Abstract
A series of polyethyleneimine (PEI) incorporated MIL-101 adsorbents with different PEI loadings were reported for the first time in the present work. Although the surface area and pore volume of MIL-101 decreased significantly after loading PEI, all the resulting composites exhibited dramatically enhanced CO2 adsorption capacity at low pressures. At 100 wt% PEI loading, the CO2 adsorption capacity at 0.15 bar reached a very competitive value of 4.2 mmol g−1 at 25°C, and 3.4 mmol g−1 at 50°C. More importantly, the resulting adsorbents displayed rapid adsorption kinetics and ultrahigh selectivity for CO2 over N2 in the designed flue gas with 0.15 bar CO2 and 0.75 bar N2. The CO2 over N2 selectivity was up to 770 at 25°C, and 1200 at 50°C. We believe that the PEI based metal-organic frameworks is an attractive adsorbent for CO2 capture.
The rapidly increasing concentration of CO2 in atmosphere, which mainly stem from the combustion of fossil fuels, is believed to be responsible for some severe global environmental crisis, such as climate change and ocean acidification. The capture and sequestration of CO2 in an energy-efficient and economical manner is deemed as a solution to mitigate the CO2 emission until a mature alternative new energy technology is sufficiently developed1. However, selective capture of CO2 from flue gas emissions still remains challenging to date. One of the major bottlenecks is the absence of CO2 adsorbent with high selectivity, rapid adsorption kinetics, high stability under practical conditions, high adsorption ability and low regeneration cost. Aqueous solutions of amines (20–30% concentration) are commonly used in large scale to capture CO2 from industrial streams because they have large capacity and high selectivity for acidic gases2. However, high heat capacity of these aqueous solutions makes the regeneration very energy intensive and costly3. Instead, solid porous adsorbents are emerging as more promising candidates because the much lower heat capacity of solids can significantly reduce the regeneration energy cost. In addition, the corrosion and volatility issues, which are intrinsic to amine solutions, could be minimized in solid adsorbents.
Recently, solid metal-organic frameworks (MOFs) adsorbents have received a great deal of attention because they exhibit remarkable CO2 adsorption capacity at high pressures owing to their extremely high surface areas and porosity4,5,6,7,8. However, the CO2 adsorption capacities of most MOFs are unsatisfying at low pressures, especially below 0.15 bar, the condition relevant to practical applications. Some attempts have been made to enhance the CO2 capture ability for MOFs at low pressure, such as developing MOFs with amine-functionality9,10. Apparently, the basic amine group is an ideal functionality to strongly interact with acidic CO2 molecules. However, amine-functionalized MOFs have been shown to be not very successful in CO2 capture. It was proposed that the covalent grafting of amine groups to the aromatic rings in MOFs cannot significantly enhance the affinity of CO2 on amine groups because of the electron withdrawing effect of benzene ring11. Alternatively, some recent studies on incorporation of diamine to the open metal sites of MOFs have been shown to dramatically enhance CO2 capture at low pressures. The open metal sites play an important role to anchor one end of the diamine groups and leave the other end (alkylamines) available to capture CO2. Here we anticipate that physical impregnation of poly-alkylamines into MOFs would afford more active amine groups than diamines and maintain the strong interaction between CO2 and alkylamine groups. Among the polyamines, polyethyleneimine (PEI) is one of the best candidates to bind CO2 due to its high amine density and accessible primary amine sites on the chain ends12,13. Indeed, some recent studies reported that PEI based silica and zeolite have excellent CO2 capture ability3,12,14,15,16,9. Ideally, the much higher pore volume, porosity and surface area of MOFs than silica or zeolite make them even more competent media to impregnate PEI for CO2 capture. However, to date, no impregnation of PEI into MOFs has been reported. This is partly because the complexity of MOFs including pore size and porosity would make it difficult to efficiently load PEI into the MOF pores.
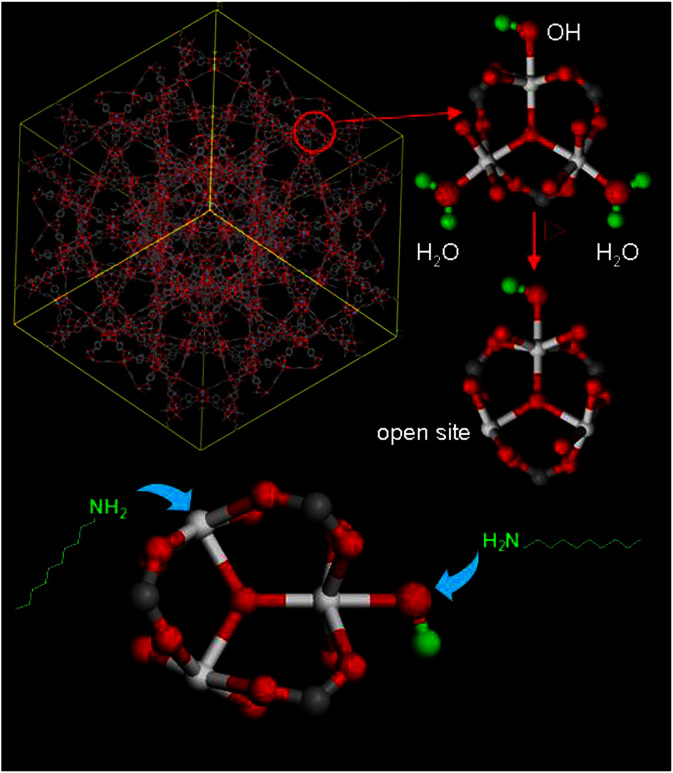
In this study, we report a series of PEI modified MOF adsorbents with low molecular-weight linear PEI loaded into MIL-101(Cr)17 for highly efficient CO2 capture. We chose MIL-101(Cr) as the model system for MOFs because it has very large surface area (above 3000 m2 g−1), high porosity, excellent thermal and chemical stability, as well as high resistance to moisture. In addition, MIL-101(Cr) possesses open metal sites, which can serve as Lewis acid to anchor the Lewis basic amine groups of PEI18,19, while leave other alkylamine groups available as reactive adsorption sites to capture CO2 (see Figure 1). Moreover, we expect that the Cr-OH groups in MIL-101 may also anchor the amine groups. Thus, the loaded PEI can interact strongly with the pore wall of MIL-101(Cr) after the removal of solvent. Considering that short-chain and linear PEI can pass the relatively narrow MIL-101 pore windows (with diameters of 1.2–1.6 nm) more smoothly, low-molecular-weight linear PEI was chosen. As a result, solid and porous PEI-MIL-101 adsorbents with moderate surface areas were successfully assembled.
