Key Points
This study identifies a calmodulin-binding sequence in Sema4D and shows that calmodulin binds to Sema4D in resting platelets.
Dissociation of the Sema4D:calmodulin complex is sufficient to trigger Sema4D cleavage and shedding of the extracellular domain.
Abstract
Semaphorin 4D (Sema4D) is a transmembrane protein that supports contact-dependent amplification of platelet activation by collagen before being gradually cleaved by the metalloprotease ADAM17, as we have previously shown. Cleavage releases a soluble 120-kDa exodomain fragment for which receptors exist on platelets and endothelial cells. Here we have examined the mechanism that regulates Sema4D exodomain cleavage. The results show that the membrane-proximal cytoplasmic domain of Sema4D contains a binding site for calmodulin within the polybasic region Arg762-Lys779. Coprecipitation studies show that Sema4D and calmodulin are associated in resting platelets, forming a complex that dissociates upon platelet activation by the agonists that trigger Sema4D cleavage. Inhibiting calmodulin with W7 or introducing a membrane-permeable peptide corresponding to the calmodulin-binding site is sufficient to trigger the dissociation of Sema4D from calmodulin and initiate cleavage. Conversely, deletion of the calmodulin-binding site causes constitutive shedding of Sema4D. These results show that (1) Sema4D is a calmodulin-binding protein with a site of interaction in its membrane-proximal cytoplasmic domain, (2) platelet agonists cause dissociation of the calmodulin–Sema4D complex, and (3) dissociation of the complex is sufficient to trigger ADAM17-dependent cleavage of Sema4D, releasing a bioactive fragment.
Introduction
Sema4D (CD100), a class IV semaphorin family member, is a 150-kDa type I membrane glycoprotein. The mature protein is a disulfide-linked homodimer that includes an NH2-terminal (N-terminal) sema domain and a cytoplasmic domain containing sites for tyrosine and serine and/or threonine phosphorylation.1-5 Sema4D was first described on T cells where it supports B-cell differentiation by binding to the low-affinity receptor CD72.3,6-8 Subsequent studies have identified roles for Sema4D in axon guidance,9-12 angiogenesis,13-17 and tumor progression18-20 and showed that some of these effects are mediated by the high-affinity receptor plexin-B120 and the related lower-affinity receptor plexin-B2.21,22
In previously published studies, we have shown that Sema4D is expressed by platelets and that it participates in the hemostatic response to injury by reinforcing collagen-initiated platelet activation in a contact-dependent manner.23,24 Deletion of Sema4D protects mice against the development of atherosclerosis by attenuating platelet hyperactivity in the setting of hyperlipidemia25 and reducing intimal neovascularization,26 which suggests that platelet Sema4D has an impact on the vessel wall as well as on platelets. Our previous studies also showed that the extracellular domain of Sema4D is gradually shed from the surface of activated platelets as a soluble 120-kDa fragment that retains biological activity.23,24 Here we have returned to the topic of Sema4D shedding by platelets, examining the mechanisms that regulate cleavage.
In the past several years, exodomain shedding has emerged as a mechanism for modulating platelet function. Our in-depth analysis of the platelet sheddome identified fragments of approximately 69 membrane proteins in the supernate of activated platelets, including Sema4D.27 A comparison of those proteins with the much larger number of membrane proteins (626) detected in a recent survey of the platelet proteome28 shows that not every platelet membrane protein is shed and suggests that platelets shed their protein exodomains selectively. Among the shed membrane proteins that have been studied the most are GPVI and GPIbα, the platelet receptors for collagen and von Willebrand factor, respectively.29-31 Shedding of both proteins involves the association of their intracellular domains with calmodulin,32-35 as does the shedding of PECAM-1 (CD31).36 It is not clear, however, that this is a general mechanism for regulating shedding in platelets.
Cleavage not only eliminates proteins from the surface of cells, it can also generate bioactive fragments. Early studies showed that soluble Sema4D is generated by a spontaneous shedding from lymphocytes.37 Receptors for Sema4D have been identified on endothelial cells13 and B cells,6 as well as on platelets. We have previously shown that Sema4D cleavage and shedding are prevented by metalloprotease inhibitors and abolished in platelets from mice that lack the metalloprotease ADAM17 (also called TACE [tumor necrosis factor-α-converting enzyme]).23 We now show that there is a regulated association of Sema4D with calmodulin in resting platelets and that this association is disrupted by agonists that cause platelet activation and Sema4D cleavage. We also show that there is a calmodulin-binding sequence in the membrane-proximal Sema4D cytoplasmic domain and that interrupting the binding of calmodulin to this sequence is sufficient to cause cleavage and shedding of the Sema4D extracellular domain, producing a fragment indistinguishable from the one produced by platelet agonists.
