Abstract
Recently, several high-impact reviews suggest that regular aerobic exercise is beneficial for maintaining cognitive function in aging adults. Higher cerebral blood flow and/or cerebrovascular reactivity may explain the favorable effect of exercise on cognition. In addition, prostaglandin-mediated vasodilator responses may be influenced by regular exercise. Therefore, our purpose was to evaluate middle cerebral artery (MCA) vasodilator responses in healthy adults before and after cyclooxygenase inhibition. A total of 16 young (26 ± 6 yr; 8 males, 8 females) and 13 older (64 ± 6 yr; 7 males, 6 females) healthy adults participated in the study. Aerobic fitness was determined by maximal aerobic capacity (V̇o2max) on a cycle ergometer. MCA velocity (MCAv) was measured at baseline and during stepped hypercapnia (2%, 4%, and 6% FiCO2) before and after cyclooxygenase inhibition using indomethacin. To account for differences in blood pressure, cerebrovascular conductance index (CVCi) was calculated as MCAv/mean arterial pressure. Cerebrovascular reactivity slopes were calculated from the correlation between either MCAv or CVCi and end-tidal CO2. Young adults demonstrated greater MCAv reactivity (1.61 ± 0.17 vs. 1.06 ± 0.15 cm·s−1·mmHg−1; P < 0.05) and CVCi reactivity (0.015 ± 0.002 vs. 0.007 ± 0.002 cm·s−1·mmHg−1; P < 0.05) compared with the older adults. There was no association between cerebrovascular reactivity and V̇o2max in the combined group of subjects; however, in older adults MCAv reactivity was correlated with maximal aerobic fitness (r = 0.64; P < 0.05). Furthermore, the change in MCAv reactivity (between baseline and indomethacin trials) was also associated with V̇o2max (r = 0.59; P < 0.05) in older adults. Cerebral vasodilator responses to hypercapnia were associated with maximal aerobic capacity in healthy older adults. These results may explain the physiological link between regular aerobic exercise and improved cognitive function in aging adults.
Keywords: cerebral blood flow, aging, aerobic fitness, prostaglandins
reductions in cerebral blood flow and cerebral vasodilator responses in aging may precede the onset of cognitive impairment in humans. Importantly, cerebral vessel responsiveness to vasodilator and vasoconstrictor stimuli is associated with known Alzheimer's disease pathologies such as white matter hyperintensities (15), and β-amyloid deposition (20). Therefore, understanding the mechanisms underlying changes in cerebral blood flow is essential for determining effective interventions to maintain or prevent the impairment in cerebral blood flow regulation.
Regular exercise and high levels of physical activity are associated with reduced rates of mild cognitive impairment (MCI) and Alzheimer's disease (12). Furthermore, in patients with MCI, standard walking training has been shown to improve cognitive function (3, 16). In addition, men who have higher aerobic capacity demonstrate slower age-related reductions in cerebral blood flow compared with untrained men (1). Regular physical activity is also associated with substantially reduced risk of vascular disease, which can be attributed to improved or maintained microvascular function (10, 11, 13). The cerebral microvasculature vasodilates in response to hypercapnia, and endothelial-derived relaxing factors likely play a key role in regulating cerebral microvascular function. The use of cyclooxygenase (COX) inhibitors in our laboratory, as well as others, has clearly shown that vasodilating prostaglandins participate in cerebral vasodilatory responses to hypercapnia (4, 22). However, whether the contribution of prostaglandins to the cerebrovascular response to hypercapnia is influenced by aerobic fitness is unknown. Therefore, our purpose was to evaluate cerebral vasodilator responses in young and older adults to explore a potential association of these responses with aerobic fitness. A secondary aim was to evaluate the association between the change in cerebral vasodilator responses after COX inhibition.
MATERIALS AND METHODS
Subjects.
