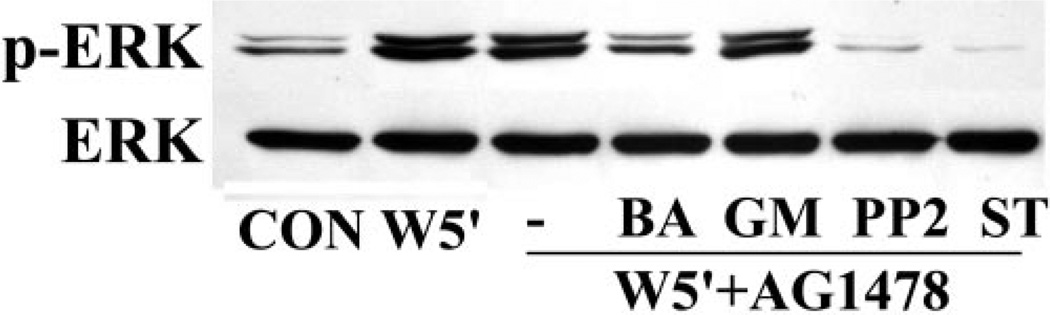Abstract
Purpose
Previous studies have shown that wounding of human corneal epithelial cells (HCECs) results in the release of G-protein-coupled receptor ligands such as ATP and lysophosphatidic acid (LPA), which in turn transactivate epidermal growth factor (EGF) receptor (EGFR) through ectodomain shedding of heparin-binding EGF-like growth factor (HB-EGF). In the present study, the role of extracellular signal-regulated kinases 1/2 (ERK1/2) in regulating EGFR transactivation was investigated.
Methods
SV40-immortalized HCECs were wounded or stimulated with ATP and LPA. EGFR and ADAM17 activation was analyzed by immunoprecipitation followed by Western blot analysis with phospho-tyrosine or phospho-serine antibodies, respectively. Phosphorylation of ERK and AKT was analyzed by Western blot analysis. HB-EGF shedding was assessed by measuring the release of alkaline phosphatase (AP) in a stably transfected human corneal epithelial (THCE) cell line expressing HB-EGF-AP. ADAM17 and ERK interaction was determined by coimmunoprecipitation.
Results
Early, but not late, ERK1/2 phosphorylation in response to wounding, LPA, and ATP was EGFR independent, but sensitive to the inhibitors of calcium influx, protein kinase C and Src kinase. Wounding-, LPA-, and ATP-induced HB-EGF shedding and EGFR activation were attenuated by the MAPK/ERK kinase (MEK) inhibitors PD98059 and U0126, as well as by ADAM10 and -17 inhibitors. ADAM17 was found to be physically associated with active ERK and phosphorylated at serine residues in an ERK-dependent manner in wounded cells.
Conclusions
Taken together, our data suggest that in addition to functioning as an EGFR downstream effector, ERK1/2 also mediates ADAM-dependent HB-EGF shedding and subsequent EGFR transactivation in response to a variety of stimuli, including wounding and GPCR ligands.
Corneal epithelium, like other epithelial barriers in the human body, is continuously subjected to physical, chemical, and biological insults, often resulting in tissue or cell injury and a loss of barrier function. Proper healing of corneal wounds is vital for maintaining a clear, healthy cornea and preserving vision. The wound repair process involves cell adhesion, migration, proliferation, matrix deposition, and tissue remodeling.1 Many of these biological processes are mediated by growth factors, cytokines, and other mediators released in the injured tissues or cells.2 We and others have shown that epithelial wounding induces epidermal growth factor (EGF) receptor (EGFR) transactivation via ectodomain shedding of heparin-binding EGF-like growth factor (HB-EGF) in human corneal epithelial cells (HCECs), and this wound-induced activation of EGFR and its coreceptor erbB2 are required for epithelial migration and wound closure.3–6
HB-EGF is synthesized as a type-1 transmembrane protein that can be cleaved to release a soluble 14- to 20-kDa growth factor via ectodomain shedding,7–9 which has emerged as an important posttranslational mechanism to regulate the functions of various membrane proteins.10,11 Several members of a family of membrane-anchored metalloproteinases (MMPs), known as ADAM (a disintegrin and metalloproteinase), have been shown to mediate ectodomain shedding of EGFR ligands and transactivation of EGFR.12–16 ADAM9, -10, -12, and -17 have been implicated in the cleavage of HB-EGF.17–20 The released HB-EGF acts via the stimulation of specific cell-surface receptors.21 Four related receptor tyrosine kinases have been identified as EGFR/erbB1/HER1, erbB2/HER2/neu, erbB3/HER3, and erbB4/HER4.21 Shed EGFR ligands such as HB-EGF act in an autocrine/paracrine fashion to stimulate its activation. Phosphorylation of EGFR creates docking sites for adaptor proteins such as Grb2, Shc, and Gab1 and leads to the activation (tyrosine phosphorylation) of effectors such as phosphatidylinositol- 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK), which have been shown to be involved in corneal epithelial wound healing.22–27
We recently showed that lysophosphatidic acid (LPA) and adenosine triphosphate (ATP), released by wounded corneal epithelial cells, promote wound healing by inducing metalloproteinase-dependent HB-EGF shedding, subsequent EGFR transactivation, and its downstream signaling.28,29 LPA is a growth factor–like lipid mediator and an important serum component that affects cell adhesion, migration, proliferation, and survival by binding to its receptors LPA1–3.30,31 ATP was first thought solely to be an intracellular energy source, but later proved to be an important extracellular signaling molecule32 that enhances wound healing via its P2Y receptors.29 LPA and P2Y receptors belong to the seven-transmembrane, G-protein-coupled receptor (GPCR) superfamily.33–35 Transactivation of EGFR by LPA and ATP represents a convergent signaling pathway accessible to stimuli, such as growth factors and ligands of GPCR in response to pathophysiological challenges. However, the intracellular signals linking GPCRs to HB-EGF shedding and EGFR signaling remain elusive.
