Abstract
Pulmonary TNFα has been linked to reduced exercise capacity in a subset of patients with moderate to severe chronic obstructive pulmonary disease (COPD). We hypothesized that prolonged, high expression of pulmonary TNFα impairs cardiac and skeletal muscle function, and both contribute to exercise limitation. Using a surfactant protein C promoter-TNFα construct, TNFα was overexpressed throughout life in mouse lungs (SP-C/TNFα+). TNFα levels in wild-type (WT) female serum and lung were two- and threefold higher than in WT male mice. In SP-C/TNFα+ mice, TNFα increased similarly in both sexes. Treadmill exercise was impaired only in male SP-C/TNFα+ mice. While increases in lung volume and airspace size induced by TNFα were comparable in both sexes, pulmonary hypertension along with lower body and muscle mass were evident only in male mice. Left ventricular (LV) function (cardiac output, stroke volume, LV maximal pressure, and LV maximal pressure dP/dt) was not altered by TNFα overexpression. Fatigue measured in isolated soleus and EDL was more rapid only in soleus of male SP-C/TNFα+ mice and accompanied by a loss of oxidative IIa fibers, citrate synthase activity, and PGC-1α mRNA and increase in atrogin-1 and MuRF1 expression also only in male mice. In situ gastrocnemius fatigue resistance, reflecting both oxygen availability and contractility, was decreased similarly in female and male SP-C/TNFα+ mice. These data indicate that male, but not female, mice overexpressing pulmonary TNFα are susceptible to exercise limitation, possibly due to muscle wasting and loss of the oxidative muscle phenotype, with protection in females possibly due to estrogen.
Keywords: angiopoietin, atrophy, chronic obstructive pulmonary disease, fatigue, PGC-1
chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in North America and Europe (21). In addition to the primary pathology that develops in the lung, patients with COPD exhibit comorbidities or extrapulmonary abnormalities in heart, bone, and skeletal muscle that may contribute to physical inactivity (9). For instance, a decrease in lean muscle mass has been reported in both smokers with normal lung function and those with obstructive lung disease. Inflammation and/or oxidative stress triggered by cigarette smoke have been suggested as one mechanism that could interfere with regeneration and/or atrophy pathways in skeletal muscle (44, 58). This is supported by the finding of Remels et al. (43) that a subgroup of COPD patients with higher expression of the inflammatory cytokine TNFα in skeletal muscle exhibit a reduced body mass index (BMI).
TNFα is also upregulated in lung and skeletal muscle of mice exposed to cigarette smoke (23, 54). TNFα overexpression in the mouse lung results in a phenotype with many similarities to human COPD. SP-C/TNFα+ mice exhibit robust lymphocytic inflammation, severe emphysema, and pulmonary hypertension (18, 19, 41). Smoke exposure also leads to early changes in body and skeletal muscle mass, an oxidative to glycolytic fiber transition, and loss of oxidative metabolic capacity that correlates with downregulation of the transcriptional coactivator PGC-1α (23, 54). Muscle wasting or a loss of muscle mass has been reported to occur in SP-C/TNFα+ mice in part due to an impaired differentiation of myoblasts (33).
We hypothesize that overexpression of TNFα in the lung leads to comorbidities in both the heart and skeletal muscle, in addition to the primary pulmonary pathology, that could contribute to exercise limitation. Therefore, exercise capacity in mice that overexpress TNFα in the lung under the control of the surfactant protein C promoter (SP-C/TNFα+ mice) was assessed in relation to the degree of emphysema, pulmonary hypertension, cardiac and skeletal muscle function and maintenance of body weight. We found that in SP-C/TNFα+ mice, exercise capacity was selectively impaired in male, but not female mice, and was accompanied by pulmonary hypertension, reduced body weight, and downregulation of the oxidative muscle phenotype, but not by physiological evidence of left ventricular dysfunction. In oxidative skeletal muscle, PGC-1α expression was reduced and the atrophy-related genes, atrogin and MurF1, upregulated. Together, these data suggest that estrogen may play a role in protecting against TNFα-induced myopathy in part by preventing the downregulation of the transcriptional co-activator, PGC-1α.
MATERIALS AND METHODS
Experimental Design
The study was designed to evaluate the role of TNFα induced loss of exercise capacity in relation to pulmonary, cardiac, and musculoskeletal function, as well as sex. Male and female SP-C/TNFα+ mice and their sex-specific littermate controls (WT) were first evaluated each month for a change in body weight up to 10–15 mo of age. Subsequently, SP-C/TNFα+ and WT mice at 6–8 mo of age were studied to determine if there was a sex-specific change in exercise capacity (maximal treadmill speed, submaximal endurance time), pulmonary structure and function (lung volume, airspace size, arterial O2 saturation), cardiac function (direct micromanometer right and left ventricular pressures and echocardiography) and skeletal muscle structure and function (in situ time to fatigue, in vitro time to fatigue, oxidative enzyme activities, fiber composition and capillarity, and atrophy-related gene expression).
Animals
SP-C/TNFα+/− mice on a C57BL6 background were obtained from Dr. Y. Miyazaki (19, 41), and bred as heterozygous mice at the University of California, San Diego. In each litter, approximately half of the mice carried the SP-C/TNFα+ gene, and the other half were negative for the transgene and used as the wild-type (WT), littermate control group. The University of California, San Diego, Institutional Animal Care and Use Committee (IACUC) approved the experimental procedures in this study.
TNFα ELISA
TNF-α was measured in serum and organs with an ELISA system that has a minimum detectable dose (MDD) of 0.36–7.21 pg/ml for mouse TNFα (R & D Systems).
Treadmill Running Tests
Mice were exercised using an Omnipacer Treadmill LC4 (Omnitech Electronics Columbus, OH) on a 10° incline in room air.
Maximal running speed.
After a low-speed warm-up (15 cm/s for 5 min), mice were subjected to an incrementally increasing speed running test. Mice ran at 50, 55, 60, 65, 70, 75, and 80 cm/s for 20 s at each speed, respectively. The fastest speed that a mouse could run was recorded as its maximal running speed.
Endurance.
Both WT and SP-C/TNFα+ mice ran at 60% of the average maximal speed of SP-C/TNFα+ mice (36 cm/s in this experiment) until exhaustion. In some mice, a clip was connected to the back of a shaved neck area and O2 saturation was continuously monitored using a MouseQ Plus Oximeter (STARR, Oakmont PA). Measurements were recorded before, during, and immediately at the end of each running bout. Total activity, rate of oxygen uptake (V̇o2), rate of carbon dioxide output (V̇co2), respiratory exchange ratio (RER), and food/drink intake in cage-confined resting mice were monitored and recorded at 12-h intervals using a Comprehensive Lab Animal Monitoring System (CLAMS) from Columbus Instruments.
Estimation of Air Space Enlargement by Mean Linear Intercept (MLI)
A point-count morphometric technique was used to assess air space enlargement from paraffin-embedded, 7-μm sections. Lungs were inflated and fixed at an airway pressure of 20 cmH2O as previously described (53). Lung volumes were estimated by fluid displacement.
Cardiac Function Measurements
Transthoracic echocardiography.
Animals were anesthetized with 5% isoflurane for 1 min and then maintained at 1% isoflurane throughout the examination. The anterior chest wall was shaved and any remaining hair removed with Nair. Small needle electrodes for simultaneous electrocardiogram were inserted into one upper and two lower limbs. Images (M-mode, 2 dimensional, and Doppler) were acquired using the VisualSonics Vevo 2100, a division of SonoSite, ultrasound system with a linear transducer of 32–55 MHz. Measurements of heart rate (HR), left ventricular end-diastolic dimensions (LVEDD) and left ventricular end-systolic dimensions (LVESD), end-diastolic interventricular septal thickness (IVSd) and LV posterior wall thickness (LVPWd) were determined from the LV M-mode tracing. Percentage fractional shortening (%FS) was used as an indicator of systolic cardiac function. In addition, interrogation with pulsed wave Doppler (PWD) of the mitral inflow and tissue Doppler imaging (TDI) of the medial mitral annulus were used to quantify diastolic performance (52).
