Abstract
Double-stranded RNA–dependent protein kinase (PKR) is implicated in inflammation and immune dysfunction through its regulation of mitogen-activated protein kinases, interferon regulatory factor 3, nuclear factorκB, apoptosis, and autophagy pathways. A study shows that PKR is also required for the activation of inflammasomes and the subsequent release of high-mobility group box 1 (HMGB1) protein, a proinflammatory cytokine. Thus, the cell stress kinase PKR has multifaceted roles in the regulation of inflammatory immune responses, and PKR and HMGB1 are attractive targets for inflammasome-associated diseases.
The inflammasome, a multiprotein oligomer, is activated by microbial pathogens, stress, and damage signals that trigger the release of proinflammatory cytokines, including interleukin-1β (IL-1β), IL-18, and high-mobility group box 1 (HMGB1) protein, as well as stimulate a form of programmed inflammatory cell death called pyroptosis, which engages immune and inflammatory responses (1, 2). The molecular mechanisms underlying the regulation of inflammasomes are still poorly defined. A study by Lu et al. (3) identified a previously uncharacterized biological function for double-stranded RNA (dsRNA)–dependent protein kinase (PKR) in promoting inflammation by sustaining inflammasome activities in response to pyroptosis-associated stimuli. This finding reveals a crucial new player in the ever-growing list of proteins that control the inflammasome and HMGB1 release (Fig. 1A) and provides a new target for the treatment of inflammasome-associated diseases, including obesity, diabetes, atherosclerosis, Alzheimer's disease, sepsis, colitis, and inflammation-associated cancer (4).
Fig. 1.
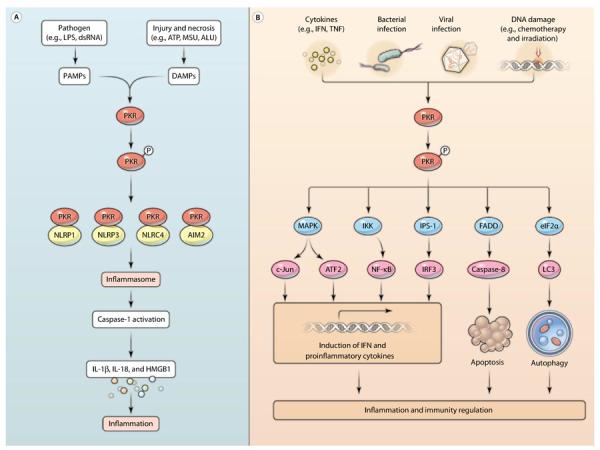
Signaling pathways involving PKR-mediated inflammation and immune regulation. (A) PKR is required for inflammasome activation. PAMPs and DAMPs promote the autophosphorylation and activation of PKR. Active PKR physically interacts with NLRP1, NLRP3, NLRC4, and AIM2 and promotes the release of inflammasome-dependent cytokines, including IL-1β, IL-18, and HMGB1. LPS, lipopolysaccharide. (B) The activation of PKR is induced by many different stimuli, such as cytokines, bacterial and viral infection, and DNA damage. Activation of PKR results in its dimerization and autophosphorylation. The activation of PKR in turn triggers the production of IFN and proinflammatory cytokines through transcriptional regulatory signals. In addition, the FADD–caspase-8 pathway mediates the proapoptotic activity of PKR, whereas the eIF2α-LC3 pathway mediates the proautophagic activity of PKR. Both apoptosis and autophagy are important in the regulation of inflammation and immunity.
PKR is a ubiquitously expressed serine and threonine protein kinase that was initially identified as an innate immune anti-viral protein that is induced by interferon (IFN) (5). In addition to its established role in the antiviral and antitumor activities of IFN (5, 6), PKR contributes to inflammation and immune regulation through several signaling pathways (Fig. 1B). PKR is activated by multiple stimuli, such as inflammatory cytokines [for example, IFN and tumor necrosis factor (TNF)]; bacterial and viral infection; and chemotherapy- and irradiation-induced DNA damage through a mechanism involving autophosphorylation (7–9). Active PKR mediates the activation of mitogen-activated protein kinases (MAPKs) (10, 11), inhibitor of κB (IκB) kinase (IKK) (12, 13), and IFN-β-promoter simulator 1 (IPS-1) signaling (14) and, then, affects diverse transcriptional factors, including interferon regulatory factor 3 (IRF3) (14), nuclear factor κB (NF-κB) (12, 13), c-Jun, and activating transcription factor 2 (ATF2) (15, 16), which are required for the expression of genes encoding proinflammatory cytokines and IFNs (7–9). The balance between apoptosis and autophagy affects the effector cells of innate and adaptive immunity that mediate the inflammatory response (17, 18). PKR triggers apoptosis through Fas-associated protein with death domain (FADD)–mediated activation of caspase-8 (19–21). In contrast, PKR triggers autophagy through eukaryotic initiation factor 2α (eIF2α)–mediated activation of the microtubule-associated protein LC3 (light chain 3) (22). Thus, PKR has multifaceted roles in the regulation of inflammatory immune responses (Fig. 1B).
