Abstract
Despite the popularity of walking as a form of physical activity for obese individuals, relatively little is known about how obesity affects the metabolic rate, economy, and underlying mechanical energetics of walking across a range of speeds and grades. The purpose of this study was to quantify metabolic rate, stride kinematics, and external mechanical work during level and gradient walking in obese and nonobese adults. Thirty-two obese [18 women, mass = 102.1 (15.6) kg, BMI = 33.9 (3.6) kg/m2; mean (SD)] and 19 nonobese [10 women, mass = 64.4 (10.6) kg, BMI = 21.6 (2.0) kg/m2] volunteers participated in this study. We measured oxygen consumption, ground reaction forces, and lower extremity kinematics while subjects walked on a dual-belt force-measuring treadmill at 11 speeds/grades (0.50–1.75 m/s, −3° to +9°). We calculated metabolic rate, stride kinematics, and external work. Net metabolic rate (Ėnet/kg, W/kg) increased with speed or grade across all individuals. Surprisingly and in contrast with previous studies, Ėnet/kg was 0–6% less in obese compared with nonobese adults (P = 0.013). External work, although a primary determinant of Ėnet/kg, was not affected by obesity across the range of speeds/grades used in this study. We also developed new prediction equations to estimate oxygen consumption and Ėnet/kg and found that Ėnet/kg was positively related to relative leg mass and step width and negatively related to double support duration. These results suggest that obesity does not impair walking economy across a range of walking speeds and grades.
Keywords: physical activity, energy expenditure, economy, metabolic rate, biomechanics
obesity prevalence and the associated risks for chronic disease are a major health concern in many parts of the world (39). Obesity is associated with heart disease, diabetes, and certain cancers and is one of the main preventable risk factors for osteoarthritis (12, 27). Obesity is typically caused by a chronic energy imbalance, where energy intake exceeds expenditure (13). As a result, individuals interested in weight management are advised to modify diet and engage in at least 30–60 min of moderate-vigorous intensity (40–60% of maximal aerobic uptake) physical activity (PA) most days of the week (18).
Walking is the most popular form of PA for obese individuals, as it is relatively easy to do, requires considerable metabolic energy expenditure, and typically meets the moderate-vigorous intensity criteria, particularly at faster speeds (4). While faster, level walking may be appropriate for some obese individuals, our laboratory has recently reported that walking relatively slowly up moderate inclines also meets the recommended PA intensity, while potentially reducing the risk of musculoskeletal injury/pathology in obese adults (11). Thus incline walking using a range of relatively slow speeds (0.5–1.0 m/s) and moderate grades (3–9°) may be a good form of PA for obese individuals.
To effectively prescribe walking as a form of PA, we need a detailed understanding of how obesity influences walking energetics. It has been well established that obese individuals expend more gross metabolic energy (Ėgross, W) during walking than do nonobese individuals, primarily because of their greater body mass. Mass-specific net metabolic rate (Ėnet/kg, W/kg) is also greater during level walking at typical self-selected speeds (1.25–1.5 m/s) in obese compared with nonobese individuals (2, 4, 15, 22, 29), suggesting that obesity impairs level walking economy at these speeds. However, Ėnet/kg is similar between obese and nonobese individuals during level walking at relatively slow speeds (0.5–1.0 m/s) (4). To date, only a few studies have measured metabolic rate in obese adults during uphill walking, and no studies have measured metabolic rate as obese adults walk downhill. Lafortuna et al. (22) reported that Ėnet/kg was similar in obese and nonobese females during level and uphill (4%) walking at 1.0 m/s, but was greater in the obese women when walking at 1.3 m/s up a 4% incline. Collectively, these findings suggest that obesity-related differences in Ėnet/kg and economy are affected by walking speed and grade. If so, estimates of energy expenditure and aerobic demand using standard prediction equations may be inaccurate for these individuals, limiting our ability to develop comprehensive walking guidelines for weight management.
During walking, metabolic energy is required to perform work to lift and accelerate the center of mass [external work (Wext)], support body weight, swing the limbs, and maintain balance. Thus differences in walking Ėnet/kg and economy due to obesity may be associated with these tasks. Although Wext/kg (J/kg) is a primary determinant of level waking metabolic rate (9, 16), Wext/kg during level walking is similar in obese compared with nonobese adults (7, 25, 33). No studies have quantified Wext/kg in obese adults during uphill or downhill walking; thus we do not know whether obese individuals will alter their gait in a way that affects Wext/kg and, consequently, Ėnet/kg. Given that obese individuals have reduced relative leg strength (23) and a greater proportion of the less efficient type IIb muscle fibers (38) compared with nonobese adults, the metabolic rate associated with supporting body weight and swinging the legs, particularly during uphill walking, may be greater in obese individuals (20). If so, body mass, body composition, and distribution of fat mass (e.g., ratio of trunk to leg fat mass) should be associated with an increase in Ėnet/kg and a decrease in walking economy across a range of speeds and grades. In addition, obese individuals walk with wider steps (5) and greater lateral leg swing circumduction compared with nonobese individuals (31), and these adaptations may also be associated with an increase in Ėnet/kg (33).
Unfortunately, we have very little information on how an obese individual walks across a range of speeds/grades. A comprehensive study investigating the metabolic and mechanical energetics of level and gradient walking will provide needed data to improve our understanding of how obesity influences gait and should allow us to improve walking-based PA/weight management recommendations and interventions. Therefore, the purpose of this study was to compare the energetics and mechanics of level and gradient walking in obese vs. nonobese adults. A secondary purpose was to develop two prediction equations: one to estimate walking mass-specific O2 consumption (V̇o2) using easily measured variables (e.g., body mass) and another to estimate walking Ėnet/kg using anthropometric and biomechanical variables thought to be related to metabolic rate. We hypothesized that 1) Ėnet/kg would be greater for obese compared with nonobese adults across a range of speed/grade combinations that elicit moderate-vigorous intensities, and that the difference between groups would be greatest at the faster walking speeds. 2) Anthropometric and biomechanical variables, including body mass, adiposity (%body fat), trunk-to-leg fat mass ratio, step width, and lateral leg swing would be significant contributors to Ėnet/kg, as evidenced by their inclusion in an equation to predict Ėnet/kg.