Cardiac micromanometer catheterization.
Hemodynamic evaluation of right and left ventricular function was performed while mice were under general anesthesia [ketamine (100 mg/kg)/xylazine (10 mg/kg)]. After connection to a ventilator (Kent Scientific TOPO pressure ventilator), a 1.4-Fr (0.46 mm) micromanometer catheter (Millar Instruments, Houston, TX) was inserted via the right jugular vein into right atrium and advanced further into the right ventricle (RV). The instantaneous pressure at RV dP/dtmax was taken as corresponding to pulmonic valve opening and the instantaneous pressure at RV dP/dtmin as corresponding to pulmonic valve closure; during ejection, the RV pressure was considered equivalent to main pulmonary artery pressure. Following measurements in the RV, the Millar catheter was withdrawn from the RV and inserted via the right common carotid artery and into the LV where LV peak and end-diastolic pressures were measured. To assess contractile reserve, LV dP/dtmax was measured during an inotropic challenge with dobutamine at dosages of 0.75, 2, 4, 6, and 8 μg·kg−1·min−1, with 3-min recordings at each dosage. All pressure recordings were continuously monitored using LabChart (ADInstruments, Colorado Springs, CO) systems (3).
Skeletal Muscle Fatigue
Ex vivo fatigue index.
Isolated soleus and EDL fatigue was measured as previously described by Zuo et al. (60). Fatigue was measured at the optimal length (Lo). The soleus was stimulated with a 500-ms train duration, 0.5-ms pulses, 80 Hz at 0.15, 0.19, 0.25, 0.33, 0.43, 0.57, 0.75, 1.0, and 1.3 Hz trains per second, 1 min each. The EDL was stimulated with a 250-ms train duration, 0.5-ms pulse, 150 Hz at 0.15, 0.19, 0.25, 0.33, 0.43, 0.57, 0.75, and 1.0 Hz trains per second, 1 min each. The fatigue index was defined as the time to reach 30% of the initial tension.
In situ fatigue.
Mice were anesthetized with isoflurane, and one sciatic nerve was exposed and connected to electrodes. The gastrocnemius complex was then separated from the bone and the Achilles tendon connected with a suture to a force transducer (Grass FT10). The gastrocnemius complex was set to L0 using single-twitch contractions and then stimulated to contract (with 10 V, 0.25 trains per second, 200-ms train duration) until the force generated fell to 50% of the initial force output and the time recorded. Mean arterial pressure was measured at the end of the contraction period and was on average 63 ± 8 mmHg.
Analysis of Myosin Heavy Chain Protein (MHC)
This method has been previously described (54). Briefly, frozen soleus samples were homogenized in a buffer containing 250 mM sucrose, 2 mM EDTA, 10 mM Tris, pH 7.4 at a volume of 50 μl/mg followed by sonication for 1 min. Homogenates were centrifuged at 10,000 g at 4°C for 10 min. The supernatant from this spin was used for citrate synthase and β-HAD assays. The remaining pellet was resuspended in 50 μl of ice-cold extraction buffer (100 mM Na4O7P2 10 H2O, 5 mM EDTA, 1 mM DTT, pH 8.5), incubated for 30 min on ice, and centrifuged at 10,000 g at 4°C for 10 min. Supernatants from the second spin were electrophoresed on an SDS-PAGE gel and silver stained. ImageJ software (http://www.nih.gov) was used to measure the density of individual MHC bands in each lane. The data are expressed as percentage of MHC I, IIa, or IIb of the total density of MHCs detected per lane.
Citrate Synthase (CS) and β-Hydroxyacyl CoA Dehydrogenase (β-HAD) Activity Assays
Enzyme activity assays have been described previously (54). Briefly, the supernatant from the first spin of the above-mentioned muscle sample preparation was assayed using the oxaloacetate method with a CS Assay Kit (Sigma, St. Louis, MO). The activity of β-HAD was measured using the NADH method.
NFκB p65 Binding Activity
The muscle samples were homogenized with passive lysis buffer (Promega, Madison WI). NFκB p65 DNA binding activity was measured using an ELISA-based kit (TransAM NF-κB p65, no. 40096 Active Motif, Carlsbad, CA).
Real-Time RT-PCR Assays
RNA isolation, reversed transcription, and PCR amplification were described previously (54). Changes in mRNA level were measured for the following genes: TNFα, TNFR-1, TNFR-2, VEGF, VEGFR-1, VEGFR-2, Ang-1, Ang-2, β-FGF, IL-6, IL-1β, INFγ, C-reactive protein, TGFβ, Atrogin-1, MuRF1, PGC-1α, PPARα, PPARγ1, and PPARδ. The PCR primers for the above-mentioned genes were designed with Vector NTI Deluxe (Tucows Toronto, Canada).
Statistics
Because it became evident prior to making any measurements that body weight in SP-C/TNFα+ mice was reduced in males but unaffected in females, all subsequent data from the male and female mice were analyzed separately. An ANOVA was used to compare body weights in WT and SP-C/TNFα+ at multiple time points and a Tukey post hoc test used to determine differences at each time point. A two-way ANOVA was used to compare type I and type II fiber sizes in soleus from SP-C/TNFα+ and WT male mice. Differences in physiological outcomes between WT and SP-C/TNFα+ groups within the same sex were analyzed with an unpaired Student's t-test. P < 0.05 was considered significant.
RESULTS
TNFα Levels in Lung, Serum, Heart, and Skeletal Muscle of SP-C/TNF+ Mice
TNFα protein levels were substantially increased in lungs, serum, and skeletal muscle but not in heart in SP-C/TNFα+ mice compared with WT mice of the same sex (Table 1). In the lungs, TNFα levels were higher in both SP-C/TNFα+ female and male mice compared with WT female and male mice (P < 0.01). Baseline TNFα levels in the lung of female mice were elevated compared with the WT male group (P < 0.05). In serum, TNFα levels were increased in both the female (P < 0.05) and male (P < 0.001) SP-C/TNFα+ groups compared with the respective sex WT group. TNFα+ levels in the serum of WT female mice were increased compared with WT male mice (P < 0.05). In soleus, TNFα was also elevated in both female (1.3-fold, P = 0.02) and male (1.6-fold, P = 0.001) SP-C/TNFα+ mice compared with WT. Similarly, TNFα levels in the gastrocnemius were elevated in both female (1.4-fold, P = 0.01) and male (1.7-fold, P < 0.001) SP-C/TNFα+ mice compared with WT. In the EDL, TNFα was also elevated in female (1.5-fold, P = 0.04) and male (1.7-fold, P = 0.003) SP-C/TNFα+ mice compared with WT.
Table 1.
