Abstract
A profound remodeling of the diaphragm and vastus lateralis (VL) occurs in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). In this mini-review, we discuss the following costal diaphragm remodeling features noted in patients with moderate-to-severe COPD: 1) deletion of serial sarcomeres, 2) increased proportion of slow-twitch fibers, 3) fast-to-slow isoform shift in sarco(endo)plasmic reticulum Ca2+-ATPase, 4) increased capacity of oxidative metabolism, 5) oxidative stress, and 6) myofiber atrophy. We then present the sole feature of diaphragm remodeling noted in mild-to-moderate COPD under the heading “MyHC and contractile remodeling noted in mild-to-moderate COPD.” The importance of VL remodeling in COPD patients as a prognostic indicator as well as a major determinant of the ability to carry out activities of daily living is well accepted. We present the remodeling of the VL noted in COPD patients under the following headings: 1) Decrease in proportion of slow-twitch fibers, 2) Decreased activity of oxidative pathways, 3) Oxidative and nitrosative stress, and 4) Myofiber atrophy. For each of the remodeling features noted in both the VL and costal diaphragm of COPD patients, we present mechanisms that are currently thought to mediate these changes as well as the pathophysiology of each remodeling feature. We hope that our mechanistic presentation stimulates research in this area that focuses on improving the ability of COPD patients to carry out increased activities of daily living.
Keywords: diaphragm, vastus lateralis, chronic obstructive pulmonary disease, fiber type transformation, slow-twitch fiber, fast-twitch fiber
recently, we have learned a great deal about the remodeling of the diaphragm and limb muscles in patients with chronic obstructive pulmonary disease (COPD). Indeed, for additional perspectives in this area, we refer the reader to several excellent recent review articles (10, 14, 24, 25, 39, 41, 47, 62, 65, 72, 74, 75). In this mini-review, we present selective aspects of this remodeling noted in the human diaphragm and vastus lateralis (VL)–the most commonly studied limb muscle–of COPD patients. For each feature of remodeling, we first provide a description of the particular characteristic(s) noted in the human diaphragm or VL; subsequently, we detail experimental animal and in vitro studies to attempt to elucidate the mechanisms underlying this remodeling, and we then provide our thoughts regarding the pathophysiological relevance of the remodeling feature (where appropriate). Although the features of COPD-induced remodeling of the diaphragm are numerous, many appear to represent the transformation of protein isoforms noted in fast-twitch fibers to those noted in slow-twitch fibers; we use the “term fast-to-slow transformation” to describe these changes. In contrast, COPD-induced remodeling of the VL is characterized by the transformation of many protein isoforms noted in slow-twitch fibers to those isoforms noted in fast-twitch fibers; accordingly, we use the term “slow-to fast transformation” to describe these latter changes.
Diaphragm
Currently, it is common to organize features of COPD-induced diaphragm remodeling into those that occur in moderate-to-severe COPD and those that occur in mild-to-moderate COPD. In this mini-review, we follow this convention by first presenting those features of remodeling noted in moderate to severe COPD; we present these under the following headings: 1) Deletion of sarcomeres from the costal diaphragm, 2) Increased proportion of slow-twitch fibers, 3) Increased capacity for oxidative metabolism, 4) Fast-to-slow isoform shift in sarco(endo)plasmic reticulum Ca2+-ATPase, 5) Oxidative stress, and 6) Myofiber atrophy. We then present features of diaphragm remodeling noted in mild-to moderate COPD under the single heading titled “MyHC and contractile remodeling noted in mild-to-moderate COPD.”
Deletion of sarcomeres from the costal diaphragm.
Briefly, due to the length-tension relationship, the length of costal diaphragm fibers is greatest at functional residual capacity (FRC) and then progressively decreases until total lung capacity (TLC) is reached. At FRC, the individual sarcomeres in the muscle fibers are at the optimum length for force generation (i.e., Lo), and, as the diaphragm contracts to TLC, a progressive increase in distance from Lo occurs in each of the sarcomeres. Therefore, if there were no chronic muscle length adaptations to hyperinflation, the contribution of the diaphragm to inspiratory pressure as lung volume increases from FRC to TLC would be severely reduced. In seminal experiments in the early 1980s, two research groups (11, 12, 44, 66) demonstrated that >3 mo after intratracheal administration of elastase to the hamster, hamsters developed marked thoracic hyperinflation and the maximum tension generated by these hamster diaphragm fibers showed a prominent shift to the left of the length-tension relationships, i.e., maximum tension occurred at a shorter fiber length than in control hamsters. Moreover, the number of sarcomeres in series in the diaphragms of these emphysematous hamsters was markedly reduced in a manner such that the length-tension relationships of the emphysematous diaphragm sarcomeres did not differ from controls (who had been given intratracheal injections of saline). At the present time, there has been widespread confirmation of these findings in experimental animals, but the relevance of these studies to humans with COPD is still somewhat uncertain (2, 57). Additionally, we do not know the molecular mechanism effecting this phenomenon. Therefore, we suggest that the various signaling and proteolytic diaphragm pathways eliciting sarcomere deletions in the elastase-treated group can be elucidated by serial observations during the time that this remodeling is occurring, e.g., the first 3 mo after intratracheal instillation of elastase or saline. We refer the reader to our prior review (10) for a more detailed discussion of this topic.
Increased proportion of slow-twitch fibers.
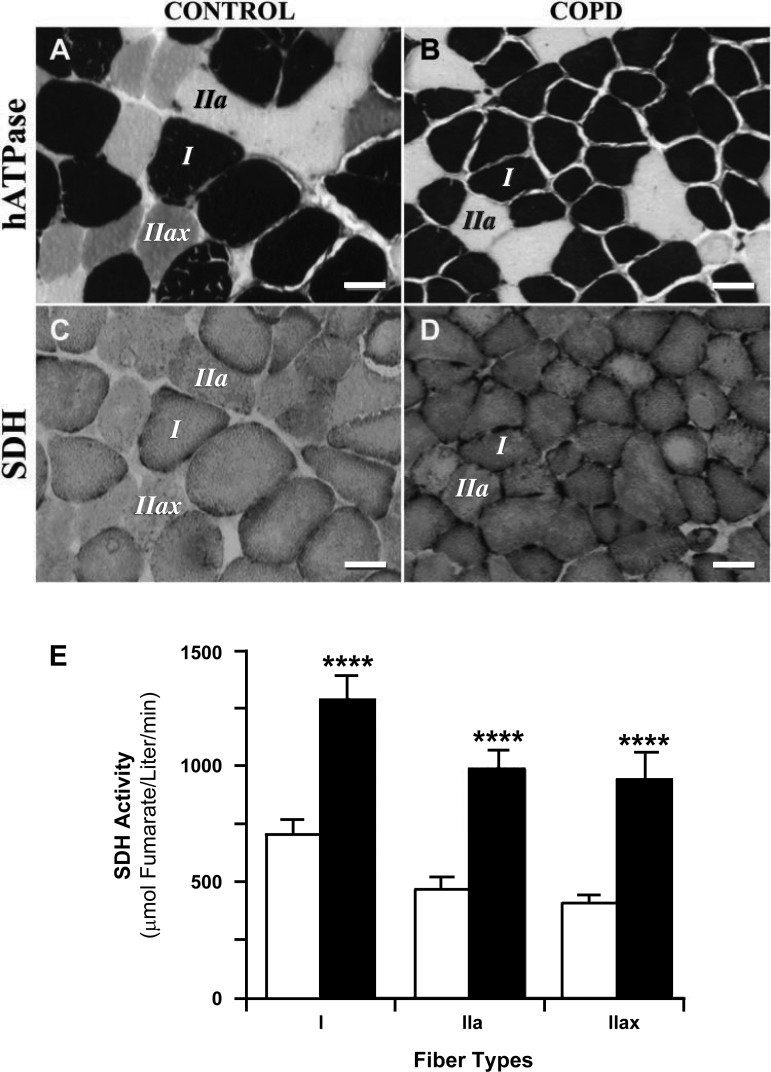
Over the past decade, multiple investigators (27–30, 40) have demonstrated that the diaphragms of patients with severe COPD exhibit an increased proportion of slow-twitch fibers and decreases in the proportion of fast-twitch fibers. Figure 1, A and B, shows representative slides prepared from diaphragm biopsies followed by histochemical staining for myosin heavy chain (MyHC)-determined fiber types (6). The section from COPD patients (i.e., Fig. 1B) shows an increased proportion of slow fibers and a decrease in fiber size of both type I and II fibers compared with the control section (i.e., Fig. 1A). Since it appears that this COPD-related muscle fiber remodeling is due to fast fibers changing their phenotype to slow fibers, we will refer to this aspect of remodeling as fast-to-slow fiber transformation. Additionally, biochemical analyses of diaphragm homogenates indicated that severe COPD diaphragms had higher expression of slow MyHCs as well as the slow isoforms of myosin light chains, troponins, and tropomyosins, whereas diaphragm fibers from control subjects had higher percentages of the fast isoforms of these proteins (28). These data suggest that COPD diaphragms had a higher proportion of slow-twitch motoneurons and a decreased proportion of fast-twitch motoneurons.