Materials and methods
Materials
Mouse monoclonal Sema4D carboxyl-terminal (C-terminal) antibody (30/CD100) was from BD Biosciences (San Jose, CA). Rabbit monoclonal Sema4D N-terminal antibody (EP3569) was from Epitomics (Burlingame, CA). A monoclonal Sema4D N-terminal antibody (1E8G9) was generated in mice immunized with the full-length extracellular domain of human Sema4D. Phorbolmyristate acetate (PMA), W7, calmodulin (CaM) -agarose, avidin-agarose, and protease inhibitor cocktail were from Sigma-Aldrich (St. Louis, MO). The CaM antibody (Cat #05-173), TACE Substrate II (MCA-PLAQAV(Dpa)RSSSR-NH) and ADAM-17 Substrate II (fluorogenic) were from Millipore (Billerica, MA). GM6001 and TAPI-2 were from Calbiochem (La Jolla, CA). Recombinant protein G-agarose was from Invitrogen (Carlsbad, CA). The actin antibody was from Beyotime (Jiangsu, China). Anti-CD100-phycoerythrin (PE) (A8) was from Abcam (Cambridge, UK). Monoclonal antibody SZ2 against GPIbα was described previously.38 Monoclonal antibody (6B12-3) against human platelet GPVI was a generous gift from Dr. Mark Kahn (University of Pennsylvania, Philadelphia, PA).39
Preparation of platelets
Venous blood was obtained from healthy donors with informed consent in accordance with the Declaration of Helsinki and permission from local ethical committees and was anticoagulated 1:5 with 65 mM Na3 citrate, 70 mM citric acid, and 100 mM dextrose [pH 4.4]. Platelet-rich plasma was obtained by centrifuging at 900 rpm for 20 minutes. Washed platelets and gel-filtered platelets were prepared as described40 and maintained at 37°C for 1 hour prior to assay.
Co-immunoprecipitation
Washed platelets (2 to 5 × 108/mL) were resuspended in Tyrode’s buffer (137 mM NaCl, 2 mM KCl, 0.3 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.1% bovine serum albumin [BSA] [pH 7.4]) and treated with 10 μg/mL collagen, 1.0 μM PMA, 5 μM calmodulin-binding peptide of Sema4D (CBPS) or 5 μM vCBPS [a variant peptide in which Ala was substituted for 4 basic residues] for 30 seconds at 37°C. Platelets were lysed by the addition of an equal volume of ice-cold lysis buffer (1% vol/vol Triton X-100, 40 mM Tris-HCl, 10 mM EGTA [pH 7.3]) containing a protease inhibitor cocktail, centrifuged at 5000 rpm for 10 minutes at 4°C, and precleared with 30 μL of protein G-agarose for 3 hours. Lysates were immunoprecipitated by using 3 μg/mL 1E8G9 overnight at 4°C. After 3 washes with phosphate-buffered saline (PBS; pH 7.4), proteins bound to the beads were eluted with Laemmli sample buffer (Bio-Rad, Hercules, CA) and analyzed by immunoblotting using the anti-calmodulin antibody.
Peptide synthesis
The CBPS corresponding to residues Arg762-Lys779 (NH2-RQCLKFRSALLIGKKKPK-COOH) of the C-terminal portion of Sema4D (NCBI Reference Sequence: NP_006369.3) with or without myristoylated or biotinylated tags was synthesized by Shanghai Bootech BioScience & Technology Co., Ltd. (Shanghai, China) and is designated CBPS. A variant peptide in which Ala was substituted for 4 basic residues is designated vCBPS. Purity was >99% in all cases.
Calmodulin-binding assay
CBPS or vCBPS (10 μg) in PBS (pH 7.4) was incubated with 20 μL calmodulin-agarose beads at 4°C for 4 hours. After washing 3 times with PBS (pH 7.4), bound peptide was eluted with Laemmli sample buffer and analyzed by Tris-tricine polyacrylamide gel electrophoresis and silver staining (Beyotime). Alternatively, platelet cell lysate was incubated with biotinylated CBPS or vCBPS (10 μg/mL) at 4°C overnight and further incubated with 50 μL avidin-agarose beads at 4°C for 3 hours. After washing 3 times with PBS (pH 7.4), proteins bound to the beads were eluted with Laemmli sample buffer and analyzed by immunoblotting using the anti-calmodulin antibody.