Mitogen-activated protein kinases (MAPK) are serine-threonine protein kinases that are activated by diverse stimuli ranging from cytokines, growth factors, neurotransmitters, hormones, cellular stress, to cell adhesion.36 Several recent studies have shown that MAPK cascades contribute to corneal wound healing by promoting cell proliferation and migration.37–40 The ERK1/2 pathway is a major downstream signaling pathway of receptor tyrosine kinase or growth factor receptors and is involved in the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells.41 Recently, the ERK1/2 pathway has been implicated in regulating ectodomain shedding of transmembrane proteins.9,42,43 In these studies, exogenous phorbol esters were used as stimuli to induce ectodomain shedding; however, the role of the ERK pathway in HB-EGF shedding under normal pathophysiological circumstances, such as mechanical injury, needs further investigation.
In the present study, we demonstrated that ERK activation, in response to wounding, ATP, and LPA, was insensitive to EGFR inhibition. This EGFR-independent ERK activity was regulated by calcium influx, Src kinase, and PKC. The MEK inhibitors, PD98059 and U0126, attenuated wound-, ATP- and LPA-induced HB-EGF shedding and EGFR activation. ADAM17 was involved in wound-induced HB-EGF shedding, whereas ADAM10 and -17 participated in ATP- and LPA-induced HB-EGF cleavage. Moreover, ADAM17 was phosphorylated at serine residue(s) and was found to be associated with active ERK in response to wounding, and its activity was regulated by ERK. Our results indicate that in addition to its EGFR effector function, the ERK pathway also contributes to corneal epithelial wound healing by regulating ADAM17 activity, HB-EGF shedding, and EGFR activation.
Materials and Methods
Defined keratinocyte SFM was purchased from Invitrogen (Grand Island, NY); keratinocyte basal medium (KBM) from BioWhittaker (Walkersville, MD); human recombinant HB-EGF from R&D Systems (Minneapolis, MN); LPA, phorbol 12-myristate 13-acetate (PMA), Ca2+ chelator BAPTA/AM, the MEK inhibitors U0126 and PD98058, the PI3K inhibitor LY294002, the matrix metalloproteinase (MMP) inhibitor GM6001, the Src kinase inhibitor PP2, the p38 inhibitor SB203580, the JNK inhibitor SP600125, and the PKC inhibitor staurosporine from Calbiochem (La Jolla, CA); LPA, ATP-γ-S, and the EGFR inhibitor tyrphostin AG1478 from Sigma-Aldrich (St. Louis, MO). Hydroxamate GW280264X, capable of blocking ADAM10 and -17, and GI254023X, preferentially blocking ADAM10 but not ADAM17, were kindly provided by Andreas Ludwig (Christian-Albrechts-University, Kiel, Germany).44 Human EGFR, ERK2 (p42 MAPK), phospho-ERK1/2 (p42/p44), phospho-tyrosine (PY99), and phospho-EGFR (tyrosine 1068) antibodies and protein A/G-agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against AKT and phospho-AKT from Cell Signaling Technology (Beverly, MA); ADAM17 antibody from Triple Point Biologics (Forest Grove, OR); and phosphoserine antibody from Zymed Laboratories, Inc. (San Francisco, CA). All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
THCE cells, an SV40-immortalized HCEC line,45 were grown in defined keratinocyte SFM in a humidified 5% CO2 incubator at 37°C and growth-factor starved in KBM for 16 hours before experiments. Primary HCECs were isolated from human donor corneas obtained from the Michigan Eye Bank. The epithelial sheet was separated from underlying stroma after overnight Dispase treatment at 4°C. The dissected epithelial sheet was trypsinized, and the cells were then collected by centrifugation. Primary HCECs were grown in defined keratinocyte SFM in a humidified 5% CO2 incubator at 37°C and then used at passage 3.
Determination of EGFR, AKT, and ERK Activation by Western Blot Analysis
To determine EGFR tyrosine phosphorylation, growth factor–starved THCE cell monolayers on 100-mm dishes were pretreated with different inhibitors for 1 hour and then stimulated with extensive wounding, ATP-γ-S, or LPA for the indicated time. Wounding was created by multiple linear scratches with a cut of 48-well shark’s tooth comb for DNA sequencing (Bio-Rad, Hercules, CA), going from one side of the dish to the other. The dish was then rotated, and scrapes were made the same way at 45°, 90°, and 135° to the original scrapes. Cells with no scrape wounds were used as the control. Damaged cells were washed away before the cells were fed with fresh KBM. The cells were lysed with RIPA buffer (150 mM NaCl, 100 mM Tris-HCl [pH 7.5], 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined using a protein assay kit (Micro BCA; Pierce, Rockford, IL). For each sample, 600 µg protein was immunoprecipitated with 10 µg antibody against EGFR followed by the addition of 20 µL protein A/G-agarose beads. Precipitants were immunoblotted with an antibody against PY99. The membrane was then stripped and reprobed with an antibody against EGFR to evaluate the total amount of EGFR precipitated. Phosphorylation of ERK1/2 and AKT was determined using monoclonal antibodies against phospho-ERK1/2 and phospho-AKT; antibodies against ERK2 and AKT were used to detect equal protein loading of the respective phosphoproteins.
Measurement of HB-EGF-AP Shedding
Growth factor–starved THCE cells expressing HB-EGF-AP were cultured in six-well plates, pretreated with different inhibitors for 1 hour, and then stimulated with extensive wounding, ATP-γ-S, or LPA for 15 minutes. Culture medium was collected and alkaline phosphatase (AP) activities in the collected media were measured by chemiluminescence detection (Great EscAPe SEAP Kit; BD Biosciences, San Diego, CA) according to the manufacturer’s instructions. Briefly, 15 µL of the collected culture medium was heated with dilution buffer at 65°C for 30 minutes in a 96-well plate, followed by addition of assay buffer and substrate. Chemiluminescence was quantified on a fluorometer (GENios; Tecan Ltd., Durham, NC). The readings, after background luminescence in KBM was subtracted, were normalized against those of cellular protein concentration and expressed as the increased level versus the control level. The results are presented as the mean ± SEM (n = 3). Statistical parameters were ascertained by commercial software (SigmaStat, Tulsa, OK) with Student’s t-test; P < 0.05 was considered significantly different.