Fig. 1.
Staining of serial diaphragmatic sections for fiber typing (A and B) and succinate dehydrogenase (SDH) activity (C and D). Labeled fibers in SDH sections are the same fibers labeled in the histochemical myosin ATPase (hATPase) sections (6). Calibration bars = 50 μm. A and B: fiber type stains. Dark-stained fibers are type I fibers, light-stained fibers are type IIa fibers, and intermediate-stained fibers are type IIax fibers. C and D: quantitative SDH stain. The intensity of the stain is directly related to SDH activity, i.e., the darker the fiber, the greater the SDH activity (77). E: fiber type-specific activities of SDH. In each of the fiber types (i.e., I, IIa, and IIax), SDH activity in chronic obstructive pulmonary disease (COPD) diaphragms was increased ∼100% over controls (****P < 0.0001). [Reproduced, with permission, from Ref. 27.]
Since slow-twitch motoneurons are more fatigue resistant than fast-twitch motoneurons (31), this feature of remodeling suggests that the diaphragms of severe COPD patients should be more fatigue resistant than control diaphragms. We recognize that this statement pertains only to in vitro fatigue measurements using the nerve-muscle preparation as described by Leiber et (31). However, in vivo, fatigue can be initiated at many points in the motor pathway from cortical neurons to various intracellular processes in the myofiber. A discussion of this topic is contained in another article in this series entitled “Neuromotor control of skeletal muscle in COPD.” However, considering only diaphragmatic motoneurons, the maximum isometric force generated by slow motoneurons is less than that of fast-twitch motoneurons (17); therefore, this postulated increase in fatigue resistance comes at the expense of decreased strength.
MECHANISMS OF FAST-TO-SLOW FIBER TRANSFORMATION.
Calcineurin (Cn) is a Ca2+/calmodulin-regulated protein phosphatase that acts on transcription factors that were first noted in the nucleus of activated T cells; indeed, the abbreviation NFAT (for nuclear factor of active T cells) is used to describe these factors. Cn is a heterodimer of catalytic (CnA) and regulatory (CnB) subunits. Both Cn subunits and NFATs are composed of various isoforms. All of the four Cn-dependent NFATs (7) are expressed in skeletal muscle.
Several lines of evidence indicate that transcription factors of the NFAT family act as nerve activity sensors in skeletal muscle and control activity-dependent fiber type specialization (9, 32). First, when NFATc1-green fluorescent protein (GFP) fusion protein is expressed in isolated, unstimulated fibers from the adult mouse flexor digitorum brevis, a predominantly fast-twitch muscle, it shows a cytoplasmic localization but translocates to the nucleus in fibers stimulated with a low-frequency pattern typical of slow motor units (9). Second, in vivo studies have revealed that NFATc1-GFP has a predominantly cytoplasmic localization in the fast tibialis anterior muscle but a predominantly nuclear localization in the slow soleus muscle (70). Finally, NFATc1 nuclear import is rapidly induced in fast tibialis anterior muscle fibers by low-frequency electrical stimulation, whereas nuclear export is rapidly induced in slow soleus muscle fibers by inactivity consequent to denervation or anesthesia.
The increased neural activity to the COPD diaphragm changes cytoplasmic Ca2+ activity from high-amplitude pulses of short duration to low-amplitude pulses of long duration (see Fig. 2A). The literature indicates that due to this change, activated Cn then binds to a combination of transcription factors that code for various aspects of the “slow fiber developmental” program. It is important to note that these slow program transcription factors are essential to the fast-to-slow fiber type MyHC remodeling since Cn alone does not activate this transformation (9). This postulated mechanism is important in explaining the finding that fast-to-slow MyHC transformation in the COPD diaphragm is accompanied by similar fast-to-slow transformation in all of the following myofibrillar proteins: myosin light chains 1, 2, and 3, α- and β-units of tropomyosin, and troponins C, T, and I (28).
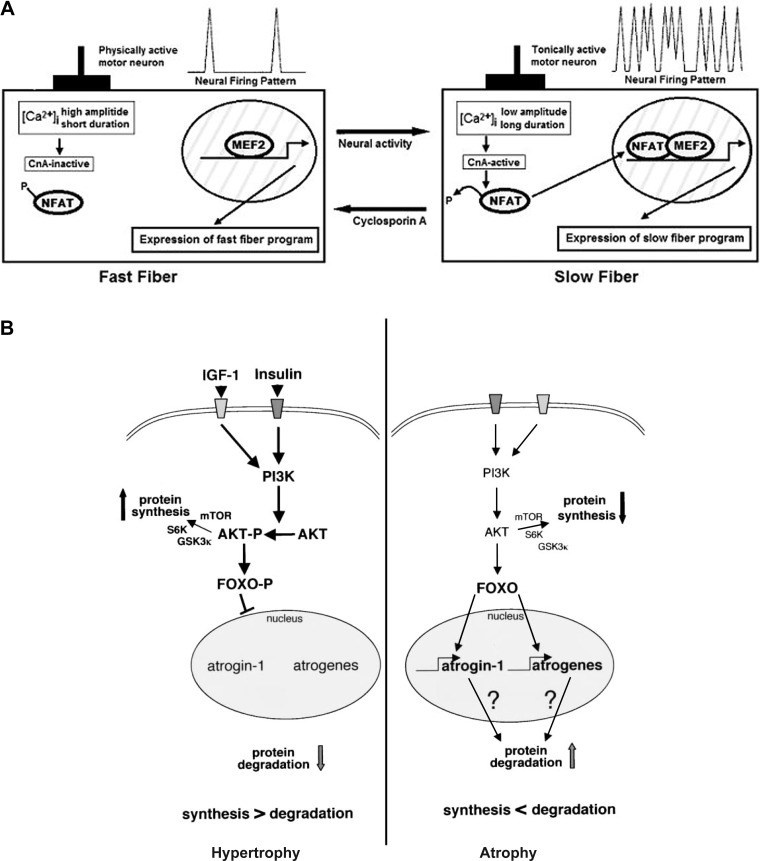
Fig. 2.