METHODS
Subjects.
Sixty-nine individuals were recruited and screened, and 32 obese (18 women) and 19 nonobese (10 women) participants met the inclusion criteria and were included in this study (Table 1). Subjects were in good health [no known acute/chronic disease or PA limitations (18)], sedentary to lightly active (<4 h of PA/wk), and were not taking any medications known to alter metabolism, and body mass was stable (<2.5 kg net change during the previous 3 mo). All nonobese subjects had a body mass index (BMI) <25 kg/m2, while obese subjects had a BMI between 30 and 50 kg/m2 (Table 1). Subjects gave written, informed consent that followed the guidelines of, and was approved by, the Colorado State University Human Research Institutional Review Board.
Table 1.
Physical characteristics of participants
| Subject Characteristics | Obese | Nonobese |
|---|---|---|
| n | 32 | 19 |
| Age, yr | 28.5 (7.6) | 22.8 (3.6) |
| Height, m | 1.73 (0.09) | 1.72 (0.09) |
| Body mass, kg | 102.1 (15.6) | 64.4 (10.6) |
| BMI, kg/m2 | 33.9 (3.6) | 21.6 (2.0) |
| Body fat, % | 39.7 (6.8) | 24.7 (7.5) |
| Lean body mass, kg | 59.4 (12.9) | 45.5 (10.1) |
| Trunk-to-leg fat mass ratio | 1.61 (0.46) | 1.11 (0.29) |
| V̇o2peak, ml·kg−1·min−1 | 28.9 (6.6) | 40.3 (4.6) |
| Standing metabolic rate, W/kg | 1.15 (0.15) | 1.54 (0.20) |
Values are means (SD); n, no. of subjects. BMI, body mass index; V̇o2peak, maximal oxygen uptake.
Experimental protocol.
Each subject completed three experimental sessions. Details of these sessions have been described previously (11), but are outlined briefly here. The first visit included a physical examination, body composition analysis using dual-energy X-ray absorptiometry (Hologic Discovery, Bedford, MA) and a graded exercise test to determine maximal V̇o2 (V̇o2 peak). We determined percent body fat and percent lean mass for the entire body and three regions of interest (thigh, shank, and foot) from the dual-energy X-ray absorptiometry image. To estimate the distribution of adipose tissue, we also calculated the ratio of trunk to leg fat mass for each individual. We used a modified Balke treadmill protocol to determine each subject's V̇o2 peak. We determined V̇o2 via open-circuit respirometry (Oxycon Mobile, Yorba Linda, CA), with expired gas data averaged every 30 s.
During the second and third sessions, we collected metabolic and biomechanics data as subjects stood and walked (with shoes) at 11 speed/grade combinations (5–6/session). Treadmill speeds ranged from 0.50 to 1.75 m/s in increments of 0.25 m/s (six total), and grades were −3, 0, 3, 6, and 9° (Table 2). Treadmill speed/grade combinations were selected to elicit moderate-vigorous intensities (18). Trials were 6 min in duration, and subjects were allowed 5 min of rest between trials. Subjects were also given an acclimatization period before data collection by walking at a comfortable pace on the level treadmill for up to 10 min.
Table 2.
Oxygen consumption and metabolic rate for each speed/grade combination
| V̇o2, l/min |
Ėgross, W |
Ėgross/kg, W/kg |
Ėnet/kg, W/kg |
|||||
|---|---|---|---|---|---|---|---|---|
| Speed, m/s, Grade, ° | Obese | Nonobese | Obese | Nonobese | Obese | Nonobese | Obese | Nonobese |
| 0.50, 9 | 1.42 ± 0.04 | 1.04 ± 0.05 | 482.3 ± 12.7 | 350.5 ± 16.9 | 4.86 ± 0.06 | 5.46 ± 0.11 | 3.70 ± 0.06 | 3.91 ± 0.09 |
| 0.75, 6 | 1.51 ± 0.04 | 1.08 ± 0.04 | 513.5 ± 14.8 | 363.2 ± 14.8 | 5.06 ± 0.06 | 5.67 ± 0.09 | 3.91 ± 0.06 | 4.13 ± 0.08 |
| 0.75, 9 | 1.91 ± 0.05 | 1.34 ± 0.05 | 658.0 ± 18.5 | 455.4 ± 17.8 | 6.47 ± 0.08 | 7.08 ± 0.12 | 5.32 ± 0.08 | 5.55 ± 0.12 |
| 1.00, 3 | 1.37 ± 0.04 | 1.02 ± 0.04 | 466.0 ± 13.3 | 343.2 ± 14.8 | 4.60 ± 0.07 | 5.33 ± 0.12 | 3.45 ± 0.06 | 3.81 ± 0.13 |
| 1.00, 6 | 1.83 ± 0.04 | 1.34 ± 0.05 | 630.0 ± 15.1 | 454.7 ± 16.9 | 6.30 ± 0.09 | 7.07 ± 0.10 | 5.17 ± 0.09 | 5.54 ± 0.11 |
| 1.25, −3 | 1.10 ± 0.05 | 0.70 ± 0.03 | 310.2 ± 9.2 | 235.8 ± 11.2 | 3.06 ± 0.07 | 3.68 ± 0.12 | 1.91 ± 0.06 | 2.15 ± 0.11 |
| 1.25, 0 | 1.18 ± 0.04 | 0.85 ± 0.04 | 398.5 ± 13.3 | 287.1 ± 13.9 | 3.92 ± 0.07 | 4.46 ± 0.09 | 2.77 ± 0.07 | 2.92 ± 0.09 |
| 1.25, 3 | 1.64 ± 0.04 | 1.18 ± 0.05 | 559.7 ± 14.7 | 399.1 ± 16.7 | 5.50 ± 0.07 | 6.20 ± 0.11 | 4.36 ± 0.06 | 4.67 ± 0.11 |
| 1.50, 0 | 1.45 ± 0.04 | 1.01 ± 0.05 | 493.3 ± 14.4 | 341.9 ± 15.3 | 4.88 ± 0.10 | 5.29 ± 0.10 | 3.73 ± 0.09 | 3.78 ± 0.10 |
| 1.50, 3 | 1.98 ± 0.06 | 1.40 ± 0.05 | 684.9 ± 18.8 | 476.9 ± 18.7 | 6.80 ± 0.10 | 7.45 ± 0.13 | 5.65 ± 0.10 | 5.91 ± 0.13 |
| 1.75, 0 | 1.87 ± 0.06 | 1.26 ± 0.05 | 643.9 ± 21.6 | 428.4 ± 18.1 | 6.23 ± 0.12 | 6.70 ± 0.17 | 5.04 ± 0.12 | 5.16 ± 0.16 |
Values are means ± SE. V̇o2, gross oxygen consumption; Ėgross, gross metabolic rate; Ėgross/kg, mass-specific gross metabolic rate; Ėnet/kg, mass-specific net metabolic rate. Bold indicates that obese value is significantly different than nonobese for that speed/grade combination.
