Abstract
Reduced respiratory neural activity elicits a rebound increase in phrenic and hypoglossal motor output known as inactivity-induced phrenic and hypoglossal motor facilitation (iPMF and iHMF, respectively). We hypothesized that, similar to other forms of respiratory plasticity, iPMF and iHMF are pattern sensitive. Central respiratory neural activity was reversibly reduced in ventilated rats by hyperventilating below the CO2 apneic threshold to create brief intermittent neural apneas (5, ∼1.5 min each, separated by 5 min), a single brief massed neural apnea (7.5 min), or a single prolonged neural apnea (30 min). Upon restoration of respiratory neural activity, long-lasting (>60 min) iPMF was apparent following brief intermittent and prolonged, but not brief massed, neural apnea. Further, brief intermittent and prolonged neural apnea elicited an increase in the maximum phrenic response to high CO2, suggesting that iPMF is associated with an increase in phrenic dynamic range. By contrast, only prolonged neural apnea elicited iHMF, which was transient in duration (<15 min). Intermittent, massed, and prolonged neural apnea all elicited a modest transient facilitation of respiratory frequency. These results indicate that iPMF, but not iHMF, is pattern sensitive, and that the response to respiratory neural inactivity is motor pool specific.
Keywords: apnea, iPMF, phrenic, plasticity, respiratory control
the neural network controlling breathing expresses remarkable plasticity in response to changing environmental or physiological conditions (7, 13, 19, 36, 39, 44, 53). We recently demonstrated that reduced respiratory neural activity (without hypoxia or hypercanpia) elicits rebound plasticity in respiratory activity (7, 31, 52), a feature that is not necessarily surprising since this critical, homeostatic control system must be continuously active to maintain life. Briefly, a prolonged (30 min) central neural apnea in ventilated rats elicits a rebound increase in inspiratory motor output, known as inactivity-induced phrenic (iPMF) (31) and hypoglossal (iHMF) motor facilitation (7). Since iPMF is induced by multiple forms of central apnea, including hyperventilation, increased inhibitory sensory feedback, and anesthesia-induced respiratory depression (31), we hypothesized that iPMF (and by extension, iHMF) arises from a common factor: reduced respiratory neural activity (7). Although initiated by similar stimuli, inactivity-induced facilitation is differentially expressed in the phrenic vs. hypoglossal motor pools after prolonged neural apnea. Specifically, iPMF is long lasting (>60 min) (31), whereas iHMF is more modest and transient (∼15 min) (7).
Many forms of respiratory plasticity are sensitive to stimulus pattern (2, 3, 29, 53). For example, intermittent hypoxia (IH) elicits a serotonin-dependent increase in phrenic and hypoglossal inspiratory motor output known as phrenic and hypoglossal long-term facilitation (pLTF and hLTF, respectively) (1, 23). By contrast, a similar cumulative duration of sustained hypoxia does not elicit pLTF (3, 16) or hLTF (7). Similarly, intermittent, but not sustained, disruptions in vagal feedback elicit long-lasting increases in genioglossus activity (53). Mechanisms giving rise to pattern sensitivity in respiratory plasticity are unknown, but may involve differential activation patterns of protein kinases/phosphatases during intermittent vs. sustained stimuli (61).
Here, we tested the hypothesis that, similar to other forms of respiratory plasticity, iPMF and iHMF are pattern sensitive. Although we know that prolonged neural apnea (∼30 min) elicits iPMF and iHMF (7, 31, 52), we now report that intermittent exposure to five brief (1.5 min) neural apneas (separated by ∼5 min) elicits iPMF, whereas sustained exposure to a single “massed” apnea of a similar cumulative duration (7.5 min) does not. By contrast, iHMF is not elicited by brief neural apnea when presented in either intermittent or massed patterns. Thus, with short neural apneas, iPMF exhibits pattern sensitivity, whereas iHMF does not. Further, we demonstrate that patterns of neural apnea that induce long-lasting iPMF also increase phrenic responses to severe hypercapnia. Last, brief massed, brief intermittent, and prolonged neural apnea all elicit a modest facilitation of respiratory frequency, whose magnitude appears to be sensitive to apnea duration. Collectively, these data demonstrate that the response to reduced respiratory neural activity is motor pool specific, and expand our understanding of inactivity-induced respiratory motor plasticity.
METHODS
Animals.
Experiments were performed on 2.5- to 5-mo-old male Sprague-Dawley rats obtained from Harlan Laboratories (n = 33; colony 217; Indianapolis, IN). All experimental protocols were approved by the Animal Care and Use Committee at the University of Wisconsin, Madison.
Experimental preparation.
Isoflurane anesthesia was induced in a closed container. Rats were then transferred to a nose cone with 2.5–3.5% isoflurane (50% O2-N2 balance). A tail vein catheter (Surflo iv catheter and injection plug) was placed for delivery of intravenous fluids (lactated Ringer solution; ∼20% sodium bicarbonate, as necessary) to maintain blood pressure and blood pH homeostasis throughout surgery and experimental protocols. Body temperature was monitored with a rectal thermometer (Physitemp, model 700 1H) and maintained near 37.0°C with a custom heated surgery table. The trachea was isolated and cannulated and mechanical ventilation was begun (∼70 breaths/min, tidal volume = 2.5–3.5 ml; Harvard Apparatus, model 683; 50% O2-N2 balance). End-tidal CO2 (ETCO2) was measured continuously from the expired limb of the ventilator circuit as an index of arterial pCO2 with a flow-through capnograph (Capnogard, Respironics). In preliminary studies, we found that, under our anesthesia protocol, spontaneously breathing rats typically have an ETCO2 of ∼45 mmHg; thus ETCO2 was maintained near 45 mmHg throughout the surgery by adding CO2 to the inspired gas mixture to prevent suppression of respiratory neural activity during mechanical ventilation. Tracheal pressure was monitored, and inspired CO2 and/or ventilator frequency was adjusted to ensure the animal continued to generate inspiratory efforts. The vagus nerve was cut bilaterally at the cervical level to prevent entrainment of respiratory neural activity with the ventilator. The right femoral artery was isolated and catheterized to measure arterial blood pressure and draw blood samples (∼0.3 ml) for pH and blood gas analysis (ABL800; Radiometer, Copenhagen, Denmark). Urethane anesthesia was then begun (1 ml/100 g of 0.175 g/ml infused at 6 ml/h iv), and isoflurane was slowly withdrawn. Adequate depth of anesthesia was confirmed by testing pressor responses to toe-pinch. The left hypoglossal (XII) and phrenic nerves were dissected via a dorsal approach, cut distally, de-sheathed, placed on bipolar silver electrodes, and submerged in mineral oil. After conversion to urethane anesthesia, pancuronium bromide was infused (2.5 mg/kg iv) to induce neuromuscular paralysis.
