Abstract
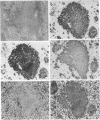
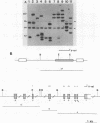
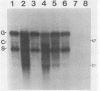
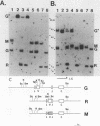
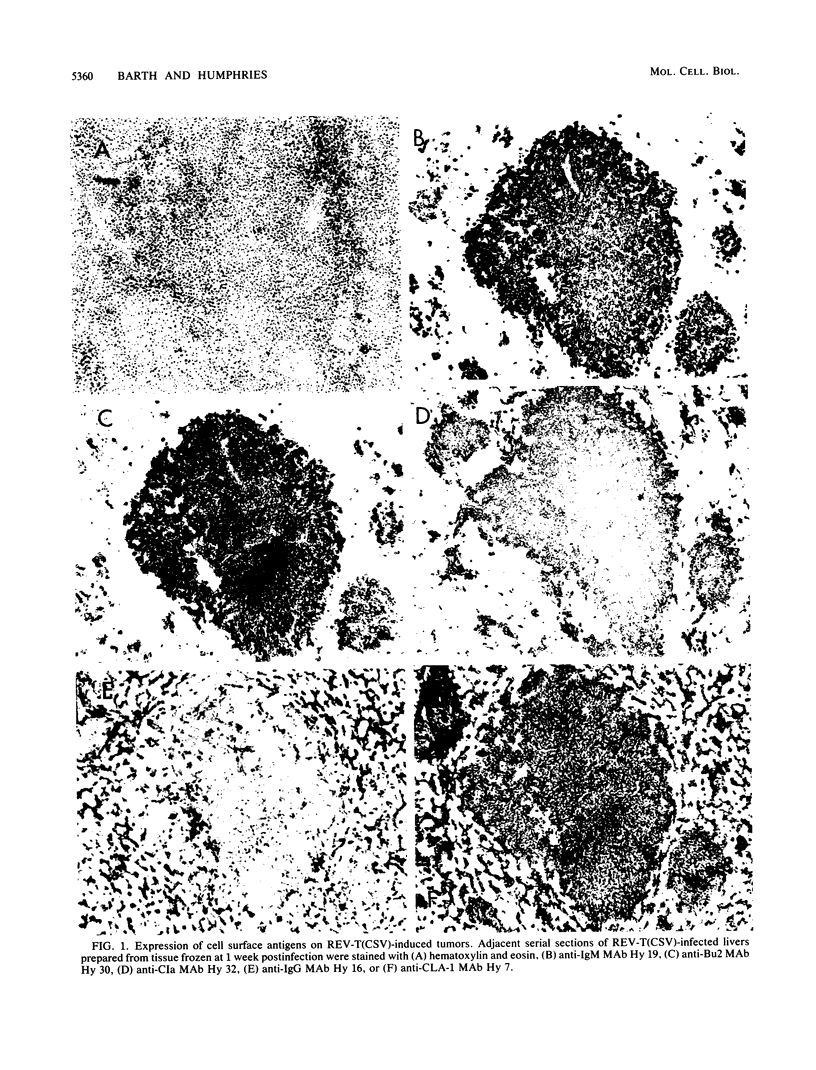
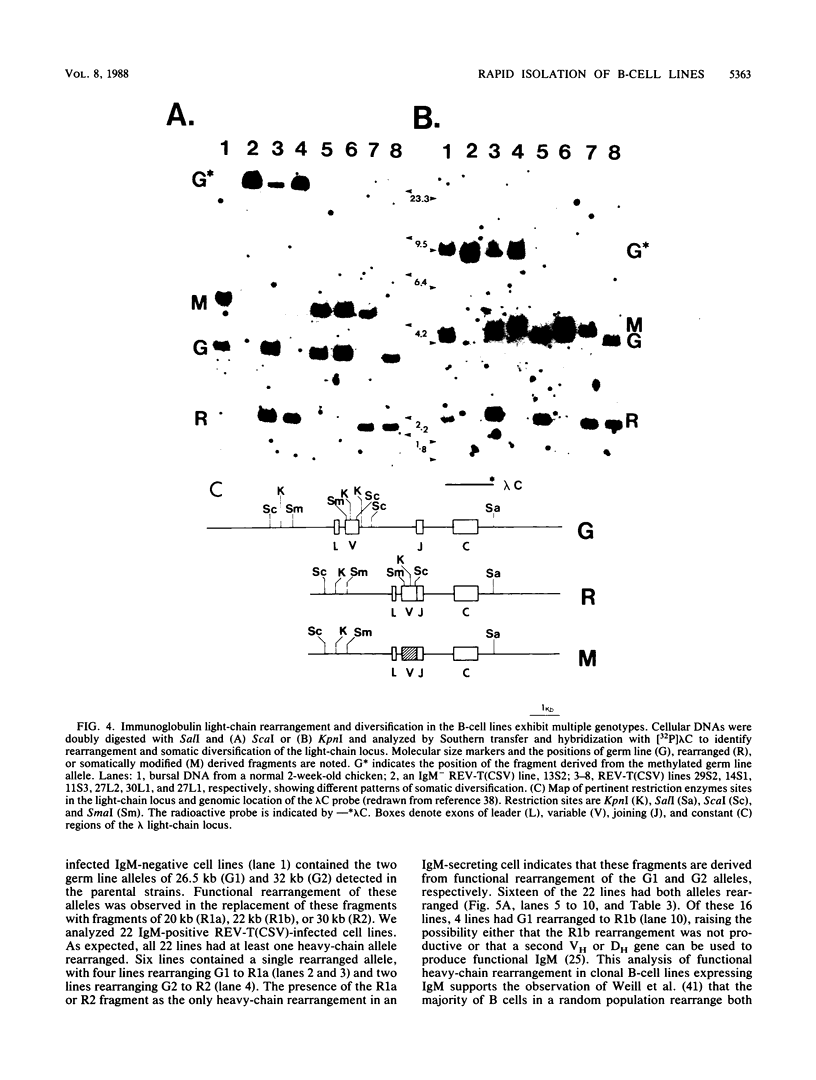
The infection of newly hatched chickens with reticuloendotheliosis virus strain T (REV-T) and a nonimmunosuppressive helper virus, chicken syncytial virus, induces rapidly metastatic B-cell lymphomas. In vivo analysis of these tumors with monoclonal antibodies detected the expression of the B-cell surface markers immunoglobulin M (IgM), CIa, Bu2, and CLA-1, but not IgG, Bu1, or a T-cell surface marker, CT-1. Cell lines derived from tumors exhibited the same pattern of staining, suggesting that expression of cell surface markers does not change during in vitro cell line development. All cell lines examined synthesized IgM in varying amounts. Northern (RNA blot) analysis confirmed abundant expression of v-rel mRNA, and Southern analysis revealed rearrangement of both heavy- and light-chain immunoglobulin loci. Analysis of the light-chain locus demonstrated that 20 of 22 lines contained a single rearranged allele. With respect to specific restriction enzyme sites within the V lambda 1 gene, the active allele in any given clone was either diversified or nondiversified. In contrast, examination of the heavy-chain loci within these lines demonstrated that 16 of the 22 had both alleles rearranged. Further diversification of the V lambda 1 locus did not occur after prolonged in vitro passage of the cell lines. We propose that v-rel expression arrests diversification of the light-chain locus in these lymphoid cells, allowing the production of stable, clonal B-cell populations. The development of these and similar cell lines will make it possible to identify specific stages of avian lymphoid ontogeny and to study the mechanism of rearrangement and diversification in the avian B lymphocyte.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Baba T. W., Giroir B. P., Humphries E. H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology. 1985 Jul 15;144(1):139–151. doi: 10.1016/0042-6822(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Avian leukosis virus infection: analysis of viremia and DNA integration in susceptible and resistant chicken lines. J Virol. 1984 Jul;51(1):123–130. doi: 10.1128/jvi.51.1.123-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A. 1985 Jan;82(1):213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C. F., Humphries E. H. A nonimmunosuppressive helper virus allows high efficiency induction of B cell lymphomas by reticuloendotheliosis virus strain T. J Exp Med. 1988 Jan 1;167(1):89–108. doi: 10.1084/jem.167.