Energetic measurements.
To determine metabolic rate during standing and walking, we measured the rates of V̇o2 and carbon dioxide production (V̇co2) using the same metabolic system described above. We calibrated the system and measured standing metabolic rate for 6 min before collecting the walking data. Subjects were allowed 4 min to reach steady state (no significant increase in V̇o2 during the final 2 min and a respiratory exchange ratio <1.0), and we calculated the average V̇o2 and V̇co2 (l/min) for the final 2 min of each trial. Relative aerobic intensity was determined by dividing the measured V̇o2 by the V̇o2 peak of each individual. We then calculated Ėgros (W) and mass-specific gross metabolic rate (Ėgross/kg, W/kg) from V̇o2 and V̇co2 using a standard equation (3) and subtracted standing metabolic rate from the walking values to derive mass-specific Ėnet/kg (W/kg). We also calculated mass-specific V̇o2/kg (W/kg) from measured V̇o2 using a conversion of 20.1 kJ/l O2.
Biomechanics measurements.
To quantify stride kinematics, step width, lateral leg swing, and Wext/kg, we used a dual-belt, inclinable, force-measuring treadmill (Fully Instrumented Treadmill, Bertec, Columbus, OH). Ground reaction forces (GRF) and moments were recorded at 1,000 Hz by force platforms embedded under each treadmill belt. We also recorded lower extremity kinematics via a seven-camera motion capture system operating at 100 Hz (Nexus, Vicon, Centenial, CO). Passive reflective markers were placed on each subject according to a modified Helen Hayes marker set (21). Data were collected for 30 s during the final minute of each trial. Raw kinetic and kinematic data were smoothed using a fourth-order, zero-lag, digital Butterworth filter with a cutoff frequency of 12 and 5 Hz, respectively. We used the vertical GRF and a 15-N threshold to determine heel-strike and toe-off and then determined stride kinematics [stride length, stride frequency, double support, and duty factor (percentage of stride spent in stance)] for each trial. Step width was calculated as the distance between the midstance center of pressure of the right and left leg during consecutive steps of each trial. We defined lateral leg swing as the medial-lateral distance between the lateral knee marker at toe-off and midswing. We quantified individual limb Wext/kg, as described in detail by Donelan et al. (10). Briefly, we calculated center-of-mass velocities from the GRF data and computed mechanical power as the dot product of the individual limb GRF and center-of-mass velocities. We then calculated Wext/kg by integrating the mechanical power for each step. We report total positive (Wstep/kg+) and negative Wext/kg per step (Wstep/kg−), which is the sum of the Wext/kg performed by leading and trailing limb during double support and the Wext/kg performed by the stance limb during single support. Finally, we calculated the normalized rate of total positive external work (Pstep/kg+, W/kg), by dividing Wext/kg by step period.
Statistical analysis.
Statistical analysis was performed with SAS version 9.2 (SAS Institute, Cary, NC). A separate repeated-measures ANOVA was fit for each energetic and biomechanical response variable (e.g., V̇o2, Ėnet/kg, Wext/kg) using the SAS mixed procedure. The models included fixed effects corresponding to obesity status, sex, speed/grade, and all interactions of these terms. The model also included a random subject effect to account for repeated measures for each subject. Linear contrasts were used to test for differences in means of obese vs. nonobese participants at each speed grade. The P values corresponding to these tests were adjusted using Bonferroni multiple testing adjustment to account for the 11 speed/grade combinations. Residual diagnostics plots were examined. Some of the variables showed evidence that assumptions of equality of variance and normality may not be met. However, the departures were not severe. We considered using a log (natural log) transformation, which did help satisfy assumptions, but did not change the main conclusions. Hence, for simplicity, we present the results of the analysis on the original scale.
To develop equations to estimate mass-specific V̇o2/kg and Ėnet/kg, we computed hierarchical linear regression of treadmill, anthropometric, and biomechanical variables onto V̇o2/kg and Ėnet/kg. For estimating V̇o2/kg, we entered treadmill speed and grade into the first block, body mass into the second block, and age into the third block. For estimating Ėnet/kg, the first two blocks were the same as for V̇o2/kg, and then we entered %body fat, trunk-to-leg fat mass ratio, step width, lateral leg swing, double support, and age into the third to ninth blocks, respectively. BMI was not included in either model due to its strong correlation with body mass. Age was included because it is known to be related to metabolic rate (28). We also cross-validated the V̇o2/kg and Ėnet/kg prediction equations by using the predicted residual sum of squares (PRESS) method (19). This method uses the prediction error for each case when only that case is deleted during the model-generating process, and the PRESS statistic is used to calculate an adjusted R2 and standard error of the estimate (SEE) (26). The criterion for statistical significance was set at P < 0.05.