TNFα levels in the lung, heart, skeletal muscle, and circulation
| Female |
Male |
|||
|---|---|---|---|---|
| Wild type (n = 8) | SP-C/TNFα+ (n = 8) | Wild type (n = 8) | SP-C/TNFα+ (n = 8) | |
| Lung, pg/mg | 30.6 ± 6.8 | 895 ± 223* | 10.6 ± 2.9‡ | 818 ± 21* |
| Serum, pg/ml | 6.8 ± 1.8 | 31.3 ± 10.4* | 3.7 ± 1.1‡ | 29.6 ± 8.9* |
| Heart, pg/mg | 7.5 ± 1.9 | 7.8 ± 1.1 | 7.1 ± 2.2 | 7.9 ± 2.0 |
| Gastrocnemius, pg/mg | 2.8 ± 0.8 | 3.9 ± 0.9# | 2.7 ± 0.8 | 4.7 ± 1.2* |
| Soleus, pg/mg | 3.6 ± 0.9 | 4.8 ± 0.9# | 3.2 ± 0.9 | 5.1 ± 0.7* |
| EDL, pg/mg | 2.1 ± 0.5 | 3.1 ± 1.1# | 2.5 ± 0.8 | 4.4 ± 1.3* |
Values represent means ± SD. EDL, extensor digitorum longus. Significant differences compared with the same-sex wild type (WT) are indicated (
P < 0.01,
P < 0.05) and between the male and female WT mice (
P < 0.05).
Body Weight and Muscle Mass
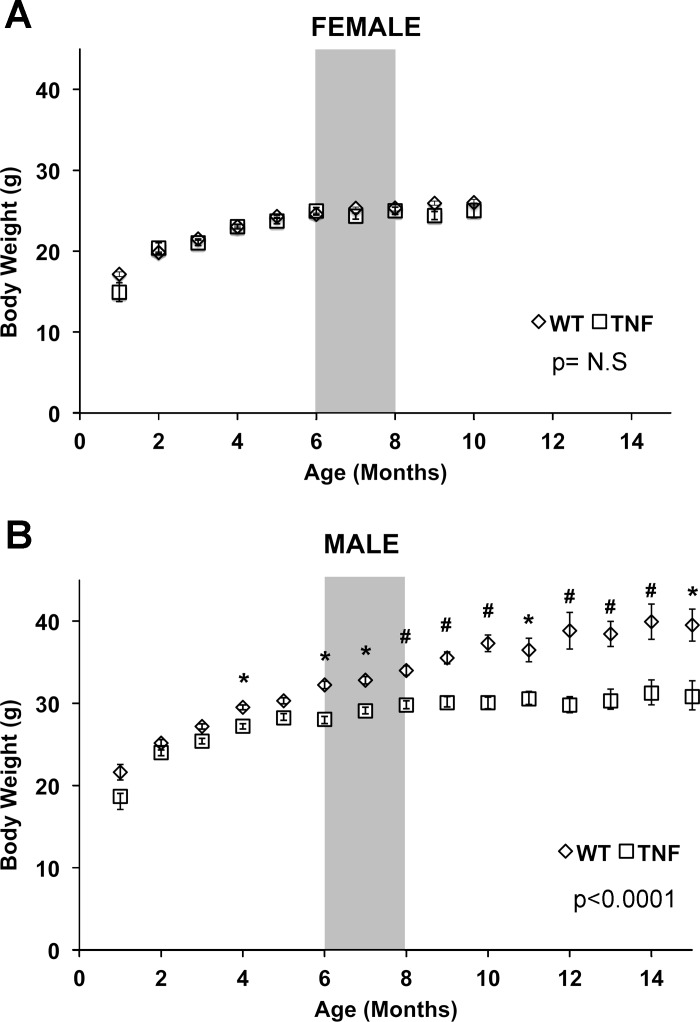
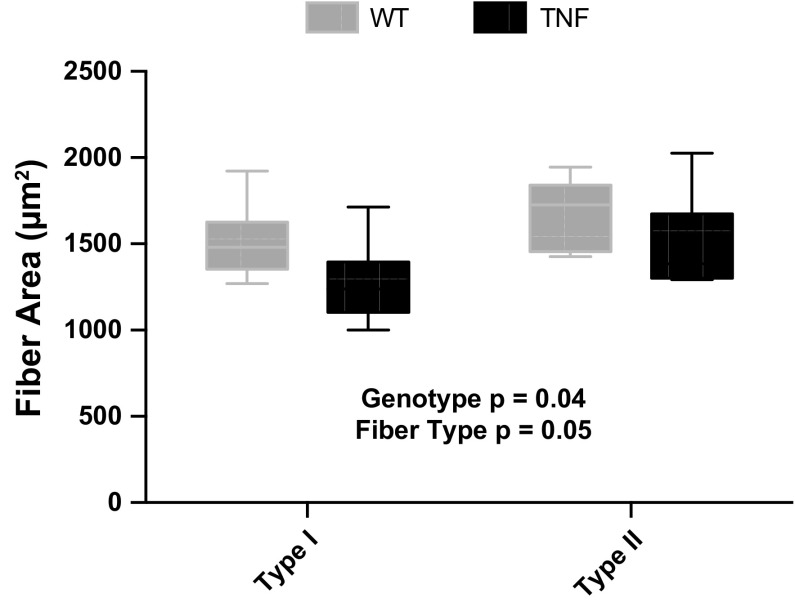
Female WT and SP-C/TNFα+ mice did not differ in body weight over 10 mo (Fig. 1). Body weights of male SP-C/TNFα+ mice were detectably lower than the male WT group starting at 4 mo of age, and this difference progressively increased over the next months. At 12 mo of age, the male SP-C/TNFα+ mice weighed 23% less than the WT mice (P < 0.001). The weight of the soleus (WT, 9.22 ± 1.3 mg; SP-C/TNFα+, 7.68 ± 1.1 mg, P = 0.01), and both soleus type I and II fiber sizes (Fig. 2) were significantly reduced in male SP-C/TNFα+ mice but not in females. Gastrocnemius and EDL muscle mass and fiber size were not altered.
Fig. 1.
Body weight gain is attenuated in adult male SP-C/TNFα+ mice. Body weights recorded at 1-mo intervals from female (A) and male (B) wild-type (WT) (◇) and SP-C/TNFα+ (□) mice. Values represent means ± SE. Differences between WT and SP-C/TNFα+ mice at each time point are indicated: *P < 0.05, #P < 0.01. WT-Female, n = 7–37; SP-C/TNFα+-Female, n = 6–40; WT-Male, n = 7–58; SP-C/TNFα+-Male, n = 5–36. The shaded gray area represents the 6- to 8-mo time interval in which mice were analyzed for changes in cardiac and skeletal muscle function.
Fig. 2.
Soleus fiber size is decreased in male SP-C/TNFα+ mice. Fiber cross-sectional area (CSA) was measured from 10-μm cryosections stained with ATPase according to the Rossenblatt method (47). Values represent the average CSA in type I and type II fibers in one soleus per mouse from 6 mice/group.