Determination of ADAM17 Serine Phosphorylation and Association with Active ERK
Growth factor–starved THCE cell monolayers on 100-mm dishes were pretreated with different inhibitors for 1 hour and then extensively wounded, as described earlier. The cells were lysed with RIPA buffer and precleared with protein A/G-agarose beads. For each sample, 1 mg protein was immunoprecipitated with 4 µg rabbit antibody against ADAM17, followed by addition of 20 µL protein A-agarose beads. The Precipitates were immunoblotted with antibodies against phosphoserine and phospho-ERK1/2. Membrane was then stripped and reprobed with ADAM17 antibody, to evaluate the total amount of ADAM17 precipitated.
Results
Effect of EGFR Inhibition on Wound-, ATPγS-, and LPA-Induced ERK Activation
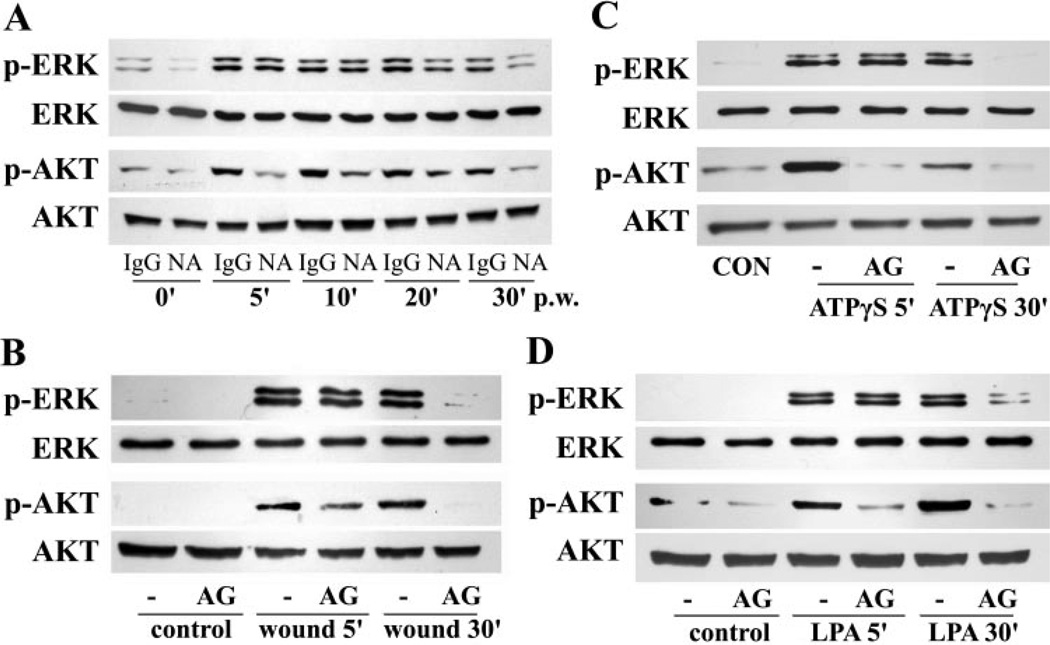
We have shown in previous work that wounding, ATPγS, and LPA triggers rapid activation of EGFR, ERK1/2, and AKT in transfected human corneal epithelial (THCE) cells.3,28,29 To determine the role of EGFR in the activation of ERK1/2 and AKT, we pretreated cells with EGFR neutralizing antibody or control mouse IgG antibody (Fig. 1A). Phosphorylation of ERK1/2 and AKT was rapidly increased as early as 5 minutes after wounding (pw) and was still elevated 30 minutes pw. Enhanced AKT phosphorylation was significantly attenuated by EGFR neutralizing antibody at all time points. ERK phosphorylation at an early stage (5 and 10 minutes pw), however, was unaffected by EGFR neutralizing antibody, whereas its later activation (20 and 30 minutes pw) was sensitive to EGFR inhibition. To confirm the insensitivity of early ERK activation to EGFR inhibition, the cells were pretreated with EGFR inhibitor AG1478 and then stimulated with wounding, ATPγS, and LPA (Figs. 1B, 1C, 1D, respectively). As we reported previously, ATPγS and LPA induced rapid ERK1/2 and AKT phosphorylation, seen at 5 and 30 minutes post stimulation. All three stimulus-induced AKT activation was attenuated by AG1478, whereas ERK activation at 5, but not 30, minutes after stimulation was insensitive to AG1478, confirming that ERK, but not PI3K, can be activated independently of EGFR in response to wounding signals.
Figure 1.
Wound-, ATPγS-, and LPA-induced ERK activation was insensitive to EGFR inhibition. (A) Growth factor–starved THCE cells were pretreated with 4 µg/mL EGFR neutralizing antibody (NA) or control mouse IgG1 antibody (IgG) for 2 hours, wounded, and incubated for the indicated times (5 to 30 minutes). (B–D) Growth factor–starved THCE cells were pretreated with 1 µM EGFR inhibitor AG1478 and stimulated with extensive wounding (B), 100 µM ATPγS (C), or 10 µM LPA (D) for 5 or 30 minutes. The cell lysates were subjected to Western blot analysis with antibodies against phospho-AKT (p-AKT), phospho-ERK 1/2 (p-ERK), AKT, and ERK2.
Regulation of Wound-Induced, EGFR-Independent ERK Phosphorylation by Ca2+, Src Kinase, and PKC
We next examined the regulatory mechanisms of wound-induced, EGFR-independent ERK activation. Because we had demonstrated that cell activation in response to wounding and ATPγS requires Src46 and Ca2+ signaling,29 respectively, and that selective PKC isoforms may be involved in corneal injury,47,48 we next evaluated the effects of the Ca2+ chelator BAPTA/AM, the Src inhibitor PP2, and the broad-spectrum PKC antagonist staurosporine on ERK activation in the presence of AG1478 (Fig. 2). Consistent with Figure 1, wounding-enhanced ERK phosphorylation was unaffected by AG1478, but was attenuated by inhibiting Ca2+, Src, and PKC. Interestingly, the EGFR-independent ERK activation was insensitive to MMP inhibitor GM6001, suggesting that the pathway is upstream of EGFR transactivation.
Figure 2.
EGFR-independent ERK phosphorylation in response to wounding was regulated by Ca2+, Src kinase, and PKC. Growth factor–starved THCE cells were pretreated with 50 µM Ca2+ chelator BAPTA/AM, 50 µM MMP inhibitor GM6001, 25 µM Src inhibitor PP2, or 0.4 µM staurosporine in the presence of 1 µM AG1478 for 1 hour before wounding for 5 minutes. Cell lysates were subjected to Western blot analysis with antibodies against phospho-ERK 1/2 (p-ERK) and ERK2.