RESULTS
Energetics.
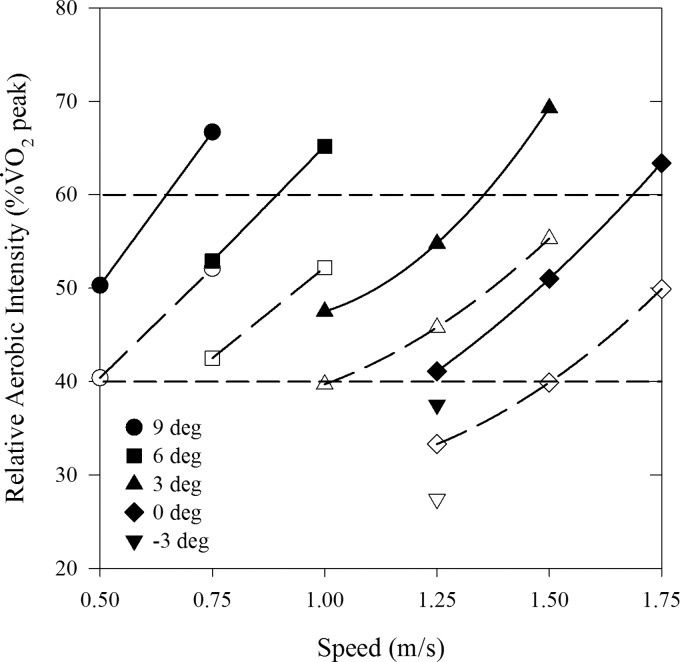
Most trials required a moderate relative aerobic intensity of between 40 and 60% of subjects' V̇o2 peak (Fig. 1). However, when the obese individuals walked at the fastest speeds at each grade, their relative aerobic intensity exceeded the moderate threshold. Relative aerobic intensities were ∼8–15% greater across all speeds/grades (P < 0.001) for obese compared with nonobese individuals, due primarily to the lesser V̇o2 peak of the obese participants.
Fig. 1.
Relative aerobic intensity (%V̇o2 peak) for obese (solid symbols) and nonobese (open symbols) adults. The relative aerobic intensity was significantly greater for obese vs. nonobese at all speed/grade combinations (P < 0.001). Dashed lines indicate lower (40% V̇o2 peak) and upper (60% V̇o2 peak) thresholds for moderate intensity. Lines are linear or second-order regressions for obese (solid) and nonobese (dashed) based on grades at each speed.
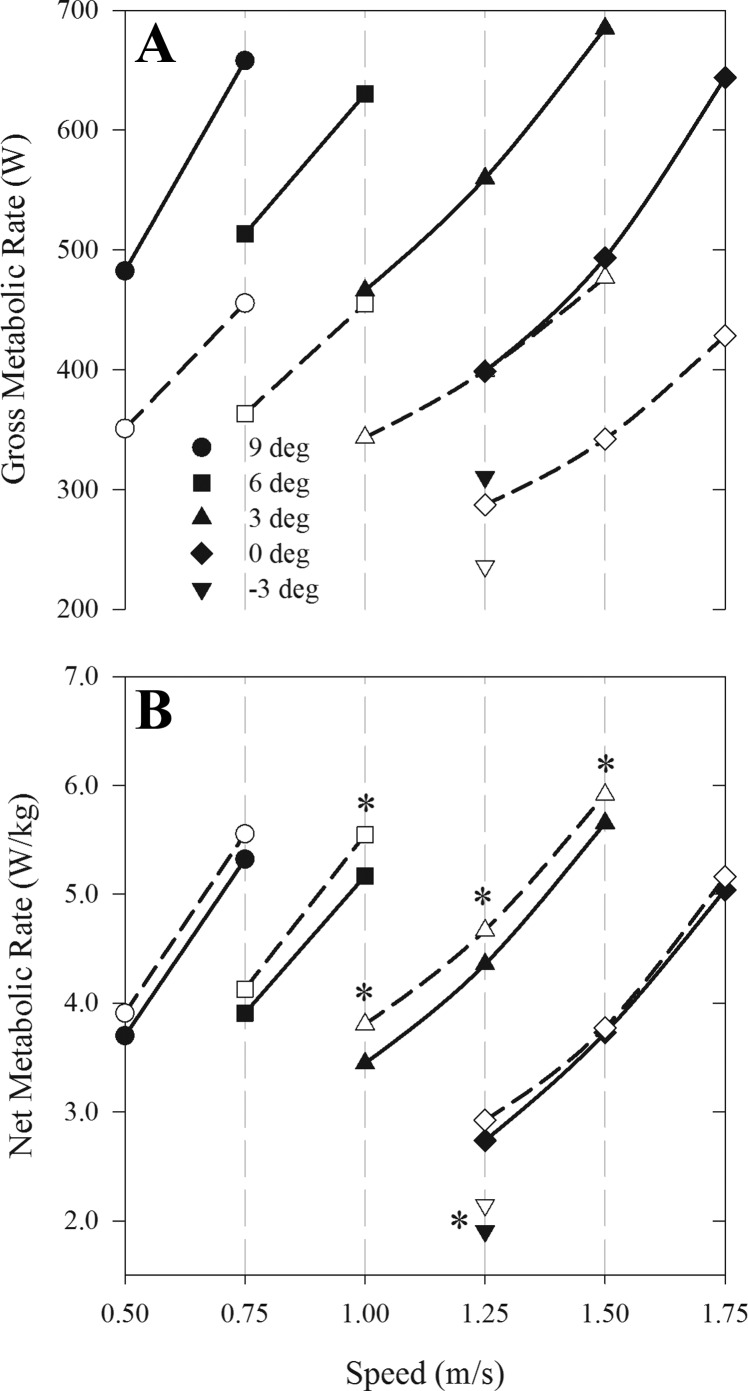
All metabolic variables (V̇o2, Ėgross, Ėgross/kg, and Ėnet/kg) significantly increased with walking speed and/or grade (P < 0.001, Table 2). Obese individuals walked with significantly greater V̇o2 and Ėgross compared with nonobese individuals across all speeds and grades (P < 0.001, Table 2, Fig. 2A). However, Ėgross/kg was significantly less in obese vs. nonobese individuals across all speeds and grades (P < 0.001), and Ėnet/kg was significantly less in obese vs. nonobese individuals at 5 of the 11 speed/grade combinations (P = 0.014 for main effect of obesity, Table 2, Fig. 2B). Men had significantly greater V̇o2 and Ėgross compared with women across all speeds and grades (P = 0.002 for both), but this was due to their greater body mass, as there was no significant effect of sex on Ėgross/kg and Ėnet/kg (P = 0.869 and P = 0.377, respectively). In addition, there were no significant interactions between the fixed effects (sex, obesity, and speed/grade) and Ėgross/kg and Ėnet/kg. Ėgross/kg was ∼7% greater in nonobese individuals compared with obese individuals during level walking at 1.50 m/s and ∼20% greater when walking at 1.25 m/s, −3°. Differences in Ėnet/kg between obese and nonobese individuals were not significant during level walking (1.25, 1.50, and 1.75 m/s) and uphill walking at the slowest speeds (0.50 and 0.75 m/s), but values were ∼4–12% less in obese compared with nonobese individuals during the gradient walking trials at the faster speeds (1.00, 1.25, and 1.50 m/s).
