Abstract
The adult zebrafish retina continuously produces rod photoreceptors from infrequent Müller glial cell division, yielding neuronal progenitor cells that migrate to the outer nuclear layer and become rod precursor cells that are committed to differentiate into rods. Retinal damage models suggested that rod cell death induces regeneration from rod precursor cells, whereas loss of any other retinal neurons activates Müller glia proliferation to produce pluripotent neuronal progenitors that can generate any other neuronal cell type in the retina. We tested this hypothesis by creating two transgenic lines that expressed the E. coli nitroreductase enzyme fused to EGFP (NTR-EGFP) in only rods. Treating transgenic adults with metronidazole resulted in two rod cell death models. First, killing all rods throughout the Tg(zop:nfsB-EGFP)nt19 retina induced robust Müller glial proliferation, which yielded clusters of neuronal progenitor cells. In contrast, ablating only a subset of rods across the Tg(zop:nfsB-EGFP)nt20 retina led to rod precursor, but not Müller glial, cell proliferation. We propose that two different criteria determine whether rod cell death will induce a regenerative response from the Müller glia rather than from the resident rod precursor cells in the ONL. First, there must be a large amount of rod cell death to initiate Müller glia proliferation. Second, the rod cell death must be acute, rather than chronic, to stimulate regeneration from the Müller glia. This suggests that the zebrafish retina possesses mechanisms to quantify the amount and timing of rod cell death.
Keywords: nitroreductase, metronidazole, Müller glia, neuronal progenitor cell, rod precursor cell, retinal regeneration, Müller glia, stem cell, nfsB
Zebrafish is a popular model to study retinal regeneration, as its retina produces a robust regenerative response following multiple forms of injury (Hitchcock and Raymond, 2004). Conventional models propose that regenerated retinal neurons derive from two sources, depending on the cell class lost (Otteson and Hitchcock, 2003; Morris et al., 2008). Müller glia possess qualities of multipotent stem cells, as they are the source of many regenerated neuronal cell classes in the retina (Fausett and Goldman, 2006; Bernardos et al., 2007; Fimbel et al., 2007; Stenkamp, 2007; Thummel et al., 2008a,b). A variety of damage paradigms induce the Müller glial cells to reenter the cell cycle and divide asymmetrically to produce neuronal progenitor cells, which continue to proliferate and migrate radially to the damaged retinal layer where they differentiate into the neuronal cell classes that are missing (Yurco and Cameron, 2005; Fausett and Goldman, 2006; Raymond et al., 2006; Fimbel et al., 2007; Thummel et al., 2008a,b). New retinal neurons are also produced during persistent neurogenesis, which occurs as the fish eye grows throughout its life and new retinal neurons are produced to accompany this growth. During this process, Müller glia proliferate at a slow rate to produce neuronal progenitor cells that migrate to the outer nuclear layer (ONL). Upon reaching the ONL, they become rod precursor cells that are committed to differentiating into only rod photoreceptors (Otteson and Hitchcock, 2003; Raymond et al., 2006). Thus, the damaged retina possesses two classes of cells with a regenerative capacity, the pluripotent Müller glia and the committed rod precursors.
Different damage models helped to elucidate the conditions involved in inducing a regenerative response from either the Müller glia or rod precursors. Surgical lesion of a portion of the retina and retinal puncture both induce Müller glial cell proliferation and formation of neuronal progenitor cell clusters in the inner nuclear layer (INL; Yurco and Cameron, 2005; Fausett and Goldman, 2006). Intravitreal injection of the neurotoxin ouabain, which kills neurons in all retinal layers at high doses and primarily removes INL neurons and ganglion cells at low doses, also produces a regeneration response that originates with Müller glial cell division (Fimbel et al., 2007; Sherpa et al., 2008). Thus, loss of non-photoreceptor neurons requires proliferation of the pluripotent Müller glia. Similarly, light-induced apoptosis of the rod and cone photoreceptors yields a robust regeneration response involving proliferation of the Müller glia (Vihtelic and Hyde, 2000; Bernardos et al., 2007; Kassen et al., 2007; Thummel et al., 2008a,b). In contrast, the light-damaged ventral retina exhibits rod photoreceptor cell death without any apparent cone cell loss, which results in significant ONL rod precursor cell proliferation and minimal Müller glial cell division (Vihtelic et al., 2006).
This suggests that if the damage is restricted to rod photoreceptors, regeneration requires only increasing rod precursor cell proliferation. This was further examined in the pde6cw59 mutant, which is unable to maintain cones, and the Tg(Xops:mCFP) transgenic line, which exhibits chronic rod cell death (Morris et al., 2008). Whereas the pde6cw59 mutant possesses Müller glial proliferation, the Tg(Xops:mCFP) fish exhibit increased rod precursor cell division without greater Müller glial cell proliferation (Morris et al., 2008). These studies suggest that all regenerated retinal neurons originate from the Müller glial cells, whereas loss of only rod photoreceptors can be regenerated from the resident rod precursors. However, none of these damage models examined regeneration following acute and specific loss of all mature rod photoreceptors, which may require accelerated production of additional rod precursor cells that would necessitate increased proliferation of the Müller glia.
To address this hypothesis, we utilized the bacterial nitroreductase (NTR)/metronidazole cell ablation system to specifically induce death of all rod photoreceptors. The NTR enzyme reduces nitroimidazole prodrugs (Lindmark and Müller, 1976), such as metronidazole, into cytotoxins that generate DNA interstrand crosslinking and cell death (Edwards, 1977; Roberts et al., 1986). Whereas many prodrugs exhibit a bystander effect, in which they cause the death of adjacent cells that do not express the activating enzyme, the NTR/metronidazole system does not display such an effect (Roberts et al., 1986; Edwards, 1993; Bridgewater et al., 1997). It was previously demonstrated that metronidazole treatment of zebrafish expressing the E. coli nitroreductase (nfsB) gene under control of known promoters specifically ablated pancreatic β cells in larval (Curado et al., 2007; Pisharath et al., 2007) and adult (Moss et al., 2009) fish, as well as larval cardiomyocytes, hepatocytes (Curado et al., 2007), and cells of the developing notochord and ventral CNS (Davison et al., 2007). Thus, unlike previous methods used, this system holds great promise for inducing rod cell death without damaging cone photoreceptors.
We describe the generation of two transgenic zebrafish lines that express an NTR-EGFP fusion protein from the zebrafish rod opsin promoter (zop). The Tg(zop:nfsB- EGFP)nt19 line expresses NTR-EGFP uniformly in all rod photoreceptors, whereas Tg(zop:nfsB-EGFP)nt20 displays a non-uniform expression of NTR-EGFP in a subset of rods. Treatment of both transgenic lines with metronidazole resulted in the death of all cells expressing the NTR-EGFP fusion protein, followed by regeneration of the lost photoreceptors. In the Tg(zop:nfsB-EGFP)nt19 line, prodrug treatment resulted in the loss of all rods in the retina. In contrast, metronidazole induced death in only a subset of rods in the Tg(zop:nfsB-EGFP)nt20 line. Unexpectedly, metronidazole treatment increased Müller glial cell proliferation in the Tg(zop:nfsB-EGFP)nt19 retina, but only increased rod precursor cell proliferation in the Tg(zop:nfsB-EGFP)nt20 retina. Thus, acute loss of a large number of only rod photoreceptors is sufficient to induce a Müller glial regeneration response, possibly due to an insufficient number of rod precursor cells in the ONL to effectively regenerate the full rod photoreceptor cell population.