Figure 1. The illustration of the interactions between PEI and MIL-101.
Results
Characterization of PEI-MIL-101
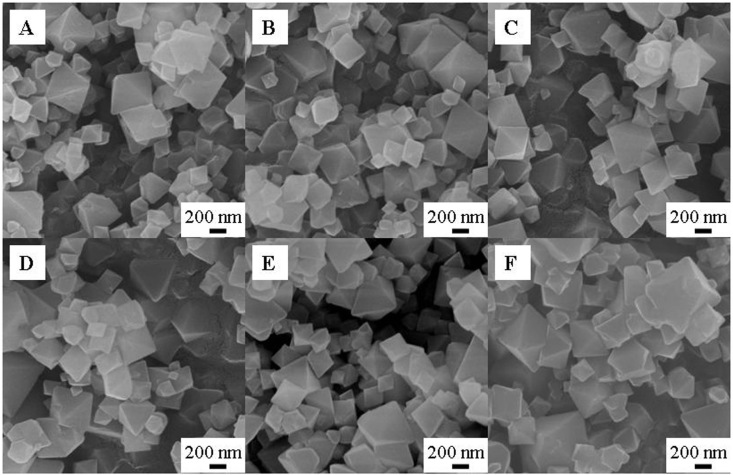
PEI-MIL-101 with different PEI loadings, corresponding to 50%, 75%, 100% and 125%, were prepared and characterized by the combination of PXRD, SEM, IR, TGA and N2 adsorption/desorption at 77 K measurements. As shown in the PXRD patterns (Figure S1), the Bragg diffraction angles in MIL-101 and PEI-MIL-101 with different PEI loadings were essentially identical, confirming that the MIL-101 crystalline structure was preserved after loading PEI. However, we observed that the intensity of the peaks below 7° was largely reduced as the PEI loading increased. In particular, the peak at 3.44°, corresponding to the (2 2 2) plane of MIL-101, was nearly invisible in PEI-MIL-101-125. These changes are ascribed to the filling of MIL-101 pores, and indicate that PEI is not simply coated on the outer surface of MIL-101. Actually, similar phenomenon has also been observed on the SBA-15 supported PEI12. Figure 2 shows the morphologies of MIL-101 before and after loading PEI. The MIL-101 octahedral particle size was rather small (with diameters of 100–500 nm), which can sufficiently facilitate the diffusion of PEI into the MIL-101 pores. Indeed, after loading the PEI, no particle stacking was found, even at 160% loading, indicating that PEI was really loaded into MIL-101 pores. In addition, the morphologies and sizes of MIL-101 after loading the PEI remained nearly intact. To some extent, it also suggested that the MIL-101 structure was preserved after loading PEI.
Figure 2. The SEM images of (A) original MIL-101, (B) PEI-MIL-101-50, (C) PEI-MIL-101-75, (D) PEI-MIL-101-100, (E) PEI-MIL-101-125 and (F) PEI-MIL-101-160.
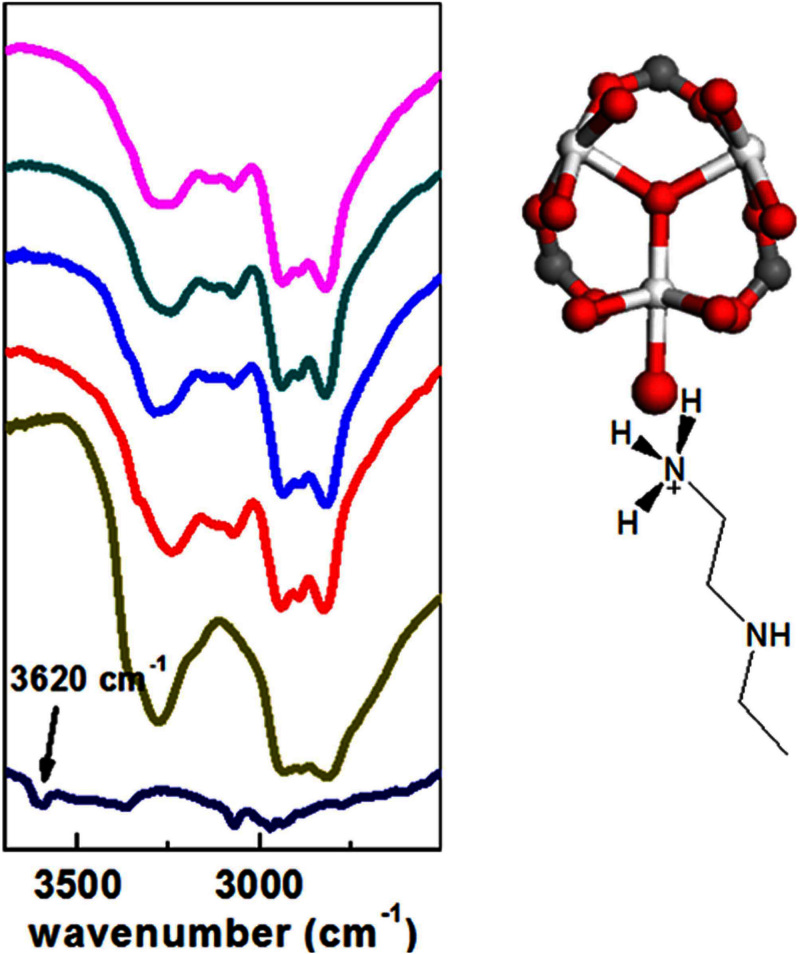
To ensure the presence of PEI and detect the interactions between PEI and MIL-101, IR spectra were collected on activated PEI-MIL-101 under vacuum condition. As shown in Figure 3, the representative peaks between 3500~2800 cm−1 corresponding to ν(NH) and ν(CH) stretching vibrations can be clearly observed in the IR of PEI-MIL-101 samples, which were consistent with that of pure PEI sample. The N-H bending vibration was also discernable at 1570 cm−1 (Figure S2). The band at 3620 cm−1, which can be assigned to Cr-OH20, was clearly observed in MIL-101. Notably, after PEI loading, this band completely disappeared. It indicated a chemical interaction between the Cr-OH groups and PEI amines, and possible formation of Cr-O-NH3R3. In addition, it was reported that amine groups can bind to open Cr sites, resulting in a slight blue-shift of C-H stretching vibration adjacent to the interacting amine groups. However, in the present study, the abundant C-H groups not adjacent to the interacting amine groups would make it hard to detect the slight blue-shift of C-H stretching vibration. Nevertheless, we believe that open metal sites still play an important role to glue PEI on the MIL-101 surface.