Fig. 2.
Mean gross and net metabolic rate during treadmill walking in obese (solid symbols) and nonobese (open symbols) adults. The average gross metabolic rate (A) and mass-specific net metabolic rate (B) plotted as a function of walking speed and gradient are shown. All values are means ± SE, but the error bars are obscured by the size of the symbols. Differences between obese and nonobese are significant at all speed/grade combination in A. *Significant difference between nonobese and obese in B.
Biomechanics.
Temporal-spatial kinematics were affected by speed/grade and, in some cases, obesity. As walking speed decreased, stride length and stride frequency decreased, while double support time and duty factor increased (main effect of speed/grade for all variables, P < 0.001). Compared with nonobese individuals, obese individuals walked across all speed/grade combinations with similar stride length/frequency (P = 0.66 and 0.99, respectively), longer double support time, greater duty factor, wider steps, and greater lateral leg swing (all P < 0.001, Table 3).
Table 3.
Temporal stride kinematics
| Kinematics Variable | Obese | Nonobese |
|---|---|---|
| Stride length, m | 1.31 ± 0.02 | 1.31 ± 0.02 |
| Stride frequency, Hz | 0.85 ± 0.01 | 0.85 ± 0.01 |
| Duty factor, %stance | 65.8 ± 0.2 | 63.4 ± 0.2 |
| Double support, %stride | 31.0 ± 0.4 | 26.6 ± 0.4 |
| Step width, m | 0.207 ± 0.002 | 0.167 ± 0.002 |
| Lateral leg swing, m | 0.040 ± 0.001 | 0.035 ± 0.001 |
Values are means ± SE for all speeds/grades. Bold indicates that obese value is significantly different than nonobese (all P < 0.001).
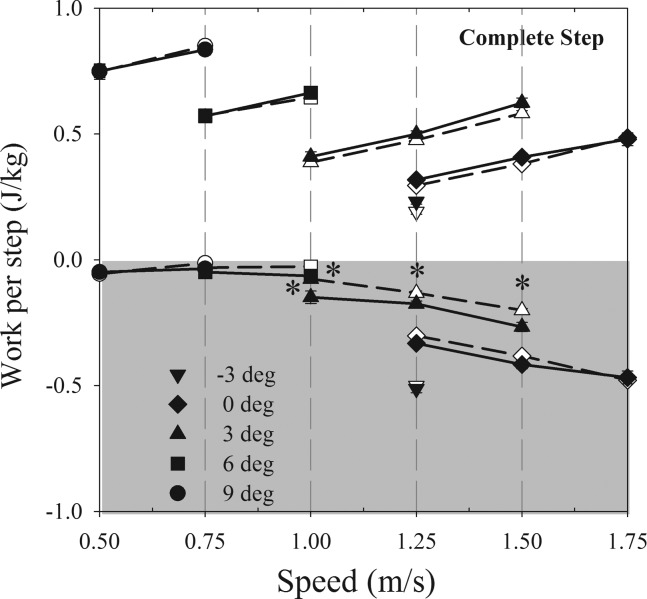
Individual limb Wext/kg was affected by walking speed/grade and obesity (Fig. 3). Wstep/kg+ was not different in obese compared with nonobese participants (P = 0.304), but increased with grade and walking speed in both groups (P < 0.001). As expected, the rate of Wstep/kg+ (i.e., Pstep/kg+) was strongly correlated to Ėnet/kg (r = 0.85, P < 0.001). Wstep/kg− was significantly greater in obese at intermediate speeds/grades (Fig. 3).
Fig. 3.
Mean external mechanical work per step, Wext/kg, during treadmill walking in obese (solid symbols) and nonobese (open symbols) adults. All values are means ± SE, but error bars are obscured by the size of the symbols. Shaded region indicates negative work. *Significant difference between nonobese and obese.
Prediction of V̇o2 and Ėnet/kg.
