Abstract
Background/Aims
Endothelial dysfunction is associated with mitochondrial alterations. We hypothesized that uric acid, which can induce endothelial dysfunction in vitro and in vivo, might also alter mitochondrial function.
Methods
Human aortic endothelial cells were exposed to soluble uric acid and measurements of oxidative stress, nitric oxide, mitochondrial density, ATP production, aconitase-2 and enoyl co-A hydratase-1 expression, and aconitase-2 activity in isolated mitochondria were determined. The effect of hyperuricemia upon renal mitochondrial integrity was also assessed in rats treated with oxonic acid that inhibits the enzyme uricase that degrades uric acid.
Results
Uric acid induced endothelial dysfunction was associated with reduced mitochondrial mass and ATP production. Uric acid also decreased aconitase-2 activity and lowered enoyl CoA hydratase-1 expression. Hyperuricemic rats showed increased mitDNA damage in association with higher levels of intrarenal uric acid and oxidative stress.
Conclusions
Uric acid induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP. These studies provide additional evidence for a deleterious effect of UA on vascular function that could be important in the pathogenesis and progression of hypertension, vascular disease and renal disease.
Keywords: nitric oxide, mitochondria, endothelial dysfunction, uric acid
Introduction
Mitochondrial dysfunction characterized by the altered expression of the enzymes aconitase-2 (ACO-2) and enoyl CoA hydratase 1(ECoH-1), as well as a significant reduction of mitochondrial mass could be a key feature of endothelial dysfunction [1].
Rats made hyperuricemic by the inhibition of uricase with oxonic acid develop endothelial dysfunction, hypertension and renal damage [2, 3] with L-arginine supplementation, antioxidants and treatment with the xanthine oxidase inhibitor allopurinol preventing these alterations [2–4]. UA induces endothelial dysfunction by increasing oxidative stress and reducing nitric oxide (NO) bioavailability [4–7]. Moreover, a large number of studies links UA with endothelial dysfunction in humans with many reports indicating that lowering UA with allopurinol can improve endothelial dysfunction [8–15].
No studies have investigated whether endothelial dysfunction induced by UA may be associated with mitochondrial alterations such as observed by Addabbo et al [1] We therefore tested whether UA might alter mitochondrial mass, as well as ACO-2 and ECoAH-1 levels in cultured human aortic endothelial cells. We also investigated whether experimental hyperuricemia in rats might reduce mitochondrial DNA or induce mitochondrial DNA damage in kidney tissue, a target organ of hyperuricemia.
Methods
Cell culture
Human aortic endothelial cells (HAEC, Lonza, Walkersville, MD USA) were grown in EGM-2 plus bullet kit media (Clonetics Walkersville, MD USA). Cells between passages 4–7 were used in the experiments.
Experimental conditions and viability assay
UA solutions were prepared as previously described [16]. In order to simulate human conditions, low normal (208 μM, 3.5 mg/dL), high normal (416 μM, 7 mg/dL) and hyperuricemic (714 μM, 12 mg/dL) concentrations of UA were used in these experiments. After incubation with or without UA, viability analysis was done by trypan blue dye-exclusion.
Measurement of reactive oxygen species (ROS) generation and the effect of apocynin
ROS generation was determined using the 2′7′-dichlorofluorescein diacetate (DCF-DA) dye (Molecular Probes, Eugene OR USA). Preparations were imaged using a laser scanning confocal microscope (LSM510; Zeiss) and data analyzed by using the LSM Image Analyzer postacquisition software (Zeiss). The effect of the NADPH oxidase inhibitor apocynin (100 μM) was also determined.
Determination of nitric oxide production
NO production was assessed using the NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA, Cayman Chemical, Ann Arbor, MI USA) and examined using a laser-scanning confocal microscope. Total amount of NOx (nitrate and nitrite) was also determined using a commercial Kit (Active Motif Carlsbad CA USA).