A: model for a calcineurin-dependent pathway linking specific patterns of motor nerve activity to distinct programs of gene expression that establish phenotypic differences between slow and fast myofibers. MEF2 is shown to represent the requirement for collaboration between activated nuclear factor of activated T cells (NFAT) proteins and muscle-restricted transcription factors in slow fiber-specific gene transcription, but other proteins (not shown) also are likely to participate. [Ca2+]i, intracellular Ca2+ concentration; CnA, catalytic calcineurin subunit. [Reproduced, with permission, from Ref. 9.] B: summary of the roles of the IGF-I/Akt pathway and forkhead box O (FOXO) in muscle atrophy (right) and hypertrophy (left). The schematics indicate that a decrease in Akt phosphorylation elicits increased binding of FOXO1 to nuclear DNA, which results in increased transcription of atrogin-1 and muscle-specific RING finger protein 1 (i.e., ubiquitin ligases), thereby increasing protein degradation. Additionally, a decrease in the phosphorylation level of Akt is associated with a decrease in protein synthesis due to dephosphorylation of glycogen synthase kinase (GSK)-3, mammalian target of rapamycin (mTOR), and S6 kinase (S6K). PI3K, phosphatidylinositol 3-kinase. Factors and pathways in bold are activated. [Reproduced, with permission, from Ref. 60.]
Increased capacity for oxidative metabolism.
Due to the increase in type I fibers and the accompanying decrease in type II fibers (see above), one would expect an increase in oxidative capacity and a decrease in glycolytic capacity in the COPD diaphragm. Indeed, the study by Wijnhoven et al. (79) demonstrated that diaphragms from COPD patients exhibit increases in 3-hydroxyacyl-CoA dehydrogenase (HADH; a biomarker for β-oxidative capacity), and a study by Sanchez et al. (59) showed decreases in both lactate dehydrogenase and hexokinase (biomarkers for glycolytic capacity). Importantly, Levine et al. (27) used quantitative histochemical determinations of succinic dehydrogenase (SDH) activity (77) to compare fiber type-specific SDH activity in COPD and control diaphragm biopsies (Fig. 1, C–E). A comparison of Fig. 1, C and D, indicates that COPD diaphragms exhibited appreciably greater SDH activity than control diaphragms. Moreover, Fig. 1E shows that in each of the fiber types (i.e., I, IIa, and IIax), SDH activity in COPD diaphragms was increased ∼100% over control diaphragms. These latter observations indicate that the increase in oxidative enzyme activity in COPD diaphragm homogenates cannot be solely due to the fast-to-slow fiber type transformation; rather, they suggest that in severe COPD patients, all fiber types become more oxidative.
Other investigators have obtained more direct mitochondrial measurements on diaphragm biopsies from COPD patients and control subjects. Wijnhoven et al. (79) noted an increase in mitochondrial electron transport system complexes III and IV and a statistically significant increase in the capacity for pyruvate oxidation as the severity of COPD increased. Moreover, an electron microscopy study by Orozco-Levi et al. (45) demonstrated that compared with control diaphragms, COPD diaphragms exhibited an increase in the volume fraction of mitochondria; indeed, these authors showed a statistically significant negative correlation between the volume fraction of mitochondria and forced expiratory volume in 1.0 s (FEV1.0; expressed as percent predicted). Finally, Ribera and coworkers (56) carried out a study on in situ mitochondrial preparations prepared from COPD and control diaphragm biopsies, and they noted that COPD mitochondria exhibited increases in both maximum O2 consumption and acceptor control of respiration. Therefore, their work suggests that COPD mitochondria exhibited increased oxidative capacity as well as increased coupling of oxidative phosphorylation.
Recent work by Rasbach et al. (55) demonstrated that a necessary component of the peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α-induced switch to oxidative fiber types is the PGC-1α induction of hypoxia-inducible factor-2α. This pathway is also dependent on the activity of sirtuin 1, a redox-sensitive (NAD+ dependent) deacetylase enzyme (69, 78). The sensitivity of these systems to both tissue oxygenation and the redox state (NAD+/NADH) of the cytosol raises interesting possibilities for future research in understanding why respiratory muscles and limb muscles undergo different fiber type programs in COPD.
Fast-to-slow isoform shift in sarco(endo)plasmic reticulum Ca2+-ATPase.
Previous workers have noted that after the MyHC, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) uses more ATP than any other ATPase enzyme during isometric contraction (67). Additionally, investigators in the area of muscle remodeling have indicated that a decrease in diaphragm ATP utilization would help prevent low-frequency fatigue of this organ. Therefore, Nguyen et al. (43) hypothesized that a fast-to-slow isoform transformation of SERCA would be present in the diaphragms of patients with severe COPD because it would decrease ATP utilization.
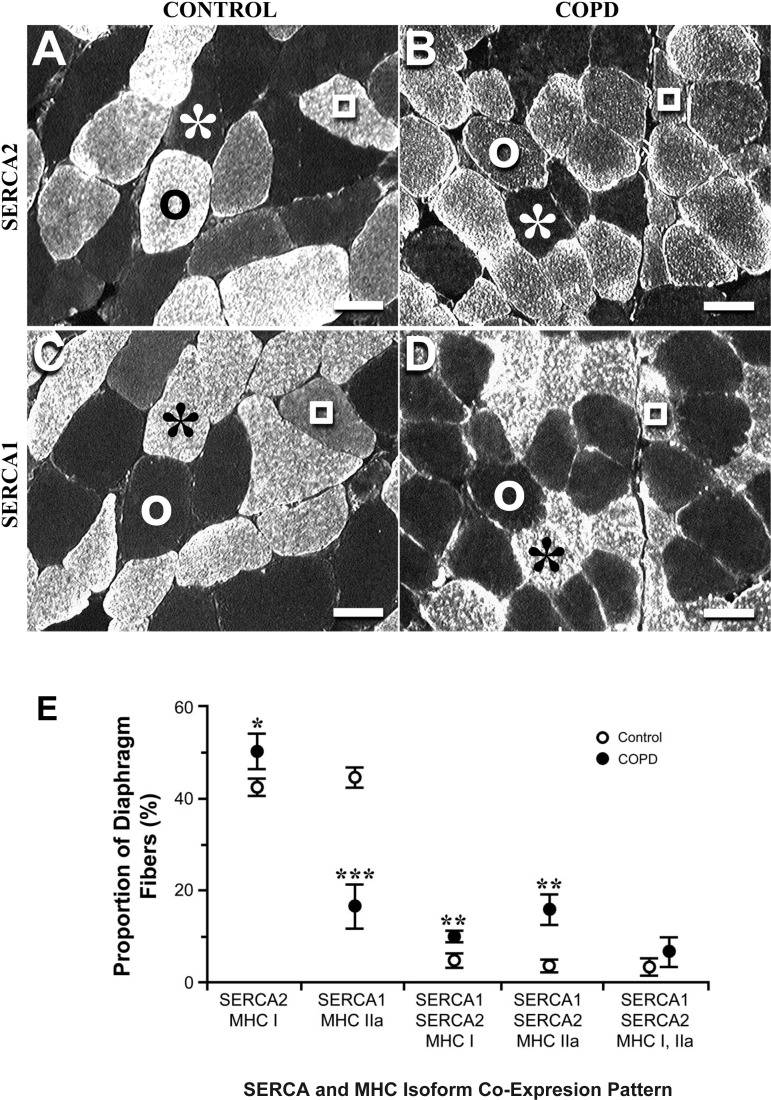
Using isoform-specific antibodies (43), they showed that compared with control diaphragms, severe COPD diaphragms exhibited a large decrease in fibers expressing only SERCA1 (the fast isoform), a large increase in hybrid fibers containing both isoforms of SERCA, and a small increase in fibers containing only SERCA2 (the slow isoform). These features of COPD remodeling are shown in Fig. 3, A–D. Additionally, immunoblot experiments carried out on diaphragm homogenates demonstrated that severe COPD diaphragms expressed only one-third the SERCA1 content noted in control diaphragms, whereas the two groups did not differ with respect to SERCA2 content (43). The combination of these histological and immunoblot results are consistent with the hypothesis that diaphragm remodeling elicited by severe COPD is characterized by a fast-to-slow SERCA isoform transformation.