Twenty-nine volunteers, including 16 young (aged 18–34 yr) and 13 older (aged 55–77 yr) healthy adults, participated in the study. Subjects were nonsmoking, nonobese (body mass index <30 kg/m2), normotensive, and did not have any underlying cardiovascular, metabolic, or other chronic pathologies (as determined by a health questionnaire and a brief clinical assessment). Blood samples were obtained after a 12-h fast. Prescription and over-the-counter medications were reviewed by a physician. Subjects were not taking antihypertensive medication or any other vasoactive medications other than statins (2 older male subjects). In addition, the use of any vitamins, antioxidant supplements, and nonsteroidal anti-inflammatory drugs (NSAIDs) were restricted for 10 days prior to the screening blood draw and experimental study day. All subjects were inactive or recreationally active (no structured training in the past 3 mo). Older female subjects were postmenopausal and were not on hormone replacement therapy. Young female subjects were studied in the early follicular phase of the menstrual cycle or the low-hormone phase of oral contraceptives. Informed written and verbal consent was obtained and subjects were familiarized with experimental conditions during an initial screening visit. All procedures had ethical approval from the Institutional Review Board of the Mayo Clinic and were performed according to the Declaration of Helsinki, including written informed consent.
Maximal aerobic capacity/V̇o2max test.
All healthy young and older subjects completed a maximal aerobic capacity test (V̇o2max test) using cycle ergometry (Lode B.V., Groningen, the Netherlands). V̇o2 was measured using a calibrated pneumotach to measure ventilation and mass-spectrometer determinations of gas concentrations (Ultima Series; Medgraphics, St. Paul, MN). Exercise workload was increased by 20–30 watts every minute until exhaustion. The criteria for an acceptable test were as follows: failure to increase V̇o2 with an increasing workload, a respiratory exchange ratio greater than 1.10, and a maximal heart rate value within 10 beats of age-predicted maximum. All subjects met the criteria for an acceptable V̇o2max test.
Experimental protocol.
The cerebral blood flow velocity experiments were conducted at least 48 h after V̇o2max testing. Subjects arrived in the morning to the Clinical Research Unit after an overnight fast. Arterial blood pressure was monitored noninvasively using finger photoplethysmography (Finometer; TPD Biomedical Instrumentation, Amsterdam, the Netherlands). Heart rate using a standard three-lead ECG and oxygen (O2) saturation using pulse oximetry were monitored continuously throughout the study (Cardiocap/5; Datex-Ohmeda, Louisville, CO). During the stepped hypercapnia trials, breath-by-breath end-tidal CO2 (ETCO2; Cardiocap/5, Datex-Ohmeda) was recorded.
Cerebral blood flow velocity.
Subjects were imaged using a 2-MHz Doppler probe (Transcranial Doppler, Neurovision System; Multigon, Yonkers, NY) to estimate right middle cerebral artery (MCA) blood flow velocity and to determine the optimal placement and settings for continuous monitoring. The probe was secured with a headband device to maintain the proper position and angle throughout the protocol (4).
Stepped hypercapnia trials.
The hypercapnic responses were assessed using a steady-state, open-circuit technique (4a). Subjects were in a semi-reclined position on a hospital bed with a mask covering their nose and mouth; the mask was attached to a one-way Hans Rudolph valve to prevent rebreathing. In each hypercapnia trial, after breathing room air, three stepwise ETCO2 elevations were applied to each subject by adding 2%, 4%, and 6% fractional concentrations of inspired CO2 (FiCO2) for 3 min each, while the oxygen content was maintained at 21% and balanced by nitrogen as described previously (4, 22). Cerebrovascular reactivity was calculated from the slope of the relationship between MCA velocity (MCAv) and ETCO2. In our laboratory, the coefficient of variation in calculated cerebrovascular reactivity between trials was 15 ± 4% (4). Cerebrovascular conductance index (CVCi) reactivity was calculated from the slope of the relationship between CVCi [MCAv/mean arterial pressure (MAP)] and ETCO2.
Drug administration.
Indomethacin, a COX inhibitor, was given orally at 1.2 mg/kg along with 10 ml simethicone (Maalox) to reduce possible stomach irritation. One hypercapnia trial was completed after an initial 3-min baseline MCAv assessment, and the second hypercapnia trial was completed 90 min after drug administration (22).
Data analysis and statistics.