Quantification of mitochondrial DNA
Mitochonrial DNA was analysed by real-time quantitative PCR. The primer sequences for mtDNA, and those for nDNA for loading normalization, 18SrRNA gene were reported previously [17]. Quantitative PCR was performed using a SYBR green master mix (JumpStart® TaqReadymix®, Sigma) on a BioRad I-Cycler. Nuclear internal control DNA and the amount of mitochondrial DNA were calculated by the comparative CT method.
Mitochondrial labeling in live cells
Mitochondrial labeling was assessed using the specific mitochondrial dye MitoTracker Orange CMTMRos (Molecular Probes, Eugene OR USA) with staining performed according to the manufacturer’s instructions. Cells were examined using a laser-scanning confocal microscope with a peak excitation wavelength of 554 nm and a peak emission wavelength of 576 nm.
Immunoblotting
The following primary antibodies were used: anti-aconitase 2 (Sigma-Prestige St Louis MO USA), anti-enoyl CoA hydratase 1 (Protein Tech Group, Chicago IL USA), and anti-beta-actin (Cell Signaling Technology Beverly MA USA). Blots were visualized using a horseradish peroxidase secondary antibody. Chemiluminescence was recorded and results analyzed with the 1D Image Software (Kodak Digital Science, Rochester, NY USA).
Aconitase activity assay
Pure mitochondrial fractions were isolated using a reagent-based method that enables the isolation of intact mitochondria by differential centrifugation (Thermo Scientific No 89874, Rockford IL USA) and assessed for ACO-2 activity using a commercial kit (Biovision. Milpitas CA USA).
Measurement of Intracellular ATP Content
Intracellular ATP was measured using an ATP bioluminescence assay kit (CLS II; Roche, Mannheim, Germany).
Animal model
Ten male Sprague-Dawley rats were studied. 5 rats received oxonic acid (750 mg/kg/BW by gavage) for 8 weeks whilst 5 animals received vehicle and served as controls. Awake systolic blood pressure (XBP1000, Kent Scientific, Torrington CT USA), proteinuria (Bradford method) and plasma UA (Amplex-Red Kit Molecular Probes, Eugene OR USA) were measured at the end of 8 weeks. At sacrifice, right kidney was extirpated and renal cortex separated from medulla, frozen in liquid nitrogen and stored at −80°C until processed. Left kidney was fixed in 4% paraformaldehyde for further processing and histological analysis. All animal experiments and procedures were approved by the INC Ignacio Chávez Ethics Committee.
Renal cortex UA measurement
UA was extracted as previously described [18] and measured using Amplex-Red Kit (Molecular Probes, Eugene OR USA).
Renal cortex protein carbonyls measurement
Tissue was homogenized in phosphate buffer containing a cocktail of proteases inhibitors. Protein carbonyls reacted with dinitrophenylhydrazine to form protein hydrazones which was measured at 370 nm.
Evaluation of glomerulosclerosis and tubulointerstitial fibrosis
Two μm sections were stained with Masson’s trichrome. Ten non-overlapping fields of cortex (640 X 477 mm, 10X) per biopsy were analyzed by light microscopy (Olympus BX51. Olympus American, Melville NY) and captured with a digital camera (VF Evolution. Media Cybernetics, Silver Spring MD). Positive blue color areas (excluding glomeruli and vessels) were analyzed in Image Pro Plus (Media Cybernetics, Silver Spring MD). Glomerulosclerosis was also evaluated in Masson’s trichrome-stained slides divided in four quadrants. Segmental and globally sclerosed glomeruli were reported as percent of the total number of glomeruli counted in one quadrant.
Evaluation of renal inflammatory cell infiltration
Leukocyte infiltration was assessed by indirect peroxidase immunostaining using an antibody against the common leukocyte antigen (CD45) with counterstaining with hematoxylin. Negative control consisted of slides incubated with irrelevant antisera. Inflammatory cell infiltartion was evaluated in 30 non-overlapping X400 cortical fields, excluding glomeruli, per biopsy. Results were expressed as positive cells per 0.074 mm2 using the mean of the 30 fields.