Fig. 3.
Serial sections from control (A and C) and COPD (B and D) diaphragms. A and B: sections stained with monoclonal antibody to the sarco(endo)plasmic reticulum Ca2+ATPase (SERCA) slow isoform (SERCA2). C and D: sections stained with monoclonal antibody to the fast SERCA isoform (SERCA1). In A–D, the light fibers are the ones that reacted with the antibody. Both diaphragms contained three types of fibers; the representative fibers of these types are indicated by the following symbols: *, fiber expressing only SERCA1; ○, fiber expressing only SERCA2; and □, fiber expressing both SERCA isoforms (i.e., a hybrid fiber). The comparison of A and B indicates that the COPD diaphragm contained a larger proportion of SERCA2 fibers than the control diaphragm, whereas the comparison of C and D indicates that the control diaphragm contained a larger proportion of SERCA1 fibers than the COPD diaphragm. Calibration bars = 50 μm. E: comparison of COPD and control diaphragms with respect to SERCA and myosin heavy chain (MyHC) coexpression patterns. These data indicate coordinate changes in the expression of MyHC and SERCA. Multivariate ANOVA showed highly statistically significant differences between COPD and control data sets, and differences between groups for each of the comparisons were as follows: *P < 0.05, **P <0.01, and ***P <0.001. [Reproduced, with permission, from Ref. 43.]
Additionally, using serial section histochemistry, Nguyen et al. (43) determined the coexpression pattern of SERCA and MyHC; the results of this study are shown in Fig. 3E. Figure 2E shows coordinate remodeling of SERCA and MyHC isoforms, and these data are once again consistent with the hypothesis that diaphragm remodeling elicited by severe COPD should decrease ATP utilization by diaphragm myofibers relative to control fibers at a given time tension index.
Finally, these changes in SERCA expression should decrease the rate of pumping Ca2+ from the myoplasm into the sarcoplasmic reticulum (at the termination of the contractile phase), and this should shift the force-frequency curve to the left, i.e., this will permit a given level of force generation with a lower frequency of stimulation, which, in turn, will reduce the ATP cost of Ca2+ pumping. These phenomena are known to occur in laboratory experiments, and they may be pertinent to the in vivo human diaphragm as well. Nonetheless, more studies are needed to elucidate this area.
Oxidative stress.
Oxidative stress is commonly defined as an imbalance of prooxidants and antioxidants with this inequality documented by the accumulation of oxidized molecules in tissue (23, 53). To evaluate the possibility that diaphragms of severe COPD patients exhibited oxidative stress, Barreiro and colleagues (3) compared diaphragm biopsies from groups of subjects afflicted with severe COPD, moderate COPD, or no COPD (i.e., controls), and they noted that severe COPD patients exhibited higher levels of protein carbonyls compared with controls. Indeed, in the two COPD groups, they noted a statistically significant negative correlation between the carbonylation level and airway obstruction (assessed by the percent predicted FEV1.0). In contrast to these finding, Barreiro and coworkers (3) noted no differences in 3-nitrotyrosine levels between the diaphragms of severe COPD subjects and those of controls, and they also noted no upregulation of any of the three nitric oxide synthases (NOSs) in COPD diaphragms. These latter data indicate that nitrosative stress does not occur in the COPD diaphragm. More importantly, this study demonstrated that oxidative stress does occur and that the severity of this latter stress is related to the degree of airway obstruction.
Subsequently, from the same laboratory, Marin-Corral et al. (36) noted that four proteins (i.e., MyHC, creatine kinase, α1-sarcomeric actin, and carbonic anhydrase) were carbonylated in all diaphragms from each group; however, they noted no differences in the levels of carbonylation between controls and patients with moderate COPD. In contrast, severe COPD diaphragms exhibited a fivefold increase in MyHC carbonylation above controls, and this was accompanied by a decrease in noncarbonylated MyHCs to one-third of that noted in controls. These data suggest that in vivo, MyHC was being rapidly carbonylated and that this carbonylated MyHC was undergoing rapid degradation. The magnitude of the observed decreases in noncarbonylated MyHC appeared to be more than sufficient to account for the decreases in maximal transdiaphragmatic pressure and maximum inspired mouth pressure noted in these severe COPD patients. Importantly, this decrease in functional MyHC is consistent with our overall hypothesis: that the severe COPD diaphragm exhibits decreased strength compared with control diaphragms.
Myofiber atrophy.
Muscle fiber cross-sectional area (CSA) is commonly used to quantify the magnitude of fiber atrophy. Multiple studies (27–29) by the Philadelphia group have indicated that CSAs of all fiber types are decreased ∼30–40%, and a recent study by Testelmans et al. (68) showed similar myofiber atrophy in the severe COPD diaphragm. Skeletal muscle fiber size depends on a dynamic balance between anabolic (hypertrophic) and catabolic (atrophic) processes. The results shown in Fig. 2B (taken from Ref. 60) indicate that a decrease in the phosphorylation level of cytoplasmic PKB (Akt) elicits myofiber atrophy by eliciting increases in proteolysis and decreases in protein synthesis. First, the decrease in the Akt phosphorylation level effects increased binding of forkhead box O (FOXO1) to nuclear DNA [including the consensus sequence coding for atrogin-1 and muscle-specific RING finger protein (MuRF)-1]. This results in increased transcription of atrogin-1 and MuRF-1, which increases the proteolytic activity of the ubiquitin-proteasome pathway (UPP), thereby increasing protein degradation via the UPP. Additionally, the results shown in Fig. 2B demonstrate that a decrease in the phosphorylation level of Akt is associated with a decrease in protein synthesis due to dephosphorylation of glycogen synthase kinase 3, mammalian target of rapamycin, and S6 kinase.
It is generally accepted that the atrophy mechanism (as described above and shown in Fig. 2B) is operative in COPD diaphragm fibers exhibiting atrophy. However, the literature does not contain any reports documenting increased activity of this pathway in human COPD diaphragms. However, we (26) recently demonstrated that this pathway plays an important role in effecting the marked fiber atrophy noted by human diaphragm myofibers in ventilator-induced diaphragm atrophy. We believe that similar types of measurements are needed on COPD diaphragm biopsies.