Hierarchical linear regression resulted in the following equations to estimate V̇o2/kg (W/kg) and Ėnet/kg (W/kg):
where G is treadmill grade (degrees); V is treadmill velocity (m/s); mbody is body mass (kg); A is age (yr); T is trunk-to-leg fat mass ratio; SW is step width (m); and DS is double support (%stride time). Body mass, %body fat, and lateral leg swing did not significantly contribute to the model and were not included in the final Ėnet/kg prediction equation. The proportion of variance in V̇o2/kg predicted by the model (R2 adjusted) was 0.77 (SEE = 0.56 W/kg). The PRESS-derived R2 (0.76) and SEE (0.56 W/kg) were nearly identical to those of the V̇o2/kg prediction model. The proportion of variance in Ėnet/kg predicted by the model (R2 adjusted) was 0.84 (SEE = 0.48 W/kg). The PRESS-derived R2 (0.83) and SEE (0.48 W/kg) were nearly identical to those of the Ėnet/kg prediction model. Regression coefficients for prediction equations (W/kg and ml O2·kg−1·min−1) are provided in Table 4.
Table 4.
Regression coefficients for prediction equations
| Variable Coefficients |
||||||||
|---|---|---|---|---|---|---|---|---|
| Prediction Equation | Constant | Grade, ° | Velocity, m/s | Body mass, kg | Age, yr | Trunk-to-leg fat mass ratio | Step width, m | Double support, %stride |
| V̇o2/kg, W/kg | 1.40 | 0.42 | 3.68 | −0.01 | −0.03 | |||
| Ėnet/kg, W/kg | −0.65 | 0.49 | 3.78 | −0.01 | −0.15 | 3.30 | −4.24 | |
| V̇o2/kg, ml·kg−1·min−1 | 4.17 | 1.24 | 10.99 | −0.03 | −0.07 | |||
| ACSM V̇o2/kg, ml·kg−1·min−1 | 3.5 | 1.8* | 0.1* | |||||
V̇o2/kg, mass-specific V̇o2; ACSM, American College of Sports Medicine. The ACSM prediction equation is: V̇o2/kg = 0.1 (speed) + 1.8 (speed) × (fractional grade) + 3.5, where speed has units of m/min.
DISCUSSION
Our results confirm that obese individuals can achieve recommended exercise intensities while walking uphill at relatively slow speeds. In addition, we have developed a practical prediction equation that can be used to estimate V̇o2 across a range of speeds and grades. We reject our hypothesis that mass-specific Ėnet/kg would be greater for obese adults compared with nonobese adults across the range of speeds and grades used in this experiment. Surprisingly, Ėgross/kg and Ėnet/kg were less than or equal in obese adults compared with their nonobese counterparts. Regardless of walking speed/grade, obese individuals spent a greater percentage of the stride in stance and double support, walked with wider steps, and had a greater lateral leg swing compared with nonobese individuals. These changes in gait biomechanics did not result in differences in Wstep/kg+ between the obese and nonobese groups, although Pstep/kg+ was strongly correlated with Ėnet/kg. We partially accept our hypothesis that several anthropometric and biomechanical variables would be related to Ėnet/kg. According to the equation developed to estimate Ėnet/kg, step width was positively related to Ėnet/kg, while trunk-to-leg fat mass ratio, double support duration, and age were negatively related to Ėnet/kg.
As expected, metabolic rate was influenced by walking speed and grade. Obese individuals had a much greater gross V̇o2 and metabolic rate compared with their nonobese counterparts, a finding supported by other studies (4, 22, 33). We found that V̇o2 (l/min) and Ėgross (W) were 35–50% greater in obese vs. nonobese participants. These results support our recent finding that obese individuals can achieve recommended exercise intensities while walking slowly up a moderate incline (11). For example, walking at 0.75 m/s up a 6° incline required ∼52% V̇o2 peak in our obese participants, whereas level walking at 1.50 m/s required ∼50% V̇o2 peak. These results also highlight the difficulty obese individuals may have if attempting to walk up moderately steep grades at faster speeds (>1.25 m/s), as the relative aerobic intensity could easily exceed a sustainable intensity. Somewhat surprisingly, when obese individuals walked down a 3° grade at 1.25 m/s, they still required nearly 40% V̇o2 peak, suggesting that moderate downhill grades may also offer an alternative form of moderate-intensity PA, although the risks of musculoskeletal injury may increase due to the large eccentric muscle forces required to maintain a steady walking speed.
Our data set allowed the development of an equation to estimate V̇o2/kg across a range of body size/composition, walking speeds, and positive and negative grades. This equation may be useful to estimate both relative aerobic effort, as well as associated energy expenditure during treadmill walking, particularly because it includes easily determined variables. We found that treadmill speed and grade were the primary contributors to the prediction model (R2 = 0.69), but that body mass and age were also significant predictors of V̇o2/kg [change (Δ) in R2 of 0.06 and 0.02, respectively]. The finding that body mass and age were negatively associated with V̇o2/kg was unexpected, and it is possible that heavier and/or older (e.g., middle aged) individuals may become adapted to walking with the added mass, presumably because they have had more time to do so. However, our data did not include individuals older than 50 yr. Therefore, our prediction equation may not estimate V̇o2/kg accurately for older individuals (>50 yr), particularly given that older individuals have a greater walking metabolic rate than younger individuals (24). We used the PRESS method to cross-validate our prediction equation, and, while the results suggest that the equation has good validity, the PRESS method does not guard against a Type II error or assign causation. Future studies are needed to validate this new equation using an independent sample. We also used the American College of Sports Medicine prediction equation [see Table 4 (14)] to estimate V̇o2/kg for our data, but we found that this equation only explained 50% of the variance in V̇o2/kg (compared with 77% in the new equation). This suggests that the new prediction equation may offer a more accurate estimate of V̇o2 during gradient and level treadmill walking than the commonly used in the American College of Sports Medicine prediction equation.