Experimental protocols.
One hour after conversion to urethane anesthesia, phrenic and hypoglossal nerve burst amplitude and frequency were monitored under isocapnic conditions until activity remained stable for at least 15–20 min (baseline). At the end of baseline, an arterial blood sample was drawn to determine baseline partial pressure of arterial CO2 (PaCO2), partial pressure of arterial O2 (PaO2), pH, and standard base excess (SBE; temperature corrected) measurements. Rats were then exposed to one of three patterns of reduced respiratory neural activity: five brief intermittent neural apneas (∼1.5 min each, separated by 5 min of baseline conditions; n = 9), a single brief massed neural apnea of a similar cumulative duration (7.5 min; n = 9), or a single prolonged neural apnea (30 min; n = 8). A fourth group underwent similar surgical procedures but received no neural apnea to control for potential time-dependent changes in neural activity over the course of the protocol (time controls; n = 6). Central neural apnea was induced by lowering inspired CO2 until all rhythmic phrenic and hypoglossal activity ceased (CO2 apneic threshold); occasionally, it was necessary to also increase ventilator frequency to lower PaCO2 enough to fully induce neural apnea. In rats receiving brief intermittent neural apnea, neural apnea was quickly reversed shortly after induction by increasing inspired CO2 until respiratory neural activity resumed (CO2 recruitment threshold) and baseline PaCO2 was restored. This process was repeated five times. In rats receiving brief massed or prolonged neural apnea, central neural apnea was induced in a similar manner but was then maintained by holding ETCO2 ∼2 mmHg below the CO2 apneic threshold for 7.5 or 30 min (brief massed or prolonged neural apnea, respectively) and then reversed as described above. Importantly, in all groups, PaO2 was maintained with the ventilator during neural apnea.
Arterial blood samples were drawn before, during (prolonged neural apnea group only) and 5, 15, 30, and 60 min following central neural apnea to confirm PaCO2 was within 1.5 mmHg of baseline and to ensure maintenance of PaO2, pH, and SBE throughout the protocol. At the end of each protocol, the response to a hypercapnic challenge (90 mmHg < ETCO2 < 100 mmHg) was tested to determine whether the ability to respond to a respiratory challenge was preserved in the face of plasticity (i.e., no “ceiling” effect). To be included in the study, PaCO2 had to be maintained within 1.5 mmHg and MAP maintained within 50 mmHg from baseline at all time points postneural apnea; 33 of 35 protocols met these criteria.
Data analysis.
Phrenic and hypoglossal burst activity was amplified (×10,000), band-pass filtered (0.3–10 kHz; AM Systems), integrated (time constant 50 ms), and rectified. The resulting signal was digitized and analyzed with PowerLab (AD Instruments; Lab Chart 7.0 software). Sixty-breath bins were taken immediately prior to blood gas sampling at baseline and 5, 15, 30, and 60 min postneural apnea and analyzed for peak amplitude and burst frequency (neural correlates of tidal volume and breathing frequency, respectively). Phrenic and hypoglossal amplitude was also quantified during the hypercapnic challenge at the end of each protocol. Phrenic and hypoglossal nerve burst amplitude was expressed as a percent change from baseline (% baseline) and burst frequency was expressed as an absolute change from baseline (Δ baseline). Statistical differences between groups and individual time points were determined using two-way repeated measures ANOVA and Bonferroni post hoc tests, respectively (Prism 5, Graph Pad software). Group differences in maximum phrenic and hypoglossal amplitude elicited during hypercapnic challenge were determined using a one-way ANOVA and Fisher's least significant difference post hoc test (Sigma Plot 12 software). Linear regression analysis was used to determine the relationship between iPMF magnitude and the magnitude of the phrenic response to a hypercapnic challenge (Prism 5, Graph Pad software). A significance level of 0.05 was set for all comparisons. Data are shown as means ± SE.