Data were collected at 250 Hz and analyzed off-line using signal processing software (Windaq; DATAQ Instruments, Akron, OH). Beat-by-beat hemodynamic measurements were averaged over the final minute of room air breathing and at each level of hypercapnia. The linear slopes of the relationships between ETCO2 and MCAv or cerebrovascular conductance index (CVCi = MCAv/MAP) were calculated to estimate cerebrovascular reactivity using linear regression. Subject demographics and baseline characteristics were compared using a one-way ANOVA. Primary variables of interest were compared between groups (young vs. old) and condition (control vs. indomethacin) using a two-way repeated-measures ANOVA followed by Tukey's post hoc analysis. Pearson product moment correlations were used to determine the association between V̇o2max and measures of cerebrovascular reactivity. Significance was set a priori at P < 0.05.
RESULTS
As shown in Table 1, older subjects had higher fasting blood glucose levels compared with younger subjects (P < 0.05); however, these values were still within the normal range. As expected, V̇o2max values were greater in young subjects compared with older subjects. Also, a subset of this subject group had participated in a previously published study (4).
Table 1.
Subject Characteristics
| Young | Older | |
|---|---|---|
| n, M/F | 8M/8F | 7M/6F |
| Age, yr | 26 ± 1 | 64 ± 2* |
| Height, cm | 175 ± 3 | 171 ± 2 |
| Weight, kg | 72 ± 3 | 76 ± 4 |
| BMI, kg/m2 | 23.3 ± 0.6 | 25.8 ± 0.8 |
| MAP, mmHg | 83 ± 3 | 84 ± 4 |
| Hemoglobin, g/dl | 14.1 ± 0.4 | 13.8 ± 0.4 |
| Glucose, mg/dl | 80 ± 2 | 93 ± 2* |
| Cholesterol, mg/dl | 172 ± 9 | 185 ± 8 |
| HDL cholesterol, mg/dl | 56 ± 2 | 57 ± 4 |
| LDL cholesterol, mg/dl | 98 ± 8 | 111 ± 7 |
| Triglycerides, mg/dl | 90 ± 11 | 86 ± 8 |
| V̇o2max, ml · kg−1 · min−1 | 38 ± 3 | 27 ± 2* |
| MCAv, cm/s | 55 ± 5 | 39 ± 4* |
| MCAv reactivity, cm · s−1 · mmHg−1 | 1.61 ± 0.17 | 1.06 ± 0.15* |
| CVCi, cm · s−1 · mmHg−1 | 0.68 ± 0.06 | 0.47 ± 0.06* |
| CVCi reactivity, cm · s−1 · mmHg−1 | 0.015 ± 0.002 | 0.007 ± 0.002* |
Values are means ± SE. M, men; F, women; BMI, body mass index; MAP, mean arterial pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; V̇o2max, maximal oxygen consumption; MCAv, middle cerebral artery velocity; CVCi, cerebrovascular conductance index.
P < 0.05 vs. young.
MCAv and CVCi during baseline (breathing room air) were lower in healthy older adults compared with young adults (Table 1). Similarly, MCAv and CVCi reactivity to hypercapnia (cerebrovascular reactivity) were both significantly lower in older adults (Table 1). After COX inhibition with indomethacin, both the young and older adults demonstrated reduced MCAv (55 ± 5 vs. 35 ± 3 cm/s in young; 39 ± 4 vs. 28 ± 3 cm/s in older; P < 0.05), CVCi (0.68 ± 0.06 vs. 0.42 ± 0.03 cm·s−1·mmHg−1 in young; 0.47 ± 0.06 vs. 0.33 ± 0.04 cm·s−1·mmHg−1 in older; P < 0.05), MCAv reactivity (1.61 ± 0.17 vs. 0.54 ± 0.09 cm·s−1·mmHg−1 in young; 1.06 ± 0.15 vs. 0.39 ± 0.09 cm·s−1·mmHg−1 in older; P < 0.05), and CVCi reactivity (0.015 ± 0.002 vs. 0.004 ± 0.001 cm·s−1·mmHg−1 in young; 0.007 ± 0.002 vs. 0.002 ± 0.001 cm·s−1·mmHg−1 in older; P < 0.05).