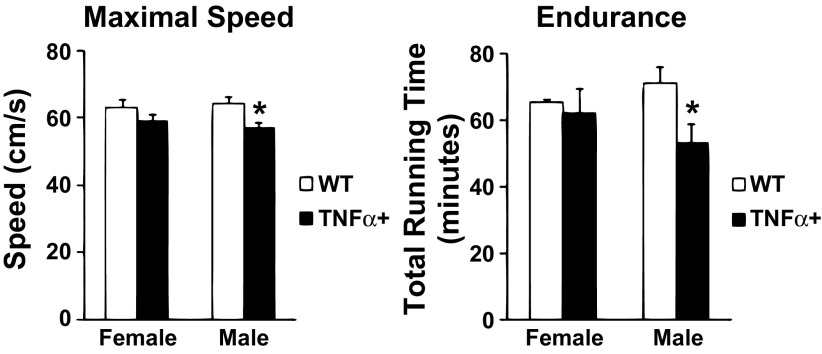
Impaired Exercise Capacity in Male SP-C/TNFα+ Mice
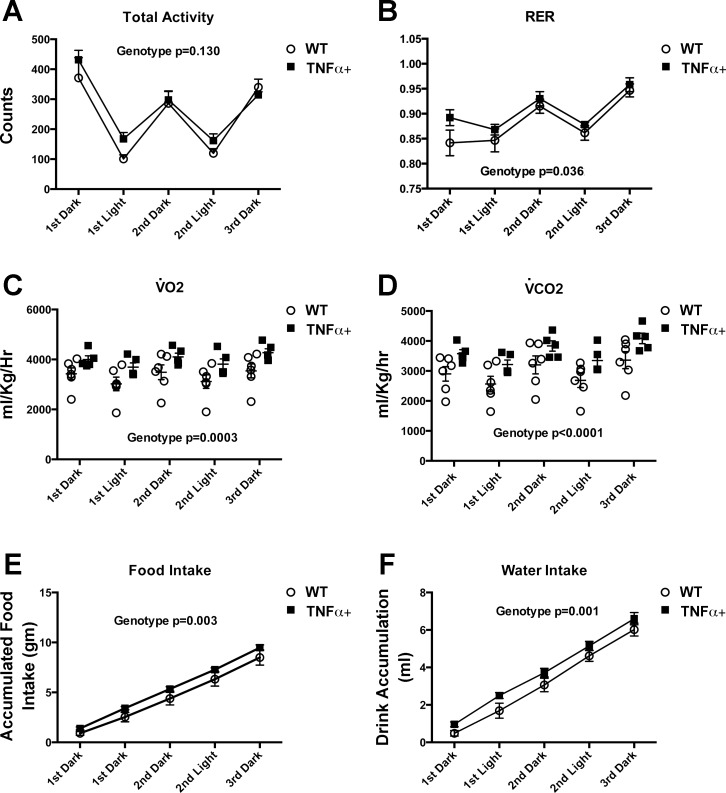
Maximal treadmill running speed was reduced by 11% in male SP-C/TNFα+ mice compared with WT (P = 0.03) (Fig. 3). Exercise endurance (time to exhaustion when run at a constant speed) was reduced by 24% only in male SP-C/TNFα+ mice (P = 0.04). Total activity in cage-confined mice was not different in male SP-C/TNFα+ mice compared with WT (Fig. 4A). However, there were small increases in the RER, V̇o2, and V̇co2 as well as food and water intake at rest in male SP-C/TNFα+ mice compared with WT (Fig. 4, B–F).
Fig. 3.
Exercise capacity is selectively impaired in male but not female SP-C/TNFα+ mice. In treadmill running tests (left) maximal speed achieved by SP-C/TNFα+ was decreased in male but not female mice. Endurance capacity (right) was selectively decreased in male SP-C/TNFα+ mice. Endurance times were not altered in female SP-C/TNFα+ mice. Values represent means ± SD; n = 6. *Difference between SP-C/TNFα+ and WT male mice, P < 0.05.
Fig. 4.
Activity, food and water intake, and oxygen uptake in cage-confined male SP-C/TNFα+ and WT mice. Mice were monitored in separate cages for changes in total activity (A), respiratory exchange ratio (RER) (B), rate of oxygen uptake (V̇o2) (C), rate of CO2 output (V̇co2) (D), accumulated food intake (E), and accumulated drink intake (F). Values represent the average of 6 mice/group ± SE for total activity, and food/drink intake; n =6 WT and 5 SP-C/TNFα+ for V̇o2, V̇co2, and RER.
Pulmonary and Cardiac Function in SP-C/TNFα+ and WT Mice
Lung volumes quantified by fluid displacement at the same inflation pressure were increased by 2.0- and 1.6-fold, respectively, in female and male SP-C/TNFα+ mice compared with their sex-specific control group (P < 0.001). Evidence of emphysema was sought by evaluating the mean linear intercept, which was increased in both the female and male SP-C/TNFα+ mice by 2.36-fold (WT, 35.2 ± 1.2 μm; SP-C/TNFα+ 82.9 ± 4.5 μm, P < 0.001) and 2.07-fold (WT, 35.2 ± 1.2 μm; SP-C/TNFα+ 82.9 ± 4.5 μm, P < 0.01), respectively, compared with the sex-specific WT group. In the heart, increases in RV maximal pressure (WT 28.0 ± 6.5 mmHg; SP-C/TNFα+ 38.63 ± 7.5 mmHg, P < 0.05) and RV minimal dP/dt pressure (WT −1,689 ± 326 mmHg/s; SP-C/TNFα+ −2,460 ± 583 mmHg/s, P < 0.05) were found in male, but not female, SP-C/TNFα+ mice. Calculated peak (WT 27.7 ± 6.6 mmHg/s; SP-C/TNFα+ 37.5 ± 8.5 mmHg P < 0.05) and mean pulmonary artery pressures (WT 19.5 ± 5.5 mmHg; SP-C/TNFα+ 25.3 ± 6.2 mmHg P < 0.05) were higher in the male SP-C/TNFα+ mice. Cardiac output, stroke volume, LV end-diastolic pressure, LV maximal pressure and LV peak dP/dt were not altered by TNFα overexpression in either sex. Left ventricular pressures following dobutamine challenge (simulating cardiac output increases with exercise) were also not different between male WT and SP-C/TNFα+ mice (data not shown). No significant differences in cardiac functional parameters were detected in female SP-C/TNFα+ mice. Arterial oxygen saturation, measured by pulse oximeter, both at rest and during exercise, was not different in any of the four groups.
Locomotor Skeletal Muscle Function
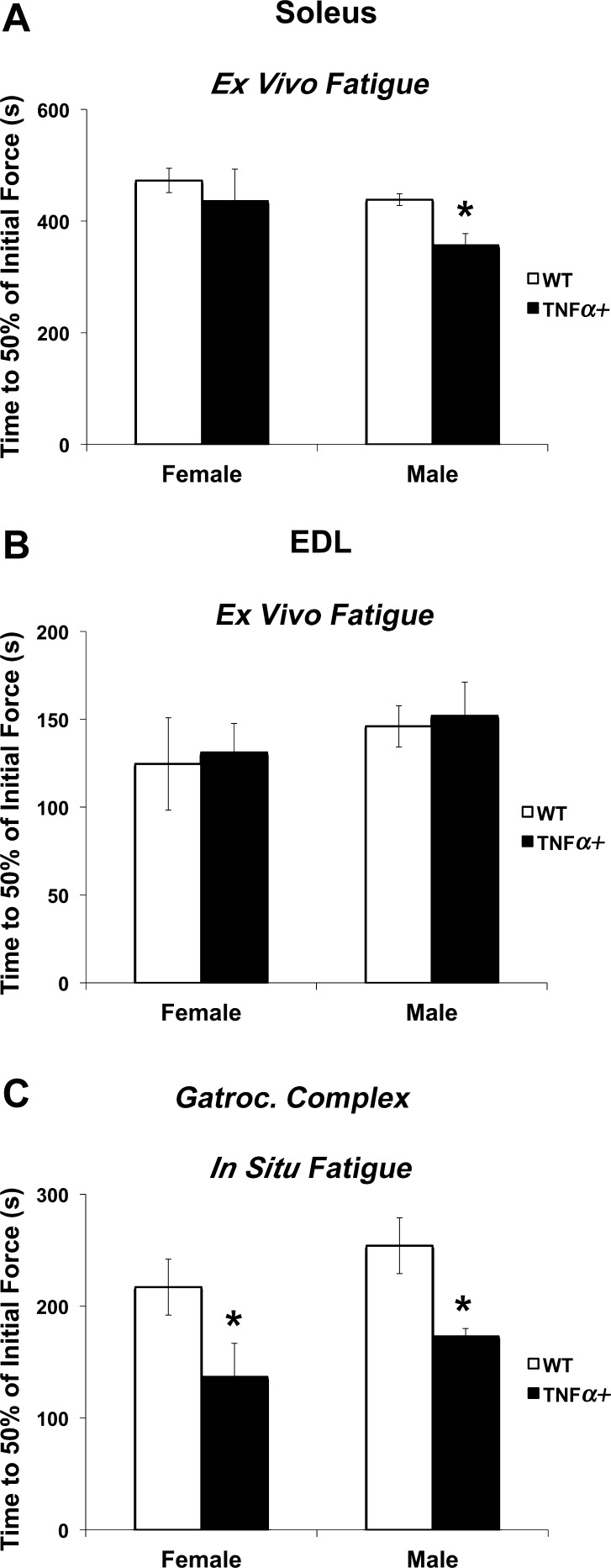
In soleus from male SP-C/TNFα+ mice studied ex vivo, the time to fatigue (a time for force to fall to 30% of initial force during a repetitive contraction protocol) was decreased compared with WT soleus (P < 0.05). However, the fatigue response in the soleus was not different between WT and SP-C/TNFα+ females (Fig. 5A). EDL muscle in either sex also did not show a difference in the time to fatigue with TNFα overexpression (Fig. 5B). In situ gastrocnemius complex (soleus, plantaris, and gastrocnemius) time to fatigue (reflecting both oxygen availability and muscle contractile function) was reduced similarly in both the female (37%) and male (32%) SP-C/TNFα+ gastrocnemius complex compared with WT (P < 0.05) (Fig. 5C).