Assay for relative mitDNA copy number and the common mitDNA deletion
Total renal cortex DNA was isolated using Quick-gDNA MiniPrep (Zymo Research, Irvine CA USA). Primers and probes for the rat D-loop (mitDNA copy number) and the rat mitDNA deletion from the rat mitochondrial genome were previously reported [19]. mitDNA was compared against the nuclear gene for 18s rRNA. Fluorescence spectra was continuously monitored by the ABI-Prism 7300 Sequence Detection System (Applied Biosystems, Carlsbad CA USA) with sequence detection software version 1.3.1. Data analysis was based on measurement of the CT and relative copy numbers were calculated as 2−ΔΔCT [19].
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). Data graphics and statistical analysis were performed using Prism 5 (Graph Pad Software, San Diego, CA USA). Multiple group analysis was done by ANOVA and post-hoc comparisons were done by Bonferroni test. Analysis of two groups was done by t-Test. In most cases experiments were performed 3 times with independent replicates. P values < 0.05 were considered statistically significant.
Results
Cell culture
UA did not affect HAEC viability
Exposure of HAEC to varying concentrations of UA or control medium for 48 h had no effect on cell viability, with 90–95% of cells remaining viable for this time period.
UA exposure increased intracellular oxidative stress
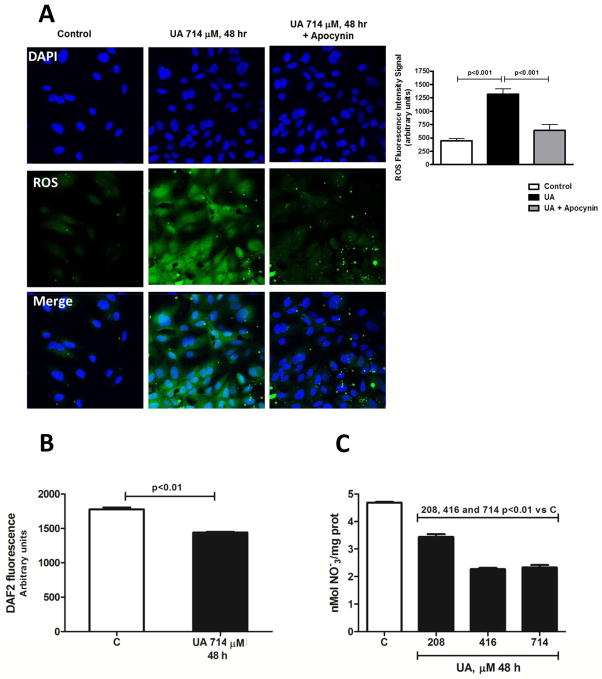
Incubation with UA for 48h produced a 200% increment in the level of intracellular oxidative stress. Co-incubation with the antioxidant apocynin almost completely prevented this effect (Fig 1A). Thus, we confirmed the pro-oxidant effect of UA inside the cell, as previously reported in other cell lines or primary cultures of both human and rodent endothelial cells [20].
Fig. 1.
Uric acid increases intracellular oxidative stress in cells incubated with UA 714 μM (12 mg/dL) for 48 h. This effect was prevented by cotreatment with apocynin suggesting the participation of NADPH oxidase in this effect (A) (n=3, triplicates). Uric acid reduces NO bioavailability in HAEC incubated with UA 714 μM (12 mg/dL) for 48 h as suggested by a significant reduction in nitrates/nitrites measured by DAF2-DA fluorescence in fixed cells (B) (n=3, triplicates) and as secreted nitrates/nitrites in the cell culture media (C) (n=2, triplicates).
UA incubation decreased eNOS-mediated NO synthesis
As shown to Figure 1B, incubation of HAEC with 714 μM of UA for 48 h significantly decreased DAF2-labeled NO level in response to the combination of insulin and A23187 that robustly stimulates NO production from eNOS. Likewise, the accumulation of NOx products (nitrates plus nitrites) in culture media after activation of eNOS with insulin-A23187 showed the same trend (Fig 1C). These findings are in agreement with previous reports [20, 21].