MATERIALS AND METHODS
Fish maintenance
Zebrafish were raised and maintained by using standard techniques (Westerfield, 2000) at the Center for Zebrafish Research in the Freimann Life Science Center at the University of Notre Dame. Fish were fed a diet of brine shrimp and flake food three times daily and maintained at 28.5°C with a light cycle of 14 hours light (250 lux):10 hours dark. All experiments were approved by the animal use committee at the University of Notre Dame and were in compliance with the provisions set forth by the Association for Research in Vision and Ophthalmology (ARVO) for the use of animals in vision research.
Generation of transgenic lines
The 1.2-kb zebrafish rod opsin (zop) promoter was amplified by polymerase chain reaction (PCR) from a plasmid template (Kennedy et al., 2001) by using Platinum Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, CA). BamHIand NcoI restriction sites were incorporated into the forward (5′-GGATCCGAATTCTTGTGCTTCAATCAGATGCG-3′) and reverse (5′-CCATGGTCTAGATGTCTGGGCAGGAC-3′) PCR primers, respectively (restriction sites are underlined). PCR was conducted with the following parameters: 94°C for 2 minutes, 35 cycles of 94°C for 30 seconds, 60°C for 1 minute, and 68°C for 4 minutes, with a final extension of 68°C for 10 minutes. The PCR product was cloned into the pCR8/GW/TOPO vector (Invitrogen) and was then subcloned into the T2KXIG-ins:nfsB-EGFP plasmid (Pisharath et al., 2007), by using a BamHI/NcoI digest to remove the preproinsulin (insa) promoter and ligate the amplified zop promoter. Tol2 transposase mRNA was in vitro transcribed from the pCSTZ2.8 plasmid (Kawakami et al., 1998) by using the SP6 mMessage mMachine kit (Ambion, Foster City, CA). The T2KXIG-zop:nfsB-EGFP expression construct was co-injected with Tol2 transposase mRNA into one-to four-cell stage AB wild-type embryos as described (Thummel et al., 2005) at a concentration of 25 ng/μl each. F0 adults were out-crossed to the AB strain to identify founders. EGFP-expressing F1 carriers were again out-crossed to generate independent transgenic lines. The Tg(zop:nfsB-EGFP)nt19 and Tg(zop:nfsB-EGFP)nt20 transgenic lines, used in the present study, have been outcrossed to the F3 generation, and transmission of the transgene in 50% of the progeny is consistent with Mendelian ratios for a single insertion of the transgene.
Metronidazole treatment and retinal preparation
Metronidazole treatments were performed on adult Tg(zop:nfsB-EGFP)nt19 and Tg(zop:nfsB-EGFP)nt20 zebrafish between 4 and 6 months post fertilization. Metronidazole (M3761, Sigma-Aldrich, St. Louis, MO) was dissolved at a concentration of 10 mM in system water (40 gr Instant Ocean Sea Salts in 1.0 L distilled water) from the University of Notre Dame Center for Zebrafish Research. Fish from each transgenic line were immediately placed into treatment tanks containing 5.0 liters of 10 mM metronidazole for 24 hours at 28°C in the dark. Fish were removed and placed in recovery tanks containing system water. A minimum of five fish from each line were deeply anesthetized in 0.2% 2-phenoxyethanol and sacrificed at each of eight time points: untreated (control), immediately following 24 hours of metronidazole treatment, and following 24, 48, 72, 96, and 168 hours and 28 days in the recovery tanks. Additional eyes were collected from the Tg(zop: nfsB-EGFP)nt19 line after 120 and 216 hours of recovery.
Eyes were enucleated, fixed, washed, cryoprotected, and embedded as previously described (Thummel et al., 2008b). Left eyes were fixed in 4% paraformaldehyde and right eyes were fixed in 9:1 ethanolic formaldehyde. Eyes were cryosectioned in the dorsal-ventral plane, with a thickness of 16 μm. Slides were dried at 50°C for 2 hours and stored at −80°C.
Immunolabeling of retinal sections
Immunolabeling protocols were followed as previously described (Thummel et al., 2008b). Primary antibodies used in the study are described below. Secondary antibodies used include Alexa Fluor goat anti-primary 488, 568, 594, and 647 (1:500; Molecular Probes, Eugene, OR). The secondary antibodies failed to stain tissue sections when the primary antibody was omitted. TO-PRO-3 Iodide (1: 750; Molecular Probes), which labels nuclei, was added to most of the secondary antibody incubations. Slides were mounted with coverslips by using ProLong Gold antifade reagent (Molecular Probes).
Primary antibody characterization
The mouse monoclonal anti-PCNA antibody (1:1,000, P8825, clone PC10; Sigma-Aldrich) was raised against a recombinant rat PCNA-Protein A fusion protein. The antibody immunoprecipitated PCNA from cell extracts, bound PCNA in competition assays, and showed immunofluorescence staining that was identical to other commercially available PCNA antibodies (Waseem and Lane, 1990). This antibody produced identical labeling in cryosections as the rabbit polyclonal anti-PCNA antibody in the present study and as described in previous studies (Vihtelic and Hyde, 2000; Raymond et al., 2006).
Rabbit polyclonal anti-PCNA antibody (1:750, ab2426; Abcam, Cambridge, MA) was raised against a synthetic peptide (DMGHLKYYLAPKIEDEEGS) corresponding to the carboxyl terminal amino acids 243–261 of human PCNA. In Western blots, the antibody detected the predicted 29- kDa molecular weight protein in HeLa nuclear and whole cell lysates (manufacturer’s datasheet). In the current study, this antibody produced the same staining pattern as the mouse monoclonal anti-PCNA antibodies in retinal cryosections and as described in previous studies (Vihtelic and Hyde, 2000; Raymond et al., 2006).
Rabbit polyclonal anti-GFP (green fluorescent protein) antibody (1:1,500, ab6556; Abcam) was raised against recombinant full-length GFP purified from E. coli. The antibody detected a primary protein on Western blots of 32 kDa and a weaker secondary protein of 20 kDa (manufacturer’s datasheet). The anti-GFP antibody staining reproduced the EGFP fluorescence expression pattern exactly in both the Tg(zop:nfsB-EGFP)nt19 and Tg(zop:nfsB-EGFP)nt20 transgenic fish and did not label retinal cryosections from wild-type AB strain zebrafish (data not shown).
Mouse monoclonal anti-glutamine synthetase antibody (1:500, mAb302, clone GS-6; Chemicon, Temecula, CA) was raised against glutamine synthetase purified from sheep brain. The Protein A-purified antibody detects a sin-gle 44-kDa protein on Western blots of human and mouse brain extracts (manufacturer’s datasheet). In the present study, the antibody produced the expected Müller glial cell labeling pattern as previously described (Thummel et al., 2008b), based on morphology and distribution of the labeled cells. Specificity was further confirmed through colabeling experiments with the Tg(gfap:EGFP)nt11 transgenic zebrafish line, which expresses EGFP specifically in all Müller glial cells (Kassen et al., 2007).
The monoclonal zs-4 antibody (1:10; Zebrafish International Resource Center, Eugene, OR) was produced by immunizing mice with homogenized adult zebrafish hindbrain membranes (Trevarrow et al., 1990). The zs-4 antibody binds an uncharacterized epitope in the rod inner segments and is a marker of rod photoreceptors (Vihtelic and Hyde, 2000).