RESULTS
Respiratory neural activity was reversibly reduced by lowering PaCO2 below the CO2 apneic threshold for breathing (neural apnea). Reduced respiratory neural activity was presented in three different patterns: five brief neural apneas (∼1.5 min each) separated by 5 min of resumed respiratory neural activity (brief intermittent neural apnea), a single brief massed neural apnea of similar cumulative duration (7.5 min), or a prolonged neural apnea (30 min) which approximated the duration of the stimulus period (neural apneas plus intervening rest periods). The total cumulative duration of brief intermittent neural apnea (8:43 ± 0:48, min:s) was not significantly different from the duration of brief massed neural apnea (7:30 ± 0:39, min:s; P > 0.05), and the total stimulus period of brief intermittent neural apnea (28:56 ± 0:59, min:s) was not significantly different from the duration of prolonged neural apnea (30:05 ± 0:19, min:s; P > 0.05). There were no differences in ETCO2 during neural apnea between groups (in mmHg: brief intermittent 27.8 ± 1.6, brief massed 26.2 ± 1.5, prolonged 28.4 ± 1.6; P > 0.05). Following intermittent, massed, or prolonged neural apnea, PaCO2 was restored to baseline levels and phrenic/hypoglossal nerve activity was monitored for 60 min. Table 1 shows PaO2, PaCO2, pH, and mean arterial pressure (MAP) during baseline and 60 min postneural apnea. There were no time-dependent changes in PaCO2 in any group (P > 0.05). There were significant time-dependent changes in PaO2 in time control rats, pH in rats receiving prolonged neural apnea, and MAP in rats receiving brief intermittent neural apnea (P < 0.05). These changes were not considered to be physiologically relevant in the context of our study since: 1) PaO2 was maintained well above (>200 mmHg) levels that stimulate breathing, 2) the drop in pH was small (from 7.34 to 7.31) and previous reports have found iPMF without a significant change in pH (31, 52), and 3) decreases in MAP were small (∼20 mmHg) and changes in MAP of this magnitude have minimal long-lasting effects on respiratory activity in rats (58). Indeed, changes in PaO2, pH, and MAP were not significantly related to the magnitude of iPMF 60 min following neural apnea (R2 = 0.308, R2 = 0.001, and R2 = 0.354, respectively; all P > 0.05). Thus these effects are not likely to have influenced our results.
Table 1.
PaO2, PaCO2, pH, and mean arterial pressure (MAP) during baseline and 60 min postneural apnea
| Prolonged |
Intermittent |
Massed |
Time Control |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 60 min | Baseline | 60 min | Baseline | 60 min | Baseline | 60 min | |
| PaCO2, mmHg | 47.6 ± 0.7 | 48.1 ± 0.7 | 45.0 ± 0.9 | 45.0 ± 0.9 | 45.4 ± 0.9 | 45.5 ± 0.9 | 46.8 ± 0.9 | 46.6 ± 1.2 |
| PaO2, mmHg | 289.9 ± 7.9 | 292.4 ± 8.0 | 296.9 ± 4.3 | 287.1 ± 5.0 | 291.9 ± 5.0 | 284.4 ± 5.8 | 296.3 ± 13.1 | 282.3 ± 16.3* |
| pH | 7.344 ± 0.01 | 7.309 ± 0.01* | 7.375 ± 0.01 | 7.362 ± 0.01 | 7.347 ± 0.01 | 7.332 ± 0.01 | 7.338 ± 0.00 | 7.323 ± 0.01 |
| MAP, mmHg | 149.3 ± 2.9 | 150.6 ± 4.5 | 144.0 ± 4.8 | 122.6 ± 5.1* | 148.3 ± 6.1 | 142.7 ± 4.7 | 151.8 ± 3.5 | 146.7 ± 3.1 |
Values are means ± SE. PaO2, partial pressure of arterial O2; PaCO2, partial pressure of arterial CO2; MAP, mean arterial pressure.
Significantly different than baseline (P < 0.05).
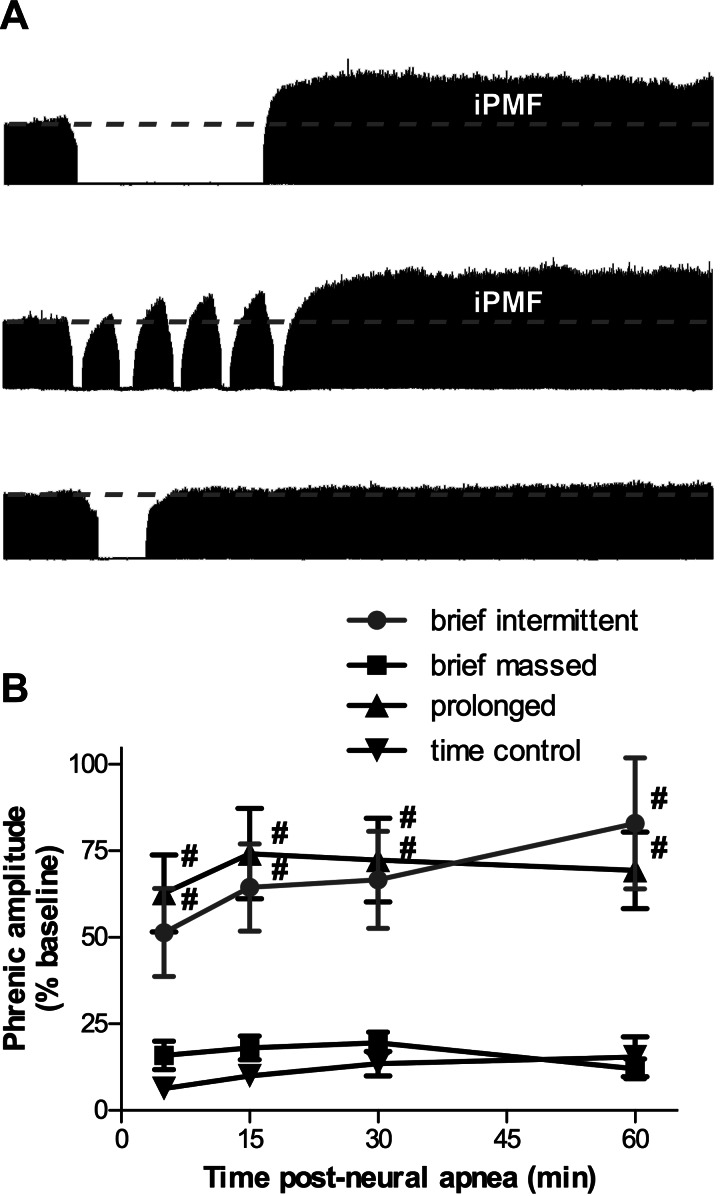
iPMF is pattern sensitive.
