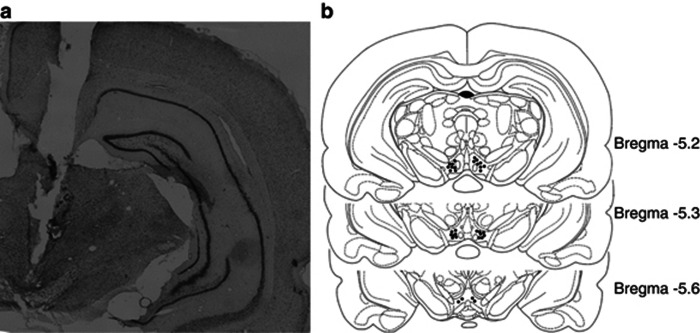
VTA cannula placement was histologically verified by Cresyl Violet staining except the animals used in the experiment of western blotting (Figure 7), in which cannula placement was verified by visualizing VTA slices under dissecting microscope. Figure 8 shows the representative coronal sections containing the VTA and the sites of the cannula tips for intra-VTA infusions from the experiments in Figures 6 and 7.
Figure 8.
Histological verification of intra-VTA cannula placements. (a) Cresyl Violet-stained coronal section of typical bilateral intra-VTA cannula placements. (b) Representative sites of cannula tips in the VTA from data shown in Figures 6 and 7.
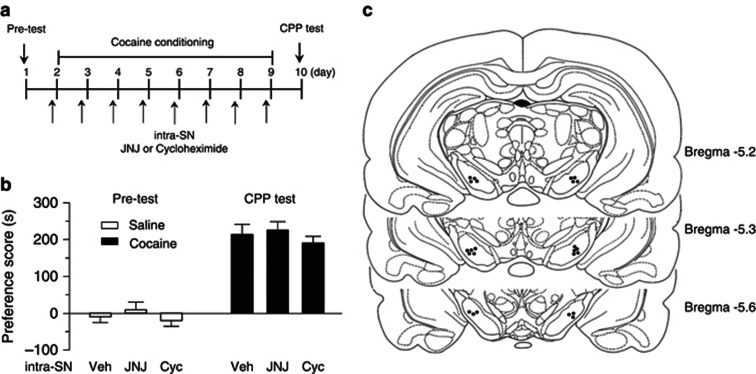
Intra-SN Infusions of JNJ16259685 and Cycloheximide Did not Affect Cocaine CPP
Intra-VTA infusions of JNJ16259685 or cycloheximide may affect the neighboring area (i.e., SN) due to drug diffusion. We examined whether intra-SN infusions of JNJ16259685 or cycloheximide affected cocaine CPP, the experiments were similar to those for intra-VTA infusions described above. Cocaine conditioning was performed as shown in Figure 9a. During pre-test, two rats showing unconditioned side preference (≥180 s disparity) were excluded. The remaining rats did not exhibit significant difference in the time spent in each chamber (p>0.05; Figure 9b). JNJ16259685 (0.1 ng, 1 μl per side) or cycloheximide (100 ng, 1 μl per side) was bilaterally microinjected into the SN via the pre-implanted cannulae 30 min prior to each cocaine- or saline-pairing with a particular chamber. Twenty-four hours after the last pairing, CPP was tested without any drug or vehicle administration. One-way ANOVA showed that intra-SN infusions of JNJ16259685 and cycloheximide had no significant effects on the acquisition of cocaine CPP compared with intra-SN infusions of vehicle (F(2,21)=0.61, p>0.05). The sites of the cannula tips for intra-SN infusions were shown in Figure 9c.
Figure 9.
Intra-SN infusions of JNJ16259685 or cycloheximide did not affect cocaine CPP. (a) Timeline of drug treatment and behavioral paradigm. (b) Pre-test indicated that rats did not exhibit baseline, non-conditioning place preference (n=7–8, p>0.05). Intra-SN infusions of JNJ16259685 (JNJ) or cycloheximide (Cyc) did not affect CPP to cocaine (n=7–8, p>0.05). The group for intra-VTA infusions of vehicle (Veh) was obtained from Figure 6 and was re-plotted here for the purpose of comparison. Error bars indicate SEM. (c) Representative sites of cannula tips in the SN.