Rabbit polyclonal antibodies were generated against carboxyl terminal peptides generated from DNA amplified from zebrafish cDNA templates of rod opsin (5′-GAATTCGGCGTGGCCTGGTACATCTT-3′ and 5′-CTCGAGGGAGAGTTTACGCCGGAGACAC-3′) and green opsin (5′-GAATTCAGCTTTGCTGCCTGGATCTTCT-3′) and amino terminal peptides amplified from blue opsin (5′-GAATTCGAAGCAACAACAGCAAACGC-3′ and 5′-CTCGAGGGTAAGAACGTTGATGGCAG-3′), red opsin (5′-GAATTCATGGCAGAGCATTGGG-3′ and 5′-CTCGAGTGAATTTGGCCGTGG-3′), and ultraviolet opsin (5′-GAATTCGCGTGGGCCGTTCAATT-3′ and 5′-CTCGAGTTCATCGTGACGAAGAGGACG-3′) cDNA (1:5,000, 1:500, 1:250, 1:500, and 1:1,000, respectively; Vihtelic et al., 1999). In Western blots of zebrafish retinal protein extract, rhodopsin and red opsin antibodies detected monomeric species of the expected sizes as well as multimeric opsin species, whereas the green, blue, and ultraviolet opsin antibodies detected single bands of the expected sizes (Vihtelic et al., 1999). Immunohistochemical labeling of zebrafish retinal cryosections showed that antibodies to rhodopsin labeled rod photoreceptor outer segments, green and red opsin labeled double cone outer segments, blue opsin labeled long single cone outer segments, and ultraviolet opsin labeled short single cone outer segments (Vihtelic et al., 1999).
TUNEL
Cell death was detected in retinal cryosections by TUNEL. Slides containing frozen retinal sections were rehydrated in 1X phosphate-buffered saline (PBS; pH 7.4) and permeabilized with 0.1% sodium citrate/0.1% Triton X-100 on ice for 2 minutes. Biotinylated dNTPs (New England Biolabs, Ipswich, MA) were incorporated into fragmented DNA by using the ApoAlert DNA Fragmentation Assay Kit (Clontech, Mountain View, CA). Biotinylated dNTPs were detected with Streptavidin-Alexa Fluor conjugates (1:200; Molecular Probes) in 1X PBS. Slides were mounted with ProLong Gold.
Microscopy and digital photography
Representative images of fluorescently labeled retinal sections were acquired by using a TCS SP5 Broadband Confocal System (Leica Microsystems, Wetzlar, Germany). Low-intensity fluorescence was enhanced and background signal was reduced in digital images through the application of the curves function by using the Jasc Paint Shop Pro (Corel, Mountain View, CA) software package. Layers were applied across the entire panel in each instance. Glutamine synthetase labeling was pseudo-colored green, and cone opsin images were converted to grayscale for improved visualization. Whole-retina composite images were produced by manually aligning two to three adjacent 20× retinal images. Red-green epifluorescence was converted to magenta-green by using Photoshop (Adobe Systems, San Jose, CA). For each image, the Channel palette was selected, and the red channel for the entire image was copied and then pasted on the blue channel. Areas of overlap between magenta and green are represented as white.
Quantification of proliferating cells and statistical analyses
Quantifications were made by using one 16-μm retinal section from each of a minimum of five fish per time point. Both individual PCNA-positive cells and clusters of PCNA- positive cells in the INL were counted in a 150-μm region 200–300 μm from the dorsal and ventral margins of metronidazole-treated Tg(zop:nfsB-EGFP)nt19 and Tg(zop: nfsB-EGFP)nt20 retinal sections. Individual cells not associated with other PCNA-positive cells were scored as one cluster. Statistical analyses were performed by using SPSS 17.0 (Chicago, IL). All data sets were analyzed with a repeated measures ANOVA (significance level P < 0.05) to compare the extent of proliferation between the dorsal and ventral regions of the retina in the Tg(zop:nfsB-EGFP)nt19 and Tg(zop:nfsB-EGFP)nt20 lines. The repeated measures ANOVA revealed that the effect of treatment was dependent on time point (i.e., significant treatment × time interaction). Therefore, two-sample t-tests were performed on individual time points and Bonferroni-corrected (0.05/ 10 tests = 0.005 as the effective α) to account for the multiple tests.
RESULTS
NTR-EGFP is expressed specifically in all rods in Tg(zop:nfsB-EGFP)nt19
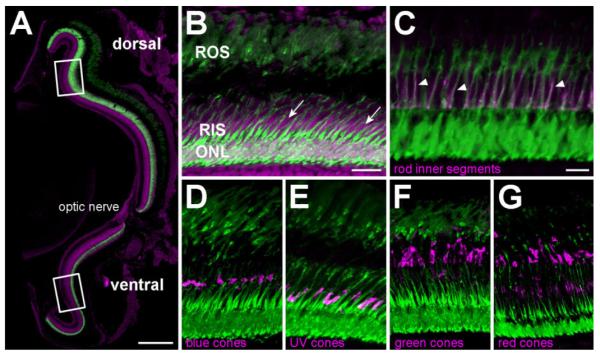
To induce rod photoreceptor cell death, transgenic zebrafish lines were generated by using the zebrafish rod opsin (zop) promoter to drive expression of the nfsB-EGFP fusion construct in rod cells (Kennedy et al., 2001; Pisharath et al., 2007). Expression of the transgene was assessed in untreated (without metronidazole) Tg(zop:nfsB-EGFP)nt19 fish by comparing EGFP fluorescence in retinal cryosections with antibodies that specifically label rod and cone photoreceptors. The transgene was expressed across both the dorsal and ventral adult retina (Fig. 1A). At higher magnification, EGFP fluorescence was visible in all cells of the outer nuclear layer (ONL), as well as in the rod inner segments and rod outer segments (Fig. 1B, RIS and ROS, respectively). Additionally, EGFP-negative cone cell nuclei were observed apical to the ONL and adjacent to the rod inner segments (Fig. 1B, arrows).
Figure 1.
NTR-EGFP expression is restricted to all rod photoreceptor cells in adult Tg(zop:nfsB-EGFP)nt19 fish. A: Confocal microscopy of TO-PRO-3 (magenta) counterstained retinal cryosections revealed fluorescent-labeled rod photoreceptors distributed throughout the entire ONL of 4–6-month-old Tg(zop:nfsB-EGFP)nt19 retinas. Rectangles represent the approximate 150 μm dorsal and ventral regions where PCNA-positive cells were quantified in metronidazole-treated fish. B: A higher magnification of the TO-PRO-3 counterstained retina revealed EGFP expression in rod nuclei in the ONL, as well as the RIS and ROS. EGFP is clearly not expressed in the cone cell nuclei adjacent to the RIS (B, arrows). C: All RIS labeled with the zs-4 marker (magenta) also expressed the transgene (arrowheads). D–G: EGFP-positive cells in the Tg(zop:nfsB-EGFP)nt19 retina clearly did not co-label with any of the cone opsins (magenta): blue (D), UV (E), green (F), and red (G). ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer. Scale bar = 150 μm in A; 20 μm in B (applies to B,D–G); 10 μm in C.
To examine penetrance and specificity of the transgene’s expression, the Tg(zop:nfsB-EGFP)nt19 line was immunolabeled for the rod inner segment marker, zs-4 (Fig. 1C). All zs-4-positive rod inner segments co-expressed EGFP (arrowheads), indicating that the nfsB-egfp transgene was expressed in all rod photoreceptors. EGFP fluorescence was further compared with immunolabeling of the four major cone opsins: blue, UV, green, and red (Fig. 1D–G). EGFP clearly did not co-label with any of the cone opsins, illustrating that NTR-EGFP fusion protein expression was restricted to all rod photoreceptors in the Tg(zop: nfsB-EGFP)nt19 line.