Effect of MEK Inhibitors on Wound-, ATPγS-, and LPA-Induced HB-EGF Shedding
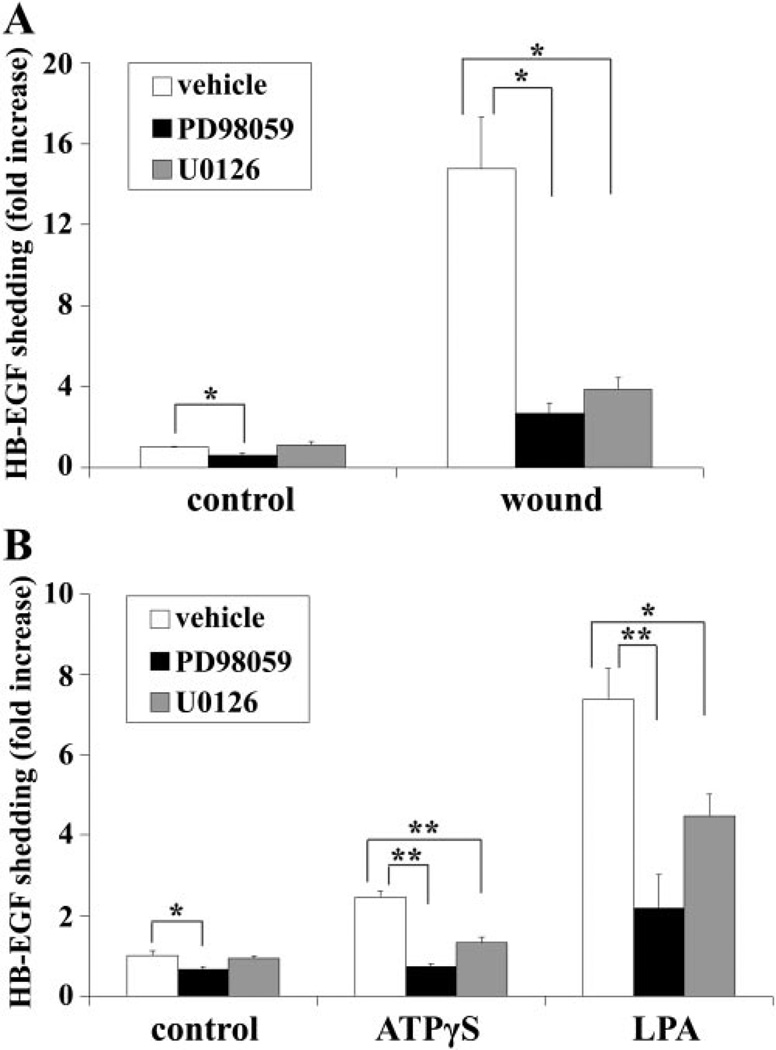
Using an epithelial wound model and a THCE cell line transfected with HB-EGF-AP,3,49 we have shown that wounding elicits HB-EGF ectodomain shedding and that the released HB-EGF acts as an endogenous agonist for EGFR activation in an autocrine/paracrine manner.3 In addition, we recently demonstrated that ATPγS and LPA induce HB-EGF shedding and subsequent EGFR transactivation.28,29 To determine whether ERK1/2 mediates the ectodomain shedding of HB-EGF in response to wounding, ATPγS, and LPA, we pretreated cells with the MEK inhibitors PD98059 and U0126 and measured AP activity in the culture media, as an indication of HB-EGF cleavage (Fig. 3). Basal HB-EGF shedding was slightly attenuated by PD98059. Wounding resulted in a 15-fold increase in HB-EGF shedding, which was significantly downregulated by PD98059 and U0126 (Fig. 3A). Similarly, ATPγS, and LPA induced a 2.5-and a 7.4-fold increase in HB-EGF shedding, which was attenuated by both MEK inhibitors (Fig. 3B), confirming that MEKERK1/2 may regulate the cleavage of HB-EGF.
Figure 3.
Wound-, ATPγS-, and LPA-induced HB-EGF shedding was sensitive to MEK inhibitors. Growth factor-starved HB-EGF-AP cells were pretreated with vehicle, 50 µM PD98059, or 10 µM U0126 for 1 hour before stimulated with extensive wounding (A), 100 µM ATPγS, or 10 µM LPA (B) for 15 minutes. HB-EGF shedding was measured and is expressed as mean increase ± SEM (n = 3). *P < 0.05 and **P < 0.01.