HMGB1 is a chromatin-associated nuclear protein, which functions as a damage-associated molecular pattern molecule (DAMP) when released from cells under stressful conditions. HMGB1 is actively secreted by innate immune inflammatory cells in response to pathogen-associated molecular patterns (PAMPs) and DAMPs and passively released by damaged or necrotic cells (23–25). Once released, HMGB1 is specifically recognized by several cell surface receptors—including the receptor for advanced glycation end products (RAGE), Toll-like receptor 4 (TLR4), CD24, and T cell immunoglobulin (Ig)–and mucin domain–containing molecule-3 (TIM-3)—and thereby mediates inflammation, immunity, chemotaxis, and other cell processes (23, 26). Targeting HMGB1 or its receptors represents an important potential application for the treatment of cancer and infectious, inflammatory, and autoimmune diseases (23, 24). It is thus critical to investigate the potential mechanism of HMGB1 release, biological activity, and receptor signal transduction.
Lu et al. first tested the possibility that PKR was involved in the regulation of HMGB1 release. They analyzed HMGB1 release in macrophages derived from wild-type (PKR+/+) and PKR-deficient (PKR−/−) mice in response to poly (I:C) and other pyroptosis-associated stimuli, including adenosine triphosphate (ATP), monosodium urate (MSU), adjuvant aluminum (ALU), and live Escherichia coli (3). They found that PKR was required for pyroptosis-mediated HMGB1 release (Fig. 1A), because inactivation of PKR by genetic deletion or pharmacological inhibition in macrophages substantially impaired HMGB1 hyperacetylation and release (3). In activated inflammatory cells, HMGB1 is hyperacetylated and relocated from the nucleus to the cytoplasm for exocytosis (27).
The inflammasome is a large multiprotein complex, the assembly of which leads to the activation of caspase-1, an aspartate-specific cysteine protease that cleaves its substrates, including pro–IL-1β (1). Similarly, caspase-1 is responsible for the processing and secretion of IL-18, as well as the secretion of other proteins, such as HMGB1, through an unconventional protein secretion pathway (28, 29). Lu et al. observed that PKR was required for inflammasome activation in response to several pyroptosis-associated stimuli (3). Overexpression of PKR substantially enhanced caspase-1 activation and IL-1β cleavage, whereas knockdown of PKR by short hairpin RNA (shRNA) inhibited caspase-1 activity and IL-1β cleavage in several different cell types, including macrophages, dendritic cells, and embryonic kidney cells (3). PKR regulated the release of inflammasome-dependent cytokines (for example, IL-1β, IL-18, and HMGB1) but not inflammasome-independent cytokines (for example TNF-α and IL-6) cytokines in vitro and in vivo (3). Inflammasome activation occurs in a pathogen-specific manner, although different inflammasomes may have redundant roles during infection (6, 18). Inflammasomes are essential for host defense against bacterial and viral infection. Both effector mechanisms activated by inflammasomes (the production of proinflammatory cytokines and the induction of pyroptosis) protect against bacterial infections (4, 30). However, Lu et al. observed that PKR−/− mice had substantially reduced bacterial titers when compared with those of PKR+/+ controls, which suggested that PKR deficiency impaired bacterial infection (3).