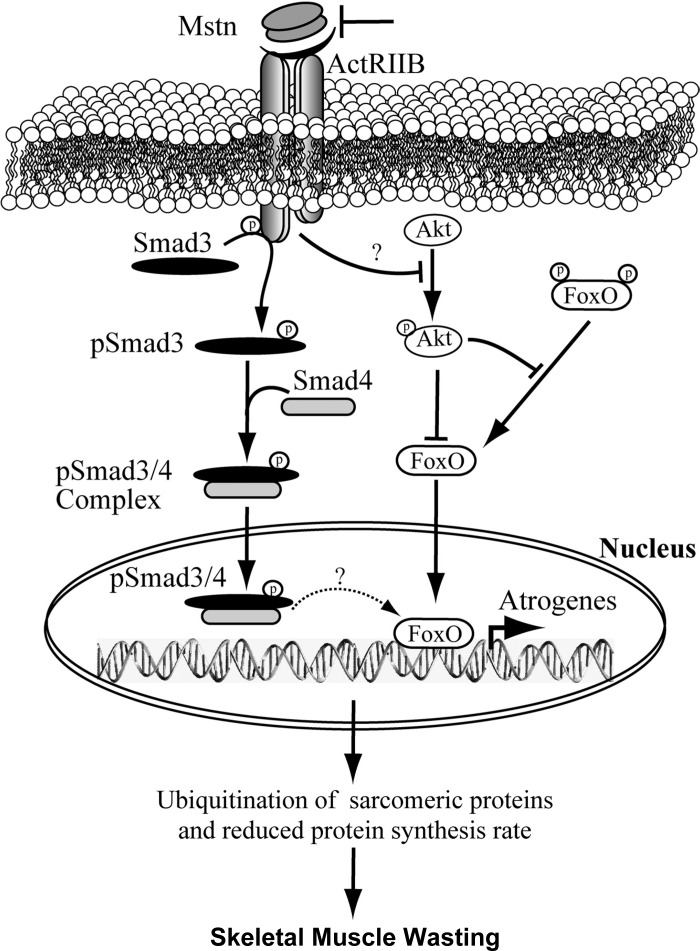
Additionally, recent work by Testelmans et al. (68) on human COPD diaphragms has indicated that several other signaling pathways (i.e., myostatin and NF-κB) are involved in the myofiber atrophy of moderate-to-severe COPD. A proposed mechanism for myostatin-induced atrophy modified from Ref. 33 is shown in Fig. 4. It shows that myostatin upregulates components of the ubiquitin proteolysis system, including atrogin-1, through a FOXO1- and Smad3-dependent signaling mechanism. Enhanced activation of the ubiquitination system leads to degradation of the majority of sarcomeric proteins, which are required for normal muscle growth and development. Myostatin also inhibits protein synthesis by decreasing the phosphorylation of Akt and thereby reduces protein synthesis, and this leads to enhanced progression of skeletal muscle atrophy. Finally, despite the myostatin-induced proteolysis, the IGF-I-phosphatidylinositol 3-kinase pathway can still stimulate Akt; indeed, laboratory experiments (18, 71) have indicated that sufficiently intense stimulation of Akt by this growth pathway can overcome the effects of myostatin.
Fig. 4.
Overview of the myostatin (Mstn) pathway. The schematic shows that Mstn upregulates genes coding for components of the ubiquitin-proteasome pathway via FOXO and Smad3 signaling pathways. Additionally, the increased level of FOXO transcription factors decreases protein synthesis (see Fig. 3B). ActRIIB, activin receptor type IIB. See text for description. [Reproduced, with permission, from Ref. 33.]
Additionally, in their landmark study, Testelmans and coworkers (68) noted changes in the NF-κB signaling pathway(s) that can effect myofiber atrophy in COPD diaphragms (22). Specifically, Testelmans and coworkers reported decreased cytoplasmic levels of the inhibitor proteins IκB-α and IκB-β, decreased NF-κB p50 DNA-binding capacity, and increased atrogin-1 transcripts. These data indicate that NF-κB (i.e., the systemic pathway) was upregulated and played a role in the atrophy of diaphragm myofibers.
Furthermore, Testelmans et al. reported decreases in mRNA coding for myoD as well as decreases in myoD protein levels that were accompanied by no changes in myogenin at either the transcript or protein level. They also noted a negative correlation between the proportion of slow myofibers and myoD protein levels. We agree with these authors that the remodeling changes in myoD are probably related to the fast-to-slow fiber type transformation.
MyHC and contractile remodeling noted in mild-to-moderate COPD.
As previously noted, the earliest studies on remodeling of the COPD diaphragm focused on moderately severe-to-severe COPD (28, 40). However, relatively early in the studies on remodeling of the human diaphragm, Ottenheijm et al. (50) used the permeabilized single-fiber preparation to test the hypothesis that contractile abnormalities exist in mild-to-moderate COPD. Specifically, they compared fiber types I and IIa from diaphragm biopsies of COPD patients with those of normal controls. Among other observations, Ottenheijm and colleagues noted that compared with control fibers, COPD fibers exhibited reduced maximum force generation per unit CSA (i.e., specific force) and reduced MyHC content and concentration. These findings suggested that decreases in MyHC concentration (per half sarcomere) accounted for the decreases in specific force. They also performed biochemical measurements on diaphragm homogenates and noted that diaphragms from COPD patients contained high levels of ubiquitin-protein conjugates. Ottenheijm et al. concluded that the contractile abnormalities in mild-to-moderate COPD were largely due to increased degradation of MyHC by an upregulation of the UPP, and they provided strong support for this conclusion in a subsequent study (49). However, the authors did not present any history of smoking or smoke exposure data for their patients. This is important because recent work by Barreiro et al. (4) has raised the possibility that smoking-induced oxidative stress per se, and not COPD, may have elicited the findings of Ottenheijm et al.
VL
In this section, we will discuss the muscle fiber remodeling that occurs in the VL muscle of COPD patients under the following headings: 1) Decrease in proportion of slow-twitch fibers; 2) Decreased activity of oxidative pathways, 3) Oxidative and nitrosative stress, and 4) Myofiber atrophy.
Decreased proportion of slow-twitch fibers.
Gosker and colleagues (19) carried out a systematic review and meta-analysis of fiber types in patients with various severities of COPD; they noted a slow-to-fast fiber type shift in COPD patients (i.e., a decrease in the proportion of type I fibers and an increase in the proportion of type II fibers). Indeed, after results from 11 previous studies were analyzed, their data indicated that in 60-to 70-year-old men, a proportion of type I fibers of <27% and/or a proportion of type IIx of >29% should be considered as pathological. Importantly, these authors concluded (in this subset of COPD patients) that the proportion of type I fibers was highly negatively correlated with the severity of airway obstruction assessed by either percent predicted FEV1.0 or the ratio of FEV1.0 to FVC (expressed as a percentage). These latter observations have been confirmed by several other investigators (42, 73).
After reviewing the literature, Caron et al. (8) suggested that these slow-to-fast VL fiber type changes cannot be due to age. In 2001, Gea and colleagues (15) concluded that much greater COPD-induced muscle remodeling occurs in the lower limbs than in the upper limbs. In contrast to the marked remodeling noted in the lower limbs, they concluded that upper limb muscle structure and function were relatively well preserved due to the maintenance of some daily activities involving the arms or even the use of some of these muscles in ventilatory efforts. While this postulate of Gea et al. is intuitively attractive, over a decade later, we still do not have adequate biopsy-derived information on upper limb muscles in COPD patients to test their hypothesis.
MECHANISMS MEDIATING SLOW-TO-FAST FIBER TRANSFORMATION.