Effect of MEK Inhibitors on Wound-, ATPγS-, and LPA-Induced EGFR Activation
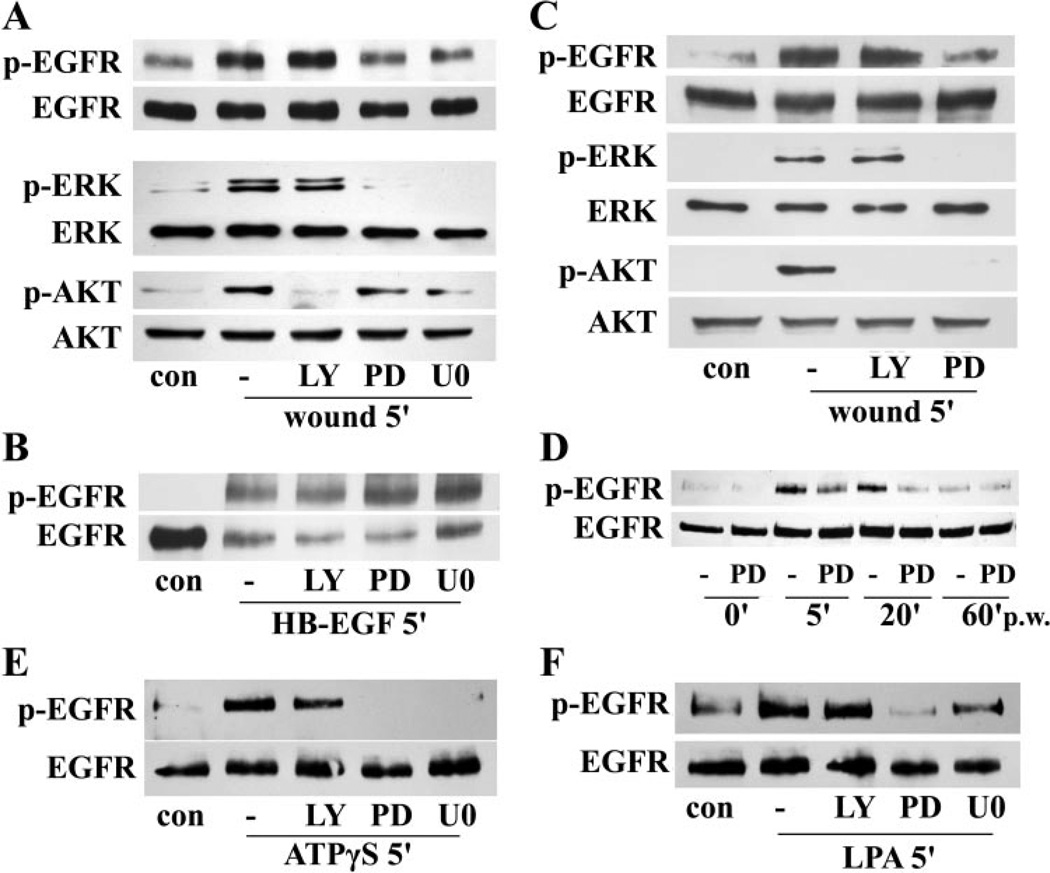
We next sought to determine the effects of MEK inhibitors on EGFR activation, indicated by its tyrosine phosphorylation. Because the PI3K-AKT pathway is another known EGFR downstream effector, the PI3K inhibitor LY294002 was included to compare with MEK inhibition. As shown in Figure 4A, wounding-induced rapid EGFR phosphorylation (5 minutes pw), as well as ERK activation, was attenuated by PD98059 and U0126, but not by LY294002 in THCE cells. AKT phosphorylation, however, was almost completely inhibited by LY294002 and was also downregulated by inhibiting MEK. When the cells were treated with HB-EGF, EGFR phosphorylation and degradation46 were insensitive to inhibitor treatment, suggesting that HB-EGF bypasses MEK inhibition and functions downstream of ERK activation after wounding (Fig. 4B). Similar results were observed in primary HCECs (Fig. 4C). Inhibitors of p38 (SB203580, 10 µM) and JNK (SP600125, 10 µM) exhibited no effects on wound-induced EGFR phosphorylation (data not shown), suggesting that these two MAPK pathways are not involved in wound-induced EGFR activation. To determine whether the effect of MEK inhibition on wounding-induced EGFR activation is transient or sustained, a time-course study was performed with PD98059 (Fig. 4D). Consistent with a previous report,3 wounding induced a prominent increase in EGFR phosphorylation at 5 and 20 minutes pw, which was significantly attenuated by PD98059. EGFR activity then declined to a level similar to that in the control at 60 minutes pw. The EGFR activation at this time seemed unaffected by PD98059. The inhibition of MEK, but not PI3K, also attenuated ATPγS- and LPA-enhanced EGFR activation (Figs. 4E, 4F).
Figure 4.
MEK inhibitors attenuated wounding-, ATPγS-, and LPA-induced EGFR activation. Growth factor-starved THCE cells (A, B, D, E, F) or primary HCECs (C) were pretreated with 50 µM of the MEK inhibitor PD98059, 10 µM of the MEK inhibitor U0126, or 40 µM of the PI3K inhibitor LY294002 for 1 hour before stimulation with wounding (A, C, D), 50 ng/mL HB-EGF (B), 100 µM ATPγS (E), or 10 µM LPA (F) for the indicated time. THCE cell lysates were immunoprecipitated with EGFR antibody, immunoblotted with anti-PY99 antibody (p-EGFR), and reprobed with EGFR antibody (EGFR) to assess the amount of EGFR precipitated. Cell lysates were also subjected to Western blot analysis with anti-phospho-AKT1/2 (p-AKT), phospho-ERK1/2 (p-ERK), AKT, and ERK2 antibodies.
Effect of ADAM Inhibition on Wound-, ATPγS-, and LPA-Induced HB-EGF Shedding
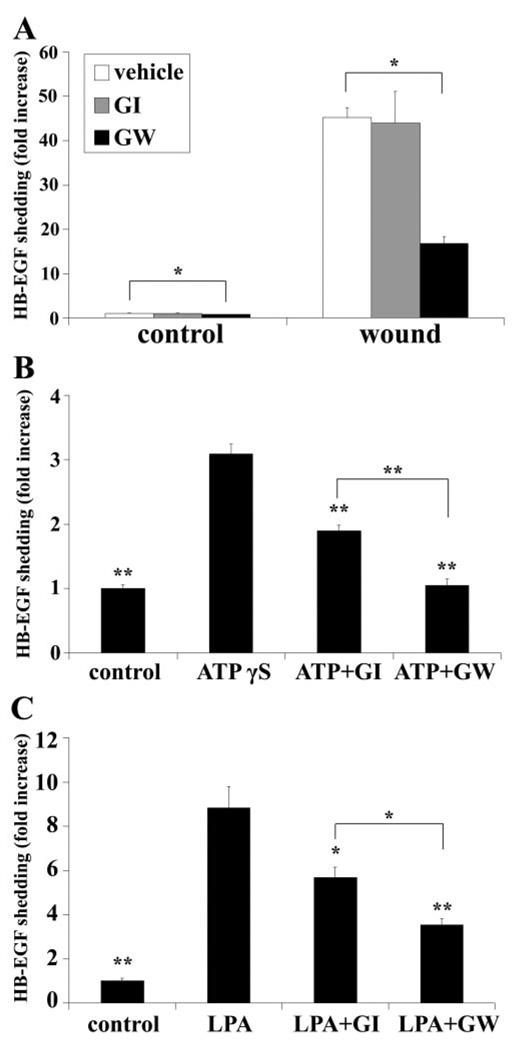
Shedding of EGFR ligands such as HB-EGF and TGF-α has been suggested to be mediated by ADAMs.12 To determine the involvement of ADAMs in HB-EGF shedding, two ADAM inhibitors were used: GI254023X (GI), which preferentially blocks ADAM10, and GW280264X (GW), which inhibits ADAM10 and -17 activities with equal potency.44 As shown in Figure 5A, extensive wounding resulted in a substantial increase in AP activity, and both basal and enhanced HB-EGF shedding were significantly downregulated by GW, but not by GI, suggesting that ADAM17 may be involved in HB-EGF cleavage in response to injury. HB-EGF-AP cells were also challenged with ATPγS (Fig. 5B) and LPA (Fig. 5C), which resulted in 3- and 8.8-fold increases in AP activities, respectively. The GPCR ligand-induced HB-EGF shedding was attenuated by GI and GW by approximately 30% and 60%, respectively, indicating that both ADAM10 and -17 are involved in THCE cell response to ATPγS and LPA.