Assembly of inflammasomes depends on the NOD-like receptor (NLR) family members, including NLR family pyrin domain–containing 1 (NLRP1), NLRP3, NLR family CARD domain–containing protein 4 (NLRC4), NLRP6, and NLRP12, or pyrin domain (PYD)– and HIN domain–containing (PYHIN) family members, including absent in melanoma 2 (AIM2) and IFN-γ-inducible protein 16 (IFI16). Many regulatory mechanisms have been identified that act as checkpoints for attenuating inflammasome signaling at multiple steps (1, 2). Lu et al. demonstrated that PKR physically interacted with several inflammasome components, including NLRP3, NLRP1, NLRC4, and AIM2 (3). This interaction was mediated by autophosphorylation of PKR, because a kinase-defective mutant PKR protein failed to bind to NLRP3. In addition, PKR failed to directly interact with other cytosolic receptors or inflammasome family members, including NOD1, NLRP12, and NLRX1 (3). The authors concluded that PKR selectively regulates the activation of the NLRP3, NLRP1, AIM2, and NLRC4 inflammasomes through direct physical interactions.
Several questions remain unresolved regarding the role of PKR in the regulation of inflammasomes. First, like many protein kinases, PKR is regulated through direct interactions with activating and inhibitory molecules, such as the 52-kD repressor of the inhibitor of the protein kinase (P52rIPK) and the 58-kD inhibitor of the protein kinase (P58IPK) (31). PKR has previously been linked with MAPKs, IRF-3, and the NF-κB pathway (Fig. 1B). There is great interest in determining whether these regulators and pathways are involved in the regulation of PKR-mediated inflammasome activity. Second, PKR is a mediator of stress-induced apoptosis (21) and autophagy (22) in immunity (Fig. 1B). Autophagy regulates not only PAMP- and DAMP-associated innate and adaptive immunity (25) but also inflammasome activity (32) and HMGB1 release (33). Such findings may help us to study the molecular mechanism by which PKR signaling regulates inflammation. Finally, reduced and oxidized HMGB1 have different roles in extracellular signaling and regulation of immune responses (34, 35). The influence of PKR on the biological activities and pathological effects of HMGB1 remains to be explored.
Acknowledgments
We thank H. Wang (the Feinstein Institute for Medical Research) and C. Heiner (University of Pittsburgh) for critical reading of the manuscript.
Funding: This work was supported by NIH (1R01CA160417-01A1 to D.T.) and a grant from the University of Pittsburgh (to D.T.).
References and Notes
- 1.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat. Immunol. 2012;13:333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 5.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 6.Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 7.Balachandran S, Barber GN. PKR in innate immunity, cancer, and viral oncolysis. Methods Mol. Biol. 2007;383:277–301. doi: 10.1007/978-1-59745-335-6_18. [DOI] [PubMed] [Google Scholar]
- 8.Williams BR. Signal integration via PKR. Sci. STKE. 2001;2001:re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 9.García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamanian-Daryoush M, Mogensen TH, Di-Donato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol. Cell. Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnet MC, Weil R, Dam E, Hovanessian AG, Meurs EF. PKR stimulates NF-kappaB irrespective of its kinase function by interacting with the IkappaB kinase complex. Mol. Cell. Biol. 2000;20:4532–4542. doi: 10.1128/mcb.20.13.4532-4542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Samuel CE. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J. Biol. Chem. 2008;283:34580–34587. doi: 10.1074/jbc.M807029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J. Virol. 2010;84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA- dependent protein kinase. J. Biol. Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vernon PJ, Tang D. Eat-Me: Autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2012.4810. 10.1089/ars.2012.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil J, Esteban M. The interferon-induced protein kinase (PKR), triggers apoptosis through FADD-mediated activation of caspase 8 in a manner independent of Fas and TNF-alpha receptors. Oncogene. 2000;19:3665–3674. doi: 10.1038/sj.onc.1203710. [DOI] [PubMed] [Google Scholar]
- 20.Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–345. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 22.Tallóczy Z, Jiang W, Virgin 4th HW, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat. Immunol. 2012;13:808–810. doi: 10.1038/ni.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahoo M, Ceballos-Olvera I, del Barrio L, Re F. Role of the inflammasome, IL-1β, and IL-18 in bacterial infections. ScientificWorldJournal. 2011;11:2037–2050. doi: 10.1100/2011/212680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale M, Jr., Blakely CM, Hopkins DA, Melville MW, Wambach M, Romano PR, Katze MG. Regulation of interferon-induced protein kinase PKR: Modulation of P58IPK inhibitory function by a novel protein, P52rIPK. Mol. Cell. Biol. 1998;18:859–871. doi: 10.1128/mcb.18.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]