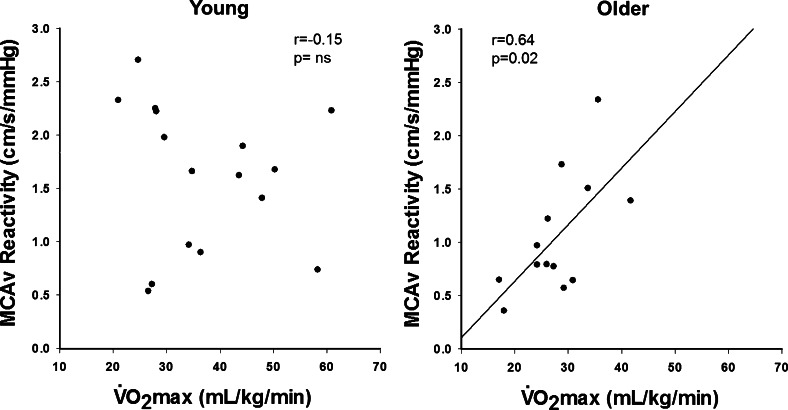
There was no association between maximal aerobic capacity and baseline MCAv or CVCi values at rest in young or older adults (although the correlation with MCAv in older adults was approaching significance; P = 0.09). In addition, there were no associations between maximal aerobic capacity and MCAv reactivity to hypercapnia in young adults; however, there was a significant positive correlation between maximal aerobic capacity and MCAv reactivity in older adults (Fig. 1). The association between aerobic capacity and CVCi reactivity in older adults only approached significance (r = 0.32, P = 0.09).
Fig. 1.
Maximal aerobic capacity (V̇o2max) is positively associated with cerebrovascular reactivity [middle cerebral artery velocity (MCAv) reactivity to hypercapnia] in older adults (right) but not young adults (left).
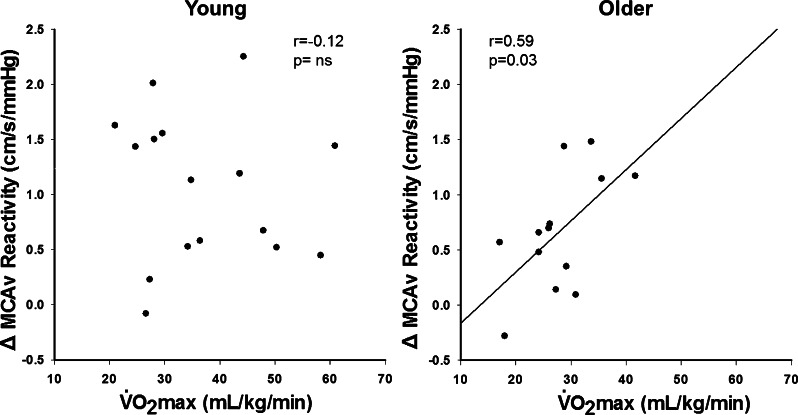
To look at the potential association between the role of prostaglandins in cerebrovascular reactivity and aerobic fitness, we examined the change in reactivity between the control and indomethacin conditions. In the combined group, ΔMCAv reactivity and ΔCVCi reactivity were not correlated with V̇o2max. However, in older adults, V̇o2max was positively associated with ΔMCAv reactivity (r = 0.59; P < 0.05; Fig. 2) but not ΔCVCi reactivity (r = 0.54; P = 0.06). Thus the older individuals with higher fitness demonstrated a larger reduction in cerebrovascular reactivity after blunting prostaglandin production with indomethacin.
Fig. 2.
Maximal aerobic capacity (V̇o2max) is positively associated with the change in cerebrovascular reactivity between the control and indomethacin conditions (ΔMCAv reactivity) in older adults (right) but not young adults (left). We examined the potential role of prostaglandins to determine whether individuals with higher aerobic fitness also had the largest decrease in cerebrovascular reactivity after cyclooxygenase inhibition.