Figure 5.
Wound-, ATPγS, and LPA-induced HB-EGF shedding was sensitive to ADAM inhibitors. Growth factor-starved HB-EGF-AP cells were pretreated with vehicle, 4 µM GI254023X (ADAM10 inhibitor), or GW280264X (ADAM10 and -17 inhibitor) for 1 hour before stimulation with extensive wounding (A), 100 µM ATPγS (B), or 10 M LPA (C) for 20 minutes. HB-EGF shedding was measured and expressed as the mean increase ± SEM (n = 3). *P < 0.05 and **P < 0.01 in (B) and (C) indicate a significant decrease in HB-EGF shedding compared with ATPγS and LPA, respectively.
Association of Serine-Phosphorylated ADAM17 with Active ERK after Wounding
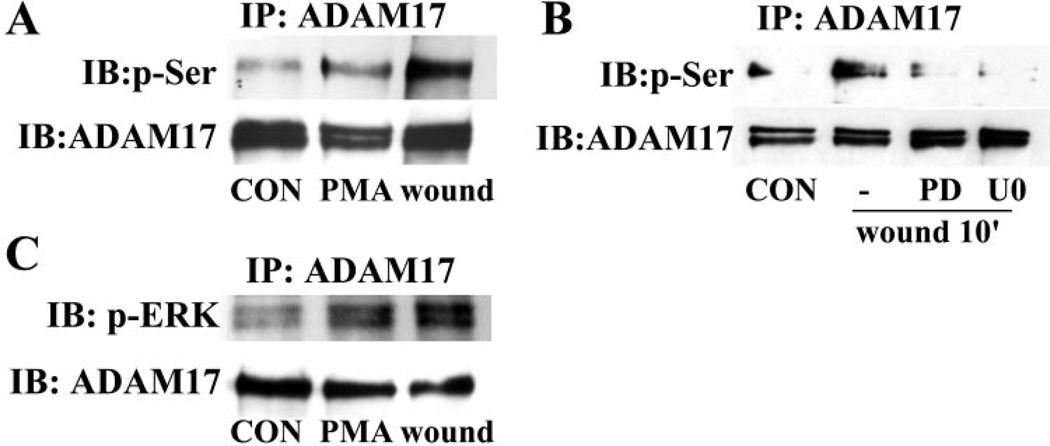
ADAM17 can be phosphorylated at its serine, threonine, or tyrosine residues.50–52 To determine whether ADAM17 is activated in response to wounding, its serine phosphorylation was assayed by immunoprecipitation. As shown in Figure 6A, wounding and PMA (serving as a positive control) induced strong serine phosphorylation of ADAM17, whereas the total ADAM17 protein precipitated remained unchanged. ERK1/2 phosphorylates substrate proteins on serine or threonine residues36 and has been shown to mediate ADAM17 phosphorylation in transfected cell lines stimulated with PMA or growth factors.50–52 We next sought to determine whether ERK1/2 regulates ADAM17 serine phosphorylation after wounding by using the MEK inhibitors PD98059 and U0126. As shown in Figure 6B, inhibiting ERK1/2 activity significantly attenuated wounding-induced ADAM17 serine phosphorylation, raising the possibility that ERK1/2 may directly use ADAM17 as a substrate. Furthermore, we showed that substantial more phospho-ERK was coimmunoprecipitated with ADAM17 in wounded or PMA-treated HCECs (Fig. 6C), confirming a role of ERK1/2 in wounding-induced ADAM17 activation.
Figure 6.
ADAM17 was serine-phosphorylated and associated with active ERK after wounding. Growth factor-starved THCE cells were pretreated with 50 µM PD98059 or 10 µM U0126 for 1 hour (B) before stimulation with extensive wounding or 1 µM PMA (A, C) for 10 minutes. Cell lysates were immunoprecipitated with ADAM17 antibody, immunoblotted with phospho-serine antibody (A, B) or phospho-ERK antibody (C), and re-probed with ADAM17 antibody, to assess the amount of ADAM17 precipitated.
Discussion
We have demonstrated that wounding of HCECs induces HBEGF shedding and subsequent EGFR activation.3 We have also shown that ligands for GPCR including ATP and LPA contribute to the regulation of wound healing through EGFR transactivation.28,29 To identify the intracellular pathway(s) participating in the activation of EGFR, we showed in the present study that ERK1/2 mediates HB-EGF shedding and EGFR activation in response to wounding, ATP, and LPA. We first observed that wounding-, ATP-, and LPA-induced early ERK1/2 activation was insensitive to EGFR inhibition by its neutralizing antibody or antagonist AG1478. This transient ERK activation, however, was sensitive to the inhibition of calcium influx, Src kinase, and PKC, but not to the inhibition of MMP. We then demonstrated that HB-EGF shedding and subsequent EGFR activation in response to wounding and GPCR ligands were inhibited by MEK inhibitors PD98059 and U0126. Although ADAM17 mediated wounding-induced HB-EGF shedding, both ADAM10 and -17 were involved in ATP- and LPA-induced HB-EGF cleavage. ADAM17 was found to be phosphorylated and physically associated with active ERK in wounded cells. Taken together, these findings provide new insights into the regulatory role of ERK1/2 in transmembrane protein shedding and EGFR activation in response to wounding and GPCR ligands.