Representative compressed phrenic neurograms at baseline, during, and 60 min following either prolonged, brief intermittent, or brief massed neural apnea are shown in Fig. 1A. The average change in phrenic burst amplitude for 60 min following prolonged, brief intermittent, or brief massed neural apnea, or an equivalent duration in time control rats not receiving neural apnea is shown in Fig. 1B. There were no time-dependent changes in phrenic burst amplitude from baseline in time controls (5 min: 6 ± 2, 15 min: 10 ± 2, 30 min: 13 ± 4, 60 min: 16 ± 6% baseline, P > 0.05; Fig. 1B), suggesting our preparation was stable during the recording period. As previously reported (31, 52), a 30-min prolonged neural apnea elicited a long-lasting (>60 min) increase in phrenic burst amplitude at all time points following resumption of respiratory activity (5 min: 63 ± 11, 15 min: 74 ± 13, 30 min: 72 ± 12, 60 min: 69 ± 11% baseline), which was significantly greater than time controls (P < 0.01; Fig. 1, A and B). Similarly, five brief neural apneas (∼1.5 min) presented intermittently over a 30-min period resulted in increased phrenic burst amplitude at all time points postneural apnea (5 min: 51 ± 13, 15 min: 64 ± 13, 30 min: 67 ± 14, 60 min: 83 ± 19% baseline), which was significantly greater than time controls (P < 0.01; Fig. 1, A and B). There were no significant differences in phrenic burst amplitude postneural apnea between rats receiving prolonged or brief intermittent neural apnea (P > 0.05). By contrast, a brief (7.5 min) massed neural apnea did not result in increased phrenic burst amplitude at any time point postneural apnea (5 min: 16 ± 4, 15 min: 18 ± 3, 30 min: 20 ± 3, 60 min: 12 ± 3% baseline), compared with time controls (P > 0.05). Further, phrenic burst amplitude following a brief neural apnea was significantly less than phrenic burst amplitude following brief intermittent or prolonged neural apnea (P < 0.05). Collectively, these data indicate that, although iPMF is observed following a prolonged period of reduced respiratory activity, iPMF is more efficiently induced by repeated reductions in respiratory neural activity than by a similar cumulative duration of sustained reduced activity.
Fig. 1.
Inactivity-induced phrenic motor facilitation (iPMF) is pattern sensitive. A: representative compressed phrenic neurograms depicting integrated nerve burst amplitude before, during, and 60 min following either prolonged (top), brief intermittent (middle), or brief massed (bottom) neural apnea. B: mean integrated phrenic amplitude 5, 15, 30, and 60 min following brief intermittent, prolonged, brief massed, or no neural apnea (time control). Changes are expressed as a percentage of baseline amplitude. Brief intermittent and prolonged neural apnea elicit an increase in phrenic amplitude (i.e., iPMF) with a similar magnitude and duration, whereas brief massed neural apnea has no effect. #Significantly different from time control and brief massed neural apnea.
iHMF is not pattern sensitive.
Representative compressed hypoglossal neurograms at baseline, during, and 60 min following either a prolonged, brief intermittent, or brief massed neural apnea are shown in Fig. 2A. The average change in hypoglossal burst amplitude for 60 min following prolonged, brief intermittent, or brief massed neural apnea, or an equivalent duration in time control rats not receiving neural apnea is shown in Fig. 2B. There were no time-dependent changes in hypoglossal burst amplitude from baseline in time controls (5 min: −5 ± 6, 15 min: −6 ± 7, 30 min: −5 ± 7, 60 min: −4 ± 4% baseline; P > 0.05; Fig. 2B). Consistent with previous reports (7), a 30-min prolonged neural apnea elicited a transient increase in hypoglossal burst amplitude, which was only significantly different from time controls for ∼15 min following the resumption of respiratory neural activity (5 min: 27 ± 12, 15 min: 36 ± 12% baseline; P < 0.05); by 30 min postneural apnea, hypoglossal burst amplitude had returned to baseline levels (30 min: 13 ± 9, 60 min: 0 ± 8% baseline; P > 0.05; Fig. 2, A and B). No change in hypoglossal burst amplitude was observed following brief intermittent neural apnea (5 min: 3 ± 6, 15 min: 7 ± 4, 30 min: −1 ± 4, 60 min: −3 ± 3% baseline) or brief massed neural apnea of an equivalent cumulative duration (5 min: 0 ± 6, 15 min: 12 ± 5, 30 min: 15 ± 5, 60 min: 6 ± 5% baseline), compared with time controls (P > 0.05). These data suggest that prolonged, but not brief (intermittent or massed), neural apnea elicits iHMF. Thus, unlike iPMF, iHMF may not be sensitive to the pattern of reduced respiratory neural activity.
Fig. 2.
Inactivity-induced hypoglossal motor facilitation (iHMF) is not pattern sensitive. A: representative compressed hypoglossal (XII) neurograms depicting integrated nerve burst amplitude before, during, and 60 min following either prolonged (top), brief intermittent (middle), or brief massed (bottom) neural apnea. B: mean integrated hypoglossal amplitude 5, 15, 30, and 60 min following brief intermittent, prolonged, brief massed, or no neural apnea (time control). Changes are expressed as a percentage of baseline amplitude. Hypoglossal amplitude is transiently increased following prolonged neural apnea, but is unchanged following brief intermittent or brief massed neural apnea. #Significantly different from time control and brief massed neural apnea; *significantly different from time control; ϕsignificantly different from prolonged neural apnea.
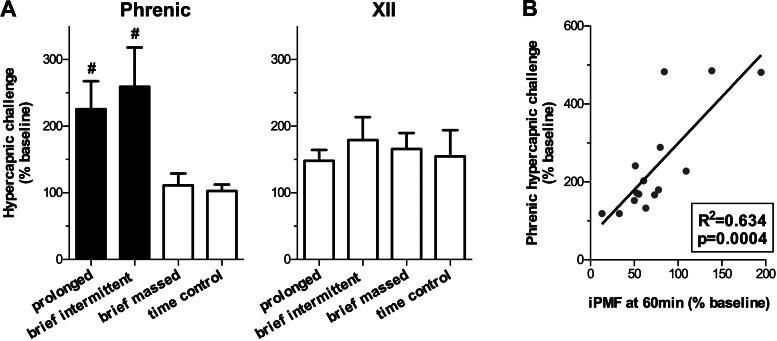
iPMF is associated with increased maximum phrenic burst amplitude.