Figure 3. IR spectra of the pure PEI and MIL-101 before and after loading PEI.
From bottom to top: MIL-101, pure PEI, PEI-MIL-101-50, PEI-MIL-101-75, PEI-MIL-101-100, PEI-MIL-101-125.
TGA measurement was employed to evaluate the thermal stability of MIL-101 before and after loading PEI (Figure S3). Interestingly, when PEI was loaded into MIL-101, the sharp weight loss of PEI took place at higher temperatures (~225°C). Moreover, the stability of MIL-101 was also increased slightly. It suggested that the strong interactions between PEI and MIL-101, most likely on the open Cr sites and Cr-OH groups, were formed and enhanced the stability of PEI-MIL-101 composite. Indeed, no elevated decomposition temperature of PEI was found when silica was used as the support21. However, incorporation of Zr into the silica support can dramatically stabilize the PEI-silica composite12.
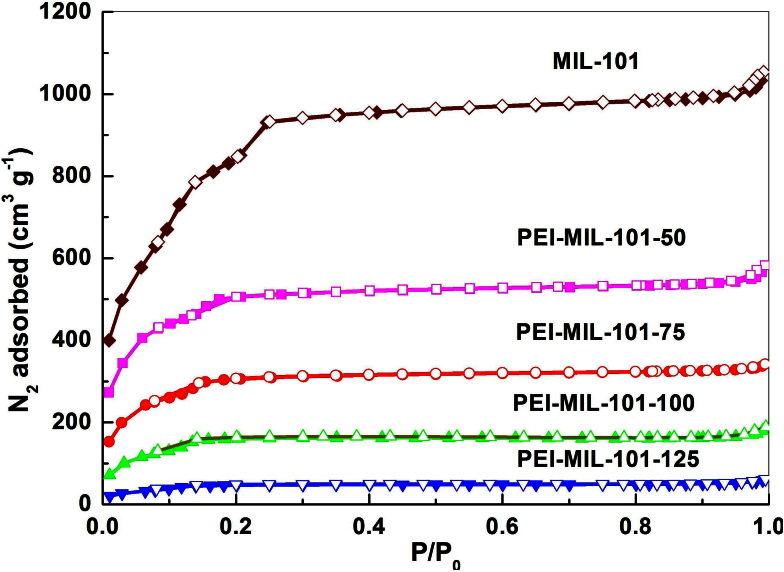
The N2 adsorption/desorption isotherms of MIL-101 at 77 K before and after loading PEI were measured to evaluate their surface area and pore volume. As shown in Figure 4, the original MIL-101 exhibited very high N2 uptakes with a saturated uptake of 1000 cm3 g−1, which was well consistent with the values in literatures22,23. The corresponding BET surface area and pore volume were calculated to be 3225 m2 g−1 and 1629 cm3 g−1, respectively. After PEI was loaded into MIL-101, the N2 uptake, surface area and pore volume decreased dramatically. As shown in Figure 5 and Table 1, the saturated N2 uptake, surface area and pore volume of PEI-MIL-101-50 were roughly half that of pure MIL-101. Particularly, the reduced pore volume confirmed that the PEI was loaded into MIL-101 pores. When the PEI loading was continuously increased to 125 wt%, the saturated N2 uptake drastically dropped to only 50 cm3 g−1. Since the pore volume of the bare MIL-101 is 1.629 cm3 g−1 and the density of PEI is 1.0 g/ml, the theoretical maximum PEI loading in MIL-101 pores should be approximately 160 wt%. Indeed, in the prepared PEI-MIL-101–160 sample, the resulting surface area and pore volume were only 7.4 m2 g−1 and 0.019 cm3 g−1 (Figure S4), respectively, indicating the complete pore filling of MIL-101 by PEI. Clearly, such material is incapable for gas adsorption. Therefore, in the following gas adsorption experiments, we discarded the PEI-MIL-101-160 sample and focused on the PEI-MIL-101 with 50–125 wt% loadings. Nevertheless, to the best of our knowledge, 125 wt% PEI (and correspondingly amine groups) is already the highest amount that can be loaded into the porous materials. It was expected that the more amount of PEI loaded would result in higher CO2 adsorption capacity24,25.