Shi et al. (64) carried out a seminal series of experiments to test the hypothesis that activation of the ERK1/2 subfamily of the MAPK signaling pathway mediates a slow-to-fast fiber transformation, whereas blockade of this pathway elicits a fast-to-slow fiber shift. First, in tissue culture experiments, these investigators demonstrated that pharmacological blocking of the ERK1/2 pathway increased slow-twitch fiber type-specific reporter activity and repressed that associated with the fast-twitch fiber phenotype; in contrast, overexpression of constitutively active ERK2 had the opposite effect. Second, inhibition of ERK signaling in cultured myotubes increased slow-twitch fiber-specific protein accumulation while repressing those characteristic of fast-twitch fibers. Third, overexpression of MAPK phosphatase-1 in mouse and rat muscle fibers containing almost exclusively type IIb or IIx fast MyHC isoforms induced de novo synthesis of the slower, more oxidative type IIa and I MyHCs in a time-dependent manner. Similar in vivo experiments were also performed, and this work revealed the conversion of fast fibers to the slower phenotype, as indicated by an upregulation of slow reporter gene activity and a downregulation of fast reporter activity. Finally, they noted that activation of ERK2 signaling induced upregulation of the fast-twitch fiber program in the slow-twitch soleus muscle. These experiments of Shi and colleagues (64) demonstrated that the ERK1/2 subfamily of the MAPK family can elicit a slow-to-fast fiber type transformation in experimental studies. Therefore, a similar mechanism may be responsible for the slow-to-fast fiber type transformation noted in the VL of COPD patients, whereas blockade of this pathway may eliminate or attenuate the slow-to-fast fiber transformation in the VL of patients with COPD.
Decreased activity of oxidative pathways.
Several studies have demonstrated that the activity of oxidative enzymes such as HADH (21, 34, 35) and citrate synthase (34, 35) is reduced in the VL of patients with moderate-to-severe COPD. Cytochrome c oxidase, a component of the electron transport system, is also decreased in these individuals (21), although the current literature is not in full agreement in this regard (54, 61). Glycolytic enzymes are not unambiguously affected in the presence of COPD (21). However, when oxidative-to-glycolytic enzymatic ratios are considered, predominance of a glycolytic metabolism appears to be a common feature in the quadriceps of patients with COPD (21). This metabolic pattern of the lower limbs differs from what is seen in the upper extremity muscles, in which increased citrate synthase and lactate dehydrogenase activity is observed in severe COPD patients (16). These observations corroborate the putative importance of local factors in the development of muscle dysfunction in the VL of COPD patients.
Oxidative and nitrosative stress.
The relationships among oxidative stress, nitrosative stress, and exercise are important since regular exercise is usually a component of rehabilitation programs for COPD. We believe that these relationships can be best understood by comparing two studies carried out 3 yr apart in patients with severe COPD by the Barcelona group. In the first study, Barreiro and colleagues (5) analyzed biopsies of severe COPD and control VLs before and after a 3-wk cycle ergometer exercise training program. Before the exercise program, protein carbonylation levels, hydroxynonenal-protein adducts, SOD activity, and inducible NOS were higher in patients than in control subjects. Importantly, 3-nitrotyrosine immunoreactivity levels were also statistically significantly increased in the quadriceps of patients compared with control subjects. In patients, the 3-wk daily training period induced a significant rise in inducible NOS levels and a fourfold increase in protein nitration. Specifically, the proteins that underwent nitration were some involved in glycolysis (enolase, aldolase A, and triosephosphate isomerase), ATP distribution (creatine kinase), muscle O2 transfer (myoglobin), CO2 hydration (carbonic anhydrase III), and DNA repair (uracil DNA glycosylase). Additionally, the contractile protein α1-actin was nitrated only in patients exhibiting muscle loss, whereas SOD increased only in control subjects. These findings argue against including daily cycle ergometer training as part of a rehabilitation program for patients with severe COPD.
However, a 2012 study by Rodriguez et al. (58) tested the hypothesis that high-intensity exercise training of long duration (i.e., 8 wk) with a cycle ergometer does not cause a deterioration in muscle redox status of severe COPD patients. At baseline, compared with control subjects, COPD subjects exhibited greater levels of both muscle protein carbonylation and muscle protein nitration. Nonetheless, after the 8-wk training period, the levels of both protein carbonylation and nitration did not change in either COPD or control subjects. Moreover, these authors noted that both COPD and control subjects exhibited increases in peak work rate, peak O2 consumption, and distance walked in 6 min and decreases in arterial lactate concentration. Rodriguez and colleagues concluded that high-intensity cycle ergometer exercise training of long duration improves exercise capacity in patients with severe COPD in the absence of any increases in muscle protein oxidation or muscle protein nitration. Therefore, the results of this latter study indicate that daily high-intensity exercise training for >8 wk with a cycle ergometer should be included in rehabilitation programs for patients with severe COPD.
We postulate that the combination of these two studies with disparate results have physiological relevance. That is, the dose-response curve for their exercise intervention could be time dependent; the early response seems deleterious, but a longer training period engages additional mechanisms that are adaptive and/or mechanisms are activated to prevent the deleterious aspects of muscle remodeling. We believe that uncovering the physiology underlying these mechanisms is important and warrants further investigation.
ROLES OF INACTIVITY AND PULMONARY PATHOLOGY IN PRODUCING OXIDATIVE STRESS AND DECREASES IN OXIDATIVE CAPACITY IN THE VL.
Severe COPD patients are more sedentary than healthy age- and sex-matched subjects, and, therefore, the possibility exists that inactivity per se might account for some of the remodeling noted in the VL of these COPD patients. Since muscle inactivity can elicit remodeling (51, 52), Mattson et al. (37, 38) developed a protocol for producing elastase-induced emphysema in hamsters that elicted 100% increases in lung volumes, but the carefully measured activity level of these hamsters did not differ from those of control hamsters. Surprisingly, these emphysematous hamsters exhibited statistically significant decreases in VL citrate synthase (a biomarker for oxidative metabolism), increases in malondialdehyde (a biomarker for lipid peroxidation), and statistically significant decreases in glutathione peroxidase (an antioxidant buffer). Although these authors did not demonstrate a mechanistic explanation for these results, their publications raise the possibility that, at least in this animal model of emphysema, lung pathology can elicit decreases in oxidative capacity and oxidative stress in leg muscles.
Myofiber atrophy.
A landmark study by Fermosele and colleagues (13) provided much human information about the relationships among severity of COPD, muscle wasting, protein carbonylation, redox status, the UPP, superoxide anion production, and FOXO and NF-κB transcription factors. They carried out comparisons of VL biopsy features among the following three groups: 1) a group of severe COPD patients without muscle wasting, 2) a group of severe COPD patients with muscle wasting, and 3) an age-matched healthy control group.
Compared with control subjects, in the VL of muscle-wasted COPD patients, levels of protein carbonylation, oxidation of MyHC and myonuclei, superoxide anion production, SOD, total ubiquitin-protein conjugates, E214k, atrogin-1, FOXO1, and p65 were higher, whereas the contents of MyHC, creatine kinase, carbonic anhydrase-3, myogenin, and fast-twitch fiber size were decreased. Importantly, in nonwasted COPD patients, whereas MyHC was more oxidized than in control subjects, its content was preserved. Muscle inflammation and glutathione levels did not differ between patients and control subjects. In all patients, muscle structure abnormalities were increased, whereas muscle force and exercise capacity were reduced.