Fig. 5.
Skeletal muscle fatigue is accelerated in SP-C/TNFα+ mice. The time to reach 30% of the initial force produced by soleus (A) and extensor digitorum longus (EDL) (B) muscles measured in vitro; n = 5. C: fatigue of the gastrocnemius complex stimulated to contract in situ. WT-female, n = 10; SP-C/TNFα+-female, n = 8; WT-male, n = 10; SP-C/TNFα+-male, n = 4. Values are means ± SE. *P < 0.05: difference between SP-C/TNFα+ and WT groups of the same sex.
Skeletal Muscle Oxidative Phenotype
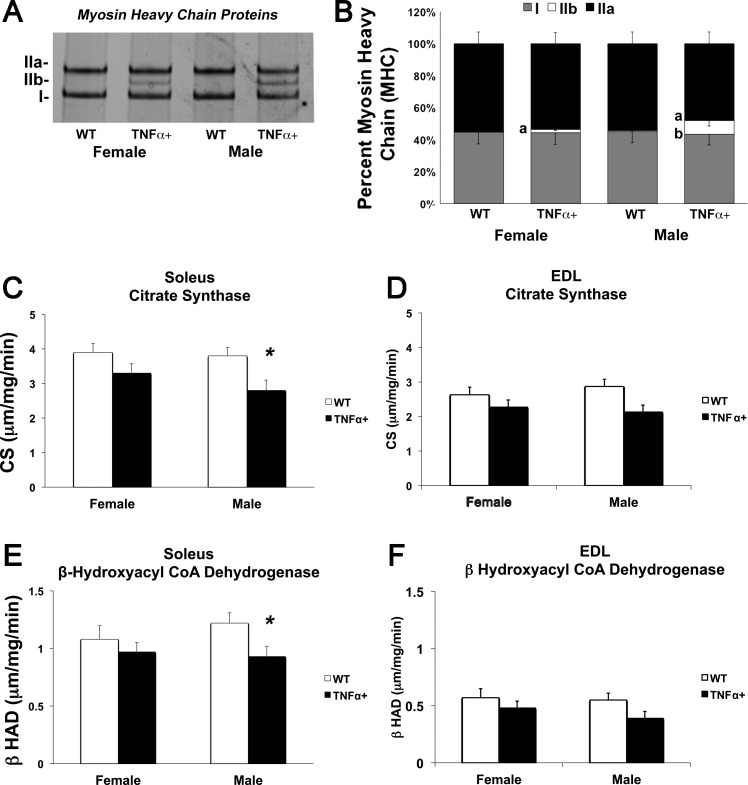
As shown in Fig. 6, A and B, in the soleus of female SP-C/TNFα+ mice, the percentage of MHC IIb was fourfold higher than in WT females (2.1 ± 0.8% vs. 0.5 ± 0.2%, P < 0.05). In the soleus of male SP-C/TNFα+ mice, the percentage of MHC IIb was 17-fold higher than in WT males (8.6 ± 3.5% vs. 0.5 ± 0.2%, P < 0.001). These changes were accompanied by 27% and 24% decreases in the activity level of the oxidative enzymes, CS (P < 0.02, Fig. 6C) and β-HAD (P < 0.045, Fig. 6E), respectively, in the soleus of SP-C/TNFα+ male mice. No significant changes in citrate synthase activity and β-HAD activity levels were detected in the female soleus and EDL of either sex (Fig. 6, D and F). Interestingly, measuring total abundance of oxidative phosphorylation proteins by immunoblot in the soleus did not show any significant difference between groups (data not shown). Skeletal muscle capillary-to-fiber ratio, measured in the soleus and EDL, was not different between any of the groups (data not shown).
Fig. 6.
Altered oxidative muscle phenotype in SP-C/TNFα+ male mice. The percentage of myosin heavy chain (MHC) types in the soleus from WT and SP-C/TNFα+ female and male mice. A: silver-stained gel of myosin chains separated by electrophoresis. B: densitometric analysis representing the proportion of type I, IIa, and IIb chains in each soleus protein extract. Means ± SD; n = 6. aP < 0.05 between SP-C/TNFα+ and WT groups of the same sex; bP < 0.05 between male and female of the same gene type; n = 6. C and D: citrate synthase enzyme activity in the soleus and EDL. E and F: β-HAD activity level in the soleus and EDL. Means ± SE; n = 6. *Difference between WT and SP-C/TNFα+ groups of the same sex: P < 0.05.