Figure 4. N2 adsorption-desorption isotherms at 77 K. The symbols: Filled, adsorption; Blank, desorption.
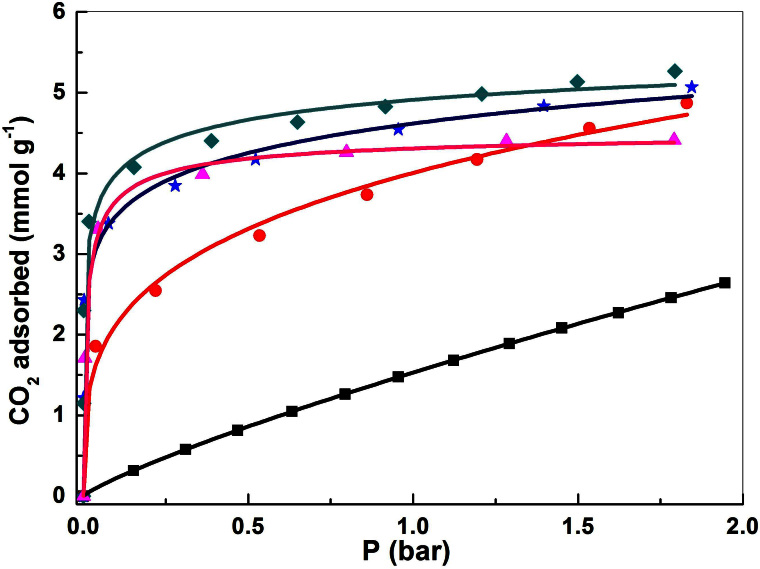
Figure 5. The CO2 adsorption isotherms of the MIL-101 before and after loading PEI at 25°C. Symbol:  MIL-101,
MIL-101,  PEI-MIL-101-50,
PEI-MIL-101-50,  PEI-MIL-101-75,
PEI-MIL-101-75,  PEI-MIL-101-100,
PEI-MIL-101-100,  PEI-MIL-101-125.
PEI-MIL-101-125.
Lines are the fitted isotherms.
Table 1. The measured properties of the PEI-MIL-101 adsorbents at 0.15 and 1 bar.
| CO2 adsorbed (mmol g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 25°C | 50°C | |||||||
| PEI loading (wt%) | SBET (m2 g−1) | Vtotal (cm3 g−1) | Occupancy a (%) | 0.15 bar | 1 bar | 0.15 bar | 1 bar | |
| MIL-101 | 0 | 3125.4 | 1.629 | 0 | 0.33 | 1.60 | 0.20 | 1.00 |
| PEI-MIL-101-50 | 50 | 1802.7 | 0.901 | 31 | 2.40 | 4.00 | 1.86 | 3.07 |
| PEI-MIL-101-75 | 75 | 1112.6 | 0.526 | 46 | 3.67 | 4.64 | 3.17 | 4.02 |
| PEI-MIL-101-100 | 100 | 608.4 | 0.292 | 61 | 4.20 | 5.00 | 3.40 | 4.14 |
| PEI-MIL-101-125 | 125 | 182.9 | 0.095 | 77 | 3.85 | 4.35 | 3.95 | 4.51 |
aThe total volume of MIL-101 divided by the volume of loaded PEI, the density of PEI was 1 ml/g.
CO2 uptake
The CO2 isotherms of MIL-101 and PEI-MIL-101 samples obtained at low-pressures and 25°C are presented in Figure 5. All isotherms were fitted with the Langmuir-Freundlich model26,27,28. It can be seen that all PEI-MIL-101 samples exhibited significantly enhanced CO2 adsorption compared to the original MIL-101. At 1 bar, PEI-MIL-101-50 can adsorb 4.0 mmol g−1 of CO2, representing a ~150% improvement in gravimetric capacity relative to MIL-101. When the PEI loading was increased to 75 and 100 wt%, the CO2 adsorption uptake was further enhanced at all tested pressures. An outstanding uptake of 5.0 mmol g−1 was achieved at 1 bar, which is on par with that of recently reported MOFs appended with diamines29,30. However, when the PEI loading was further increased to 125 wt%, decreased CO2 adsorption uptakes were found. The PEI-MIL-101-125 can adsorb 4.35 mmol g−1 of CO2 at 1 bar, about 13% lower than that of PEI-MIL-101-100. At higher pressure, the decrease is even more significant. Actually, as aforementioned, the surface areas of PEI-MIL-101-125 dropped to only 182.9 m2 g−1, indicating that some pores were completely filled and thus not accessible to CO2 molecules at room temperature. Furthermore, incorporation of PEI into MIL-101 would increase the framework density, which is also negative to the improvement in CO2 gravimetric capacity. Although we do not show the data at higher pressures (>2 bar), but the trend can be clearly seen that the adsorption uptake at higher pressures would follow the sequence: MIL-101 > PEI-MIL-101-50 > PEI-MIL-101-75 > PEI-MIL-101-100 > PEI-MIL-101-125 (i.e., proportional to the surface area). Therefore, it is necessary to carefully balance the PEI loading and adsorbents surface area to achieve the desired CO2 adsorption capacity at specific pressures.