A fluorescence-based assay for the binding of the Sema4D peptides to calmodulin
Calmodulin conjugated with 5-({2-[(iodoacetyl)amino]ethyl}amino)naphthalene-1-sulfonic acid (IAEDANS) has been described previously.41 The stock solution of IAEDANS-labeled calmodulin (I-CaM) was added to ∼3 mL of 10 mM 4-morpholinepropanesulfonic acid buffer (pH 7.4) that also contained 100 mM NaCl, 0.1 mg/mL BSA, and 0.1 mM CaCl2 to achieve a final protein concentration of 50 nM. Ligand peptide solutions were prepared with the same buffer containing I-CaM so that the I-CaM concentration was kept constant during titration. All the solutions were passed through a 0.2-μm filter prior to the experiments. The steady-state IAEDANS fluorescence of the peptide–calmodulin mixture was acquired on a SpectraMax M Series Microplate Reader (Molecular Devices, Sunnyvale, CA). The excitation and emission wavelengths were 340 nm and 460 nm, respectively. The slit widths for excitation and emission were adjusted to minimize photobleaching of the samples and to achieve sufficient fluorescent signal intensity. When applicable, the fluorescence measurements as a function of peptide concentration were fitted with the hyperbolic function as described.41
Immunoblotting
Aliquots of gel-filtered platelets (100 μL) were incubated with PMA or CBPS at 37°C. The reactions were stopped by the addition of radioimmunoprecipitation assay buffer (1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 10 mM Tris, 150 mM NaCl) with protease inhibitors. After heating to 97°C for 10 minutes, proteins were separated by using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Bio-Rad). The membrane was incubated with antibodies at 4°C overnight followed by the corresponding secondary antibodies (goat anti-rabbit IRDye 800CW and goat anti-mouse IRDye 800CW). Densitometric band scanning was performed by using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
ADAM17 activity
Gel-filtered platelets were treated with collagen (10 μg/mL), CBPS (50 μM), or vCBPS (50 μM) for 30 minutes at 37°C and then incubated with 10 μM fluorogenic quenched TACE substrate peptide ((7-methoxycoumarin-4-yl)acetyl-PLAQAV-N-3-(2,4-dinitrophenyl)- L-2,3-diaminopropionyl-RSSSR-NH2) (Calbiochem) at 37°C. ADAM17 activity was measured in a 96-well white plate (OptiPlate; PerkinElmer, Melbourne, Australia) at 37°C using a SpectraMax M Series Microplate Reader (Molecular Devices) set at 320/405 nm.
Confocal fluorescence microscopy
Glass slides were coated with fibrinogen (20 μg/mL) overnight at 4°C, blocked with 1% BSA in PBS for 2 hours, and then washed 3 times with PBS prior to use. Gel-filtered platelets were treated with fluorescein isothiocyanate (FITC) -myr-CBPS, FITC-myr-vCBPS, FITC-CBPS, or FITC-vCBPS for 30 minutes at room temperature and allowed to spread on immobilized fibrinogen for 60 minutes. Slides were analyzed by using a Fluovier FV1000 laser confocal microscope.
Flow cytometry
Gel-filtered platelets were incubated with 10 μM PMA, 10 μΜ FITC-myr-CBPS, FITC-myr-vCBPS, FITC-CBPS, or FITC-vCBPS for 15 minutes. Platelets were fixed with formaldehyde and stained with PE-conjugated mouse anti-human CD62P antibody (eBioscience, San Diego, CA). PE-conjugated mouse immunoglobulin G was used as an isotype control (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were analyzed by using a BD FACSCalibur Flow Cytometer (BD Biosciences).
Mutational analysis
Full-length complementary DNA of Sema4D (OriGene Technologies, Rockville, MD) was cloned into pSecTag2 B vector. Two truncation mutants were generated by inserting a premature stop codon in place of P761 (P761Δ) or D781 (D781Δ) by polymerase chain reaction. All constructs were verified by using DNA sequencing prior to transfection. pSecTag2 B vectors expressing wild-type or mutant Sema4D were transfected into Chinese hamster ovary cells by using X-tremeGENE HP DNA transfection reagent following the supplier’s instruction (Roche) and incubated for 48 hours. Soluble Sema4D in supernatants was immunoprecipitated with 1E8G9 and immunoblotted with EP3569. Sema4D on the platelet surface was monitored by flow cytometry.
Statistical analysis
Data were analyzed by using GraphPad Prism 5.0 software and presented as means ± standard error of the mean. The statistical significance was determined by one-way analysis of variance with the Bonferroni post test for multiple groups.