To determine if expression of inactivity-induced plasticity occluded further increases in inspiratory burst amplitude (i.e., a ceiling effect), rats were exposed to severe hypercapnia at the end of the protocol to elicit a maximum phrenic and hypoglossal burst amplitude response. Average phrenic and hypoglossal burst amplitude during a severe hypercapnic challenge following prolonged, brief intermittent, or brief massed neural apnea, or an equivalent duration in time controls is shown in Fig. 3A. There were no significant differences in hypoglossal burst amplitude in severe hypercapnia relative to time controls following brief intermittent, brief massed, or prolonged neural apnea (179 ± 34, 166 ± 23, 148 ± 16, and 155 ± 39% baseline, respectively; P > 0.05), treatments that do not result in long-lasting facilitation. Similarly, no significant difference was observed in phrenic burst amplitude during severe hypercapnia in rats receiving brief massed neural apnea, relative to time controls (111 ± 18 and 103 ± 10% baseline, respectively; P > 0.05). By contrast, rats receiving either prolonged or brief intermittent neural apnea had significantly increased phrenic burst amplitude during severe hypercapnia (226 ± 42 and 259 ± 59% baseline, respectively), relative to time controls or rats receiving brief massed neural apnea (both P < 0.05), suggesting that the expression of iPMF is associated with an increase in the maximum phrenic response to a respiratory challenge. Indeed, a significant positive relationship between the magnitude of iPMF 60 min following neural apnea and the maximum phrenic amplitude elicited during hypercapnia was observed following prolonged or brief intermittent neural apnea (% baseline; R2 = 0.634, P < 0.001; Fig. 3B). Collectively, these data suggest that the expression of iPMF is associated with a proportional increase in the phrenic dynamic range.
Fig. 3.
Reduced respiratory neural activity increases maximum phrenic burst amplitude during hypercapnia. A: average maximum integrated phrenic and hypoglossal (XII) amplitude elicited during a hypercapnic challenge ∼60 min following brief intermittent, brief massed, prolonged, or no neural apnea (time control). Changes are expressed as a percentage of baseline amplitude. Rats exposed to brief intermittent or prolonged neural apnea (inducing iPMF) have a significantly greater maximum phrenic amplitude during hypercapnic challenge than rats receiving brief massed neural apnea or time controls. By contrast, no differences in maximum hypoglossal amplitude were observed between any treatment groups. Shaded bars indicate patterns of neural apnea that result in significant iPMF. #Significantly different from time control and brief neural apnea. B: linear regression of maximum phrenic amplitude elicited during a hypercapnic challenge vs. the magnitude of iPMF expressed 60 min following brief intermittent and prolonged neural apnea.
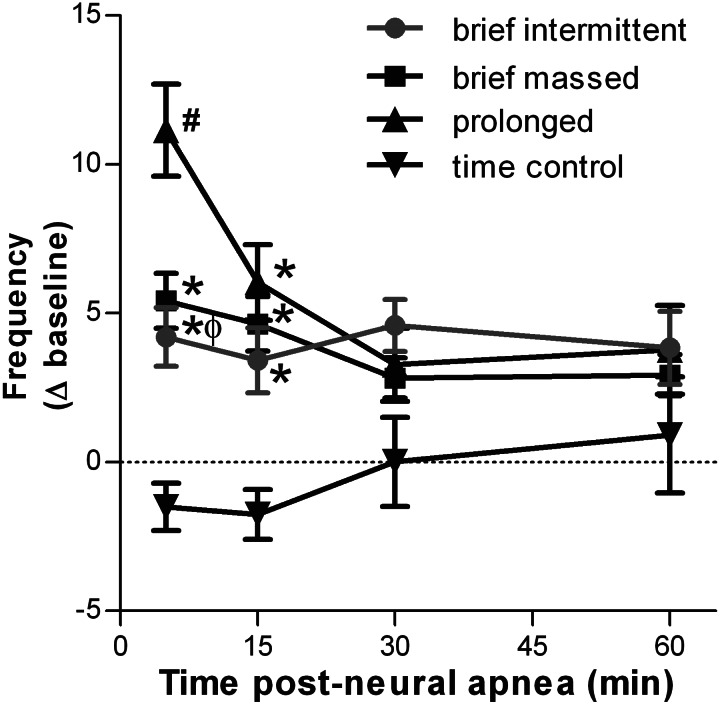
Frequency facilitation is sensitive to the duration, but not the pattern, of neural apnea.
The average change in respiratory burst frequency at 5, 15, 30, and 60 min following either a prolonged, brief intermittent, or brief massed neural apnea, or an equivalent duration in time control rats is shown in Fig. 4. No time-dependent changes in burst frequency were observed in time control rats not receiving neural apnea (5 min: −2 ± 1, 15 min: −2 ± 1, 30 min: 0 ± 1, 60 min: 1 ± 2 change from baseline; P > 0.05). Following prolonged neural apnea, burst frequency was significantly increased at 5 and 15 min (11 ± 2 and 6 ± 1 change from baseline, respectively) relative to time controls (P < 0.05); however, by 30 min following restoration of respiratory neural activity, burst frequency was no longer significantly different from time controls (30 min: 3 ± 1, 60 min: 4 ± 1 change from baseline; P > 0.05). Modest transient increases in burst frequency were observed following brief intermittent (5 min: 4 ± 1, 15 min: 3 ± 1 change from baseline) and brief massed (5 min: 5 ± 1, 15 min: 5 ± 1 change from baseline) neural apnea; both responses were significantly greater than equivalent time points in time controls (P < 0.05), but were significantly less than those observed following prolonged neural apnea (P < 0.05). By 30 min following brief neural apnea, burst frequency had returned to levels not significantly different from time controls (brief intermittent: 5 ± 1 and 4 ± 1; brief massed: 3 ± 1 and 3 ± 1 change from baseline, 30 and 60 min, respectively; P > 0.05). Collectively, these data suggest that the magnitude of inactivity-induced facilitation of respiratory frequency is sensitive to the cumulative duration of neural apnea, but not its pattern.