Metronidazole treatment ablated all Tg(zop: nfsB-EGFP)nt19 rods
To induce rod photoreceptor cell death in the Tg(zop: nfsB-EGFP)nt19 line, transgenic fish were placed in a treatment tank containing 10 mM metronidazole for 24 hours. Five fish were sacrificed after the 24 hours of metronidazole treatment (24h Met.) for analysis, whereas the remaining fish were transferred to a recovery tank that did not contain prodrug. Additional eyes were collected from a minimum of five fish per time point after 24, 48, 72, 96, and 168 hours and 28 days in the recovery tank (24h Rec., 48h Rec., 72h Rec., 96h Rec., 168h Rec., and 28d Rec.). Control Tg(zop:nfsB-EGFP)nt19 fish, which had not been exposed to metronidazole, exhibited EGFP fluorescence in both the ONL and ROS of rod photoreceptors (Figs. 2A, 3A,I). As shown previously, the dorsal ONL contained more nuclei than the ventral ONL (Vihtelic et al., 2006). After 24 hours of metronidazole treatment, the condensation and retraction of the ROS basally toward the cell bodies suggested that the rods were disrupted, although there was no apparent loss of EGFP-expressing cells (Figs. 2B, 3B,J).
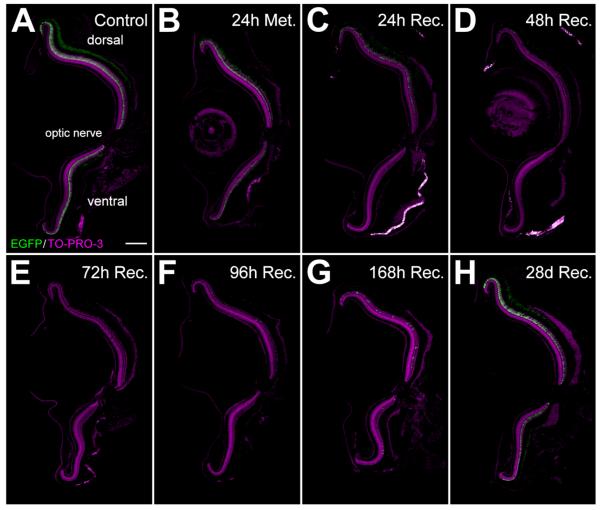
Figure 2.
Rod photoreceptor cell loss and regeneration following metronidazole treatment of Tg(zop:nfsB-EGFP)nt19 zebrafish. Adult fish were treated with 10 mM metronidazole for 24 hours. Following treatment, a minimum of five fish were sacrificed (24h Met., B); the remaining fish were transferred to a recovery tank and collected following 24, 48, 72, 96, and 168 hours and 28 days of recovery (C–H, respectively). A: Control Tg(zop:nfsB-EGFP)nt19 fish were not treated with metronidazole. B: After 24 hours of metronidazole treatment, rod photoreceptors throughout the retina appeared disorganized. C: After 24 hours of recovery, there was an obvious reduction in the number of EGFP-expressing dorsal and ventral ONL cells, consistent with rod photoreceptor death. D–F: After 48 hours of recovery, a maximal amount of rod cell loss was achieved in the ventral retina (D), based on the absence of EGFP expression, whereas the dorsal retina retained some EGFP-expressing rods through 72 hours of recovery (E). Regenerated ventral rods were first observed following 72 hours of recovery (E) and regenerated dorsal rods were found following 96 and 168 hours of recovery (F,G). H: By 28 days of recovery, rod photoreceptors had fully regenerated throughout the retina. ONL, outer nuclear layer. Scale bar = 150 μm in A (applies to A–H). ONL, outer nuclear layer.
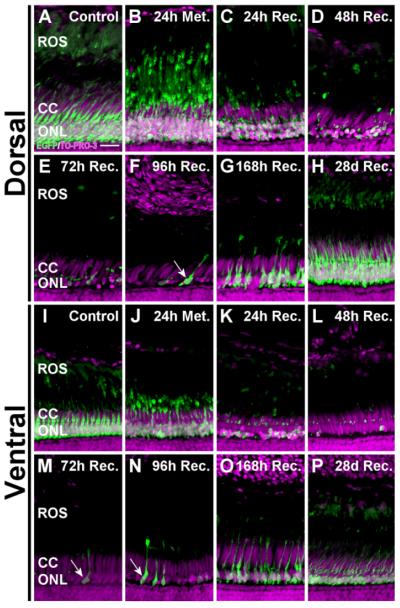
Figure 3.
Dorsal-ventral differences in photoreceptor loss following metronidazole treatment. Metronidazole-treated Tg(zop:nfsB- EGFP)nt19 retinal cryosections were stained with TO-PRO-3 and examined for loss of EGFP fluorescence. A,L: The ONLs of control retinas were comprised of healthy, EGFP-positive rod photoreceptors in the dorsal (A) and ventral (I) retinal regions. B,J: At the end of the 24-hour treatment, the ROS appeared to condense and retract basally, toward the ONL. C,K,D,E: Loss of EGFP-positive cells was apparent by 24 hours of recovery (C,K) and continued in the dorsal retina until virtually no EGFP-labeled nuclei were detected in the ONL after 72 hours of recovery (D,E). F,G: Newly regenerated, EGFP-expressing immature rods were observed at 96 (F) and 168 (G) hours of recovery (arrow). L: In the ventral retina, maximal cell loss was achieved by 48 hours of recovery. M–O: At 72–168 hours of recovery, EGFP-expressing immature rods were detected in the ventral retina (arrows). H,P: By 28 days of recovery, both the dorsal and ventral ONL had regenerated to a thickness comparable to the control. The NTR-EGFP-negative cone cell nuclei, located apical to the rod nuclei in the cone cell layer, were retained throughout this time course. ROS, rod outer segments; CC, cone cell layer; ONL, outer nuclear layer. Scale bar = 15 μm in A (applies to A–P).
After 24 hours of recovery, the metronidazole-dependent ablation had clearly reduced the number of EGFP-positive cells in the ONL relative to the control, indicative of rod cell death (Figs. 2C, 3C,K). After 24 hours of recovery, the ventral retina exhibited more rapid rod cell loss than the dorsal retina. Rod photoreceptor loss continued in the dorsal retina until virtually no EGFP-positive rod cells remained in the dorsal ONL at 72 hours of recovery (Figs. 2D,E, 3D,E). In contrast, the TO-PRO-3-labeled cone cells, located apically to the rod nuclei, remained intact following treatment (Fig. 3B–G), which confirmed that the cone photoreceptors were not lost in the dorsal Tg(zop:nfsBEGFP)nt19 retina. This was consistent with the absence of NTR-EGFP expression in any cone cells. In contrast, maximal rod cell loss was achieved in the ventral Tg(zop:nfsB- EGFP)nt19 retina by 48 hours of recovery (Figs. 2D, 3L), with TO-PRO-3-labeled cone cell nuclei persisting throughout the time course (Fig. 3J–O).
Newly regenerated EGFP-positive rods were discernable in the dorsal retina by 96 hours of recovery (Figs. 2F, 3F, arrow). The ventral retina, however, began exhibiting EGFP-positive, newly regenerated rods after 72 hours of recovery (Fig. 3M, arrow). The rods appeared to be fully regenerated in both aspects of the retina by 28 days of recovery (Figs. 2H, 3H, P), with the ONL of comparable thickness to the control. Thus, the dorsal and ventral retinal regions exhibited different rates of rod cell loss and regeneration.
As seen in other ablation experiments, fluorescent markers can outlast the cells being killed. Therefore, we independently assessed the extent of rod cell death following metronidazole treatment by using TUNEL labeling in the dorsal retina. TUNEL signal was not detected in the ONL of untreated control retinas (Fig. 4A). TUNEL-positive cells were observed in the ONL immediately following prodrug treatment (Fig. 4B) and increased in number through 24 and 48 hours of recovery (Fig. 4C and D, respectively). By 72 and 96 hours of recovery, little TUNEL signal remained, as essentially all the EGFP-expressing rods had died and were being regenerated (Fig. 4E and F, respectively).
Figure 4.