Results
Sema4D is constitutively bound to calmodulin, forming a complex that dissociates during platelet activation
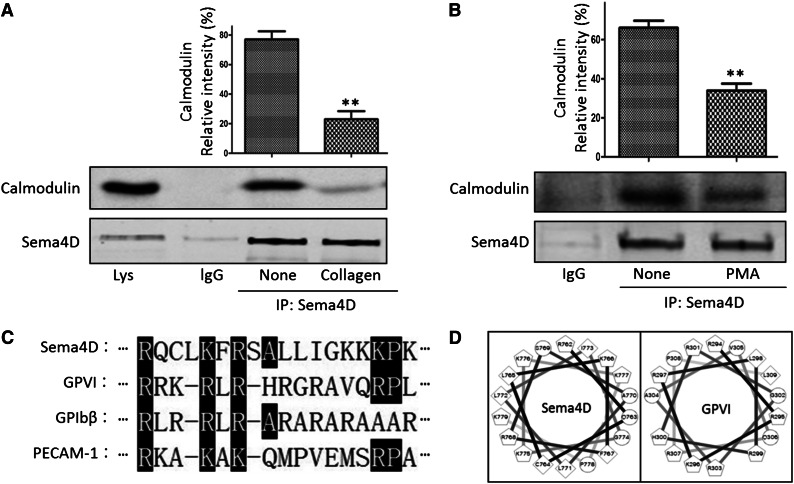
Our previous studies showed that Sema4D is gradually cleaved and shed in an ADAM17-dependent manner during platelet activation but left open the mechanism by which cleavage is regulated.23 Although the impact of knocking out expression of Sema4D on platelets seemed to be limited to events mediated by the platelet collagen receptor GPVI, all of the agonists that were tested, including thrombin, thromboxane A2 mimetics, and PMA as well as collagen, were equally effective in causing cleavage of Sema4D. Cleavage releases a soluble 120-kDa extracellular domain fragment, leaving behind a fragment that contained the remaining extracellular domain, the transmembrane domain, and the cytoplasmic domain. Cleavage did not occur as quickly as platelet aggregation but was detectable within 5 minutes and reached completion within 30 to 45 minutes.23 The data in Figure 1A-B show that calmodulin co-precipitates with Sema4D in resting platelets. Preincubating the platelets with collagen for 30 seconds at 37°C reduced the amount of calmodulin that precipitated with Sema4D by 75%. Preincubating the platelets with PMA for 30 seconds reduced it by 51%. The decrease was not caused by Sema4D cleavage, which is barely detectable within this time frame.23 Therefore, these data show that Sema4D is bound to calmodulin in resting platelets, but this complex dissociates during platelet activation at a rate that precedes Sema4D cleavage.
Figure 1.
Co-immunoprecipitation of Sema4D and calmodulin from platelet lysates and identification of a calmodulin-binding sequence in Sema4D. (A) Platelets (5 × 108/mL) were treated with 10 μg/mL collagen for 30 seconds and lysed. Proteins were immunoprecipitated with anti-Sema4D N-terminal antibody (1E8G9) and immunoblotted with anti-calmodulin and an anti-Sema4D C-terminal antibody (mean ± standard error of the mean; **P < .01; n = 3). (B) Platelets were treated with 1.0 μΜ PMA for 30 seconds and immunoprecipitated with the anti-Sema4D N-terminal antibody. (C) An alignment of the putative calmodulin binding sequence (Arg762-Lys779) of Sema4D with known calmodulin-binding sequence of other platelet surface molecules GPVI (Arg294-Pro309), GPIbβ (Arg149-Arg164), and PECAM-1 (Arg599-Ala614). Identical amino acids or conserved substitutions are highlighted. (D) Helical wheel representation of the Sema4D sequence (Arg762-Lys779) and the GPVI sequence (Arg294-Pro309). Circles, hydrophilic residues; diamonds, hydrophobic residues; pentagons, potentially positively charged.
A calmodulin binding site is present in the membrane-proximal region of Sema4D cytoplasmic domain
A search of the Calmodulin Target Database42 identified a potential binding site for calmodulin in the membrane-proximal Sema4D cytoplasmic domain (RQCLKFRSALLIGKKKPK, Arg762-Lys779). Figure 1C shows a sequence alignment of this polybasic region with the cytoplasmic domains of three other platelet surface proteins that have been shown to bind to calmodulin. A helical wheel projection suggests that the charged residues in this region of the Sema4D cytoplasmic domain are arranged to form a predicted calmodulin-binding site in the shape of an α-helix similar to that of GPVI34 (Figure 1D).