Fig. 4.
Mean respiratory nerve burst frequency 5, 15, 30, and 60 min following intermittent, prolonged, brief, or no neural apnea (time control). Changes are expressed as an absolute change from baseline (Δ baseline). All neural apnea transiently increases nerve burst frequency; however, a continuous 30-min neural apnea elicits a greater increase in nerve burst frequency than 5 min of intermittent or massed neural apnea. #Significantly different from time control and brief neural apnea; *significantly different from time control; ϕsignificantly different from prolonged neural apnea.
DISCUSSION
Here we demonstrate that a novel form of respiratory plasticity induced by reduced respiratory neural activity (i.e., iPMF) is pattern sensitive. Although a prolonged central neural apnea is sufficient to elicit iPMF (7, 31, 52), iPMF is more efficiently induced by repeated (intermittent) neural apneas vs. a sustained neural apnea of similar cumulative duration. Further, iPMF is associated with a proportional increase in the phrenic burst amplitude response to a hypercapnic challenge, suggesting that the ability to respond to a respiratory challenge is preserved in the presence of inactivity-induced plasticity. The effects of neural apnea appear to be motor pool specific since facilitation in hypoglossal activity is either absent or transient following brief intermittent or prolonged neural apnea, respectively. Last, we show that changes in respiratory frequency following reduced respiratory neural activity are transient and sensitive to the duration of neural apnea, but not its pattern. These results extend our understanding of plasticity induced by reduced activity within the respiratory neural network and may have implications for conditions that result in episodic reductions in central respiratory drive, such as sleep at altitude, chronic heart failure, and central sleep apnea.
Methodological considerations.
To assess the role of reduced respiratory neural activity in eliciting phrenic and hypoglossal inspiratory motor plasticity, we used a well-established anesthetized rat preparation commonly used to study respiratory plasticity (20, 37). This preparation offers distinct advantages that enable investigations of the neural control of breathing independent from the process of blood gas homeostasis. For example, mechanical ventilation enables strict control over arterial blood gases and avoids attendant hypercapnia and hypoxia—stimuli that induce plasticity in and of themselves (1, 2)—that would normally accompany reduced respiratory neural activity. Further, afferent feedback is minimized by vagotomy, paralysis, and cutting the recorded phrenic and hypoglossal nerves. Collectively, these techniques enable isolation of the effects of reducing brainstem respiratory neural output from many other factors that modify it. However, anesthesia (12) and afferent feedback from the vagus (22) and phrenic (49) nerves are capable of modulating the expression of plasticity. Nevertheless, our findings demonstrate that neural networks underlying breathing sense and respond rapidly to reduced respiratory neural activity, independent of the effects of hypercapnia and hypoxia. The conditions under which these mechanisms manifest as a phenotypic physiological response are of clear clinical and biological interest.
Expression of iPMF is sensitive to the pattern of inactivity.
Plasticity within neural networks controlling motor behaviors is often more effectively induced by intermittent than sustained stimuli (3, 9, 10, 35, 53). For instance, LTF of sensorimotor synapses in Aplysia is induced by both intermittent (five applications over 1.5 h) and prolonged (1.5 h) application of serotonin (18, 27, 38), whereas a relatively brief (∼25 min) application of serotonin is less likely to induce LTF (34).
In many studies describing pattern-sensitive plasticity, the relevant stimulus is often directly or indirectly associated with an increase in neuronal and/or synaptic activity. However, reduced neuronal activity also elicits plasticity, typically by altering the properties of a neuron or network of neurons in a non-synapse specific manner (15, 57). This “synaptic scaling” is thought to have a compensatory or homeostatic function since prolonged reductions in neuronal activity alter cellular properties to increase excitation and/or decrease inhibition (25, 26, 50, 55, 56, 60), presumably to restore a target level of activity and preserve “normal” function. For example, in cultured hippocampal neurons subjected to a prolonged (48 h) activity blockade via application of tetrodotoxin (blocking axon conduction), postsynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor expression is increased through a tumor necrosis factor alpha-dependent mechanism (51). Although it is clear that long-lasting plasticity can be induced by a single sustained period of reduced activity within a neural network, few studies have tested the effect of repeated or intermittent inactivity. Our data suggest that, in some motor pools (but not in others), intermittent patterns of reduced respiratory neural activity may be more efficient than prolonged reductions to induce plasticity. The detailed, and potentially unique, mechanisms that impart pattern sensitivity on inactivity-induced plasticity of phrenic motor output remain unknown.
Expression of iPMF increases phrenic dynamic range.
Respiratory motor output operates within a limited dynamic range beginning at zero (neural apnea) and spanning to a maximum value that can be elicited during a potent respiratory challenge, such as high CO2. Under normal conditions, only a fraction of this range is utilized (32). Our observation that iPMF proportionally increases the maximum phrenic response to high CO2 is consistent with other reports (53), and suggests that the ability of the respiratory control system to respond to a respiratory challenge is preserved in the face of at least certain forms of plasticity. Thus we hypothesize that the iPMF phenotype is the result of a compensatory shift in phrenic excitability to restore motor output when respiratory neural activity is reduced. Because the dynamic range of all inspiratory motor output was not increased (i.e., hypoglossal maximum output remains constant), it is likely that inactivity-induced changes in phrenic dynamic range occur downstream of sites of chemosensation, central respiratory rhythm generation, and (brainstem) premotor processing. Together, these data are consistent with the idea that iPMF is not due to an increase in brainstem respiratory drive, but is a process occurring locally within individual respiratory motor pools.