Inter-receptor cross talk is a well established concept in understanding complex signaling networks and in the translation of environmental conditions into appropriate cell responses.12,53 Transactivation of EGFR by GPCR combines the broad diversity of GPCRs with potent EGFR signaling and serves as a paradigm for inter-receptor cross talk.12,16,53–56 We previously demonstrated that EGFR and its coreceptor ErbB2 are critical for corneal epithelial wound healing.3,4 More recently, we showed that ATP and LPA, released on corneal wounding, enhance wound closure via transactivation of EGFR.28,29 ATP P2Y and LPA receptors belong to the GPCR superfamily.35,57 However, the initial mediators that link wounding, ATP, and LPA receptors to EGFR signaling remain elusive. In this study, we hypothesized that the ERK1/2 pathway is one of the missing links and plays a dual role as an upstream activator and a downstream effector of EGFR signaling.
MAPKs are serine-threonine protein kinases that are activated by diverse stimuli ranging from cytokines, growth factors, neurotransmitters, hormones, cellular stress, to integrinmediated cell adherence.36 The mammalian MAPK can be subdivided into five families: ERK1/2, p38, JNK, ERK3/4, and ERK5, among which ERK1/2 is the best characterized.36 The ERK1/2 pathway includes three kinases establishing a sequential activation pathway of Raf-MEK-ERK, which is believed to be a major signaling route of EGFR.58 However, several studies revealed that ERK1/2 mediates the shedding of transmembrane proteins including HB-EGF,43,50–52,59 raising the possibility that ERK1/2 may function upstream of EGFR and regulate its activation. Using an EGFR-neutralizing antibody, we showed that wound-induced ERK phosphorylation is not affected by EGFR inhibition within 20 minutes after wounding. Since PI3KAKT pathway is another EGFR downstream signaling pathway56 and wounding, LPA, and ATP triggered rapid phosphorylation of AKT in HCECs,28,29,46 activation of AKT was compared with that of ERK. Contrary to the insensitivity of ERK to EGFR neutralizing antibody, AKT phosphorylation was inhibited at all time points. To shed light on the cross talk between GPCR and EGFR, we also challenged cells with ATPγS and LPA in the presence of the EGFR inhibitor AG1478 and found that early (5 minutes), but not late (30 minutes), phosphorylation of ERK was unaffected by EGFR inhibition. We have shown elsewhere that Src kinase, calcium, and PKC mediate wound- and ATP-induced EGFR transactivation.29,46 Using respective inhibitors, we demonstrated in this study that Src, calcium, and PKC may also be involved in wound-induced, EGFR-independent ERK activation. The fact that the MMP inhibitor GM6001 failed to attenuate ERK activation suggests that ERK may act upstream to the sheddases.
To elucidate the role of ERK1/2 activation in HB-EGF shedding and EGFR activation, we used two specific MEK inhibitors PD98059 and U0126. Using stably transfected THCE cells expressing HB-EGF-AP,3 we showed that wounding, ATPγS, and LPA induced HB-EGF shedding to various degrees and both MEK inhibitors greatly attenuated the induced release of AP. Consistently, EGFR activation, assessed by its tyrosine phosphorylation, was also attenuated by MEK inhibitors, but not by PI3K, p38, or JNK inhibitors. These data suggest that ERK1/2, but not PI3K, p38, or JNK, indeed act upstream to HB-EGF shedding and EGFR activation, which was further confirmed by the fact that HB-EGF treatment activated EGFR in the presence of the MEK inhibitor. Although PI3K is not involved in wound-induced HB-EGF shedding, it has been found to be the link between insulin receptor and shedding of transmembrane proteins, including amyloid precursor protein and the antiaging protein Klotho.60,61 Hence, pathways leading to the ectodomain shedding of membrane proteins may be cell-type– and substrate-specific and related to the activation of different sheddases. Raf has been suggested to play a role in the EGFR autocrine loop by inducing HB-EGF expression and secretion. However, such regulation occurs at the transcription level at a much later time (in a matter of hours) in transformed cells.62–64 In the pathophysiological wounding and GPCR ligand stimulation situations of the present study, ERK-mediated HB-EGF shedding and EGFR activation did not require new protein synthesis and happened much faster (in a matter of minutes). In addition, contrary to the EGFR overexpression and autocrine loop signaling commonly seen in cancer cells,58 wounding-induced EGFR phosphorylation was rapid and transient in wounded HCECs, as at 60 minutes pw the level of EGFR phosphorylation was similar to that of the control. The fact that PD98059 had no effect on EGFR phosphorylation at 0 and 60 minutes pw suggests that ERK1/2 is not involved in the maintenance of basal EGFR activity.
Ectodomain shedding has emerged as an important post-translational mechanism to regulate the functions of various membrane proteins.10,11 Ectodomain shedding of EGFR ligands has been linked to the ADAM family of proteins and among the family members, ADAM10 and ADAM17/TACE (tumor necrosis factor-α–converting enzyme) are particularly important in the context of ectodomain generation.10,14,65 Using GI254023X, which preferentially blocks ADAM10 but not -17, and GW280264X, which antagonizes both ADAM10 and -17 with equal potency,44 we showed that both basal and wounding-induced release of HB-EGF was sensitive to GW280264X but not GI254023X, suggesting that constitutive and wound-induced ectodomain shedding of HB-EGF requires ADAM17 but not -10 in HCECs. ATPγS- and LPA-enhanced HB-EGF cleavage, on the other hand, is diminished by both GI254023X and GW280264X, with the latter to a greater extent, indicating the involvement of both ADAM10 and -17 in mediating GPCR ligand-induced sheddase activity. The underlying mechanism for differential regulation of ADAMs by wounding and by GPCR ligands remains elusive, and siRNA targeting ADAMs yields no conclusive evidence for the involvement of these enzymes in EGFR transactivation (data not shown).
ADAM17 has been shown to be phosphorylated at its serine or threonine residues by ERK1/2 in transfected cells treated with PMA or growth hormones.50–52 More recently, Staphylococcus aureus protein A was found to activate ADAM17 in an ERK-dependent manner.43 Our study is the first to document that wounding of HCECs is more potent than PMA in inducing ADAM17 serine phosphorylation in a MEK inhibitor-sensitive manner. In addition, we demonstrated that ADAM17 is associated with phosphorylated ERK in response to wounding and PMA, confirming that ERK may interact with and phosphorylate ADAM17 and therefore regulate HB-EGF shedding.