UA induced mitochondrial alterations in HAEC (Fig 2)
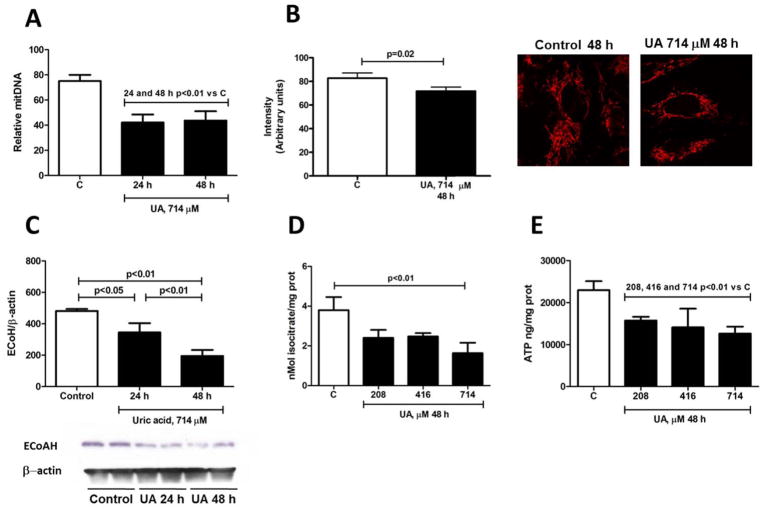
Fig. 2.
Uric acid reduces mitochondrial mass in HAEC incubated with UA 714 μM (12 mg/dL) as showed by a significant decrement of mitDNA at 24 and 48 h (A) (n=3, triplicates). This effect was in association with a lower fluorescence intensity using the specific mitochondrial dye Mitotracker Orange™ (B) at 48 h of incubation with UA (n=2, triplicates). Uric acid also alters the expression and bioactivity of mitochondrial enzymes and reduces ATP levels in HAEC incubated with UA 714 μM (12 mg/dL) for 48 h. Mitochondrial ECoAH-1 expression (C) and ACO-2 activity (D) were significantly reduced in UA treated cells (n=3, triplicates). UA incubation for 48 h also reduced basal intracellular ATP concentration (E) (n=3, triplicates).
Since it was recently reported that specific mitochondrial alterations are an early marker of endothelial dysfunction [1], we determined whether UA exposure might induce the same effect in HAEC. Endothelial cells incubated with UA for 24 and 48 h showed a 50% reduction in mitochondrial DNA levels (Fig 2A). Quantification of mitochondrial labeling intensity with MitoTrakerOrange™ showed a significantly lower fluorescence in cells treated with UA (714 μM) for 48 h compared to control cultures (Fig 2B) indicating a decrease in mitochondrial mass. In addition we found a significant reduction in the protein expression of the mitochondrial enzyme ECoAH-1 at 24 and 48 h (Fig 2C), while ACO-2 protein expression was modestly and transiently reduced at 24 h but not at 48 h (data not shown). Nevertheless, incubation of HAEC with uric acid for 48 h resulted in a significant reduction in ACO-2 bioactivity (Fig 2D).