To investigate the influence of elevated temperature on CO2 adsorption behaviors of the PEI-MIL-101 adsorbents, we further measured the CO2 adsorption isotherms of the PEI-MIL-101 samples at 50°C (Figure S5). Interestingly, at 50°C, PEI-MIL-101-125 displayed the highest CO2 uptakes at all tested pressures. Moreover, the CO2 adsorption capacity of PEI-MIL-101-125 at 50°C was also higher than its adsorption capacity at 25°C. Actually, a similar phenomenon has also been observed when PEI was loaded into SBA-153. Indeed, due to the high elasticity of PEI, some pores in PEI-MIL-101-125 that were not accessible to CO2 molecules at low temperature, would become accessible at elevated temperatures. Furthermore, the high kinetic barrier for the diffusion of CO2 from the surface into the filled pores would be overcome by the intense thermal motion at higher temperature. Apparently, upon PEI impregnation, the original window size (1.2–1.6 nm) and pore size (2.9–3.4 nm) of MIL-101 were reduced so that CO2 diffusion would be more difficult. On the other hand, it is worth mentioning that the small and unstacked PEI-MIL-101 particles in this study would sufficiently facilitate the diffusion of CO2 from the gas phase to the surface and inner pores of the sorbents.
Above we discussed the CO2 adsorption uptakes of PEI-MIL-101 materials at 1 bar. Actually, the CO2 uptake at near 0.15 bar is a more important parameter because the flue gas usually contains ~15% CO231. Hence, the CO2 adsorption uptakes of the PEI-MIL-101 samples at 0.15 and 1 bar are summarized in Table 1 for comparison. Fortunately, it is found that the CO2 adsorption uptakes of PEI-MIL-101 increase extremely fast with respect to pressure in the range of 0–0.2 bar, which make them very advantageous for low-pressure CO2 capture. On the PEI-MIL-101 samples with higher loadings (>75 wt%), CO2 uptakes rapidly reached a considerable amount at 0.15–0.2 bar. At 0.15 bar and 25°C, PEI-MIL-101-100 displayed the best CO2 adsorption performance with a value of 4.2 mmol g−1. Under the same conditions, the original MIL-101 can only capture 0.33 mmol g−1 CO2, respectively. Thus, PEI-MIL-101-100 can adsorb about 12 times more CO2, even though the surface is reduced by 80%. The capacity value of PEI-MIL-101-100 is ~220% higher than the saturation absorption capacity (1.32 mmol g−1 CO2) of 30% MEA solution at 25°C and 0.15 bar, which is commonly used in coal-fired power plants2,29,32. Very recently, PEI/Zr-SBA-15 was reported to show excellent CO2 adsorption performance, with a CO2 capacity of 1.65 mmol g−1 at a partial pressure of 0.1 bar and 25°C. Here, at 0.1 bar and 25°C, PEI-MIL-101-100 can adsorb about 140% more amount (4.0 mmol g−1) of CO2 owing to the higher loading of PEI. The performance of PEI-MIL-101-100 for CO2 capture at 0.15 bar is on par with that of the best functionalized solid materials reported to date, particularly the MOF-based materials3,29,30,33,34. In addition, we also measured the CO2 adsorption capacity of humid PEI-MIL-101-100, which was exposed to moisture. No decreased CO2 adsorption capacity was observed, indicating the high moisture stability of the PEI-MIL-101 materials.
CO2 adsorption Kinetics and cycling measurements
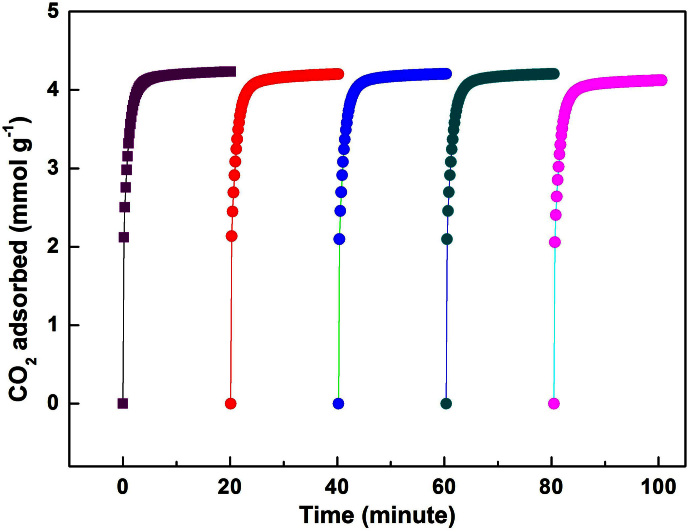
As mentioned, one of the major bottlenecks for deliverable CO2 adsorbents is the slow adsorption kinetics. Thus, it is very necessary to measure the CO2 adsorption kinetics of PEI-MIL-101 materials. As shown in Figure 6, CO2 adsorption capacity in PEI-MIL-101-100 could reach 4.2 mmol g−1 within the first 5 minutes. Afterwards, the PEI-MIL-101-100 sample seemed completely saturated. The corresponding pressure drop (to around 0.15 bar) in the sample holder also indicated a very rapid adsorption behavior (Figure S7). The CO2 adsorption kinetics for PEI-MIL-50, 75 and 125 (Figure S8–S10) were similar to that of PEI-MIL-101-100. Hence, PEI-MIL-101 materials exhibited rather good kinetics for CO2 adsorption.
Figure 6. Cycling CO2 adsorption kinetics of PEI-MIL-101-100.
For clarity, the kinetics for the cycle 2, 3, 4 and 5 were shifted horizontally by 20, 40, 60 and 80.
To ensure the regenerability of PEI-MIL-101 materials, we further performed CO2 adsorption cycling measurements. In our experiments, we found that the CO2 captured in PEI-MIL-101 materials can be completely desorbed at 110°C under vacuum condition for 1 hour, which is confirmed by the equal mass of PEI-MIL-101 materials before adsorption and after desorption measurements (Table S1). Therefore, between each cycle measurement, the sample was degassed at 110°C for 1 hour under vacuum condition. It can be obviously observed that, after five adsorption/desorption cycles, the CO2 adsorption capacity of the prepared PEI-MIL-101 was well retained.