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mathieson B., Young H. A., Keller J., Ihle J. N., Rice N. R. Detection of c-rel-related transcripts in mouse hematopoietic tissues, fractionated lymphocyte populations, and cell lines. Mol Cell Biol. 1987 Mar;7(3):1304–1309. doi: 10.1128/mcb.7.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Chanh T. C., Cooper M. D. Chicken thymocyte-specific antigen identified by monoclonal antibodies: ontogeny, tissue distribution and biochemical characterization. Eur J Immunol. 1984 May;14(5):385–391. doi: 10.1002/eji.1830140502. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Courtneidge S. A., Bishop J. M. Immunological phenotype of lymphomas induced by avian leukosis viruses. Mol Cell Biol. 1983 Jun;3(6):1077–1085. doi: 10.1128/mcb.3.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lim M. Y., Bose H., Jr, Bishop J. M. Rearrangements of chicken immunoglobulin genes in lymphoid cells transformed by the avian retroviral oncogene v-rel. Proc Natl Acad Sci U S A. 1988 Jan;85(2):549–553. doi: 10.1073/pnas.85.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert D. L., Munchus M. S., Chen C. L., Cooper M. D. Analysis of structural properties and cellular distribution of avian Ia antigen by using monoclonal antibody to monomorphic determinants. J Immunol. 1984 May;132(5):2524–2530. [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., McKearn J., Risser R. Substitution of the LTR of Abelson murine leukemia virus does not alter the cell type of virally induced tumors. Oncogene. 1988 Jun;2(6):585–592. [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Cell transformation and tumor induction by Abelson murine leukemia virus in the absence of helper virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5932–5936. doi: 10.1073/pnas.84.16.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Clonal dominance and progression in Abelson murine leukemia virus lymphomagenesis. J Virol. 1987 Jul;61(7):2192–2197. doi: 10.1128/jvi.61.7.2192-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog N. K., Bargmann W. J., Bose H. R., Jr Oncogene expression in reticuloendotheliosis virus-transformed lymphoid cell lines and avian tissues. J Virol. 1986 Jan;57(1):371–375. doi: 10.1128/jvi.57.1.371-375.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Houssaint E., Belo M., Le Douarin N. M. Investigations on cell lineage and tissue interactions in the developing bursa of Fabricius through interspecific chimeras. Dev Biol. 1976 Oct 15;53(2):250–264. doi: 10.1016/0012-1606(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Houssaint E., Tobin S., Cihak J., Lösch U. A chicken leukocyte common antigen: biochemical characterization and ontogenetic study. Eur J Immunol. 