We did not expect to find that Ėnet/kg of obese individuals would be less than or equal to Ėnet/kg of nonobese individuals. Although the significant differences in Ėnet/kg between groups were typically small (∼5%), our results suggest that obese adults walk with similar or slightly better economy compared with nonobese adults. Our results are not in agreement with the few studies that have reported or suggested that walking Ėnet/kg is ∼10% greater in obese vs. nonobese adults (4, 22, 29) at typical or fast level walking speeds (1.25–1.75 m/s). The Ėnet/kg reported here for moderately obese individuals are similar to those reported by other studies, including our own (4, 6, 22). As a result, we are confident that our metabolic data for obese individuals are representative. Our nonobese Ėnet/kg values are also within the range of values reported in the walking energetics literature (8, 34, 35), but are greater than those reported in the studies that have directly compared obese and nonobese adults (4, 6, 22). A potential explanation for the greater Ėnet/kg of nonobese adults in our study compared with other studies is the inherent variability in metabolic measures (both standing and walking). Notably, Rubenson et al. (35) recently reported that there is considerable variability in walking metabolic rates of nonobese adults, with minimum net metabolic costs ranging from 1.71 to 2.59 J·kg−1·m−1 (42% of mean value of 2.06 J·kg−1·m−1). This variability is likely due, in part, to methodological differences between studies, including population (e.g., sex, sedentary), methods of indirect calorimetry, measurement of standing metabolic rate, and acclimation to the protocol/equipment (if walking on a treadmill). Clearly, future studies that use large sample sizes, a control group, and standardized standing and walking protocol are needed to confirm our results.
Studies that have quantified the energetics of load-carrying during incline walking provide additional support for relatively good uphill walking economy in obese adults. Load-carrying studies have reported that V̇o2/kg and Ėnet/kg, when normalized by total mass (body mass plus load), are similar or smaller compared with unloaded walking (17, 36). Thus, if obesity is considered analogous to walking with added mass, one would expect that obese adults may be as or more economical than their nonobese counterparts. Further support for the finding that obese adults may have relatively good walking economy can be found in studies that have investigated habitual load carriers (e.g., porters). Two studies have reported that Himalayan porters walked with much better economy compared with their European mountaineering counterparts (nonhabituated) when carrying substantial loads (1, 30). Collectively, our results and these studies suggest that perhaps we should expect obese adults to have similar or smaller mass-specific walking metabolic rates and better economy compared with nonobese adults, particularly when walking uphill.
By simultaneously quantifying metabolic and biomechanical characteristics of walking, we gained insights into how obesity affects the energetics and economy of walking. We found a strong correlation between Pstep/kg+ and Ėnet/kg, demonstrating that work done to lift and accelerate the center of mass is an important determinant of Ėnet/kg. However, the similarity in Wext/kg in obese and nonobese individuals suggests that this work is not affected by obesity, as has been reported in other studies (7, 25, 33). Although Wstep/kg+ was not different between the groups, Wstep/kg− was slightly, but significantly, greater in the obese individuals at some same speed/grades (e.g., 3° trials). However, the net Wext/kg was similar in both groups at these speed/grades. While our results suggest that obesity does not elicit changes in walking mechanics that reduce the Wext/kg, not all Wext/kg is performed by muscles. Wext/kg may also be performed by soft tissues that rebound after the leading limb's collision with the ground and perform positive work (40). This soft tissue positive work may reduce Ėnet/kg through a reduction in muscle work and may be one explanation for the relatively good walking economy of obese individuals. Future studies that quantify both center of mass and joint work will illuminate how work done by soft tissue affects the economy of walking in obese individuals.
We included several anthropometric and biomechanical variables thought to be associated with body weight support, leg swing, and balance in a linear regression analysis to determine how obesity affected the metabolic rate and walking economy associated with these tasks. We found, as has been reported previously, that obese adults spent more time in stance and double support (relative to stride time), walked with wider steps, and had a greater lateral leg swing compared with their nonobese peers (5, 33, 37). Neither body mass nor composition were found to significantly improve the ability to predict Ėnet/kg, which we interpret to mean that the metabolic rate associated with body weight support is not affected by obesity. The inclusion of trunk-to-leg fat mass ratio (ΔR2 = 0.01) and step width (ΔR2 = 0.01) in our prediction equation suggests that swinging/lifting heavier legs and walking with wider steps is associated with a slightly greater metabolic rate, but lateral leg swing is not. In addition, double support duration was negatively associated with metabolic rate (ΔR2 = 0.02), and we hypothesize that obese individuals may increase double support time to maintain balance/stability, which may, in turn, act to reduce Ėnet/kg (32). We did not expect age to be a significant predictor of Ėnet/kg, and in fact its contribution to the ability to estimate Ėnet/kg is small (ΔR2 = 0.002). While our findings may seem at odds with studies that report older adults (>60 yr old) have a greater Ėnet/kg than younger adults (28), we did not include older individuals in this study. Therefore, the resulting prediction equation is not likely to be applicable to an older population. The inclusion of age as a predictor of Ėnet/kg suggests that, as individuals age, they are able to adapt to changes in body mass. Collectively, these results indicate that obese individuals' heavier legs and wider steps are associated with reduced walking economy, but this is offset by an increased double support time, which is associated with improved walking economy. However, our results indicate that each of these variables makes a relatively minor contribution to predicting metabolic rate and is a potential, but not proven, determinant of metabolic rate. Future studies that provide body weight and/or leg swing support and stability and quantify obesity onset are needed to confirm the roles that these variables play in walking metabolic rate and economy.
Conclusions.
Our results suggest that, while obese adults require a greater amount of metabolic energy to walk, they can easily achieve recommended exercise intensities walking uphill slowly. We have developed new prediction equations that can be used to estimate V̇o2 and metabolic rate across a range of walking speeds/grades and levels of adiposity. We found that obese adults walk with a similar or smaller mass-specific Ėnet/kg, suggesting these individuals have equivalent or better walking economy compared with their nonobese counterparts. The external work required to lift and accelerate the body, although a primary determinant of Ėnet/kg, was not affected by obesity across the range of speeds/grades used in this study. Ėnet/kg was positively related to relative leg mass and step width and negatively related to double support duration. These results suggest that obesity does not impair walking economy across a range of walking speeds and grades.