DISCUSSION
Both mGluR1 and mGluR5 antagonists reduce behavioral responses to drugs of abuse, including cocaine (Achat-Mendes et al, 2012; Bird and Lawrence, 2009; Chiamulera et al, 2001), but the underlying mechanisms remain largely unknown. We demonstrated here that cocaine conditioning activated protein synthesis machinery in the VTA through mGluR1 activation, while intra-VTA microinjections of an mGluR1 antagonist or a protein synthesis inhibitor blocked the acquisition of cocaine CPP. These results suggest that mGluR1-dependent protein synthesis in the VTA is required for acquisition of cocaine CPP. Since mGluR1 and mGluR5 belong to the same group I mGluR family and share same signal transduction mechanisms (Conn and Pin, 1997), it is likely that inhibition of de novo protein synthesis represents a common mechanism by which group I mGluR antagonists reduce addictive behavior.
Group I mGluR agonist DHPG induced protein synthesis-dependent LTD in the VTA
We showed that DHPG induced more pronounced initial depression of IPSCs followed by modest I-LTD in the VTA. The CB1 receptor antagonist AM251 significantly attenuated the early component of DHPG-induced depression of IPSCs without affecting I-LTD, whereas protein synthesis inhibitors anisomycin and cycloheximide did not affect the early component of the depression of IPSCs but attenuated I-LTD. At excitatory synapses, protein synthesis inhibitors attenuated or blocked group I mGluR-mediated LTD without affecting the early component of DHPG-induced depression (Banko et al, 2005; Hoeffer and Klann, 2010; Huber et al, 2000; Mameli et al, 2007; Mockett et al, 2011; Ronesi et al, 2012; Yin et al, 2006). In contrast, CB1 receptor antagonists blocked the early component of DHPG-induced depression, but not LTD (Bellone and Luscher, 2005; Grueter et al, 2008; Rouach and Nicoll, 2003). These results indicate that group I mGluR-LTD and I-LTD are protein synthesis-dependent, while DHPG-induced initial depression is mediated by the CB1 receptor. However, it should be noted that some forms of DHPG-induced LTD was not dependent on protein synthesis (Grueter et al, 2007; Jung et al, 2012). Brain regions and methodological approaches (Sajikumar et al, 2005) might explain the difference in protein synthesis dependence.
We have shown that pairing cocaine application (3 μM) with repetitive stimulation at 10 Hz induced synaptic activation of D2 dopamine receptor and group I mGluRs to induce I-LTD, which was also CB1-dependent (Pan et al, 2008a, 2008b). Besides synaptic activation of group I mGluRs, repetitive synaptic stimulation induced persistent increase in presynaptic Ca2+ level, which is required for CB1-dependent I-LTD (Heifets et al, 2008; Pan et al, 2008b). The 10-Hz repetitive synaptic stimulation was not applied during DHPG application, which might explain why DHPG-induced activation of CB1 receptors induced acute depression, but not I-LTD in the VTA. It is known that reduced GABAA receptor-mediated inhibition induces metaplasticity of the excitatory synapses and facilitates long-term potentiation and learning (Cui et al, 2008; Pan et al, 2011a). Dopamine neurons receive continuous synaptic bombardment in vivo; many synaptic connections are severed in the VTA slice preparation. We speculate that synaptic activation of group I mGluRs in the presence of cocaine causes I-LTD-like depression of inhibitory synaptic transmission in vivo, the resulting reduction of GABAA receptor-mediated inhibition may facilitate cue–cocaine associations and acquisition of CPP to cocaine.