DISCUSSION
We found that cerebrovascular reactivity to hypercapnia is correlated with maximal aerobic capacity in older healthy adults, but not in young adults. However, our most salient finding from the present study is that the magnitude of change in cerebrovascular reactivity after COX inhibition was positively correlated with maximal aerobic capacity in older, but not young, adults. Collectively, these results suggest higher levels of physical activity in older adults are associated with a beneficial effect on cerebral vasodilator responses, highlighting the importance of being physically active and maintaining aerobic fitness throughout the lifespan. Furthermore, these results may partially explain how regular aerobic exercise is physiologically linked to improved cognitive function.
Our finding that a greater magnitude of change in cerebral vasodilator responses to hypercapnia is associated with higher aerobic fitness in older adults is especially novel in that it provides a potential mechanism for the apparent preservation of cerebrovascular reactivity in older, more physically fit adults. Prostaglandin synthesis and availability may be upregulated or the balance of prostaglandins may favor vasodilation in more physically fit adults. Prostacyclin interacts with adenylate cyclase enzyme and is therefore important in regulating vascular tone and blood flow. Previous studies have reported that endurance training increases prostacyclin release during exercise in healthy young men (23) and lowers thromboxane in adults with hypertension (14). Additionally, prostacyclin release during exercise is associated with aerobic fitness in young men (23, 24). It should be noted that the subjects in our study were recreationally active and not engaged in any structured endurance training program. We might expect more robust associations if endurance trained older adults were included.
Along these lines, similar studies have reported that cerebral blood flow characteristics are associated with aerobic fitness. Ainslie, et al. (1) demonstrated that cerebral blood flow velocity was higher in endurance trained men and that, at any age, endurance trained men had ∼9 cm/s greater MCA velocity compared with untrained men. Similarly, in postmenopausal women, CVCi was higher in those who were more physically active, and it was associated with maximal aerobic capacity (5). In the present study, we did not see a correlation between MCAv or CVCi values at rest and aerobic capacity, although the relationship in older adults was approaching significance (P = 0.09).
The positive association between cerebral vasodilator responses to hypercapnia and aerobic fitness in older adults in this study suggests that regular exercise may affect or even improve the ability of cerebral microvessels to respond to vasodilatory stimuli. In young adults, however, cerebrovascular reactivity was not associated with aerobic fitness. It is possible that this is due to our small subject number and the fact that we did not include individuals who were endurance trained. However, similar peripheral vasodilator responses between endurance-trained and untrained young men have been shown in the forearm (8). In addition, emerging evidence suggests that short-term exercise training elevates cerebrovascular reactivity in both young and older adults, although the mechanism for improvement is unclear (17). Taken together, this suggests that young adults may already have optimal (or near optimal) vasodilator function, and therefore lifestyle-related habits have less of an influence on vasodilator responses.
In addition to vasodilating prostaglandins, other mechanisms related to the association between cerebral vasodilator responses and aerobic fitness may be involved. For example, after a year of moderate aerobic training in humans, the change in V̇o2max was positively associated with changes in hippocampal volume in older adults (9). Interestingly, the increase in hippocampal volume was associated with higher levels of brain-derived neurotrophic factor (BDNF) (9). BDNF is necessary for neuronal growth and supports existing neurons, and these neuronal effects are regulated by nitric oxide (6). After endurance training, Seifert, et al. (18) reported that training enhances the release of BDNF from the brain. When BDNF is blocked in rats, the exercise-mediated increases in learning and memory were abolished, indicating BDNF may be necessary for these exercise benefits (21). However, it is unclear how these pathways interact with prostanoid mechanisms in contributing to exercise- or fitness-related changes in the cerebral circulation.
Our findings have particular relevance to recent studies demonstrating a beneficial effect of regular exercise and high levels of aerobic fitness on cognitive function in older adults (2, 16). We speculate that enhanced cerebral blood flow at rest and cerebral vasodilator function with regular exercise may explain some of the cognitive benefits. In this context, Brown, et al. (5) reported a positive association between cerebrovascular conductance and aerobic fitness in older women and also found that cerebrovascular conductance was a predictor of cognitive function. A recent review suggested cerebral blood flow responsiveness to stimuli is a key link between aerobic fitness and cognition, promoting the usefulness of vascular reactivity measurements to assess cerebrovascular function (7); however, this has not yet been systematically investigated.