Fermosele et al. (13) concluded that in severe COPD, while muscle oxidative stress occurs regardless of the presence or absence of muscle wasting, protein ubiquitination and loss of MyHC were enhanced only in those patients exhibiting muscle atrophy. These investigators interpreted their data as evidence that oxidative stress does not directly modulate muscle loss in severe COPD patients. We believe that equally important conclusions from this study are that the UPP appears to mediate the protein degradation and that the transcription factors eliciting this upregulation of the UPP are FOXO1 and the p65 protein of the NF-κB signaling pathway.
Limitations of This Mini-Review
Due to space constraints, we limited our discussion to selected topics in the area of COPD-induced remodeling of the diaphragm and VL. We believe that virtually all other topics may be covered in other articles in this Highlighted Topic series. However, we will briefly mention two topics that may not be discussed in other articles in this series. The first topic is recent developments in the area of skeletal muscle angiogenesis, VEGF (1, 20, 76, 80), and the relationship between myofibers and capillaries (8), whereas the second topic is the vulnerability of the diaphragm to injury (46, 48, 63) and the mechanisms involved in repair of this pathology. We provide some guidance to the interested reader by the references cited above.
Conclusions
Here, we have presented the remodeling that occurs in the costal diaphragm and VL of COPD patients. Surprisingly, some aspects of remodeling in these two muscles occur in opposite directions. For example, the COPD diaphragm is characterized by a fast-to-slow fiber transformation, whereas the VL of these types of patients undergoes a slow-to-fast fiber transformation. Hopefully, we can exploit these discrepant aspects of muscle remodeling in the same patient to more fully arrive at cellular and molecular mechanisms in human experiments.
GRANTS
During the preparation of this manuscript, S. Levine was supported by National Heart, Lung, and Blood Institute Grant HL-078834.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L., M.H.B., T.L.C., S.K.P., and S.S. conception and design of research; S.L. and M.H.B. analyzed data; S.L., M.H.B., T.L.C., S.K.P., and S.S. interpreted results of experiments; S.L. and M.H.B. prepared figures; S.L. and M.H.B. drafted manuscript; S.L., M.H.B., T.L.C., S.K.P., and S.S. edited and revised manuscript; S.L., M.H.B., T.L.C., S.K.P., and S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Due to space constraints, we could only reference some of the laudatory papers in the topics that we covered; therefore, we apologize to our many colleagues whose work was not presented.
REFERENCES
- 1. Alexopoulou C, Mitrouska I, Arvanitis D, Tzanakis N, Chalkiadakis G, Melissas J, Zervou M, Siafakas N. Vascular-specific growth factor mRNA levels in the human diaphragm. Respiration 72: 636–641, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Arora NS, Rochester DF. COPD and human diaphragm muscle dimensions. Chest 91: 719–724, 1987. [DOI] [PubMed] [Google Scholar]
- 3. Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, Hussain SN, Gea J. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 1116–1124, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, Sanchez F, Gea J, Barbera JA; ENIGMA in COPD Project. Cigarette smoke-induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med 182: 477–488, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Barreiro E, Rabinovich R, Marin-Corral J, Barbera JA, Gea J, Roca J. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax 64: 13–19, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18: 670–672, 1970. [DOI] [PubMed] [Google Scholar]
- 7. Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA 106: 13335–13340, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caron MA, Debigare R, Dekhuijzen PN, Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol 107: 952–961, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12: 2499–2509, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clanton TL, Levine S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? J Appl Physiol 107: 324–335, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farkas GA, Roussos C. Adaptability of the hamster diaphragm to exercise and/or emphysema. J Appl Physiol 53: 1263–1272, 1982. [DOI] [PubMed] [Google Scholar]
- 12. Farkas GA, Roussos C. Diaphragm in emphysematous hamsters: sarcomere adaptability. J Appl Physiol 54: 1635–1640, 1983. [DOI] [PubMed] [Google Scholar]
- 13. Fermoselle C, Rabinovich R, Ausin P, Puig-Vilanova E, Coronell C, Sanchez F, Roca J, Gea J, Barreiro E. Does oxidative stress modulate limb muscle atrophy in severe copd patients? Eur Respir J 40: 851–862, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Gea J, Casadevall C, Pascual S, Orozco-Levi M, Barreiro E. Respiratory diseases and muscle dysfunction. Expert Rev Respir Med 6: 75–90, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Gea J, Orozco-Levi M, Barreiro E, Ferrer A, Broquetas J. Structural and functional changes in the skeletal muscles of COPD patients: the “compartments” theory. Monaldi Arch Chest Dis 56: 214–224, 2001. [PubMed] [Google Scholar]
- 16. Gea JG, Pasto M, Carmona MA, Orozco-Levi M, Palomeque J, Broquetas J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J 17: 939–945, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol 89: 695–703, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13: 225–229, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 62: 944–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gouzi F, Prefaut C, Abdellaoui A, Roudier E, de Rigal P, Molinari N, Laoudj-Chenivesse D, Mercier J, Birot O, Hayot M. Blunted muscle angiogenic training-response in copd patients versus sedentary controls. Eur Respir J; doi:10.1183/09031936.00053512. [DOI] [PubMed] [Google Scholar]
- 21. Green HJ, Bombardier E, Burnett M, Iqbal S, D'Arsigny CL, O'Donnell DE, Ouyang J, Webb KA. Organization of metabolic pathways in vastus lateralis of patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 295: R935–R941, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Jackman RW, Cornwell EW, Wu CL, Kandarian SC. NF-κB signaling pathway and transcriptional regulation in skeletal muscle atrophy. Exp Physiol; doi:10.1113/expphysiol.2011.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones DP. Redefining oxidative stress. Antioxid Redox Signal 8: 1865–1879, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 3: 637–658, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klimathianaki M, Vaporidi K, Georgopoulos D. Respiratory muscle dysfunction in COPD: from muscles to cell. Curr Drug Targets 12: 478–488, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Levine S, Biswas C, Dierov J, Barsotti R, Shrager JB, Nguyen T, Sonnad S, Kucharchzuk JC, Kaiser LR, Singhal S, Budak MT. Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med 183: 483–490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, Dudley G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol 92: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 28. Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 337: 1799–1806, 1997. [DOI] [PubMed] [Google Scholar]
- 29. Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 168: 706–713, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Levine S, Nguyen T, Shrager J, Kaiser L, Camasamudram V, Rubinstein N. Diaphragm adaptations elicited by severe chronic obstructive pulmonary disease: lessons for sports science. Exerc Sport Sci Rev 29: 71–75, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Lieber R. Skeletal Muscle Structure, Function, and Plasticity. Baltimore, MD: Lippincott, Williams & Wilkins, 2002, p. 369. [Google Scholar]