Effects of neural apnea on respiratory motor output are motor pool specific.
Phrenic and hypoglossal motor pools provide excitatory drive to their target muscles (diaphragm and tongue, respectively) during the inspiratory phase of respiration; however, the physiological functions of these motor pools differ greatly. Regular rhythmic activity of phrenic motor neurons is predominantly involved in breathing (negative pressure ventilation) and is essential to maintain life, while hypoglossal motor neurons are active during a variety of behaviors including chewing, swallowing, and vocalization, as well as maintaining upper airway patency during breathing (28). Further, reduced hypoglossal activity does not generally pose an immediate threat to life (42, 62).
Despite differences in functionality, phrenic and hypoglossal motor pools are both controlled by the same brainstem regions that initiate, coordinate, and modulate respiratory motor neuron activity (19). Because of this shared input, different respiratory motor pools often respond similarly when the system is challenged. However, there may also be benefits in local regulation specific to each motor pool, particularly with regard to long-lasting changes in system performance (i.e., plasticity) (3, 7, 53), a characteristic that may allow motor pools to respond in a stimulus-specific manner. For example, moderate IH elicits a serotonin-dependent increase in inspiratory motor output known as LTF (1, 6). Similar to inactivity-induced facilitation, IH-induced LTF is differentially expressed in phrenic and hypoglossal motor output (4, 7, 20, 21). One important question is: How does differential expression of plasticity arise if both phrenic and hypoglossal motor pools experience similar inducing stimuli? Evidence suggests that local mechanisms operating in or near the motor pool may ultimately underlie subsequent responses, even though central mechanisms may be an initial trigger. With regard to LTF following moderate IH, it is suggested that the stimulus (hypoxia) is “sensed” by peripheral chemoreceptor stimulation which initiates a common (centralized) trigger: episodic activation of medullary raphe neurons and subsequent release of serotonin in the phrenic and hypoglossal motor pools (in addition to other disparate sites), resulting in LTF. However, local mechanisms (e.g., serotonin receptor expression and terminal density, presence of other neuromodulator systems) then appear to modify IH-induced LTF in a motor pool specific manner (7, 24, 41).
iPMF and iHMF are also initiated by a common trigger: central neural apnea. However, we hypothesize that local mechanisms within individual respiratory motor pools sense and respond to the initiating stimulus: reduced respiratory related synaptic inputs. Mechanisms that sense reduced respiratory activity and initiate iPMF or iHMF are unknown; however, downstream mechanisms that give rise to enhanced inspiratory burst amplitude are beginning to be elucidated (52). At least for iPMF, these mechanisms ultimately activate atypical protein kinase C (aPKC) isoforms PKCζ or PKCι within or near the phrenic motor pool and induce the formation of a signaling complex between PKCζ/ι and the scaffolding molecule p62/ZIP (52). aPKCs represent one of three subfamilies of PKC (classical, novel, and atypical), and have been implicated in multiple forms of plasticity (for review, see Refs. 47, 48, 54). Mechanisms of transient iHMF are unknown, and we do not understand why inactivity-induced facilitation differs so greatly in phrenic and hypoglossal motor pools. Inactivity-induced plasticity may operate in time domains tailored to the specific characteristics of a motor pool, such as the regularity and extent of normal activity, the diversity of function, or the physiological consequences of reduced activity. Consistent with this idea, the phrenic motor pool responds to reduced respiratory neural activity fairly rapidly, whereas the hypoglossal motor pool may require a longer duration of inactivity to consolidate long-lasting plasticity. In this sense, rapid and robust iPMF may preserve a vital, time-sensitive (i.e., adjustments must be made within minutes) physiological function, whereas a similar response within the hypoglossal motor pool may hinder normal function when required for other nonrespiratory behaviors. Alternatively, it is possible that the transient nature of iHMF represents a faster induction of a counter mechanism that reduces an inappropriately facilitated motor output back toward normal levels.
Frequency facilitation and neural apnea.
All patterns of neural apnea tested here elicited frequency facilitation that differed only in magnitude, but not duration. It is noteworthy that frequency facilitation was more similar to iHMF than iPMF in its transient (∼15 min) duration; however, the reason(s) for this similarity is unknown. Because prolonged neural apnea elicited greater frequency facilitation than brief neural apnea, we hypothesize that inactivity-induced frequency facilitation is more sensitive to the duration of inactivity than its pattern. The cellular mechanisms responsible for frequency facilitation following neural apnea are not known, but likely occur in brainstem respiratory rhythm generating neurons (5, 11). Medullary neurons responsible for respiratory rhythm generation exhibit plasticity, such as frequency LTF following intermittent anoxia in vitro (11). Although it has not yet been directly shown, frequency LTF following intermittent hypoxia in vivo is hypothesized to similarly occur in medullary respiratory rhythm generating neurons (5, 11, 45). Similar to frequency facilitation following reduced respiratory neural activity, frequency LTF is small (∼10%) and relatively insensitive to the pattern of hypoxia (5).
Significance of repeated neural apnea and iPMF.
Recurrent apnea or hypopnea, particularly during sleep, is experienced by millions of people worldwide with varying degrees of severity (14, 63) and may be classified as central, obstructive, or mixed (both central and obstructive). Central apnea is characterized by a reduction in respiratory neural activity, whereas obstructive apnea is characterized by continued respiratory efforts against a collapsed airway. While the effects of intermittent hypoxia that occurs with apnea/hypopnea have received considerable attention (8, 30, 33, 40, 43, 46, 59), little work has addressed the impact of repeated fluctuations in respiratory neural activity. Both central apnea (7, 31, 52) and airway obstruction (53) elicit plasticity in respiratory control independent of hypoxia and hypercapnia that would normally accompany an apneic event. Mechanisms giving rise to these different forms of plasticity are not well known, but may involve reduced respiratory neural activity in efferent respiratory motor output (data presented here and see Refs. 31 and 52) or afferent respiratory feedback (53), respectively. Interestingly, central neural apnea appears to preferentially elicit phrenic plasticity in phrenic burst amplitude, whereas airway obstruction appears to preferentially elicit plasticity in genioglossus (and presumably hypoglossal) activity. Although the significance of these motor pool-specific responses is unknown, these differential effects may represent appropriate stimulus-specific responses to these very different threats to ventilation.