1987 Feb;17(2):287–290. doi: 10.1002/eji.1830170221. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Houssaint E., Jotereau F. V., Belo M. Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2701–2705. doi: 10.1073/pnas.72.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the bursa of Fabricius. Dev Biol. 1966 Aug;14(1):40–51. doi: 10.1016/0012-1606(66)90004-2. [DOI] [PubMed] [Google Scholar]
- Parvari R., Avivi A., Lentner F., Ziv E., Tel-Or S., Burstein Y., Schechter I. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. EMBO J. 1988 Mar;7(3):739–744. doi: 10.1002/j.1460-2075.1988.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. D., Purchase H. G., Burmester B. R., Cooper M. D., Good R. A. Relationships among visceral lymphomatosis, bursa of Fabricius, and bursa-dependent lymphoid tissue of the chicken. J Natl Cancer Inst. 1966 Apr;36(4):585–598. doi: 10.1093/jnci/36.4.585. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Kieran M. W., Rijnbeek A. M., Longenecker B. M. A monoclonal antibody against chicken MHC class I (B-F) antigens. Immunogenetics. 1985;21(3):293–297. doi: 10.1007/BF00375381. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Rijnbeek A. M. Monoclonal antibodies against chicken lymphocyte surface antigens. Hybridoma. 1983;2(3):287–296. doi: 10.1089/hyb.1983.2.287. [DOI] [PubMed] [Google Scholar]
- Premkumar E., Potter M., Singer P. A., Sklar M. D. Synthesis, surface deposition, and secretion of immunoglobulins by Abelson virus-transformed lymphosarcoma cell lines. Cell. 1975 Oct;6(2):149–159. doi: 10.1016/0092-8674(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Chen I., Howatson A., Mak T. W. Morphological, immunological, and biochemical analyses of chicken spleen cells transformed in vitro by reticuloendotheliosis virus strain T. Cancer Res. 1982 Jul;42(7):2722–2728. [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Zeigel R. F., Twiehaus M. J. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):731–743. [PubMed] [Google Scholar]
- Thompson C. B., Humphries E. H., Carlson L. M., Chen C. L., Neiman P. E. The effect of alterations in myc gene expression on B cell development in the bursa of Fabricius. Cell. 1987 Nov 6;51(3):371–381. doi: 10.1016/0092-8674(87)90633-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973 Sep;3(9):585–595. doi: 10.1002/eji.1830030912. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Temin H. M. Structure and dimorphism of c-rel (turkey), the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1984 Feb;49(2):521–529. doi: 10.1128/jvi.49.2.521-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]