The calmodulin inhibitor W7 induces Sema4D shedding in resting platelets
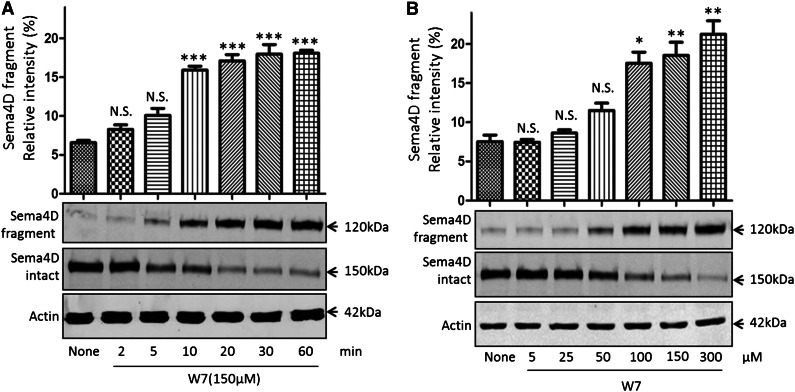
We next asked whether inhibiting the calmodulin–Sema4D interaction is sufficient to trigger Sema4D shedding, starting with a pharmacologic approach to inhibition. W7 (N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide) is a membrane-permeable compound that has been shown to induce shedding of other platelet membrane proteins.33,36,43,44 It is thought to do this either by causing a change in the structure of calmodulin45 or by occupying the hydrophobic pocket found in calmodulin.46 In the experiments summarized in Figure 2A, platelets were incubated with 150 µM W7 for up to 60 minutes. Intact Sema4D appeared as a 150-kDa protein on immunoblots with an antibody directed against the C-terminus of the protein. The appearance of a 120-kDa soluble Sema4D exodomain fragment was detected with an antibody to the N-terminus. Loss of full-length Sema4D and appearance of the soluble fragment were detectable 5 minutes after the addition of W7, reaching maximal accumulation under these conditions after approximately 30 minutes. Half-maximal cleavage at 60 minutes required 50 to 100 µM W7 (Figure 2B).
Figure 2.
The calmodulin inhibitor W7 causes Sema4D cleavage in platelets. Sema4D shedding detectable as a decrease in full-length Sema4D and an increase in soluble Sema4D. (A) Gel-filtered platelets were incubated with 150 µM W7 for 2 to 60 minutes at 37°C. Platelet pellets and supernatants were separated and immunoblotted for full-length and soluble Sema4D. Actin was used to confirm equal sample loading. *P < .05; **P < .01; ***P < .001. N.S., not significant (n = 3). (B) Platelets were incubated with 5 to 300 μΜ W7 for 60 minutes, blotted, and analyzed for intact and soluble Sema4D.
A synthetic peptide corresponding to the calmodulin-binding sequence binds to both recombinant calmodulin and platelet calmodulin
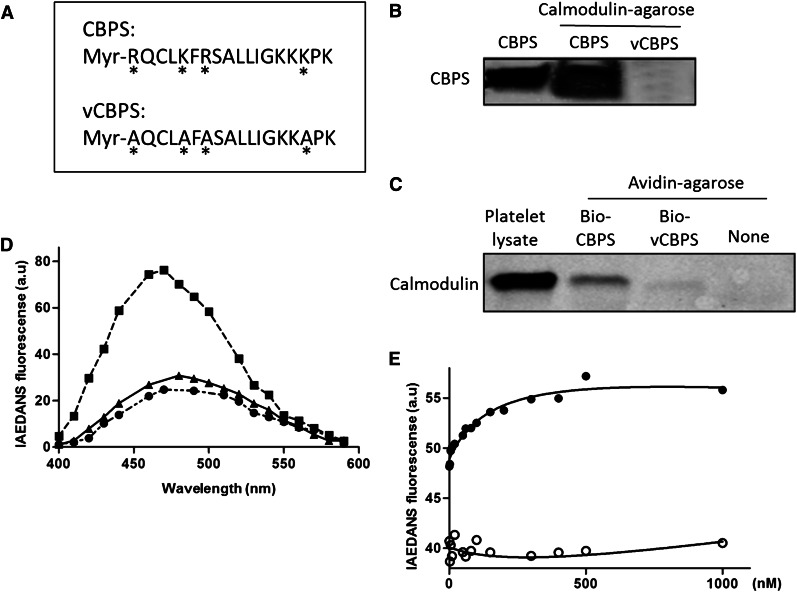
To determine whether calmodulin can actually bind to its putative binding site in Sema4D, a peptide corresponding to Arg762-Lys779 (designated CBPS) was synthesized, as was a variant peptide in which 4 basic residues were substituted with alanine residues (designated vCBPS) (Figure 3A). Two assays were used to examine whether calmodulin can bind to CBPS. In the first, recombinant calmodulin conjugated to agarose was incubated with CBPS and vCBPS. CBPS became bound; vCBPS did not (Figure 3B). In the second assay, biotinylated CBPS and biotinylated vCBPS were incubated with platelet lysate. Peptide–calmodulin complexes were isolated on avidin-conjugated agarose, subjected to electrophoresis, and then probed with anti-calmodulin. The CBPS peptide retrieved calmodulin from platelet lysates; the vCBPS peptide did not (Figure 3C).
Figure 3.