- 32. Liu Y, Shen T, Randall WR, Schneider MF. Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J Muscle Res Cell Motil 26: 13–21, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Lokireddy S, McFarlane C, Ge X, Zhang H, Sze SK, Sharma M, Kambadur R. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol Endocrinol 25: 1936–1949, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Belanger M, Breton MJ, Jobin J. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 55: 848–853, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maltais F, Simard AA, Simard C, Jobin J, Desgagnes P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 153: 288–293, 1996. [DOI] [PubMed] [Google Scholar]
- 36. Marin-Corral J, Minguella J, Ramirez-Sarmiento AL, Hussain SN, Gea J, Barreiro E. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur Respir J 33: 1309–1319, 2009. [DOI] [PubMed] [Google Scholar]
- 37. Mattson JP, Poole DC. Pulmonary emphysema decreases hamster skeletal muscle oxidative enzyme capacity. J Appl Physiol 85: 210–214, 1998. [DOI] [PubMed] [Google Scholar]
- 38. Mattson JP, Sun J, Murray DM, Poole DC. Lipid peroxidation in the skeletal muscle of hamsters with emphysema. Pathophysiology 8: 215–221, 2002. [DOI] [PubMed] [Google Scholar]
- 39. McKenzie DK, Butler JE, Gandevia SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol 107: 621–629, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, Marrash R, Pariente R, Aubier M. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol Lung Cell Mol Physiol 274: L527–L534, 1998. [DOI] [PubMed] [Google Scholar]
- 41. Miranda EF, Malaguti C, Corso SD. Peripheral muscle dysfunction in COPD: lower limbs versus upper limbs. J Bras Pneumol 37: 380–388, 2011. [DOI] [PubMed] [Google Scholar]
- 42. Montes de Oca M, Torres SH, Gonzalez Y, Romero E, Hernandez N, Mata A, Talamo C. Peripheral muscle composition and health status in patients with COPD. Respir Med 100: 1800–1806, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Nguyen T, Rubinstein NA, Vijayasarathy C, Rome LC, Kaiser LR, Shrager JB, Levine S. Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm. J Appl Physiol 98: 2004–2010, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Oliven A, Supinski GS, Kelsen SG. Functional adaptation of diaphragm to chronic hyperinflation in emphysematous hamsters. J Appl Physiol 60: 225–231, 1986. [DOI] [PubMed] [Google Scholar]
- 45. Orozco-Levi M, Gea J, Lloreta JL, Felez M, Minguella J, Serrano S, Broquetas JM. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J 13: 371–378, 1999. [DOI] [PubMed] [Google Scholar]
- 46. Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1734–1739, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respir Res 9: 12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 527–534, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ottenheijm CA, Heunks LM, Li YP, Jin B, Minnaard R, van Hees HW, Dekhuijzen PN. Activation of the ubiquitin-proteasome pathway in the diaphragm in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 997–1002, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol 102: 2389–2397, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 51: 942–950, 2011. [DOI] [PubMed] [Google Scholar]
- 54. Puente-Maestu L, Perez-Parra J, Godoy R, Moreno N, Tejedor A, Gonzalez-Aragoneses F, Bravo JL, Alvarez FV, Camano S, Agusti A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J 33: 1045–1052, 2009. [DOI] [PubMed] [Google Scholar]
- 55. Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1α regulates a HIF2α-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA 107: 21866–21871, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ribera F, N'Guessan B, Zoll J, Fortin D, Serrurier B, Mettauer B, Bigard X, Ventura-Clapier R, Lampert E. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 873–879, 2003. [DOI] [PubMed] [Google Scholar]
- 57. Rochester DF. The respiratory muscles in COPD. State of the art. Chest 85: 47S–50S, 1984. [DOI] [PubMed] [Google Scholar]
- 58. Rodriguez DA, Kalko S, Puig-Vilanova E, Perez-Olabarria M, Falciani F, Gea J, Cascante M, Barreiro E, Roca J. Muscle and blood redox status after exercise training in severe COPD patients. Free Radic Biol Med 52: 88–94, 2012. [DOI] [PubMed] [Google Scholar]
- 59. Sanchez J, Bastien C, Medrano G, Riquet M, Derenne JP. Metabolic enzymatic activities in the diaphragm of normal men and patients with moderate chronic obstructive pulmonary disease. Bull Eur Physiopathol Respir 20: 535–540, 1984. [PubMed] [Google Scholar]
- 60. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sauleda J, Garcia-Palmer F, Wiesner RJ, Tarraga S, Harting I, Tomas P, Gomez C, Saus C, Palou A, Agusti AG. Cytochrome oxidase activity and mitochondrial gene expression in skeletal muscle of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157: 1413–1417, 1998. [DOI] [PubMed] [Google Scholar]
- 62. Schols AM, Gosker HR. The pathophysiology of cachexia in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care 3: 282–287, 2009. [DOI] [PubMed] [Google Scholar]
- 63. Scott A, Wang X, Road JD, Reid WD. Increased injury and intramuscular collagen of the diaphragm in COPD: autopsy observations. Eur Respir J 27: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 64. Shi H, Scheffler JM, Pleitner JM, Zeng C, Park S, Hannon KM, Grant AL, Gerrard DE. Modulation of skeletal muscle fiber type by mitogen-activated protein kinase signaling. FASEB J 22: 2990–3000, 2008. [DOI] [PubMed] [Google Scholar]
- 65. Singh S, Harrison S, Houchen L, Wagg K. Exercise assessment and training in pulmonary rehabilitation for patients with COPD. Euro J Phys Rehab Med 47: 483–497, 2011. [PubMed] [Google Scholar]
- 66. Supinski GS, Kelsen SG. Effect of elastase-induced emphysema on the force-generating ability of the diaphragm. J Clin Invest 70: 978–988, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Szentesi P, Zaremba R, van Mechelen W, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol 531: 393–403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Testelmans D, Crul T, Maes K, Agten A, Crombach M, Decramer M, Gayan-Ramirez G. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur Respir J 35: 549–556, 2010. [DOI] [PubMed] [Google Scholar]
- 69. Tonkin J, Villarroya F, Puri PL, Vinciguerra M. SIRT1 signaling as potential modulator of skeletal muscle diseases. Curr Opin Pharmacol 12: 372–376, 2012. [DOI] [PubMed] [Google Scholar]
- 70. Tothova J, Blaauw B, Pallafacchina G, Rudolf R, Argentini C, Reggiani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci 119: 1604–1611, 2006. [DOI] [PubMed] [Google Scholar]
- 71. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296: C1258–C1270, 2009. [DOI] [PubMed] [Google Scholar]
- 72. Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, Athanasopoulos D, Roussos C, Wagner PD, Zakynthinos S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J 36: 301–310, 2010. [DOI] [PubMed] [Google Scholar]
- 73. Vogiatzis I, Terzis G, Stratakos G, Cherouveim E, Athanasopoulos D, Spetsioti S, Nasis I, Manta P, Roussos C, Zakynthinos S. Effect of pulmonary rehabilitation on peripheral muscle fiber remodeling in patients with COPD in GOLD stages II to IV. Chest 140: 744–752, 2011. [DOI] [PubMed] [Google Scholar]
- 74. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J 31: 492–501, 2008. [DOI] [PubMed] [Google Scholar]
- 75. Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology 11: 681–686, 2006. [DOI] [PubMed] [Google Scholar]
- 76. Wagner PD. Vascular endothelial growth factor and the pathogenesis of emphysema. Am J Med 114: 413–414, 2003. [DOI] [PubMed] [Google Scholar]
- 77. Watchko JF, Sieck GC. Respiratory muscle fatigue resistance relates to myosin phenotype and SDH activity during development. J Appl Physiol 75: 1341–1347, 1993. [DOI] [PubMed] [Google Scholar]
- 78. White AT, Schenk S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303: E308–E321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wijnhoven JH, Janssen AJ, van Kuppevelt TH, Rodenburg RJ, Dekhuijzen PN. Metabolic capacity of the diaphragm in patients with COPD. Respir Med 100: 1064–1071, 2006. [DOI] [PubMed] [Google Scholar]
- 80. Zanini A, Chetta A. Vascular endothelial growth factor in the human diaphragm: new insight into adaptation mechanisms in chronic obstructive pulmonary disease patients. Respiration 72: 577–578, 2005. [DOI] [PubMed] [Google Scholar]