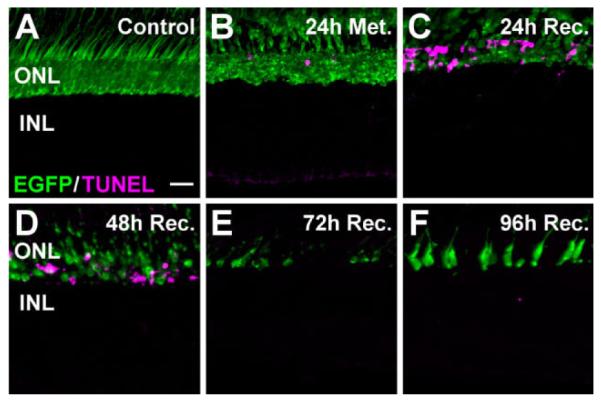
Photoreceptor apoptosis following metronidazole treatment of Tg(zop:nfsB-EGFP)nt19 fish. Cell death in the dorsal retina was assessed in metronidazole-treated Tg(zop:nfsB-EGFP)nt19 retinal cryosections using the TUNEL assay (magenta). A: TUNEL-positive nuclei were not observed in untreated control retinas. B: Immediately following metronidazole treatment (24h Met.), TUNEL-positive, EGFP-positive cells were detected in the ONL of the dorsal retina. C: The ONL TUNEL signal increased after 24 hours of recovery. D: At 48 hours of recovery, the number of TUNEL-positive, EGFP-positive dorsal cells decreased. E,F: By 72 hours of recovery, TUNEL-positive nuclei were largely absent (E) and newly regenerated EGFP-expressing immature rods were observed after 96 hours of recovery (F). ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar = 20 μm in A (applies to A–F).
To more closely examine the effect of metronidazole treatment on Tg(zop:nfsB-EGFP)nt19 cone photoreceptors, long single cones (Fig. 5A–D), short single cones (Fig. 5E–H), and double cones (Fig. 5I–L) were immunolabeled for cone opsin expression (Vihtelic et al., 1999). Control retinas exhibited fairly uniform cone opsin immunolabeling throughout the dorsal outer segments (Fig. 5A,E,I). Through 168 hours of recovery, the cone cell outer segments in the dorsal retina maintained a relatively uniform labeling pattern, suggesting that they were unaffected during the metronidazole treatment and recovery (Fig. 5B–D, F–H, J–L). The ventral retina exhibited a similar persistence of cone cell outer segments through 168 hours of recovery (data not shown). These data, taken together, show that metronidazole treatment of the Tg(zop:nfsB-EGFP)nt19 line caused loss of all rod photoreceptors, without affecting cone cell viability. These results are consistent with previous reports that metronidazole does not result in a bystander effect (Roberts et al., 1986; Edwards, 1993; Bridgewater et al., 1997; Pisharath, 2007).
Figure 5.
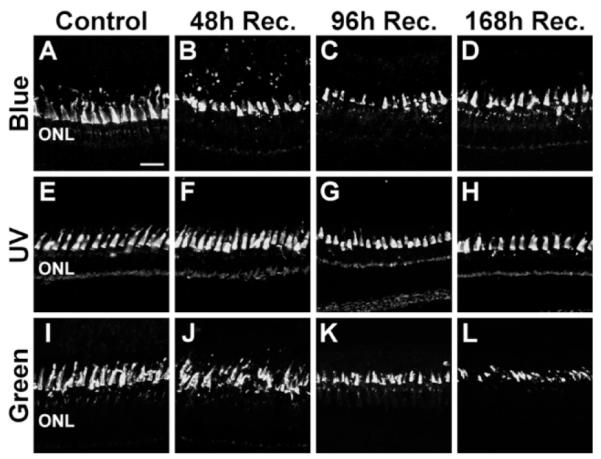
Preservation of cone cells following metronidazole treatment of Tg(zop:nfsB-EGFP)nt19 fish. Long single cones, short single cones, and double cones were immunolabeled in metronidazole- treated Tg(zop:nfsB-EGFP)nt19 retinas with antibodies to blue, UV, and green opsin, respectively. A,E,I: In control sections, the opsin antibodies evenly labeled the cone outer segments in the dorsal retina, apical to the ONL. B,F,J: After 48 hours of recovery, the opsin immunoreactivity revealed that the cone cells were retained, although they appeared to be slightly irregular in organization. C,D,G,H,K,L: A similar pattern of cone opsin immunoreactivity was observed at 96 (C,G,K) and 168 hours of recovery (D,H,L). The slight irregularities observed in the metronidazole-treated retina were also observed in various control retinal sections, and thus did not represent significant cone cell damage or death. ONL, outer nuclear layer. Scale bar = 20 μm in A (applies to A–L).
NTR/metronidazole-induced loss of all rods is sufficient to induce an INL proliferation response
Chronic loss of rod photoreceptors in the Tg(Xops: mCFP) transgenic line resulted in ONL rod precursor cell division, without Müller glial cell proliferation (Morris et al., 2008). In contrast, light-induced rod and cone cell death resulted in proliferation of both ONL rod precursors and INL Müller glia (Vihtelic and Hyde, 2000; Bernardos et al., 2007; Thummel et al., 2008a). When these data were taken together, it appeared that induction of Müller glial proliferation required the death of cone cells. This is consistent with the ONL rod precursors being committed to become rod photoreceptors. We used the metronidazole- treated Tg(zop:nfsB-EGFP)nt19 fish to test whether acute loss of rods would also induce rod precursor cell proliferation in the absence of increased Müller glial cell division. A low number of PCNA-positive rod precursor cells (Fig. 6B,I, arrowheads) was observed in the ONL of control retinas and after 24 hours of metronidazole treatment, whereas no PCNA-positive cells were detected in the INL at these time points (Fig. 6A,B,I,J).
Figure 6.
PCNA immunolocalization in metronidazole-damaged Tg(zop:nfsB-EGFP)nt19 fish. The regenerative response to rod photoreceptor ablation in the dorsal and ventral Tg(zop:nfsB-EGFP)nt19 retina was examined by immunolabeling retinal cryosections for PCNA expression (magenta) with TO-PRO-3 counterstaining the nuclei (blue). A,I, B,J: Occasional PCNA expression was observed in the ONL of control retinas (A,I) and after 24 hours of metronidazole treatment (B,J), which corresponded to the rod precursor cells (arrowheads). C,K: After 24 hours of recovery, PCNA was detected in a small number of individual INL cells in the dorsal (C) and ventral (K) retina (arrows) and in the ONL. D,L, E,M, F,N: The number of proliferating INL cells increased at 48 (D,L) and 72 hours of recovery (E,M), with clusters of PCNA-positive neuronal progenitors visible at 72 hours (F) and 96 (N) hours of recovery. G,O: At 168 hours of recovery, the majority of the PCNA-positive cells had migrated to the ONL. H,P: By 28 days of recovery, the majority of PCNA-positive cells resided in the ONL at levels slightly above the undamaged control retinas. Q,R: The number of proliferating INL cells (Q) and cell clusters (R) were quantified over a 150-μm region lying approximately 250 μm from the dorsal (blue) and ventral (yellow) retinal margins. A repeated measures ANOVA analysis revealed that the effect of treatment was dependent on the time point. Therefore, we performed two-sample t-tests on individual time points and Bonferroni-corrected (0.05/ 10 tests = 0.005 as the effective α) to account for the multiple tests. Those time points that revealed a statistically significant difference were marked with an asterisk. Error bars represent the standard error of the mean. ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar = 20 μm in A (applies to A–P).