CO2/N2 selectivity
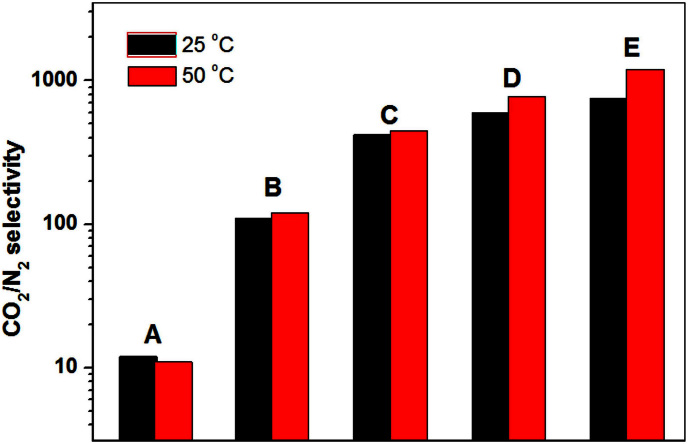
Motivated by the observation that increasing PEI loading would reduce the N2 adsorption uptake but enhance CO2 sorption uptake, we anticipate that this kind of materials could be an exceptional candidate for post-combustion CO2 capture. For coal-fired power stations, the main flue gas components by volume are N2 (~75%) and CO2 (~15%). Thus, the CO2 selectivity for a gas mixture with 0.15 bar CO2 and 0.75 bar N2 is often used to evaluate the CO2 capture ability. Therefore, the adsorption isotherms for N2 in MIL-101 before and after loading the PEI were also measured (Fig S11–12), and the molar selectivity of MIL-101 before and after loading the PEI in the designed gas mixture were further calculated according to the previously reported equation29,30,35. Similar to the N2 adsorption at 77 K, PEI-MIL-101 adsorbed less N2 than pure MIL-101 at all tested pressures and temperatures due to the significantly reduced surface area. Figure 7 depicts the calculated CO2/N2 selectivities at 25 and 50°C. Indeed, PEI-MIL-101 materials show dramatically enhanced CO2/N2 selectivities. For example, the CO2/N2 selectivities for PEI-MIL-101-100 were calculated to be 600 at 25°C, 750 at 50°C. Further, the CO2/N2 selectivities for PEI-MIL-101-125 reached 770 at 25°C, 1200 at 50°C. To the best of our knowledge, these values represent the highest CO2/N2 selectivity in flue gas by zeolite and MOF-based adsorbents reported in literatures.
Figure 7. The CO2/N2 ideal selectivity for a gas mixture with 0.15 bar CO2 and 0.75 bar N2.
(A) MIL-101, (B) PEI-MIL-101-50, (C) PEI-MIL-101-75, (D) PEI-MIL-101-100, (E) PEI-MIL-101-125.
In addition, the mole of CO2 adsorbed at 0.15 bar divided by the mole of N2 adsorbed at 0.75 bar is also often employed to evaluate CO2/N2 selectivity. Therefore, the corresponding CO2/N2 selectivities for the PEI-MIL-101 materials were calculated using this method (Table S2). The calculated selectivities at 25°C for PEI-MIL-101-100 and PEI-MIL-101-125 were 120 and 150, respectively, both of which are much higher than that of PPN-6-SO3Li34 (selectivity = 17) and metal-inserted MOF-25333 (selectivity = 12).
The separation performance of PEI-MIL-101 material for CO2/N2 was further tested in a breakthrough experiment performed at 25°C using an equimolar CO2/N2 mixture. As shown in Figure S13, N2 eluted rapidly from the column, whereas CO2 only started to elute after a period of time. Clearly, the distinct breakthrough data of the two components suggested the strong interaction between CO2 and PEI-MIL-101 materials and corroborated the high CO2/N2 selectivity calculated from single component gas adsorption.
Discussion
In conclusion, the impregnation of PEI into MOFs afforded a new and high-performance sorbent for post-combustion CO2 capture at low pressures. It possesses excellent CO2 adsorption capacity, rapid adsorption kinetics, together with very high CO2/N2 selectivity in flue gas. We believe that PEI modified MOF is a more promising MOF-based material, considering that many pure MOF adsorbents are unsatisfying at low pressures, especially below 0.15 bar, which greatly hinders the practical application. Overall, the present study has demonstrated the first successful combination of polymeric amine and MOFs for post-combustion CO2 capture. We anticipate that some others MOFs with large surface area and open metal sites, such as NU-10036 and MOF-17737, can also be excellent supports for PEI.
Methods
Materials
The chemicals are of analytical grade and used without further purification in this study. Deionized water and alcohol was employed as solvent. The PEI (purity, 99%) used in the present study was a linear polymer with an average molecular weight of 300 g/mol. Due to the hydroscopic property, the PEI was placed in glove box.
The PEI molecular formula:
Synthesis of MIL-101
MIL-101 was prepared by a hydrothermal reaction following the procedure reported by Ferey and coworkers with minor difference: the hydrofluoric acid was replaced with hydrochloric acid. The detailed synthesis was as follows: chromic nitrate (800 mg, 2 mmmol), terephthalic acid (332 mg, 2 mmol), 12 M hydrochloric acid (164 μL, 2 mmol), and deionized water (10 ml) were mixed in a Teflon-lined stainless steel autoclave and kept at 220°C for 8 h. After the synthesis, the autoclave reactor was slowly cooled down to room temperature for 3 h in order to obtain larger crystals of the unreacted terephthalic acid. After cooling, the reaction mixture was filtered using a large pore paper filter to eliminate the recrystallized free acid. Water and MIL-101(Cr) powder can pass through the filter, whereas the free acid would stay inside or on the paper filter. After that, the product was recovered by centrifugation. To remove the free acid lying in the MIL-101(Cr) pores, a solvothermal treatment of the product was employed by using hot ethanol (95% EtOH, 5% water) in an autoclave at 80°C for 8 h. The resulting solid was cooled down, centrifuged, and finally dried over night at 80°C under air atmosphere. For the following experiments, about 2 g MIL-101(Cr) samples were prepared by the above procedure.