UA treatment induced a significant reduction in the basal concentration of ATP
HAEC exposed to UA for 48 h showed a reduced basal intracellular ATP concentration of 30%, 39% and 43% for concentrations of 208, 416 and 714 μM of UA, respectively (Fig 2E)
Animal model
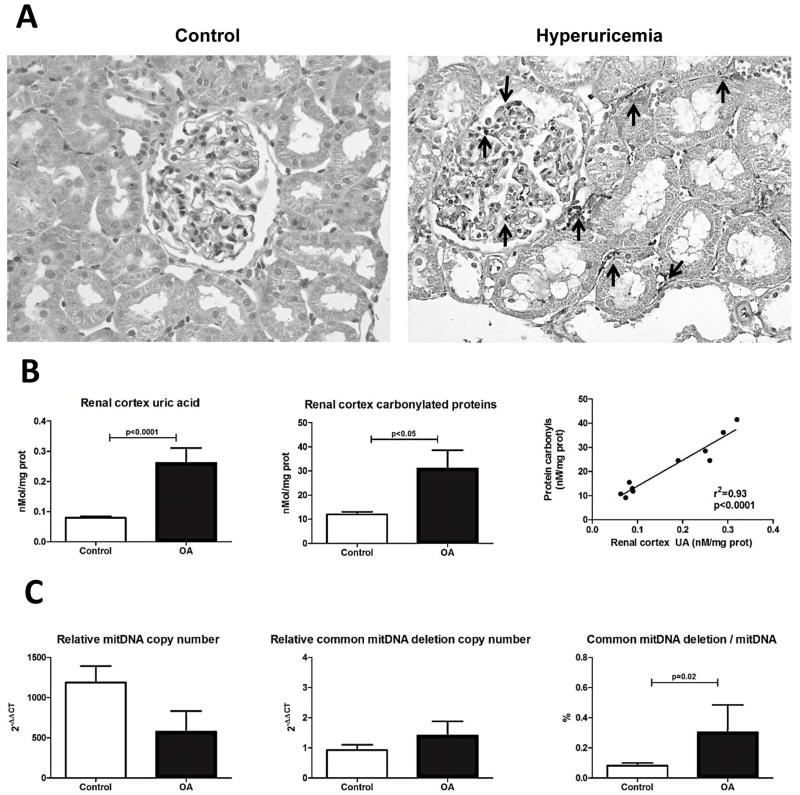
As previously shown, we confirmed that treatment with the uricase inhibitor oxonic acid (OA) could induce mild hyperuricemia and endothelial dysfunction characterized by systemic hypertension, glomerulosclerosis and renal inflammatory cell infiltration (Table 1 and Fig 3A). At this time point tubulointerstitial fibrosis was not evident (data not shown). In the present study we also report that OA-treated rats had significantly higher intrarenal levels of UA associated with greater oxidative stress (Fig 3B). The increase in plasma and intrarenal uric acid was associated with lower relative mitDNA copy number and significantly higher relative mitDNA common deletion copy number in the renal cortex, resulting in a high mitDNA deletion/mit DNA ratio in OA-treated rats (Fig 3C).
Table 1.
Parameters studied in control rats vs oxonic acid-induced hyperuricemic rats
| Parameter/Group | Control | OA | p |
|---|---|---|---|
| Body weight (g) | 337±11 | 332±23 | ns |
| SBP (mmHg) | 125±4 | 141±3 | 0.0001 |
| Plasma UA (μM) | 45±5 | 248±18 | <0.0001 |
| Urine prot (mg/d) | 7±3 | 11±2 | 0.04 |
| Glomerulosclerosis (%) | 0.50±0.41 | 4.24±2.33 | 0.02 |
| TI fibrosis | 3.0±2.4 | 5.2±4.9 | ns |
| Inflammatory cell infiltration (positive cells/X400 field) | 0.45±0.40 | 13.3±4.7 | 0.01 |
TI= Tubulointerstitial
Fig 3.
Representative micrographs of renal sections (X400) from a control and an oxonic acid (OA) treated hyperuricemic rat showing inflammatory cell infiltration (anti-CD45 antibody) (arrows) (A). The concentration of UA in the renal cortex was significantly increased in OA-treated rats and was associated with increased oxidative stress. In addition, the intra-renal UA level correlated with the degree of oxidative stress (B). OA-treated animals had less relative mitDNA copy number and a proportional increment of damaged mitDNA in the renal cortex compared to control rats, as showed by a significant increased percentage of common mitDNA deletion (C).
Discussion
We report that UA exposure reduced mitochondrial mass, decreased the expression of ECoAH1 and reduced the activity of aconitase-2 in isolated mitochondria from HAEC. Collectively these alterations are considered the mitochondrial manifestations of early endothelial dysfunction [1]. In addition UA decreased the basal concentration of ATP in these cells. Importantly, we reproduced mitDNA alterations in renal tissue of hyperuricemic rats induced by the uricase inhibitor, oxonic acid, providing additional evidence for a general deleterious effect of UA on mitDNA.
In the present studies we first corroborated the most distinctive effects of UA on endothelial cells, that consisting of increased oxidative stress partially mediated by NADPH activation, as suggested by the protective effect of apocynin, and reduced bioavailability of NO (Fig 1) (20, 21).