There are a number of methodological limitations in this study that warrant discussion. First, this study investigated potential correlations between cerebral hemodynamics and fitness before and after a drug blockade. Therefore, cause and effect cannot be determined. Second, we had a small number of nonexercise trained subjects of both sexes and varying fitness levels. We expect that the inclusion of endurance-trained older adults would strengthen the associations reported; however, including trained older adults would introduce selection bias because these individuals typically have better overall health/lifestyles and limit the generalizability of our study. Furthermore, the information on healthy, minimally active, and recreationally active older adults in our study is especially relevant to those who may be at risk for cognitive decline. The adults in our study were not competitive athletes but were likely those that strive to meet the recommended amount of physical activity. Finally, the use of transcranial Doppler does not provide direct measurements of cerebral blood flow, and we used MCAv to quantify changes in cerebral hemodynamics. However, this limitation is well known and MCAv is considered a reliable indicator of cerebral blood flow at rest and in response to hypercapnia (19). There is a substantial amount of heterogeneity within the cerebral circulation, and while the MCAv is considered an indicator of global flow, it is unclear if the associations reported are regionally specific.
In conclusion, cerebral vasodilator responses to hypercapnia were associated with maximal aerobic capacity in older healthy adults. In addition, the magnitude of change in cerebrovascular reactivity (after COX inhibition) was positively correlated with maximal aerobic capacity again in older adults. Taken together, higher levels of physical fitness are associated with a beneficial effect on cerebral vasodilator responses, which may be mediated by prostaglandin regulation. Importantly, because adequate cerebral blood flow is critical for brain metabolism, impaired cerebrovascular regulation will likely affect cognitive abilities. In this context, our results may partially explain the physiological link between exercise and improved cognitive function and promote the use of behavioral interventions to diminish the rates of cognitive decline in aging adults.
GRANTS
This work was supported by National Institutes of Health Grants RR-024150 (Center for Translational Science Activities); AR-056950 (J. N. Barnes); AG-38067 (J. N. Barnes); AG-16574–11PP2 (M. J. Joyner); and HL-46493 (M. J. Joyner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.N.B., C.P.J., and M.J.J. conception and design of research; J.N.B., J.L.T., and C.P.J. performed experiments; J.N.B., J.L.T., and B.N.K. analyzed data; J.N.B., J.L.T., and M.J.J. interpreted results of experiments; J.N.B. and B.N.K. prepared figures; J.N.B., J.L.T., and B.N.K. drafted manuscript; J.N.B., J.L.T., and M.J.J. edited and revised manuscript; J.N.B., C.P.J., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Pamela Engrav, Casey Hines, and Wayne Nicholson for technical assistance and expertise throughout the study.
REFERENCES
- 1. Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev CD005381, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 67: 71–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol 112: 1884–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a. Berkenbosch A, Bovill JG, Dahan A, DeGoede J, Olievier IC. The ventilatory CO2 sensitivities from Read's rebreathing method and the steady-state method are not equal in man. J Physiol 411: 367–377, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 31: 2047–2057, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258: 319–333, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev 40: 153–158, 2012 [DOI] [PubMed] [Google Scholar]
- 8. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108: 3017–3022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Galetta F, Franzoni F, Plantinga Y, Ghiadoni L, Rossi M, Prattichizzo F, Carpi A, Taddei S, Santoro G. Ambulatory blood pressure monitoring and endothelium-dependent vasodilation in the elderly athletes. Biomed Pharmacother 60: 443–447, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 67: 80–86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105: 766–768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension 58: 943–949, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke 40: 1229–1236, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 300: 1027–1037, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-wk exercise training. Age (Dordr). In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 298: R372–R377, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke 40: 2601–2606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20: 2580–2590, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zoladz JA, Majerczak J, Duda K, Chlopicki S. Endurance training increases exercise-induced prostacyclin release in young, healthy men—relationship with VO2max. Pharmacol Rep 62: 494–502, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Zoladz JA, Majerczak J, Duda K, Chlopicki S. Exercise-induced prostacyclin release positively correlates with VO(2max) in young healthy men. Physiol Res 58: 229–238, 2009 [DOI] [PubMed] [Google Scholar]