Gene Expression of Inflammatory, Angiogenic, and Metabolic Regulators in the Soleus and EDL of Both Male and Female WT and SP-C/TNFα+ Mice
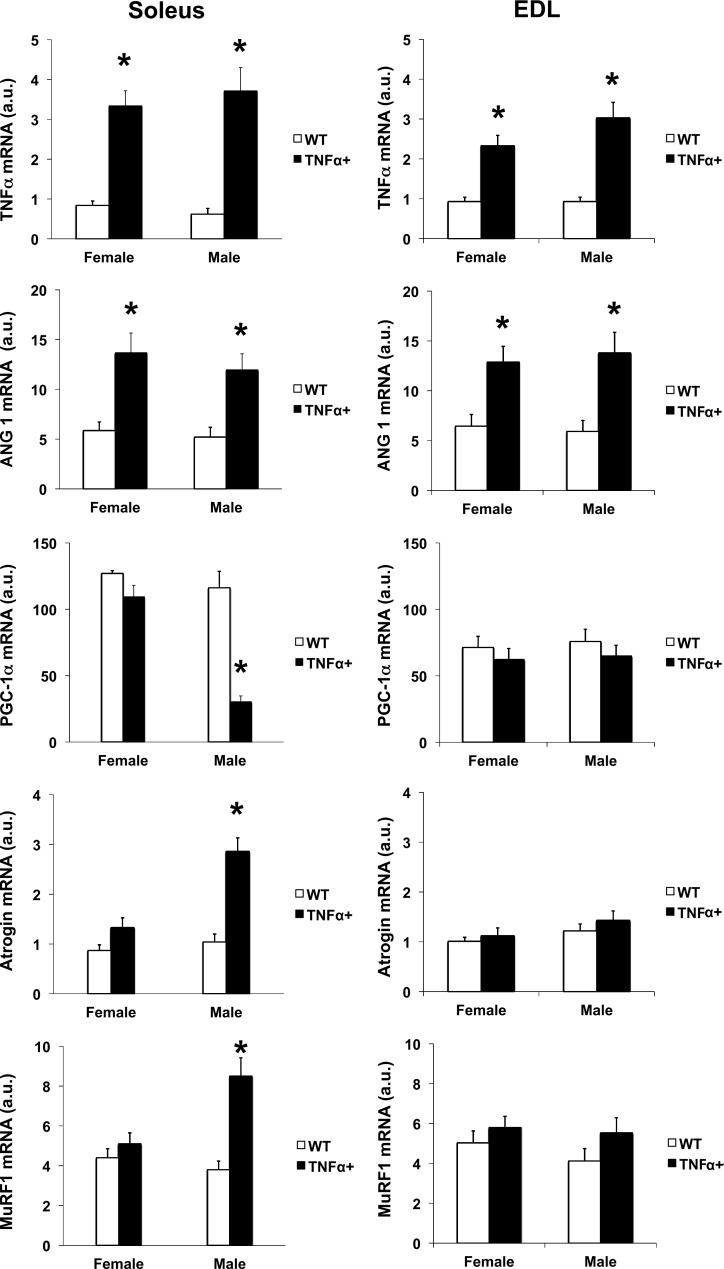
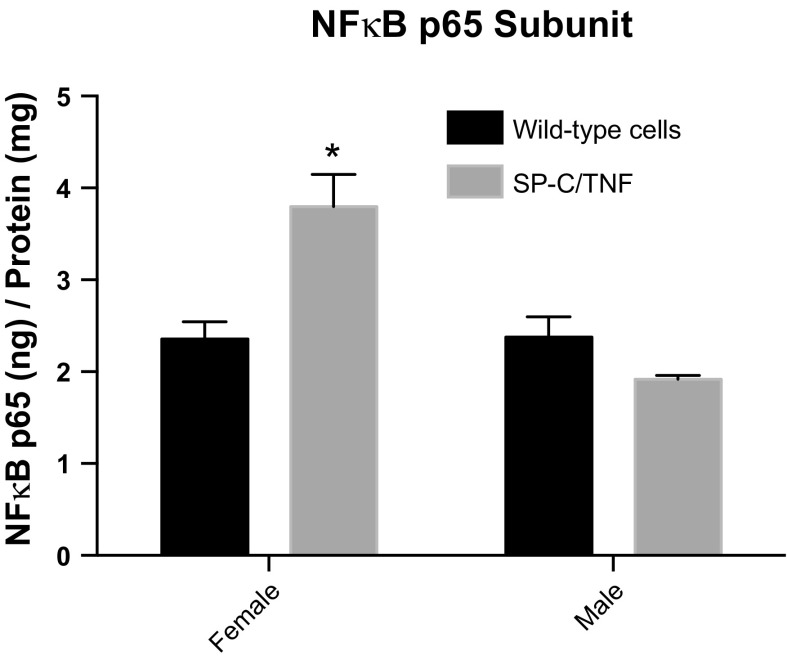
TNFα mRNA levels were increased in both female (P < 0.001) and male (P < 0.001) SP-C/TNFα+ mice compared with the sex control group (Fig. 7). Angiopoietin-1 mRNA levels were also increased by 2.4-fold in female SP-C/TNFα+ mice (P < 0.01) and 2.3-fold in male SP-C/TNFα+ (P < 0.05). PGC-1α mRNA decreased by 74% only in the soleus from male SP-C/TNFα+ mice (P < 0.001). Two genes involved in muscle atrophy, atrogin 1 and MuRF1, were upregulated in male, but not female, SP-C/TNFα+ soleus. Atrogin-1 mRNA levels were increased by 2.8-fold in SP-C/TNFα+ male soleus (P < 0.001). MuRF1 mRNA levels were increased by 2.2-fold in SP-C/TNFα+ male soleus (P < 0.0001). In contrast, the DNA binding activity of the canonical p65 subunit of NF-κB was increased in female, but not male, SP-C/TNFα mice (Fig. 8).
Fig. 7.
TNFα, angiopoietin-1, PGC-1α, atrogin-1, and MuRF1 mRNA level in the soleus and EDL of WT and SP-C/TNFα+ mice. Values represent means ± SE; n = 6. Difference between the WT and SP-C/TNFα+ group of the same sex are indicated: *P < 0.05.
Fig. 8.
NF-κB p65 subunit DNA binding activity is increased in female SP-C/TNFα+ skeletal muscle. DNA binding activity of the p65 NF-κB subunit was detected in gastrocnemius homogenates. Values represent means ± SE; n = 6. *P < 0.001 compared with all other groups.
DISCUSSION
Summary of Major Findings
In this study of a lifelong pulmonary inflammation mouse model, circulating TNFα reached very high levels in the lung and circulation similarly in both male and female mice. However, exercise limitation was selectively impaired only in male SP-C/TNFα mice. Changes in the SP-C/TNFα mouse phenotype that could contribute to this exercise limitation and which were found to be selectively altered in male mice are a decrease in overall body weight, skeletal muscle atrophy, and an impaired muscle oxidative phenotype that was accompanied by increased TNFα, MurF1, and atrogin1 and reduced PGC-1α expression.
Reduced Body Weight and Loss of the Oxidative Muscle Phenotype
A prominent change that occurred only in male SP-C/TNFα+ mice was an up to 23% lower body weight. Total activity in cage-confined mice was the same in both the SP-C/TNFα+ and WT male mice. A small increase in the RER, despite an increase in food and drink intake, suggests that the overall inflammatory state may contribute to a loss in body weight. In addition, the male soleus demonstrated a selective decrease in oxidative capacity (decreased percent of type I fibers and oxidative enzyme activity) and increased expression of two atrophy-related genes, atrogin and MurF1, even though all muscle types analyzed expressed elevated TNFα levels. This reduced oxidative capacity, detected from the reduced fatigue resistance of isolated muscles and reduced activity of oxidative enzymes, was not correlated with a reduction in the total abundance of mitochondrial proteins for oxidative phosphorylation. This finding would suggest that there might be an intrinsic impairment in the mitochondrial oxidative machinery. These data also suggest that loss in muscle mass as well as reduced muscle oxidative profile are not associated with physical inactivity, but related to the chronic pulmonary inflammatory state. Similar observations have been reported in some COPD patients that exhibit persistent weight loss despite an increase in dietary intake (10, 11).
There is evidence to support that both of these metabolic and atrophy pathways are aberrantly regulated in COPD patients and in mice exposed to cigarette smoke by the upstream metabolic regulator, PGC-1α (22, 43, 48, 54, 57). One unique function of PGC-1α is to stimulate muscle fiber remodeling. In PGC-1α-overexpressing mice, glycolytic fibers in the plantaris muscle have been reported to transition to oxidative fibers (39, 48). Inactivation of the PGC-1α gene in mice leads to the downregulation of genes involved in cellular respiration and results in decreased mitochondrial enzyme activity and ATP production in the heart (2). Selective expression of PGC-1α in oxidative but not glycolytic fibers may be one mechanism to restrict or target this muscle atrophy and/or loss of mitochondrial function to a subset of oxidative fiber types.