Mitochondrial dysfunction has recently been described as a characteristic feature associated with endothelial dysfunction and was characterized by a reduction of mitochondrial mass and selective depletion of ACO-2 and ECoAH-1 [1]. In the present study we observed similar effects induced by UA exposure: a significant reduction of mitochondrial mass coupled with reduced expression of ECoH-1 protein expression and ACO-2 bioactivity, although ACO-2 protein expression remained unchanged. Contrary to ACO-2, for ECoH-1, there is no evidence for the participation of metal ions or cofactors in its catalytic mechanism that can be inactivated by oxidative stress [22]. Moreover, ECoH-1 is one of the most proficient catalysts known such that the enzyme level needs to be down-regulated in order to accomplish a decrement in enzyme activity [23]. On the other hand, the structural predilection of iron-sulphur center in ACO-2 to ROS and peroxynitrite may in part explain its selective vulnerability [24]. In the present study we report a significantly reduced activity of the enzyme in mitochondria isolated from cells treated with UA with evidence for increased oxidative stress under the same conditions. Thus we speculate that UA-induced oxidative stress also acts to inactivate ACO-2 in this setting.
We also documented a lower basal concentration of ATP in cells exposed to UA. We can provide three mechanisms that might contribute to this effect: 1) by blocking aconitase-2 in the Krebs cycle, and by reducing β-fatty acid oxidation via reduction in ECoAH, it is possible that both effects participate to decrease ATP generation; 2) UA treatment was also associated with a slight but significant reduction in mitochondrial mass as noted by the reduction in mitochondrial DNA/nuclear DNA ratio and as determined by the Mitotracker™ assay; 3) It was showed that acute high concentrations of UA produced mitochondrial calcium overload in HUVEC resulting in a significant increment of mitochondrial membrane potential that could lead to excess generation of ROS [25]. These effects are suggestive of mitochondrial uncoupling, which can be associated with a decreased synthesis of ATP through oxidative phosphorylation. Nevertheless, we cannot rule out a detrimental effect of UA on the ATP produced by glycolysis, since endothelial cells tend to have a strong glycolytic pathway.
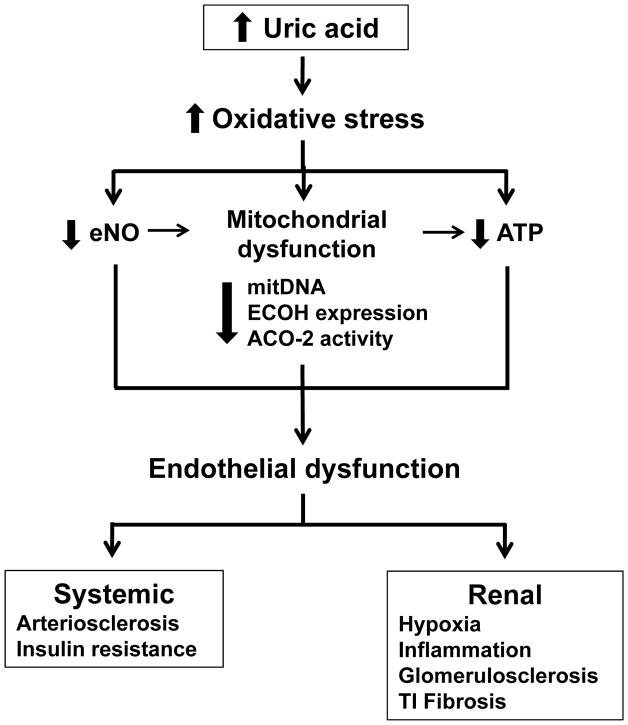
Since we previously found increased oxidative stress and endothelial dysfunction in the kidney of hyperuricemic rats, we also examined whether similar mitochondrial changes might occur in this model. To assess mitochondrial injury we evaluated both the relative mitDNA copy number and the relative mitDNA common deletion copy number. The increase of deleted mitDNA is considered a marker of increased mitDNA damage induced by oxidative stress; as such it is augmented in a number of pathological conditions and during aging [26, 27]. We found that increased levels of plasma and renal UA and augmented renal oxidative stress were associated with a decrement in the relative mitDNA copy number and an increased proportion of mitDNA common deletion. These findings are in agreement with the results in HAEC exposed to UA. Since renal tissue includes more cell types than endothelial cells, we speculate that the effect of UA on mitochondrial damage is a more general process that may affect diverse cell types. Figure 4 summarizes the mechanisms potentially involved in the endothelial dysfunction induced by uric acid.