Synthesis of PEI-MIL-101 adsorbent
The PEI-MIL-101 adsorbent was prepared by wet impregnation method. The detailed process was as follows. First, before the impregnation, the MIL-101 (Cr) powders were heated at 160°C under vacuum condition for 12 h, removing the adsorbed water and coordinated water. Second, the desired amount of PEI was dissolved in 1 mL anhydrous methanol under stirring for 10 min, and then 0.2 g MIL-101 (Cr) powders were added step by step into the PEI/methanol solution under stirring. Finally, the resulting gel was dried over night under room temperature and nitrogen protection, and then the temperature of the sample was increased at programmed rate and held at 110°C for 12 h under vacuum condition. After that, a porous and solid PEI-MIL-101 (Cr) adsorbent was obtained. Herein, four PEI-MIL-101 samples with different PEI loadings were prepared, corresponding to 50%, 75%, 100% and 125%. To confirm the accurate PEI loading, the mass of MIL-101(Cr) was measured immediately after activated, and the mass of PEI was measured in glove box. The loading of PEI was calculated by the following equation:
In addition, the PEI loading amount was further verified by element analysis (see Table S3).
Gas adsorption measurement
The adsorption isotherms and of the probe gas CO2 (purity, 99.999%) and N2 (purity, 99.999%) were measured using volumetric technique by an apparatus from SETARAM France (PCTpro-E&E). Before each measurement, the sample was evacuated at 110°C for 12 h. Pressure as a function of the amount of CO2 adsorbed was determined using the Langmuir-Freundlich fit26,27,28 for the isotherms:
Here, N is the amount of CO2 adsorbed (mmol g−1), p is the pressure, Nm is the amount of CO2 adsorbed at saturation, B and t are constants. To solve the exact pressures, p, corresponding to constant amount of CO2 adsorbed, the above equation can be rearranged to:
CO2 selectivity calculations
The CO2 selectivities (S) of samples were evaluated by the following equation which was previously reported29,30,35:
where qi is the adsorption capacity of i component, pi is the partial pressure of i component. The adsorption capacities of the components are defined to be molar excess adsorption capacities determined without correction for absolute adsorption.
CO2 adsorption kinetics measurement
The CO2 adsorption kinetics of the probe gas CO2 were also measured using volumetric technique by the apparatus from SETARAM France (PCTpro-E&E). Before each measurement, the sample was evacuated at 110°C for 12 h. In a simple procedure, the apparatus first give a desired pressure CO2 gas to the sample holder, and record the pressure values in the sample holder and calculated the adsorbed CO2 amount.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) was measured using a system provided by Mettler Toledo (model TGA/DSC1), in air at a heating rate of 5°C/min up to 600°C. Before the TGA measurement, the samples were first activated and then placed in atmosphere for the same time (~5 days). The PEI was immediately measured after moving from the glove box.
Infrared spectra (IR)
IR of the samples were recorded on KBr/sample pellets in a Thermo model Nicolet 6700 spectrometer to determine the amine. IR spectra were collected on activated PEI-MIL-101 and MIL-101 samples under vacuum condition. IR spectra of pure PEI are also shown for comparison.
Other physical measurements
For structure analysis, powder x-ray diffraction (PXRD) data of the samples was collected on a Bruker AXS D8 Advance diffractometer using Cu Kα radiation at room temperature. The 77 K nitrogen adsorption/desorption isotherm was measured on ASAP 2020 M apparatus. The Brunauer-Emmett-Teller (BET) surface area was calculated over the range of relative pressures between 0.05 and 0.20 bar. Before the 77 K N2 adsorption/desorption measurements, samples were pretreated under vacuum at 110°C for 12 h. The as-prepared samples morphologies were examined using a field emission scanning electron microscope (SEM) (Hitachi, S-4800).
Author Contributions
L.C., C.K. and Y.L. designed the project; Y.L. carried out the experiments; Y.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Supplementary Material
Supporting Information
Acknowledgments
We gratefully acknowledge the financial support by the national key basic research program of China (Grant No. 2012CB722700), national science foundation of China (Grant No.51072204, 51172249) and the aided program for science and technology innovative research team of Ningbo municipality (Grant No.2009B21005).