In contrast the canonical p65 NF-κB subunit, which is known to coordinate the regulation of inflammatory cytokines, was not upregulated in male SP-C/TNFα+ skeletal muscle. While there is evidence in muscle cell systems acutely treated with TNFα that the PGC-1α coactivator can repress NF-κB activation (16), in our study, total NF-κB DNA binding activity was increased in female SP-C/TNFα+ skeletal muscle and PGC-1α mRNA levels were unchanged. In vivo PGC-1α -NF-κB interactions were not measured. In contrast in male SP-C/TNFα+ skeletal muscle the level of PGC-1α mRNA was decreased and NF-κB DNA binding activity unaltered. Acute pulmonary inflammation, due to LPS instillation, and TNFα treatment have been reported to lead to muscle atrophy through an NF-kB mediated ubiquitin pathway. However, this does not appear to take place in response to chronic lung TNF-α expression (24, 32, 36, 37). An in vitro study performed in C2C12 cells suggests that coordinate expression of small heat shock proteins, such as αB crystalin, and NF-κB, may potentially protect myoblasts against TNF-α cytotoxicity by enhancing anti-apoptotic factors (1).
Soleus from male, but not female, TNFα overexpressing mice also demonstrate a more rapid fatigue response, as previously reported by Zuo et al. (60). However, when fatigue was assessed in situ, in the naturally perfused gastrocnemius complex, decreased fatigue resistance was observed in both female and male SP-C/TNFα+ mice. Analysis of the fatigue response in situ reflects not only the contractile ability of the muscle but oxygen available to skeletal muscle mitochondria. It should be noted that this experiment was performed in anesthetized mice. Thus anesthesia could attenuate diaphragm function in both male and female mice that already present an extensive degree of emphysema and elevation in pulmonary artery pressure. Another possibility is that the gastrocnemius is altered in both females and male TNFα mice. However, this larger muscle type cannot be accurately tested in an in vitro preparation. Experiments were limited to the smaller soleus and EDL muscles (4). Thus, under these experimental conditions, it is likely that reduced fatigue resistance in the mixed-fiber gastrocnemius complex of both sexes is potentially influenced by circulating cytokines, oxidative stress, or possible alterations in regional blood flow, noting that lung disease per se did not reduce arterial O2 saturation during exercise.
High Circulating TNF Levels and Normal Cardiac Function
Several cardiac-targeted TNFα transgenic mice have been engineered that exhibit a concentric hypertrophic cardiac phenotype or dilated cardiomyopathy depending on whether the membrane or soluble TNFα isoforms are overexpressed (6, 14, 15, 30, 38, 49). Cardiomyocyte TNFα overexpressing mice also have high circulating TNFα levels that lead to impaired skeletal muscle contractile function, particularly in the diaphragm (6, 15, 35). In contrast, in the present study TNFα expression in alveolar epithelial cells, under the control of the SP-C promoter, and the accompanying activation of inflammatory cells, resulted in an increase in serum TNFα levels (in the same ranges as the TNFα expressed by cardiomyocytes), but alterations in left ventricle structure and function at rest and in response to a dobutamine challenge were not detected (5, 6, 30, 35, 49). These observations suggest that the effect of TNFα on overall exercise capacity is not caused by cardiac dysfunction. The relatively mild increase in right ventricular pressure that was observed in male SP-C/TNFα mice likely reflects a response to the pathological vascular changes occurring in the lung. Thus these data suggest that high circulating TNFα levels alone are not necessarily a good indicator of imminent heart failure. Alternatively, the cellular source and presence or absence of additional protective factors may dictate whether cardiac dysfunction develops. Potential cardioprotective factors (i.e., TNFR2, Akt, TRAF2, STAT3, heat shock proteins) may also differ in their bioavailability depending on the sex and/or age of the mouse (7, 8, 25, 51). Male cardiomyocyte TNFα overexpression mice exhibit greater TNFR signaling and lower survival rates compared with female mice expressing the same TNFα transgene (28). However, in the lung-targeted TNFα overexpression model, female mice are protected from diminished growth and exercise capacity. One possibility is that the small elevation in wild-type female serum TNFα levels activates the TNFR2/JAK/STAT3 survival pathway (34). However, starting at 6 mo of age survival of female SP-C/TNFα+ mice rapidly declines (17), and this outcome suggests a decrease in an age-related, yet unidentified, female cardioprotective factor. Interestingly, STAT3 knockout mice and postpartum women with low serum STAT3 levels develop dilated cardiac myopathy shortly after the stress of giving birth due to high oxidative stress and premature regression of left ventricle capillaries (26). More studies will be required to understand the balance of TNFR1 and TNFR2 signaling and sex-specific protective factors that differentially regulate muscle function and overall survival in response to high TNFα expression in the lung.
Increased TNFα and Angiopoietin Expression Within Skeletal Muscle
Both female and male soleus and EDL muscles from pulmonary TNFα overexpressing mice have enhanced skeletal muscle TNFα and angiopoietin expression. While a balance or interplay between VEGF, angiopoietin 1, and angiopoietin 2 are necessary to form mature vessels during development, angiopoietin 1 is not necessary to stabilize uninjured vessels in the adult organism (27). Consistent with this finding, the number of capillaries surrounding myofibers in the soleus and EDL of adult SP-C/TNFα+ mice in the present study was not altered. These data indicate that skeletal muscle in both male and female mice are responding to pulmonary TNFα overexpression. However, the activation of pathways that protect from or signal muscle atrophy and fatigue differ depending on sex.
Limitations of the SP-C/TNFα COPD Mouse Model
TNFα is often measured as one of a set of inflammatory biomarkers in serum from COPD patients. Disease symptoms often manifest in older individuals with COPD. However, TNFα overexpression occurs early on during the lifespan of the SP-C/TNFα+ transgenic mouse line and could allow compensatory mechanisms to be initiated during development. Nevertheless, TNFα expression in several locomotor skeletal muscles from SP-C/TNFα+ mice was elevated 1.7-fold compared with the control group, and this is in the range of the study reported by Remels et al. (43), which showed that a subset of COPD patients expressing 3.9-fold higher vastus lateralis muscle TNFα mRNA levels exhibited reduced body mass. Furthermore, while the levels of serum TNFα in healthy and COPD patients varies greatly among research studies, the level expressed by the SP-C/TNFα+ mouse greatly exceeds what has been reported in patients with COPD (13, 20, 42, 55, 59).
Potential Protective Role of Estrogen
Female SP-C/TNFα+ mice showed a much milder or even no response to the elevated circulatory TNFα levels in most of the physiological parameters measured. Thus it is possible that higher steroid levels in female mice have a protective effect. Estrogen and estrogen derivatives have been reported to attenuate the effects of multiple proinflammatory factors including TNFα (29) as well as protect against pulmonary hypertension (31, 45, 56), motoneuron apoptosis (12), and immobilization-induced atrophy (50). Furthermore, healthy men supplemented with estradiol were found to increase PGC-1α expression and augment β-oxidation of lipids (40). Conversely, mice engineered with an estrogen receptor (ERα) deficiency have an impaired oxidative metabolism and inflammation that is associated with increased skeletal muscle insulin resistance and increased Hsp72 (46). The more prominent pulmonary hypertension, weight loss, and attenuated oxidative metabolism in male SP-C/TNFα+ mice compared with female mice suggest that estrogen-related pathways may partially protect female mice from the robust expression of pulmonary TNFα.