We hypothesize that iPMF represents one component in a continuum of endogenous responses to reduced respiratory neural activity. Given the prevalence of central apnea and the associated health risks (e.g., cardiovascular complications, frequent nighttime awakenings, excessive daytime sleepiness) (8, 17, 14), further investigation of the causes and potential endogenous compensatory responses to central apnea are warranted. To fully understand the physiology or pathophysiology of repeated central apnea, one must consider all associated physiological stimuli (e.g., chemical feedback, mechanical feedback, sympathoexcitation, and reduced network activity), as all are potential sources of plasticity, which may occur simultaneously (perhaps with important interactions) to benefit or hinder stable breathing. A greater understanding of conditions that efficiently recruit these mechanisms may guide treatment and prevention strategies for conditions that destabilize breathing.
GRANTS
Research support was provided by National Heart, Lung, and Blood Institute Grant HL-105511.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.A.B. and T.L.B.-H. conception and design of research; N.A.B. performed experiments; N.A.B. and T.L.B.-H. analyzed data; N.A.B. and T.L.B.-H. interpreted results of experiments; N.A.B. prepared figures; N.A.B. and T.L.B.-H. drafted manuscript; N.A.B. and T.L.B.-H. edited and revised manuscript; N.A.B. and T.L.B.-H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kristi Strey for her helpful comments on this manuscript.
REFERENCES
- 1. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol 129: 25–35, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170: 260–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179: 48–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 114: 805–816, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Baumbauer KM, Hoy KC, Jr, Huie JR, Hughes AJ, Woller SA, Puga DA, Setlow B, Grau JW. Timing in the absence of supraspinal input I: variable, but not fixed, spaced stimulation of the sciatic nerve undermines spinally-mediated instrumental learning. Neuroscience 155: 1030–1047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumbauer KM, Huie JR, Hughes AJ, Grau JW. Timing in the absence of supraspinal input II: regularly spaced stimulation induces a lasting alteration in spinal function that depends on the NMDA receptor, BDNF release, and protein synthesis. J Neurosci 29: 14383–14393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol 87: 2964–2971, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cao Y, Ling L. Urethane inhibits genioglossal long-term facilitation in un-paralyzed anesthetized rats. Neurosci Lett 477: 124–128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Dwinell MR, Janssen PL, Bisgard GE. Lack of long-term facilitation of ventilation after exposure to hypoxia in goats. Respir Physiol 108: 1–9, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 131: 595–607, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science 262: 253–256, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation in the rat requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001–2006, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Golder FJ, Martinez SD. Bilateral vagotomy differentially alters the magnitude of hypoglossal and phrenic long-term facilitation in anesthetized mechanically ventilated rats. Neurosci Lett 442: 213–218, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci 22: 1328–1337, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu QR, Hattar S, Endo S, MacPhee K, Zhang H, Cleary LJ, Byrne JH, Eskin A. A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization. J Neurosci 17: 755–764, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowe AA. The neural regulation of tongue movements. Prog Neurobiol 15: 295–344, 1980 [DOI] [PubMed] [Google Scholar]
- 29. MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mahamed S, Strey KA, Mitchell GS, Baker-Herman TL. Reduced respiratory neural activity elicits phrenic motor facilitation. Respir Physiol Neurobiol 175: 303–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem 5: 246–256, 1998 [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Mitchell GS, Terada J. Should we standardize protocols and preparations used to study respiratory plasticity? Respir Physiol Neurobiol 177: 93–97, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249–1254, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Invited review: Neural network plasticity in respiratory control. J Appl Physiol 94: 1242–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Neubauer JA. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol 90: 1593–1599, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol 112: 1678–1688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ouahchi Y, Marie JP, Verin E. Effect of lingual paralysis on swallowing and breathing coordination in rats. Respir Physiol Neurobiol 181: 95–98, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Powell FL, Garcia N. Physiological effects of intermittent hypoxia. High Alt Med Biol 1: 125–136, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir Physiol 121: 223–236, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol 281: L524–L528, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Reyland ME. Protein kinase C isoforms: multi-functional regulators of cell life and death. Front Biosci 14: 2386–2399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res 169: 27–40, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Sandhu MS, Lee KZ, Fregosi RF, Fuller DD. Phrenicotomy alters phrenic long-term facilitation following intermittent hypoxia in anesthetized rats. J Appl Physiol 109: 279–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steinmetz CC, Turrigiano GG. Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. J Neurosci 30: 14685–14690, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Strey KA, Nichols NL, Baertsch NA, Broytman O, Baker-Herman TL. Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation. J Neurosci 32: 16510–16520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tadjalli A, Duffin J, Peever J. Identification of a novel form of noradrenergic-dependent respiratory motor plasticity triggered by vagal feedback. J Neurosci 30: 16886–16895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci 17: 551–567, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264: 974–977, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity- dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walker JK, Jennings DB. Respiratory effects of pressor and depressor agents in conscious rats. Can J Physiol Pharmacol 76: 707–714, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wenner P. Mechanisms of GABAergic homeostatic plasticity. Neural Plast 2011: 489–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28: 2949–2958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilson JR, Sumner AJ, EichelmanJ Aberrant reinnervation following hypoglossal nerve damage. Muscle Nerve 17: 931–935, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Young T. Rationale, design and findings from the Wisconsin Sleep Cohort Study: toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin 4:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]