After 24 hours of recovery, a small number of proliferating cells was observed in both the dorsal and ventral INL (Fig. 6C,K, respectively, arrows). Proliferating cells were found more frequently in the INL as they began to form small clusters after 48 hours of recovery (Fig. 6D,L). The number of proliferating cells in these clusters increased in both the dorsal and ventral INL after 72 hours of recovery (Fig. 6E,M, respectively). By 72 hours of recovery, the cells appeared to align in columns, reminiscent of the clusters of neuronal progenitors that migrated from the INL to the ONL in the light-damaged retina (Vihtelic and Hyde, 2000). After 96 hours of recovery, large clusters of PCNA-positive cells were found throughout the dorsal and ventral INL (Fig. 6F,N). The regeneration response in the INL declined by 168 hours of recovery, as fewer PCNA-positive INL cells were observed, apparently having migrated to the ONL (Fig. 6G,O). INL proliferation returned to near control levels by 28 days of recovery (Fig. 6H,P).
We quantified the number of PCNA-positive cells and cell clusters in a 150-μm region located approximately 250 μm from the dorsal and ventral margins of five or more retinas per time point. Individual PCNA-positive cells not bordering other proliferating cells were scored as one cluster, providing an approximation of how many Müller glia were activated. With a repeated measures ANOVA analysis, the mean total number of proliferating INL cells in the dorsal retina was found to be significantly higher than in the ventral retina across the time points (Fig. 6Q, P < 0.001). Similarly, the mean number of proliferating cell clusters, or columns, was greater in the dorsal INL relative to the ventral INL across the different time points (Fig. 6R, P < 0.05). These differences may result from the loss of a greater number of rods in the dorsal retina relative to the ventral retina, rather than the slightly slower rod cell loss in the dorsal retina relative to the ventral retina (Fig. 3). Regardless, the robust INL proliferation response following loss of only rods in the metronidazole-damaged retina revealed that generation of Müller glial-derived neuronal progenitor cells was not dependent on cone cell loss, but did occur under acute and massive rod cell death.
To verify that the Müller glia were the source of proliferation in the INL, metronidazole-treated Tg(zop:nfsB- EGFP)nt19 retinal cryosections were double-labeled for PCNA and glutamine synthetase, a marker of Müller glial cells. Consistent with our previous observations (Fig. 6), no INL proliferation was observed in the control retinas (Fig. 7A). After 48 hours of recovery, many PCNA-positive nuclei were detected in the INL (Fig. 7B). All of these PCNA-positive nuclei were associated with glutamine synthetaseexpressing Müller glial cells (Fig. 7B, arrows). Clusters of PCNA-positive neuronal progenitor cells continued to associate with Müller glia through 72 hours of recovery (Fig. 7C). After 96 hours of recovery, the hypertrophied Müller glia had enveloped the proliferating neuronal progenitor cell clusters, with several PCNA-positive cells appearing to have migrated toward the ONL (Fig. 7D, arrowheads). These results confirmed that rod photoreceptor cell death was sufficient to induce the Müller glia to reenter the cell cycle, which produced a population of neuronal progenitor cells that continued to proliferate as they appeared to migrate to the ONL.
Figure 7.

INL PCNA expression localized to Müller glia. PCNA immunolabeling (magenta) was examined relative to the Müller glialspecific marker glutamine synthetase (GS, green). A: Any PCNA expression in the control retinas resided in the ONL. B: By 48 hours of recovery, many individual PCNA-positive nuclei were observed in the INL, all of which were localized to Müller glial cell bodies (arrows). C: At 72 hours of recovery, the Müller glia hypertrophied, engulfing clusters of PCNA-positive neuronal progenitors. D: By 96 hours of recovery, the PCNA-positive cells appeared to have migrated to the ONL (arrowheads). ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar = 10 μm in A (applies to A–D).
Incomplete ablation of rod photoreceptors in Tg(zop:nfsB-EGFP)nt20 does not cause a significant INL proliferation response
In contrast to the Tg(zop:nfsB-EGFP)nt19 line, the Tg(zop:nfsB-EGFP)nt20 transgenic line consistently displayed incomplete penetrance of the transgene expression. The NTR-EGFP protein was observed in rod photo- receptors throughout both the dorsal and ventral retina (Fig. 8A). At higher magnification, however, many To-Pro-3-positive ONL nuclei clearly did not express EGFP (Fig. 10A, arrows), which demonstrated that only a subset of rod photoreceptors in the Tg(zop:nfsB-EGFP)nt20 retina contained NTR-EGFP. The distinct expression patterns of the Tg(zop:nfsB-EGFP)nt19 and Tg(zop:nfsB-EGFP)nt20 lines permitted comparison of two independent damage models; metronidazole-inducible death of all rods and only a subset of rods, respectively.
Figure 8.
Rod photoreceptor cell loss and regeneration following metronidazole treatment of Tg(zop:nfsB-EGFP)nt20 zebrafish. Tg(zop:nfsB- EGFP)nt20 zebrafish were treated with 10 mM metronidazole for 24 hours and transferred to a recovery tank. Eye specimens were collect from untreated controls, after 24 hours of metronidazole treatment, and after 24, 48, 72, 96, 168 hours, and 28 days of recovery. Death of NTR-EGFP expressing rod photoreceptors was assessed by loss of EGFP fluorescence. A: EGFP-positive cells were observed in the dorsal and ventral ONL of adult control retinas. B: After 24 hours of metronidazole treatment, there was a reduction and disorganization of the EGFP expression in the ONL. C,D–F: Cell loss was evident by 24 hours of recovery (C), with few EGFP-positive rods detected from 48 through 96 hours of recovery (D–F). G,H: Rods were beginning to regenerate by 96 and 168 hours of recovery (G) and the metronidazole-treated retinas were comparable to controls by 28 days of recovery (H). There was no significant difference in cell loss or regeneration between the dorsal and ventral regions. ONL, outer nuclear layer. Scale bar = 150 μm in A (applies to A–H).
Figure 10.
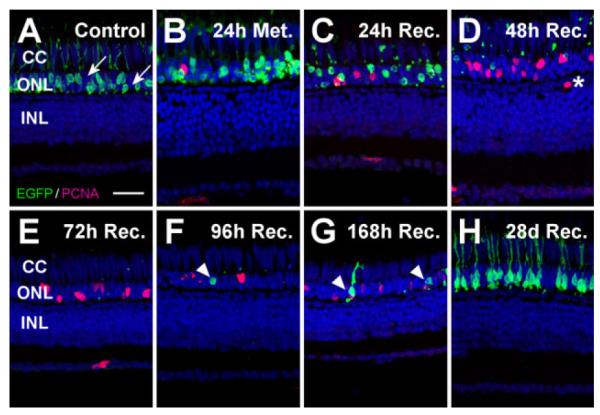
Proliferation response to ablation of a subset of rod photoreceptors. To examine regeneration following loss of a portion of rods in the dorsal metronidazole-treated Tg(zop:nfsB-EGFP)nt20 retina, proliferating cells were labeled with PCNA (magenta) and nuclei were stained with TO-PRO-3 (blue). A: Only a subset of the rods in the control retina expressed the nfsB-EGFP transgene, based on the many EGFP-negative rod cell nuclei in the ONL (arrows). B: EGFP- expressing rods became disorganized and ONL rod precursor proliferation was slightly increased after 24 hours of metronidazole treatment. C: By 24 hours of recovery, loss of EGFP-expressing rods and the presence of EGFP-positive pyknotic ONL nuclei confirmed the presence of rod cell death, while there was also an increase in the number of PCNA-positive nuclei in the ONL. D,E: Virtually all EGFP- expressing rods were absent by 48 (D) and 72 (E) hours of recovery, with even greater numbers of PCNA-expressing ONL nuclei, corresponding to committed rod precursor cells. A single PCNA-positive cell was observed in the INL at 48 hours of recovery, whose localization was not consistent with proliferating Müller glial cells (D, asterisk). F,G: Regenerated EGFP-positive rods appeared at 96 (F) and 168 (G) hours of recovery (arrowheads). H: After 28 days of recovery, EGFP expression in the ONL was similar to the control, indicating that new rod photoreceptors had fully regenerated. Notably, the ONL contained TO-PRO-3-labeled nuclei at every time point, confirming that rods lacking the transgene were not killed. Furthermore, cone cell nuclei, located apical to the ONL rod cell nuclei, were clearly discernable at all times in the cone cell layer. CC, cone cell layer; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar = 20 μm in A (applies to A–H).