Summary
In the present study, we investigated cardiac and skeletal muscle responses to lung overexpression of TNFα in male and female mice. SP-C/TNFα+ transgenic mice have a lifelong 30- to 80-fold elevation of circulatory TNFα and severe lung damage that could contribute to exercise limitation. Male (but not female) SP-C/TNFα+ mice showed significant decreases in exercise capacity and oxidative muscle fatigue resistance accompanied by a loss of whole body weight and skeletal muscle oxidative capacity. Cardiac function was, however, preserved. Although circulating TNFα levels were far higher in our mice than in patients with COPD, this phenotype reflects the situation in a subset of COPD patients (43). In female SP-C/TNFα+ mice, with similar circulating TNFα levels and lung damage, there was maintenance of body weight and little or no skeletal muscle dysfunction. These findings suggest that estrogen may have protective effects against the actions of TNFα.
GRANTS
This work was funded by Grant TRDRP 12RT-0062 and National Institutes of Health (NIH) Grant PO1-HL-17731-28.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.T., G.M., H.W., L.N., A.T., N.D.D., Y.G., and E.C.B. performed experiments; K.T., G.M., L.N., N.D.D., Y.G., K.L.P., and E.C.B. analyzed data; K.T., G.M., P.D.W., K.L.P., and E.C.B. interpreted results of experiments; K.T. and E.C.B. prepared figures; K.T., L.N., P.D.W., N.D.D., K.L.P., and E.C.B. edited and revised manuscript; K.T., L.N., P.D.W., and K.L.P. approved final version of manuscript; P.D.W., K.L.P., and E.C.B. conception and design of research; E.C.B. drafted manuscript.
REFERENCES
- 1. Adhikari AS, Singh BN, Rao KS, Rao Ch M. alphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim Biophys Acta 1813: 1532–1542, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101: 3697–3702, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil 26: 225–235, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97: 1382–1391, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 97: 1375–1381, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, Entman ML, Sivasubramanian N, Mann DL. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail 3: 157–164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai D, Xaymardan M, Holm JM, Zheng J, Kizer JR, Edelberg JM. Age-associated impairment in TNF-alpha cardioprotection from myocardial infarction. Am J Physiol Heart Circ Physiol 285: H463–H469, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 5: 549–555, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creutzberg EC, Schols AM, Weling-Scheepers CA, Buurman WA, Wouters EF. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 745–752, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 162: 1239–1245, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Das A, Acharya S, Gottipati KR, McKnight JB, Chandru H, Alcorn JL, Boggaram V. Thyroid transcription factor-1 (TTF-1) gene: identification of ZBP-89, Sp1, and TTF-1 sites in the promoter and regulation by TNF-alpha in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 301: L427–L440, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 150: 1453–1455, 1994. [DOI] [PubMed] [Google Scholar]
- 14. Dibbs ZI, Diwan A, Nemoto S, DeFreitas G, Abdellatif M, Carabello BA, Spinale FG, Feuerstein G, Sivasubramanian N, Mann DL. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation 108: 1002–1008, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, Wilson EM, Spinale FG, Mann DL. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation 109: 262–268, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J Biol Chem 288: 2246–2260, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujita M, Ikegame S, Ye Q, Harada E, Ouchi H, Inoshima I, Watanabe K, Mason RJ, Nakanishi Y. Attenuation of pulmonary hypertension, but not emphysematous change, by breeding emphysema model mice at sea level. Cytokine 41: 286–292, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Fujita M, Mason RJ, Cool C, Shannon JM, Hara N, Fagan KA. Pulmonary hypertension in TNF-alpha-overexpressing mice is associated with decreased VEGF gene expression. J Appl Physiol 93: 2162–2170, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 280: L39–L49, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Rio F, Miravitlles M, Soriano JB, Munoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Ancochea J. Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res 11: 63, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. [Available from: http://www.goldcopd.org/].
- 22. Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J 30: 73–79, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Gosker HR, Langen RC, Bracke KR, Joos GF, Brusselle GG, Steele C, Ward KA, Wouters EF, Schols AM. Extrapulmonary manifestations of COPD in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol 40: 710–716, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Haegens A, Schols AM, Gorissen SH, van Essen AL, Snepvangers F, Gray DA, Shoelson SE, Langen RC. NF-κB activation and polyubiquitin conjugation are required for pulmonary inflammation-induced diaphragm atrophy. Am J Physiol Lung Cell Mol Physiol 302: L103–L110, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Higuchi Y, Chan TO, Brown MA, Zhang J, DeGeorge BR, Jr, Funakoshi H, Gibson G, McTiernan CF, Kubota T, Jones WK, Feldman AM. Cardioprotection afforded by NF-kappaB ablation is associated with activation of Akt in mice overexpressing TNF-alpha. Am J Physiol Heart Circ Physiol 290: H590–H598, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589–600, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J Clin Invest 106: 589–597, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp Gerontol 40: 549–555, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81: 627–635, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 30: 660–667, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Langen RC, Haegens A, Vernooy JH, Wouters EF, de Winther MP, Carlsen H, Steele C, Shoelson SE, Schols AM. NF-kappaB activation Is required for the transition of pulmonary inflammation to muscle atrophy. Am J Respir Cell Mol Biol 47: 288–297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 35: 689–696, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J 32: 680–685, 2011. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Moody MR, Engel D, Walker S, Clubb FJ, Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17: 1048–1057, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12: 871–880, 1998. [DOI] [PubMed] [Google Scholar]
- 38. Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA 97: 12746–12751, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics 42: 342–347, 2010. [DOI] [PubMed] [Google Scholar]
- 41. Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest 96: 250–259, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piehl-Aulin K, Jones I, Lindvall B, Magnuson A, Abdel-Halim SM. Increased serum inflammatory markers in the absence of clinical and skeletal muscle inflammation in patients with chronic obstructive pulmonary disease. Respiration 78: 191–196, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, Galdiz J, Wouters EF, Langen RC, Schols AM. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J 24: 5052–5062, 2010. [DOI] [PubMed] [Google Scholar]
- 44. Renvall MJ, Friedman P, Ramsdell JW. Predictors of body mass index in patients with moderate to severe emphysema. COPD 6: 432–436, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 280: L88–L97, 2001. [DOI] [PubMed] [Google Scholar]
- 46. Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab 298: E304–E319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenblatt JD, Kuzon WM, Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol 62: 85–92, 1987. [DOI] [PubMed] [Google Scholar]
- 48. Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104: 826–831, 2001. [DOI] [PubMed] [Google Scholar]
- 50. Sugiura T, Ito N, Goto K, Naito H, Yoshioka T, Powers SK. Estrogen administration attenuates immobilization-induced skeletal muscle atrophy in male rats. J Physiol Sci: 393–399, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Szalay L, Shimizu T, Suzuki T, Yu HP, Choudhry MA, Schwacha MG, Rue LW, 3rd, Bland KI, Chaudry IH. Estradiol improves cardiac and hepatic function after trauma-hemorrhage: role of enhanced heat shock protein expression. Am J Physiol Regul Integr Comp Physiol 290: R812–R818, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J., Jr Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94: 1109–1117, 1996. [DOI] [PubMed] [Google Scholar]
- 53. Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63–69, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Tang K, Wagner PD, Breen EC. TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222: 320–327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm 7: 29, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol 45: 358–367, 2006. [DOI] [PubMed] [Google Scholar]
- 57. Uguccioni G, Hood DA. The importance of PGC-1alpha in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab 300: E361–E371, 2011. [DOI] [PubMed] [Google Scholar]
- 58. van den Borst B, Koster A, Yu B, Gosker HR, Meibohm B, Bauer DC, Kritchevsky SB, Liu Y, Newman AB, Harris TB, Schols AM. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax 66: 961–969, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J 31: 492–501, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Zuo L, Nogueira L, Hogan MC. Effect of pulmonary TNF-α overexpression on mouse isolated skeletal muscle function. Am J Physiol Regul Integr Comp Physiol 301: R1025–R1031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]