Fig. 4.
Potential mechanisms involved in uric acid-induced endothelial dysfunction and renal damage. Hyperuricaemia promotes augmented oxidative stress which in turn decreases endothelial NO bioactivity, induces mitochondrial dysfunction characterized by diminished mitochondrial mass and decreased expression or activity of the Kreb’s cycle enzymes ECoH and ACO-2 and reduced intracellular ATP. Reduced endothelial NO bioactivity may also reduce mitochondrial mass by altering biogenesis, and altered mitochondria may also contribute to reduced intracellular ATP levels. Therefore, reduced NO, mitochondrial alterations and decreased ATP results in the induction of endothelial dysfunction, which may promote arteriosclerosis and insulin resistance. In the kidney, endothelial dysfunction result in tissue hypoxia, inflammatory cell infiltration, and progressive renal disease as manifested by glomerulosclerosis and tubulointerstitial fibrosis.
In summary, UA induced endothelial dysfunction is associated with mitochondrial dysfunction and reduced ATP generation. These studies provide additional evidence for a deleterious effect of UA on vascular function that could be important in the pathogenesis and progression of hypertension, vascular disease and renal disease.
Acknowledgments
Support for this paper was provided by NIH grants HL-68607 and DK-90859, Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea grant A101742, and National Council of Science and Technology (CONACyT) Mexico grants 081054 and 0133232.
We thank Benito Chavez-Renteria for processing the renal sections for histological evaluation.
Footnotes
Conflict of interest statement
Dr Johnson has a patent with the University of Washington for allopurinol in the treatment of hypertension (7,799,794) and has patent applications with the University of Florida and with the University of Colorado for UA-lowering therapy and or treatments to block fructose metabolism in the treatment of metabolic syndrome or diabetic nephropathy.
References
- 1.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174:34–43. doi: 10.2353/ajpath.2009.080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik VS, Hu FB. Sugar-sweetened beverages and health: where does the evidence stand? Am J Clin Nutr. 2011;94:1161–1162. doi: 10.3945/ajcn.111.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 6.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 7.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 8.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 9.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, Sezgin N, Muderrisoglu H. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59:1276–1282. doi: 10.1111/j.1742-1241.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 11.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–439. doi: 10.3945/ajcn.2008.26980. author reply 439–440. [DOI] [PubMed] [Google Scholar]
- 12.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–289. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 15.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 16.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 17.Gianotti TF, Sookoian S, Dieuzeide G, Garcia SI, Gemma C, Gonzalez CD, Pirola CJ. A decreased mitochondrial DNA content is related to insulin resistance in adolescents. Obesity (Silver Spring) 2008;16:1591–1595. doi: 10.1038/oby.2008.253. [DOI] [PubMed] [Google Scholar]
- 18.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 21.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 22.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol. 2008;22:811–816. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnihotri G, Liu HW. Enoyl-CoA hydratase. reaction, mechanism, and inhibition. Bioorg Med Chem. 2003;11:9–20. doi: 10.1016/s0968-0896(02)00333-4. [DOI] [PubMed] [Google Scholar]
- 24.Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta. 1992;1122:178–182. doi: 10.1016/0167-4838(92)90321-4. [DOI] [PubMed] [Google Scholar]
- 25.Arima S. Role of angiotensin II and endogenous vasodilators in the control of glomerular hemodynamics. Clin Exp Nephrol. 2003;7:172–178. doi: 10.1007/s10157-003-0249-8. [DOI] [PubMed] [Google Scholar]
- 26.Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1176–1183. doi: 10.1021/tx9701790. [DOI] [PubMed] [Google Scholar]
- 27.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]