GRANTS
This work was supported in part by a Colorado State University College of Applied Human Sciences grant and National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R03-AR-059264.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C.B. conception and design of research; R.C.B., M.M.R., W.J.B., and K.A.W. performed experiments; R.C.B., M.M.R., W.J.B., and K.A.W. analyzed data; R.C.B., M.M.R., W.J.B., K.A.W., and R.F.R. interpreted results of experiments; R.C.B., M.M.R., and W.J.B. prepared figures; R.C.B. and M.M.R. drafted manuscript; R.C.B., M.M.R., W.J.B., K.A.W., and R.F.R. edited and revised manuscript; R.C.B., M.M.R., W.J.B., K.A.W., and R.F.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ann Hess for assistance with the statistical analysis. The research would not have been possible without the assistance of the Colorado State University Human Performance and Clinical Research Laboratory staff.
REFERENCES
- 1. Bastien GJ, Schepens B, Willems PA, Heglund NC. Energetics of load carrying in Nepalese porters. Science 308: 1755, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Bloom WL, Marshall FE. The comparison of energy expenditure in the obese and lean. Metabolism 16: 685–692, 1967. [Google Scholar]
- 3. Brockway JM. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr 41: 463–471, 1987. [PubMed] [Google Scholar]
- 4. Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol 100: 390–398, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Browning RC, Kram R. Effects of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc 39: 1632–1641, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res 13: 891–899, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Browning RC, McGowan CP, Kram R. Obesity does not increase external mechanical work per kilogram body mass during walking. J Biomech 42: 2273–2278, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burdett RG, Skrinar GS, Simon SR. Comparison of mechanical work and metabolic energy consumption during normal gait. J Orthop Res 1: 63–72, 1983. [DOI] [PubMed] [Google Scholar]
- 9. Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol 205: 3717–3727, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Donelan JM, Kram R, Kuo AD. Simultaneous positive and negative external mechanical work in human walking. J Biomech 35: 117–124, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Ehlen KA, Reiser RF, 2nd, Browning RC. Energetics and biomechanics of inclined treadmill walking in obese adults. Med Sci Sports Exerc 43: 1251–1259, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis: the Framingham study. Ann Intern Med 109: 18–24, 1988. [DOI] [PubMed] [Google Scholar]
- 13. Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain–a systematic review. Obes Rev 1: 95–111, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Franklin BH, Whaley GP, Howley ET. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins, 2000. [Google Scholar]
- 15. Freyschuss U, Melcher A. Exercise energy expenditure in extreme obesity: influence of ergometry type and weight loss. Scand J Clin Lab Invest 38: 753–759, 1978. [DOI] [PubMed] [Google Scholar]
- 16. Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol 98: 579–583, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Grenier JG, Peyrot N, Castells J, Oullion R, Messonnier L, Morin JB. Energy cost and mechanical work of walking during load carriage in soldiers. Med Sci Sports Exerc 44: 1131–1140, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1423–1434, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Holiday DB, Ballard JE, McKeown BC. PRESS-related statistics: regression tools for cross-validation and case diagnostics. Med Sci Sports Exerc 27: 612–620, 1995. [PubMed] [Google Scholar]
- 20. Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve 24: 654–661, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res 8: 383–392, 1990. [DOI] [PubMed] [Google Scholar]
- 22. Lafortuna CL, Agosti F, Galli R, Busti C, Lazzer S, Sartorio A. The energetic and cardiovascular response to treadmill walking and cycle ergometer exercise in obese women. Eur J Appl Physiol 103: 707–717, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A, Lafortuna CL, Sartorio A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol 101: 51–59, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, Caillaud C. Energy cost of walking and gait instability in healthy 65 and 80 yr olds. J Appl Physiol 95: 2248–2256, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Malatesta D, Vismara L, Menegoni F, Galli M, Romei M, Capodaglio P. Mechanical external work and recovery at preferred walking speed in obese subjects. Med Sci Sports Exerc 41: 426–434, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Malek MH, Housh TJ, Berger DE, Coburn JW, Beck TW. A new nonexercise-based V̇o2 max equation for aerobically trained females. Med Sci Sports Exerc 36: 1804–1810, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Malnick SD, Knobler H. The medical complications of obesity. QJM 99: 565–579, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol 73: 200–206, 1992. [DOI] [PubMed] [Google Scholar]
- 29. Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord 21: 380–386, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Minetti AE, Formenti F, Ardigo LP. Himalayan porter's specialization: metabolic power, economy, efficiency and skill. Proc Biol Sci 273: 2791–2797, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peyrot N, Morin JB, Thivel D, Isacco L, Taillardat M, Belli A, Duche P. Mechanical work and metabolic cost of walking after weight loss in obese adolescents. Med Sci Sports Exerc 42: 1914–1922, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Peyrot N, Thivel D, Isacco L, Morin JB, Belli A, Duche P. Why does walking economy improve after weight loss in obese adolescents? Med Sci Sports Exerc 44: 659–665, 2012. [DOI] [PubMed] [Google Scholar]
- 33. Peyrot N, Thivel D, Isacco L, Morin JB, Duche P, Belli A. Do mechanical gait parameters explain the higher metabolic cost of walking in obese adolescents? J Appl Physiol 106: 1763–1770, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Ralston HJ. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol 17: 277–283, 1958. [DOI] [PubMed] [Google Scholar]
- 35. Rubenson J, Heliams DB, Maloney SK, Withers PC, Lloyd DG, Fournier PA. Reappraisal of the comparative cost of human locomotion using gait-specific allometric analyses. J Exp Biol 210: 3513–3524, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Sagiv M, Ben-Gal S, Ben-Sira D. Effects of gradient and load carried on human haemodynamic responses during treadmill walking. Eur J Appl Physiol 83: 47–50, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil 72: 1065–1070, 1991. [PubMed] [Google Scholar]
- 38. Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PRG, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002. [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva: WHO, 2000. [PubMed] [Google Scholar]
- 40. Zelik KE, Kuo AD. Human walking isn't all hard work: evidence of soft tissue contributions to energy dissipation and return. J Exp Biol 213: 4257–4264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]