ERK1/2 and mTOR signaling pathways play key roles in regulating protein synthesis (Costa-Mattioli et al, 2009) and have been implicated in group I mGluR-mediated LTD (Banko et al, 2005; Cammalleri et al, 2003; Hoeffer and Klann, 2010; Huber et al, 2000; Huo et al, 2011; Ronesi et al, 2012). We found that DHPG-induced I-LTD in the VTA was blocked by the combination of MEK inhibitor U0126 and mTOR inhibitor rapamycin, but not by either inhibitor alone. Western blotting showed that DHPG increased the p-ERK1/2 and p-mTOR levels in the VTA, and these increases were attenuated by mGluR1 antagonist JNJ16259685. These results indicate that mGluR1 activation in the VTA activates both ERK1/2 and mTOR signaling pathways to induce I-LTD. We suspect that the activation of either pathway is subthreshold for the induction I-LTD in the VTA, while activation of both ERK1/2 and mTOR signaling pathways reaches the threshold for LTD induction. Previous studies have shown that group I mGluR agonist DHPG induces LTD in the hippocampus, and this LTD is blocked by either MEK inhibitor U0126 (Gallagher et al, 2004) or mTOR inhibitor rapamycin (Hou and Klann, 2004), while mGluR-LTD in the VTA was blocked by rapamycin but was unaffected by U0126 (Mameli et al, 2007). Thus, ERK1/2 and mTOR signaling pathways are recruited differentially by group I mGluR activation, depending upon the synapses (excitatory vs inhibitory), brain regions or experimental conditions. It is thus likely that group I mGluR activation increases protein synthesis in the VTA through the activation of ERK1/2 and mTOR signaling pathways.
mGluR1-dependent activation of ERK1/2 and mTOR signaling pathways induced by cocaine conditioning
We have shown that cocaine conditioning increased p-ERK1/2 and p-mTOR levels in the VTA, and this increase was blocked by intra-VTA infusions of mGluR1 antagonist JNJ16259685. Thus, ERK1/2 and mTOR signaling pathways were not only activated by exogenous mGluR agonist DHPG, but also activated by cocaine conditioning. In contrast, repeated exposure to the conditioning chambers without cocaine injections did not activate ERK1/2 or mTOR, suggesting that environmental cues become effective in activating ERK1/2 or mTOR only after being paired with cocaine injections. How did cocaine conditioning lead to mGluR1 activation in the VTA? The environmental cues associated with cocaine injection during cocaine conditioning likely activate sensory inputs through glutamatergic excitatory afferents. Repetitive synaptic activity activates group I mGluRs in VTA dopamine neurons (Fiorillo and Williams, 1998). It is likely that cocaine conditioning activates excitatory afferents to induce the release of glutamate, which activates group I mGluRs (mainly mGluR1) on VTA dopamine neurons, and mGluR1 activation in turn leads to phosphorylation and activation of ERK1/2 and mTOR.
Systemic or local administration of ERK1/2 inhibitors blocked incubation of cocaine craving and CPP to cocaine (Girault et al, 2007; Lu et al, 2006; Miller and Marshall, 2005; Pan et al, 2011b). mTOR inhibitors blocked the expression of cocaine CPP and cue-induced reinstatement of cocaine seeking (Bailey et al, 2012; Wang et al, 2010). These studies suggest that ERK1/2 and mTOR signaling pathways are critically involved in cocaine- or cue-induced addictive behavior. However, the upstream activators and downstream effectors of ERK1/2 and mTOR in mediating addictive behavior remain poorly defined. Group 1 mGluRs activate translation regulatory factors via ERK1/2 and mTOR (Page et al, 2006). The translation regulatory factor p70S6K and its downstream target ribosomal protein S6 can be phosphorylated and activated by mTOR and ERK (Cammalleri et al, 2003; Jaworski et al, 2005; Lawrence et al, 2004; Page et al, 2006). We found that cocaine conditioning increased p-p70S6K, p-S6, and p-eIF4E in the VTA, and these increases were blocked by JNJ16259685. The activation of the translation regulatory factors is indicative of de novo protein synthesis. Thus, cocaine conditioning induces mGluR1 activation, which activates ERK1/2 and mTOR signaling pathways to increase translation and protein synthesis.