Binding between calmodulin and the calmodulin-binding peptide of Sema4D (CBPS). (A) A myristoylated peptide corresponding to the calmodulin-binding sequence of Sema4D (Myr-RQCLKFRSALLIGKKKPK) was synthesized and designated as CBPS. A variant peptide in which four conserved amino acids were replaced with Ala (*) is designated as vCBPS (Myr-AQCLAFASALLIGKKAPK). (B) Ten micrograms of CBPS or vCBPS in PBS were incubated with 20 μL of calmodulin-agarose beads at 4°C for 4 hours. After washing extensively, bound peptide was eluted with Laemmli sample buffer and analyzed by gel electrophoresis followed by silver staining. (C) Platelet lysate was incubated with 10 µg of biotinylated CBPS or vCBPS overnight at 4°C. Biotinylated peptide-calmodulin complexes were captured by using avidin-agarose beads, eluted, and subjected to immunoblot analysis by using a calmodulin monoclonal antibody. (D) Fluorescence emission spectra of IAEDANS-labeled calmodulin (I-CaM). (●) I-CaM was added to 3 mL of 10 mM 4-morpholinepropanesulfonic acid (pH 7.4), 0.1 mM CaCl2, 100 mM NaCl, and 0.1 mg/mL bovine serum albumin to a final concentration of 50 nM, and an emission scan was obtained. (▪) CBPS or (▲) vCBPS was added to a final concentration of 40 μΜ, and the scans was obtained. The excitation wavelength was 340 nm. All spectra were corrected for background reading from the buffer. (E) Binding affinity of CBPS with I-CaM. Association of CBPS to I-CaM was monitored by the change in IAEDANS emission fluorescence at 460 nm. Calmodulin protein was diluted to 50 nM in a 100-μL system and added to the 96-well plate. CBPS (●) or vCBPS (○) polypeptide was added to the calmodulin solution. The buffers were the same as in (D). I-CaM denotes IAEDANS-labeled calmodulin. Data are representative of at least 3 independent experiments.
The binding affinity for the CBPS–calmodulin interaction was measured by using I-CaM.41 As shown in Figure 3D, addition of CBPS to I-CaM caused an increase in IAEDANS fluorescence; addition of vCBPS did not. Titrations of CBPS to I-CaM were monitored by the change in IAEDANS fluorescence and fitted to standard hyperbolic function (Figure 3E). The calculated equilibrium dissociation constant was 165 ± 40 nM, which is between that of GPVI and L-selectin. The vCBPS peptide did not fit the titration curve.
Membrane-permeable CBPS peptide triggers Sema4D cleavage in platelets
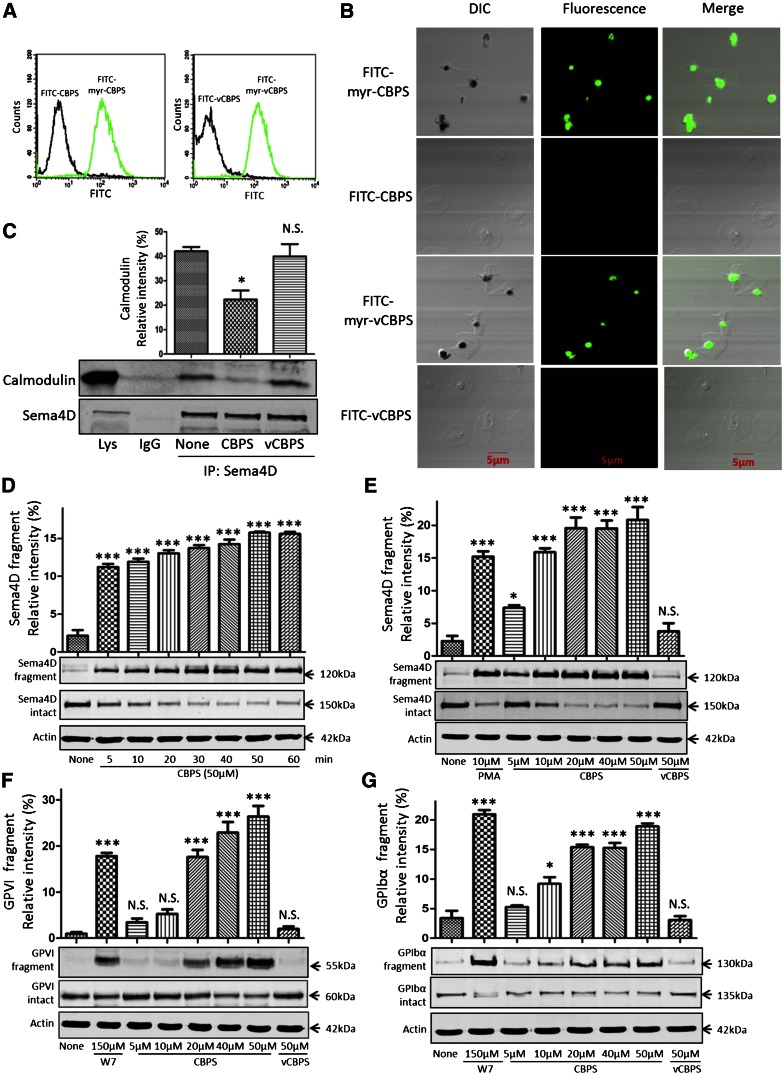
To determine whether CBPS peptide can trigger Sema4D cleavage in platelets, we first tested whether myristoylated CBPS and myristoylated vCBPS are able to penetrate the platelet plasma membrane and cause the dissociation of calmodulin from Sema4D. Both peptides were conjugated with FITC and incubated with platelets. Binding was detected by flow cytometry. Passage into the cytoplasm was detected by confocal fluorescence microscopy. The same peptides lacking myristate were used as additional controls. Only the myristoylated versions bound to and entered the platelets (Figure 4A-B). Pulldown experiments showed that myristoylated CBPS also caused dissociation of calmodulin from Sema4D, but myristoylated vCBPS did not (Figure 4C). Finally, immunoblotting showed that myristoylated CBPS, but not the myristoylated vCBPS, caused time- and concentration-dependent cleavage of Sema4D, as detected by loss of the full-length protein and the appearance in the supernatant of the 120-kDa fragment (Figure 4D-E). Thus, using the peptide to act as a decoy is sufficient to trigger cleavage and shedding of Sema4D.