After 24 hours of metronidazole treatment, the Tg(zop: nfsB-EGFP)nt20 rod outer segments appeared to condense, although only a few EGFP-positive rod cells were lost (Figs. 8B, 9B). From 24 to 72 hours of recovery, the metronidazole-treated retinas exhibited a large reduction in EGFP-expressing rods (Fig. 8C–E). However, rhodopsin expression in the ROS persisted due to the subset of rods that did not express the NTR-EGFP protein (Fig. 9B–D). Additionally, EGFP-negative rod cell nuclei were still present in the ONL after 48 and 72 hours of recovery (Fig. 10D,E), indicating that only a subset of rods was destroyed. Newly regenerated, EGFP-positive rods were beginning to develop by 72 hours of recovery (Fig. 9E, arrow), with several new, EGFP-positive rods apparent by 96 and 168 hours of recovery (Figs. 8F,G, 9F,G). A fully regenerated retinal EGFP expression pattern, equivalent to the control (Figs. 8A, 9A), was observed by 28 days of recovery (Figs. 8H, 9H).
Figure 9.

Metronidazole treatment results in the loss of only the subset of rods expressing the nfsB-EGFP transgene. Tg(zop:nfsB- EGFP)nt20 zebrafish were treated with 10 mM metronidazole for 24 hours and transferred to a recovery tank for 24, 48, 72, 96, 168 hours, and 28 days of recovery. Cell death was assessed by the loss of EGFP fluorescence, whereas rhodopsin immunolocalization was used to examine the non-transgenic rods. A: The control retina showed both EGFP-expressing rods in the ONL and rhodopsin expression in the ROS. B–D: The number of EGFP-expressing rods decreased from 24 hours of metronidazole treatment through 48 hours of recovery, whereas significant rhodopsin expression persisted in a thick layer of ROS due to the preservation of rods not expressing the nfsB-EGFP transgene. E: By 72 hours of recovery, newly regenerated EGFP-positive rods were observed (arrow). F–H: The number of regenerated EGFP-expressing rods increased through 96 (F) and 168 (G) hours of recovery until the retina, at 28 days of recovery (H), was indistinguishable from the control (A). ONL, outer nuclear layer; ROS, rod outer segments. Scale bar = 20 μm in A (applies to A–H).
TO-PRO-3-labeled cone cell nuclei were observed apical to the rod cell nuclei throughout the metronidazole treatment and recovery in both the ventral (data not shown) and dorsal retina (Fig. 10A–H), demonstrating that there was no significant loss of cones in this transgenic line. TUNEL analysis and cone opsin immunolabeling confirmed that dorsal and ventral cones were not significantly affected by the metronidazole treatment (data not shown).
To examine the regeneration response induced by loss of a subset of rod photoreceptors, we assessed PCNA expression in the dorsal region of metronidazole-treated Tg(zop:nfsB-EGFP)nt20 retinas. In control retinas, we observed no obvious proliferating cells present in the INL (Fig. 10A). Proliferation in the ONL, corresponding to rod precursor cells, increased after the metronidazole treatment and 24 hours of recovery (Fig. 10B,C, respectively). Proliferation of rod precursor cells remained elevated throughout the recovery as new, EGFP-positive rods were eventually regenerated (Fig. 10D–G, arrowheads). However, we observed only a single PCNA-positive cell in the INL at all of the time points studied (Fig. 10D, asterisk). This PCNA-positive cell was located outside the normal INL location for a Müller glial nucleus, which is consistent with the absence of a Müller glial proliferation response in the metronidazole-treated Tg(zop:nfsB-EGFP)nt20 retina. Similar observations were made in the metronidazole-treated ventral retina (data not shown). Thus, ablation of all rod photoreceptors was sufficient to elicit a robust Müller glial-derived regeneration response, whereas the loss of only a subset of rods yielded a rod precursor cell-derived regeneration response. This suggests that the magnitude of rod cell death dictates whether regeneration requires increased Müller glial cell proliferation or whether induction of resident rod precursor cell proliferation is sufficient.
DISCUSSION
Previous studies described two cell types that are capable of regenerating rod photoreceptors, the INL Müller glia and the ONL rod precursor cells (Otteson et al., 2001; Raymond et al., 2006). In the undamaged zebrafish retina, the Müller glial cells divide infrequently to generate neuronal progenitor cells that migrate to the ONL and ultimately differentiate into rod precursors. A variety of retinal damage models have been developed to destroy cells of all the retinal neuronal types (Yurco and Cameron, 2005; Fausett and Goldman, 2006; Sherpa et al., 2008), only inner retinal neurons (Fimbel et al., 2007), and specifically rod and cone photoreceptors (Vihtelic and Hyde, 2000; Raymond et al., 2006; Bernardos et al., 2007). In all of these retinal damage models, the Müller glia behave as adult stem cells, with large numbers entering the cell cycle to produce neuronal progenitors that regenerate the neuronal cell types that were lost. Recently, the Tg(Xops:mCFP) transgenic line was shown to chronically lose rods shortly after beginning to differentiate, which induced the rod precursor cells, but not the Müller glia, to continually proliferate (Morris et al., 2008). Similarly, light-induced death of a subset of rods in the ventral albino retina also yielded a rod precursor- derived regeneration response (Vihtelic et al., 2006).
These two studies suggested that loss of only rods was unable to induce a Müller glial-derived regeneration response. This seemed reasonable because the ONL rod precursor cells, which were already committed to differentiate into rod photoreceptors, only needed to continue proliferating to regenerate sufficient numbers of rods. In this model, loss of any other retinal neuronal types would require increased proliferation of the Müller glial stem cells to increase the number of pluripotent neuronal progenitors.
To examine whether different rod damage environments affected the source of the regeneration response, we developed two novel transgenic lines. The Tg(zop:nfsB- EGFP)nt19 line expressed NTR-EGFP in all rods (Fig. 1) and Tg(zop:nfsB-EGFP)nt20 expressed the transgene in only a subset of rods (Figs. 9, 10). This incomplete expression pattern was occasionally observed with the zop promoter (unpublished observations), likely due to the transgene’s insertional site. Treatment of either transgenic line with metronidazole killed all the NTR-EGFP-expressing rods. The metronidazole-treated Tg(zop:nfsB-EGFP)nt19 fish lost all of the ventral rods between 24 and 48 hours after treatment, whereas the dorsal rods were lost between 48 and 72 hours after treatment (Fig. 3). Because metronidazole was shown to have no bystander effect (Roberts et al., 1986; Edwards, 1993; Bridgewater et al., 1997), cone cells remained viable throughout the entire retina. Slight perturbations in the cone opsin immunolabeling pattern were observed in both the Tg(zop:nfsB-EGFP)nt19 line and the undamaged control retinas (Fig. 5). In contrast, the metronidazole-treated Tg(zop:nfsB-EGFP)nt20 line exhibited death in only a subset of the rod photoreceptors. This phenotype of incomplete rod cell death without damaging cone cells is similar to the light-damaged ventral retina, which exhibits damaged rod photoreceptors and no significant loss of cones (Vihtelic et al., 2006). Using these two transgenic lines allowed us to examine how differences in the extent of rod cell death could affect the regeneration response.