mGluR1-dependent protein synthesis is required for the acquisition of cocaine CPP
We found that intra-VTA infusions of mGluR1 antagonist JNJ16259685 or protein synthesis inhibitor cycloheximide significantly attenuated cocaine CPP, suggesting that mGluR1-dependent protein synthesis is required for the acquisition of cocaine CPP. mGluR5-dependent protein synthesis has been implicated in the pathology of fragile X syndrome (Krueger and Bear, 2011; Osterweil et al, 2010; Ronesi et al, 2012). Less is known whether mGluR1 activation is involved in protein synthesis and whether this process is required for the formation or drug-associated memories. Protein translation from mRNA can be divided into three critical steps: initiation, elongation, and termination (Taylor et al, 2006). During elongation, cognate aminoacyl-tRNA is delivered to the A site of the ribosome by eEF1A, which greatly speeds up the process. eEF1A is therefore required for translation elongation (Mateyak and Kinzy, 2010; Taylor et al, 2006). We found that cocaine conditioning increased the levels of eEF1A, and this increase was blocked by JNJ16259685 or cycloheximide. These results suggest that mGluR1 antagonism blocked cocaine CPP through inhibition of de novo protein synthesis. Previous studies have shown that systemic administration of mGluR5 antagonists reduces cocaine-induced locomotor sensitization, CPP, and self-administration (Herzig and Schmidt, 2004; Kenny et al, 2005; Lee et al, 2005; McGeehan and Olive, 2003). Since mGluR1 and mGluR5 share common signal transduction mechanisms (Conn and Pin, 1997), we speculate that inhibition of protein synthesis may constitute a mechanism for mGluR5 antagonism-induced reduction of addictive behavior as well. mGluR1 and mGluR5 are often expressed in non-overlapping fashion in the central nervous system (Hubert et al, 2001; Shigemoto et al, 1992, 1993). mGluR5 is abundantly expressed in the nucleus accumbens (Shigemoto et al, 1993). Inhibition of protein synthesis in the nucleus accumbens and other parts of the reward circuitry may account for the effects of mGluR5 antagonists on addictive behavior (Chiamulera et al, 2001; Herzig and Schmidt, 2004; Kenny et al, 2005; Kumaresan et al, 2009; Lee et al, 2005; McGeehan and Olive, 2003; Olive, 2009).
Intra-VTA microinjections of JNJ16259685 and cycloheximide did not significantly alter cocaine-induced increase in locomotor activities. Although it is thought that a common brain circuitry underlies both drug reward and locomotor stimulation (Wise and Bozarth, 1987), locomotor sensitization and development of CPP are dissociable effects (Shimosato and Ohkuma, 2000; Veeneman et al, 2011). The attenuation of cocaine CPP by JNJ16259685 and cycloheximide could be explained by decreased rewarding effects of cocaine or decreased ability to learn the association between environmental cues and rewarding effects of cocaine. More recent studies indicate that group I mGluRs, mGluR5 in particular, are critically involved in learning and memory. Group I mGluR antagonists attenuated the acquisition of hippocampus-dependent contextual and reference memories (Gravius et al, 2006; Naie and Manahan-Vaughan, 2004). Long-term memory requires transcription and protein synthesis as protein synthesis inhibitors disrupt memory consolidation (Morris et al, 2006; Santini et al, 2004). The decreased associative learning might explain why JNJ16259685 and cycloheximide attenuated the acquisition of CPP to cocaine. A recent study has shown that non-pharmacological manipulation of cue–drug associated memories through extinction within the reconsolidation window can reduce drug craving and seeking in rats and humans (Xue et al, 2012). Both pharmacological and non-pharmacological intervention of drug–cue memories might provide new avenues for treating drug addiction.
Acknowledgments
This work was supported by National Institutes of Health Grants DA024741 and MH095921. It was also partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and National Institutes of Health Grant UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Sciences.