Figure 4.
Addition of membrane-permeable CBPS causes dissociation of the Sema4D–calmodulin complex and cleavage of Sema4D. (A) Platelets were incubated with 10 μΜ FITC-myr-CBPS, FITC-CBPS, FITC-myr-vCBPS, or FITC-vCBPS for 15 minutes, fixed with formaldehyde, and analyzed by flow cytometry. (B) Platelets were treated with 10 μΜ FITC-myr-CBPS, FITC-CBPS, FITC-myr-vCBPS, or FITC-vCBPS peptide for 30 minutes, allowed to spread on immobilized fibrinogen for 60 minutes, and examined by differential interference contrast (DIC) and confocal fluorescence microscopy. The images are representative of at least 3 independent experiments. (C) Platelets (5 × 108/mL) were incubated with 5.0 μΜ CBPS or vCBPS for 30 seconds, lysed, and immunoprecipitated with anti-Sema4D N-terminal antibody (1E8G9). Proteins were immunoblotted with an anti-calmodulin antibody and an anti-Sema4D C-terminal antibody (n = 3). (D) Gel-filtered platelets were incubated with 50 μΜ CBPS. Soluble 120-kDa fragment was detected with an N-terminal antibody. Full-length 150-kDa Sema4D in the platelet pellets was detected by using the Sema4D C-terminal antibody. (E) Gel-filtered platelets were incubated with CBPS or vCBPS for 60 minutes at 37°C. (F,G) Gel-filtered platelets were incubated with CBPS, vCBPS, or W7 for 60 minutes at 37°C. Platelet pellets and supernatants were separated and blotted with GPVI antibody (6B-12) or GPIbα antibody (SZ2). *P < .05; ***P < .001 compared with control (n = 3). N.S., not significant (n = 3).
Myristoylated CBPS peptide also triggers cleavage of GPVI and GPIbα in platelets
In addition to Sema4D, calmodulin binds directly to other platelet receptors, including GPIbβ and GPVI, and dissociation of the calmodulin from the receptors has been shown to facilitate shedding.47 Upon entering into platelets, myristoylated CBPS may act as a calmodulin sink to break the dynamic equilibrium of free and bound calmodulin and therefore could induce cleavage of GPVI and GPIbα in platelets. To test this hypothesis, we examined the effect of CBPS on the cleavage of GPVI and GPIbα.32,35 Myristoylated CBPS, but not vCBPS, induced cleavage of GPVI and GPIbα. However, higher concentrations of the peptide were required (Figure 4F-G).