In conclusion, our results suggest that ERK1/2 mediates HB-EGF shedding and subsequent EGFR transactivation in response to a variety of stimuli including wounding and GPCR ligands via regulating phosphorylation and activation of ADAM17. The present study provides a novel understanding of ERK function and insights into the regulatory mechanisms of transmembrane protein shedding and EGFR activation. Future research is needed to address the specific roles of mediators upstream to ERK and to determine the interaction between ERK and other ADAMs.
Acknowledgments
Supported by National Eye Institute Grant EY10869 (F-SXY), Midwest Eye Banks Student’s Stipend (JY), and an unrestricted grant from the Research to Prevent Blindness to the Department of Ophthalmology, Wayne State University School of Medicine.
Footnotes
Disclosure: J. Yin, None; F.-S.X. Yu, None
References
- 1.Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 3.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu KP, Riggs A, Ding Y, Yu FS. Role of ErbB2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2004;45:4277–4283. doi: 10.1167/iovs.04-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 6.Mazie AR, Spix JK, Block ER, Achebe HB, Klarlund JK. Epithelial cell motility is triggered by activation of the EGF receptor through phosphatidic acid signaling. J Cell Sci. 2006;119:1645–1654. doi: 10.1242/jcs.02858. [DOI] [PubMed] [Google Scholar]
- 7.Raab G, Higashiyama S, Hetelekidis S, et al. Biosynthesis and processing by phorbol ester of the cells surface-associated precursor form of heparin-binding EGF-like growth factor. Biochem Biophys Res Commun. 1994;204:592–597. doi: 10.1006/bbrc.1994.2500. [DOI] [PubMed] [Google Scholar]
- 8.Goishi K, Higashiyama S, Klagsbrun M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- 10.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112:3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 11.Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflamm Res. 2002;51:83–84. doi: 10.1007/BF02684007. [DOI] [PubMed] [Google Scholar]
- 12.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta. 2005;1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 14.Sahin U, Weskamp G, Kelly K, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DC, Sunnarborg SW, Hinkle CL, et al. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann N Y Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 17.Izumi Y, Hirata M, Hasuwa H, et al. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol. 2002;158:221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 20.Sunnarborg SW, Hinkle CL, Stevenson M, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277(15):12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 21.Hynes NE, Horsch K, Olayioye MA, Badache A. The ErbB receptor tyrosine family as signal integrators. Endocr Relat Cancer. 2001;8:151–159. doi: 10.1677/erc.0.0080151. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekher G, Kakazu AH, Bazan HE. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res. 2001;73:191–202. doi: 10.1006/exer.2001.1026. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekher G, Bazan HE. Corneal epithelial wound healing increases the expression but not long lasting activation of the p85alpha subunit of phosphatidylinositol-3 kinase. Curr Eye Res. 1999;18:168–176. doi: 10.1076/ceyr.18.3.168.5372. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Akhtar RA. Epidermal growth factor stimulation of phosphatidylinositol 3-kinase during wound closure in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1139–1148. [PubMed] [Google Scholar]
- 25.Zhang Y, Akhtar RA. Effect of epidermal growth factor on phosphatidylinositol 3-kinase activity in rabbit corneal epithelial cells. Exp Eye Res. 1996;63:265–275. doi: 10.1006/exer.1996.0115. [DOI] [PubMed] [Google Scholar]
- 26.Glading A, Chang P, Lauffenburger D, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 27.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007;48:636–643. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994;1198:185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 31.Tigyi G. Physiological responses to lysophosphatidic acid and related glycero-phospholipids. Prostaglandins. 2001;64:47–62. doi: 10.1016/s0090-6980(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 32.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 33.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 34.Bandoh K, Aoki J, Hosono H, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 35.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 36.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 37.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 38.Imayasu M, Shimada S. Phosphorylation of MAP kinase in corneal epithelial cells during wound healing. Curr Eye Res. 2003;27:133–141. doi: 10.1076/ceyr.27.3.133.16055. [DOI] [PubMed] [Google Scholar]
- 39.Saika S, Okada Y, Miyamoto T, et al. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- 40.Xu K, Zoukhri D, Zieske J, et al. A role for MAP kinase in regulating ectodomain shedding of APLP2 in corneal epithelial cells. Am J Physiol Cell Physiol. 2001;280:C603–C614. doi: 10.1152/ajpcell.2001.281.2.C603. [DOI] [PubMed] [Google Scholar]
- 41.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 42.Fan H, Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez MI, Seaghdha MO, Prince AS. Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J. 2007;26:701–709. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 45.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 46.Xu KP, Yin J, Yu FS. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:2832–2839. doi: 10.1167/iovs.05-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandrasekher G, Bazan N, Bazan H. Selective changes in protein kinase C (PKC) isoform expression in rabbit corneal epithelium during wound healing. Inhibition of corneal epithelial repair by PKCalpha antisense. Exp Eye Res. 1998;67:603–610. doi: 10.1006/exer.1998.0555. [DOI] [PubMed] [Google Scholar]
- 48.Xu KP, Dartt DA, Yu FS. EGF-induced ERK phosphorylation independent of PKC isozymes in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3673–3679. [PubMed] [Google Scholar]
- 49.Dethlefsen SM, Raab G, Moses MA, Adam RM, Klagsbrun M, Freeman MR. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J Cell Biochem. 1998;69:143–153. doi: 10.1002/(sici)1097-4644(19980501)69:2<143::aid-jcb5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan H, Turck CW, Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J Biol Chem. 2003;278:18617–18627. doi: 10.1074/jbc.M300331200. [DOI] [PubMed] [Google Scholar]
- 52.Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 53.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 54.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 55.Leserer M, Gschwind A, Ullrich A. Epidermal growth factor receptor signal transactivation. IUBMB Life. 2000;49:405–409. doi: 10.1080/152165400410254. [DOI] [PubMed] [Google Scholar]
- 56.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 57.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 58.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 59.Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Mitogen-activated protein kinase-dependent and -independent routes control shedding of transmembrane growth factors through multiple secretases. Biochem J. 2002;363:211–221. doi: 10.1042/0264-6021:3630211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solano DC, Sironi M, Bonfini C, Solerte SB, Govoni S, Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 2000;14:1015–1022. doi: 10.1096/fasebj.14.7.1015. [DOI] [PubMed] [Google Scholar]
- 61.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 63.McCarthy SA, Samuels ML, Pritchard CA, Abraham JA, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 64.Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]