References
- Haszeldine R. S. Carbon Capture and Storage: How Green Can Black Be? Science 325, 1647–1652 (2009). [DOI] [PubMed] [Google Scholar]
- Rochelle G. T. Amine Scrubbing for CO2 Capture. Science 325, 1652–1654 (2009). [DOI] [PubMed] [Google Scholar]
- Ma X. L., Wang X. X. & Song C. S. "Molecular Basket" Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 131, 5777–5783 (2009). [DOI] [PubMed] [Google Scholar]
- Kauffman K. L. et al. Selective Adsorption of CO2 from Light Gas Mixtures by Using a Structurally Dynamic Porous Coordination Polymer. Angew. Chem. Int. Edit. 50, 10888–10892 (2011). [DOI] [PubMed] [Google Scholar]
- Sumida K. et al. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 112, 724–781 (2012). [DOI] [PubMed] [Google Scholar]
- Llewellyn P. L. et al. High uptakes of CO2 and CH4 in mesoporous metal-organic frameworks MIL-100 and MIL-101. Langmuir 24, 7245–7250 (2008). [DOI] [PubMed] [Google Scholar]
- Furukawa H. et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 329, 424–428 (2010). [DOI] [PubMed] [Google Scholar]
- Babarao R., Jiang J. W. & Sandler S. I. Molecular Simulations for Adsorptive Separation of CO2/CH4 Mixture in Metal-Exposed, Catenated, and Charged Metal-Organic Frameworks. Langmuir 25, 6590–6590 (2009). [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Kong C. L. & Chen L. Direct synthesis of amine-functionalized MIL-101(Cr) nanoparticles and application for CO2 capture. RSC Adv. 2, 6417–6419 (2012). [Google Scholar]
- Si X. L. et al. High and selective CO2 uptake, H2 storage and methanol sensing on the amine-decorated 12-connected MOF CAU-1. Energy Environ. Sci. 4, 4522–4527 (2011). [Google Scholar]
- Brune S. N. & Bobbitt D. R. Role of Electron-Donating Withdrawing Character, Ph, and Stoichiometry on the Chemiluminescent Reaction of Tris(2,2′-Bipyridyl)Ruthenium(Iii) with Amino-Acids. Anal. Chem. 64, 166–170 (1992). [Google Scholar]
- Kuwahara Y. et al. Dramatic Enhancement of CO2 Uptake by Poly(ethyleneimine) Using Zirconosilicate Supports. J. Am. Chem. Soc. 134, 10757–10760 (2012). [DOI] [PubMed] [Google Scholar]
- Chaikittisilp W., Khunsupat R., Chen T. T. & Jones C. W. Poly(allylamine)-Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 50, 14203–14210 (2011). [Google Scholar]
- Goeppert A. et al. Carbon Dioxide Capture from the Air Using a Polyamine Based Regenerable Solid Adsorbent. J. Am. Chem. Soc. 133, 20164–20167 (2011). [DOI] [PubMed] [Google Scholar]
- Qi G. G., Fu L. L., Choi B. H. & Giannelis E. P. Efficient CO2 sorbents based on silica foam with ultra-large mesopores. Energy Environ. Sci. 5, 7368–7375 (2012). [Google Scholar]
- Qi G. G. et al. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 4, 444–452 (2011). [Google Scholar]
- Ferey G. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 309, 2040–2042 (2005). [DOI] [PubMed] [Google Scholar]
- Hwang Y. K. et al. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angew. Chem. Int. Edit. 47, 4144–4148 (2008). [DOI] [PubMed] [Google Scholar]
- Hong D. Y., Hwang Y. K., Serre C., Ferey G. & Chang J. S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 19, 1537–1552 (2009). [Google Scholar]
- Gascon J., Serra-Crespo P., Ramos-Fernandez E. V. & Kapteijn F. Synthesis and Characterization of an Amino Functionalized MIL-101(Al): Separation and Catalytic Properties. Chem. Mater. 23, 2565–2572 (2011). [Google Scholar]
- Xu X. C., Song C. S., Andresen J. M., Miller B. G. & Scaroni A. W. Preparation and characterization of novel CO2 "molecular basket" adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Micropor. Mesopor. Mat. 62, 29-45 (2003). [Google Scholar]
- Chowdhury P., Bikkina C. & Gumma S. Gas Adsorption Properties of the Chromium-Based Metal Organic Framework MIL-101. J. Phys. Chem. C 113, 6616–6621 (2009). [Google Scholar]
- Liu Y. Y., Ju-Lan Z., Jian Z., Xu F. & Sun L. X. Improved hydrogen storage in the modified metal-organic frameworks by hydrogen spillover effect. Int. J. Hydrogen Energ. 32, 4005–4010 (2007). [Google Scholar]
- Bollini P., Didas S. A. & Jones C. W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 21, 15100–15120 (2011). [Google Scholar]
- Choi S., Drese J. H., Eisenberger P. M. & Jones C. W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 45, 2420–2427 (2011). [DOI] [PubMed] [Google Scholar]
- Sips R. On the Structure of a Catalyst Surface.2. J. Chem. Phys. 18, 1024–1026 (1950). [Google Scholar]
- Sips R. On the Structure of a Catalyst Surface. J. Chem. Phys. 16, 490–495 (1948). [Google Scholar]
- An J. & Rosi N. L. Tuning MOF CO2 Adsorption Properties via Cation Exchange. J. Am. Chem. Soc. 132, 5578–5579 (2010). [DOI] [PubMed] [Google Scholar]
- McDonald T. M., D′Alessandro D. M., Krishna R. & Long J. R. Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal-organic framework CuBTTri. Chem. Sci. 2, 2022–2028 (2011). [Google Scholar]
- McDonald T. M. et al. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal-Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 134, 7056–7065 (2012). [DOI] [PubMed] [Google Scholar]
- Granite E. J. & Pennline H. W. Photochemical removal of mercury from flue gas. Ind. Eng. Chem. Res. 41, 5470–5476 (2002). [Google Scholar]
- Peeters A. N. M., Faaij A. P. C. & Turkenburg W. C. Techno-economic analysis of natural gas combined cycles with post-combustion CO2 absorption, including a detailed evaluation of the development potential. Int. J. Greenh. Gas. Con. 1, 396–417 (2007). [Google Scholar]
- Bloch E. D. et al. Metal Insertion in a Microporous Metal-Organic Framework Lined with 2,2′-Bipyridine. J. Am. Chem. Soc. 132, 14382–14384 (2010). [DOI] [PubMed] [Google Scholar]
- Lu W. G. et al. Sulfonate-Grafted Porous Polymer Networks for Preferential CO2 Adsorption at Low Pressure. J. Am. Chem. Soc. 133, 18126–18129 (2011). [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang Z. U. & Zhou H.-C. Recent advances in carbon dioxide capture with metal-organic frameworks. Greenh. Gas.: Sci. and Technol. 2, 239–259 (2012). [Google Scholar]
- Farha O. K. et al. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2, 944–948 (2010). [DOI] [PubMed] [Google Scholar]
- Chae H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information