First, the metronidazole-treated Tg(zop:nfsB-EGFP)nt19 retina undergoes an acute loss of rods, whereas the Tg (Xops:mCFP) transgenic retina exhibits a chronic loss of rods. The rapid and sudden loss of rods could produce a signal for the Müller glia to enter into a regenerative response. Alternatively, the metronidazole-treated Tg(zop: nfsB-EGFP)nt19 retina loses fully differentiated rods, whereas the Tg(Xops:mCFP) transgenic retina loses rods that are undergoing differentiation. In this case, the fully differentiated rods could produce a different regenerative signal relative to differentiating rods. Finally, the metronidazole-treated Tg(zop:nfsB-EGFP)nt19 retina undergoes a rapid loss of a large number of rods, whereas the Tg(Xops:mCFP) transgenic retina experiences a persistent loss of a smaller number of rods. This would suggest that increasing the number of dying rods produces a different signal or a greater signal that is interpreted by the Müller glia.
In contrast to the Tg(zop:nfsB-EGFP)nt19 line, loss of only a subset of rods in the Tg(zop:nfsB-EGFP)nt20 line increased ONL rod precursor proliferation, with very limited numbers of proliferating INL cells. It is very likely that the metronidazole-dependent rod cell loss directly induced the increased number of PCNA-positive rod precursor cells in the ONL (Fig. 10). It is possible, however, that the metronidazole-dependent damage directly induced an increased number of proliferating Müller glia that, in turn, yielded the greater number of PCNA-positive rod precursor cells in the ONL. Because we did not observe an increased number of proliferating Müller glia, then all the proliferating Müller glia would have to express PCNA for less than the 24 hours between time points. This seems unlikely based on examination of 44 independent retinas and the persistent PCNA expression in the INL during the regeneration response observed in the Tg(zop:nfsB-EGFP)nt19 INL (Fig. 6) and every other zebrafish retinal regeneration model examined. The lack of INL proliferation in the metronidazole- treated Tg(zop:nfsB-EGFP)nt20 line also suggested that the Tg(zop:nfsB-EGFP)nt19 Müller glial proliferation response was not an artifact of prodrug treatment. We affirmed that metronidazole itself was not toxic to rods nor did it induce Müller glia or rod precursor cell proliferation, by exposing wild-type zebrafish to the prodrug for 24 hours. In these wild-type fish, we failed to observe either loss of rods or increased proliferation in the ONL or INL (data not shown).
The induction of rod precursor cell proliferation, in the absence of increased Müller glial cell division, in the Tg(zop:nfsB-EGFP)nt20 line suggests that the level of rod cell death is also a critical factor in determining whether the regeneration response originates with the Müller glia or the resident ONL rod precursor cells. It is possible that the resident ONL rod precursor cell population can regenerate a reduced level of rods, but is unable to regenerate all the retinal rods. However, we recently found that the Pax6b protein is required for proliferation of the INL neuronal progenitors and their migration to the ONL (Thummel and Hyde, unpublished results). If Pax6b expression is knocked down in the light-damaged retina, there is a significant increase in the number of proliferating ONL rod precursor cells that leads to a complete regeneration of rods, but not cones (Thummel and Hyde, unpublished results). This suggests that the signal for rod cell regeneration is more complex than a single molecule and that it can be modified if the intended origin of regeneration is disrupted.
Whereas previous reports of the NTR-metronidazole system focused on ablation of cells in larval and adult zebrafish (Curado et al., 2007; Pisharath et al., 2007; Pisharath, 2007; Moss et al., 2009), this report represents the first use of metronidazole-induced neuronal cell death to examine regeneration in the zebrafish. We also showed that simple immersion of adult fish into metronidazole is sufficient to induce NTR-dependent neuronal cell death. The relative ease with which transgenic lines can be generated in zebrafish makes the NTR system a powerful tool to inducibly ablate specific cell types in the central nervous system to study the potential for neuronal cell regeneration throughout the central nervous system. Because the NTR system is genetically driven, its sole limitation is the number of strong promoters available to reliably drive NTR expression in a cell-and/or tissue-specific manner. To circumvent this limitation, generation of enhancer trap lines that yield specific Gal4-VP16 expression patterns will permit NTR expression in a wider variety of cell types and tissues when crossed to UAS:nfsB-mCherry lines (Davison et al., 2007). Accordingly, the NTR/metronidazole system is readily adaptable to target numerous tissues and cell classes, which will further expand the usefulness of this system in adult zebrafish.
ACKNOWLEDGMENTS
We thank Deborah Bang and the staff at the Center for Zebrafish Research at the University of Notre Dame for animal care and maintenance, Suzyanne Guzicki for microinjection of the zop:nfsB-EGFP construct, and Dr. Ryan Thummel for his contributions and suggestions regarding this manuscript. Additional thanks to past and current members of the Hyde Lab for their input throughout the project.
Grant sponsor: the National Institutes of Health (NIH); Grant number: R21-EY017134 (to D.R.H.); Grant sponsor: the Center for Zebrafish Research at the University of Notre Dame.
LITERATURE CITED
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater JA, Knox RJ, Pitts JD, Collins MK, Springer CJ. The bystander effect of the nitroreductase/CB1954 enzyme/ prodrug system is due to a cell-permeable metabolite. Hum Gene Ther. 1997;8:709–717. doi: 10.1089/hum.1997.8.6-709. [DOI] [PubMed] [Google Scholar]
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DI. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3:43–48. doi: 10.1093/jac/3.1.43. [DOI] [PubMed] [Google Scholar]
- Edwards DI. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin- expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. The teleost retina as a model for developmental and regeneration biology. Zebrafish. 2004;1:257–271. doi: 10.1089/zeb.2004.1.257. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, Burket C, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Koga A, Hori H, Shima A. Excision of the Tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene. 1998;225:17–22. doi: 10.1016/s0378-1119(98)00537-x. [DOI] [PubMed] [Google Scholar]
- Kennedy BN, Vihtelic TS, Checkley L, Vaughan KT, Hyde DR. Isolation of a zebrafish rod opsin promoter to generate a transgenic zebrafish line expressing enhanced green fluorescent protein in rod photoreceptors. J Biol Chem. 2001;276:14037–14043. doi: 10.1074/jbc.M010490200. [DOI] [PubMed] [Google Scholar]
- Lindmark DG, Müller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976;10:476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Scholz TL, Brockerhoff SE, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev Neurobiol. 2008;68:605–619. doi: 10.1002/dneu.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes. 2009;58:1844–1851. doi: 10.2337/db08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Pisharath H. Validation of nitroreductase, a prodrug- activating enzyme, mediated cell death in embryonic zebrafish (Danio rerio) Comp Med. 2007;57:241–246. [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JJ, Friedlos F, Knox RJ. CB 1954 (2,4-dinitro-5- aziridinyl benzamide) becomes a DNA interstrand crosslinking agent in Walker tumour cells. Biochem Biophys Res Commun. 1986;140:1073–1078. doi: 10.1016/0006-291x(86)90744-8. [DOI] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Li L, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL. Neurogenesis in the fish retina. Int Rev Cytol. 2007;259:173–224. doi: 10.1016/S0074-7696(06)59005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Burket CT, Brewer JL, Sarras MP, Jr, Li L, Perry M, McDermott JP, Sauer B, Hyde DR, Godwin AR. Cremediated site-specific recombination in zebrafish embryos. Dev Dyn. 2005;233:1366–1377. doi: 10.1002/dvdy.20475. [DOI] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008a;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008b;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82:558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96:121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) 4th ed University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]