AUTHOR CONTRIBUTIONS
All authors designed the experiments. FY, PZ, XL, and DS performed the experiments, collected and analyzed the data. FY, PZ, and QSL drafted the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 receptors attenuates behavioral effects of cocaine and methamphetamine in squirrel monkeys. J Pharmacol Exp Ther. 2012;343:214–224. doi: 10.1124/jpet.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Ma D, Szumlinski KK. Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol. 2012;17:248–258. doi: 10.1111/j.1369-1600.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, et al. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity. Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schafer D, Schmidt WJ, Danysz W. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats - a comparison. Neuropharmacology. 2006;51:1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J Neurosci. 2008;28:9261–9270. doi: 10.1523/JNEUROSCI.2886-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction. Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci USA. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Iadevaia V, Proud CG. Differing effects of rapamycin and mTOR kinase inhibitors on protein synthesis. Biochem Soc Trans. 2011;39:446–450. doi: 10.1042/BST0390446. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Kauer JA. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J Neurosci. 1999;19:9780–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Liang KC, Chen HH, Cherng CG, Lee HT, Lin Y, et al. Cocaine-but not methamphetamine-associated memory requires de novo protein synthesis. Neurobiol Learn Mem. 2007;87:93–100. doi: 10.1016/j.nlm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Lawrence JC, Lin TA, McMahon LP, Choi KM. Modulation of the protein kinase activity of mTOR. Curr Top Microbiol Immunol. 2004;279:199–213. doi: 10.1007/978-3-642-18930-2_12. [DOI] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateyak MK, Kinzy TG. eEF1A: thinking outside the ribosome. J Biol Chem. 2010;285:21209–21213. doi: 10.1074/jbc.R110.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Mierzejewski P, Siemiatkowski M, Radwanska K, Szyndler J, Bienkowski P, Stefanski R, et al. Cycloheximide impairs acquisition but not extinction of cocaine self-administration. Neuropharmacology. 2006;51:367–373. doi: 10.1016/j.neuropharm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Guevremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci. 2011;31:7380–7391. doi: 10.1523/JNEUROSCI.6656-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000a;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000b;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Cohen P, Wu J, Sturgill T. MAP kinase activator from insulin-stimulated skeletal muscle is a protein threonine/tyrosine kinase. EMBO J. 1992;11:2123–2129. doi: 10.1002/j.1460-2075.1992.tb05271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O, et al. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int. 2006;49:413–421. doi: 10.1016/j.neuint.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008a;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008b;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011a;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011b;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1986The Rat Brain in Stereotaxic coordinates Compact3rd ednOrlando: Academic Press: Sydney [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15:431–440. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Nicoll RA. Endocannabinoids contribute to short-term but not long-term mGluR-induced depression in the hippocampus. Eur J Neurosci. 2003;18:1017–1020. doi: 10.1046/j.1460-9568.2003.02823.x. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Frey JU. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Curr Opin Neurobiol. 2005;15:607–613. doi: 10.1016/j.conb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Ohkuma S. Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav. 2000;66:285–292. doi: 10.1016/s0091-3057(00)00185-4. [DOI] [PubMed] [Google Scholar]
- Shin JH, Kim YS, Worley PF, Linden DJ. Depolarization-induced slow current in cerebellar Purkinje cells does not require metabotropic glutamate receptor 1. Neuroscience. 2009;162:688–693. doi: 10.1016/j.neuroscience.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Frank J, Kinzy TG.2006. In: Sonenberg N, , Hershey JWB, , Mathews MB,, (eds).. Translational control in biology and medicine Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY; 59–85. [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, et al. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2011;214:863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Yu F, Nazari M, Liu X, Liu QS. Phosphodiesterase 4 inhibition impairs cocaine-induced inhibitory synaptic plasticity and conditioned place preference. Neuropsychopharmacology. 2012;37:2377–2387. doi: 10.1038/npp.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.