CBPS induces metalloprotease-mediated Sema4D shedding without causing platelet activation or enhancing ADAM17 activity
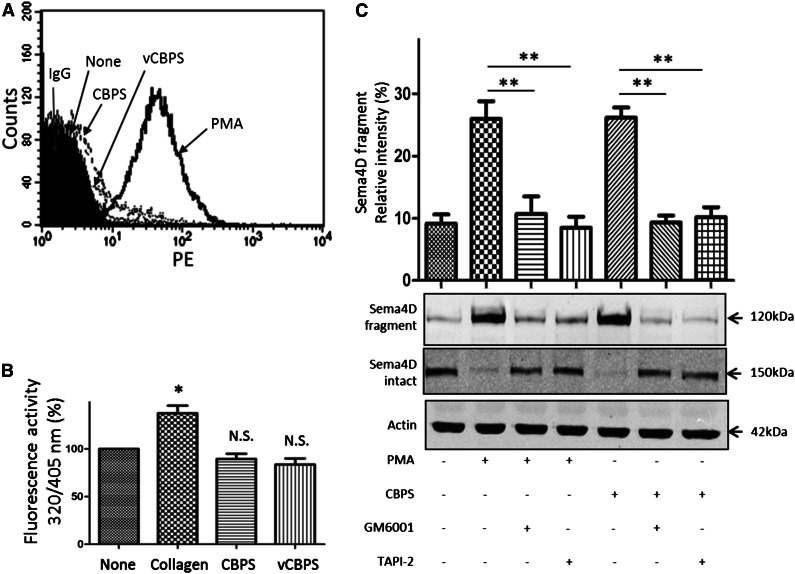
Sema4D shedding appears to be a normal consequence of platelet activation, and we have previously shown that it occurs when platelets are activated by agonists as diverse as thrombin, collagen, TxA2 mimetics, and PMA.23 To be sure that CBPS was not causing Sema4D cleavage by unexpectedly causing platelet activation, we incubated platelets with the peptide and used the appearance on the platelet surface of the α-granule membrane protein P-selectin (CD62P) as a marker of platelet activation. The results show that there is no significant increase in P-selectin expression in CBPS-treated samples compared with vCBPS-treated or untreated platelets (Figure 5A). To determine whether CBPS activates ADAM17, we measured platelet ADAM17 activity with a specific substrate. Collagen produced a measurable increase, but CBPS did not (Figure 5B). Collectively, these results suggest that CBPS-induced cleavage is not due to either platelet activation or ADAM17 activation. Additionally, we tested whether CBPS-induced Sema4D shedding, like agonist-induced shedding, is sensitive to the metalloprotease inhibitors GM6001 and TAPI-2. Both inhibitors prevented shedding (Figure 5C)
Figure 5.
Effect of CBPS on platelet activation and ADAM17 activity. (A) Detection of P-selectin surface expression by flow cytometry in platelets treated with CBPS or vCBPS. Gel-filtered platelets were incubated with PMA (10 μM), CBPS (50 μΜ), or vCBPS (50 μΜ) at 37°C for 15 minutes. Platelets were fixed with formaldehyde and stained with PE-conjugated mouse anti-human CD62P antibody. Data are representative of at least 3 independent experiments using different donors. (B) A fluorogenic substrate was used to detect ADAM17 activity on platelets incubated with 50 µM CBPS or vCBPS for 30 minutes at 37°C. Fluorescence (320/405 nm) was quantified, and all values were corrected for background fluorescence. *P < .05 compared with control (n ≥ 3). N.S., not significant. (C) Gel-filtered platelets were preincubated with GM6001 (100 μΜ) or TAPI-2 (10 μΜ), incubated with CBPS (50 μΜ) or PMA (10 μΜ), and then immunoblotted for full length and soluble Sema4D. **P < .01 (n = 3).
Deletion of the Sema4D calmodulin binding domain triggers constitutive shedding
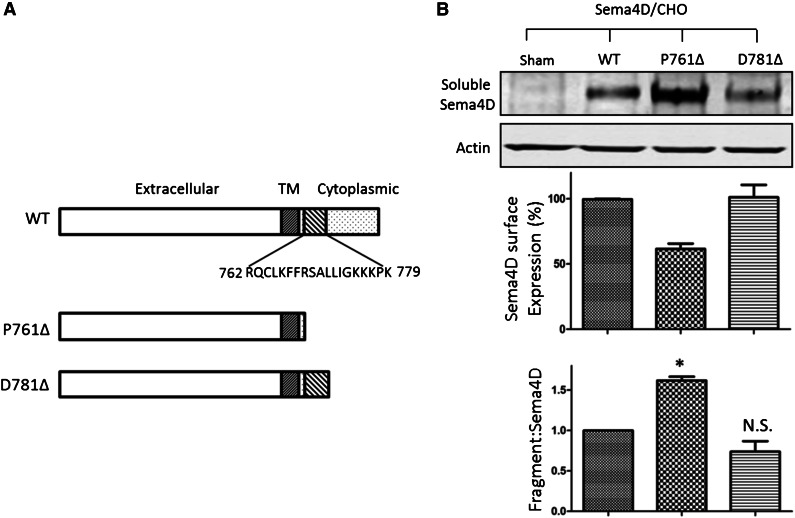
Finally, the role of calmodulin-binding domain in the membrane-proximal region in Sema4D shedding was assessed by mutational analysis. Constructs of full-length Sema4D or Sema4D truncated after either residue P761 (denoted P761Δ) or D781 (denoted D781Δ) were expressed in Chinese hamster ovary cells. These two truncation mutants bracket the newly identified calmodulin-binding site (Figure 6A). The amount of soluble Sema4D constitutively released into the culture medium was monitored by immunoblotting (Figure 6B, upper panel). Surface expression was measured by flow cytometry (middle panel). The ratio of soluble Sema4D to surface Sema4D was normalized against the corresponding ratio observed in cells expressing wild-type Sema4D and was used to denote the extent of Sema4D shedding (lower panel). The results show that all three constructs produce measurable amounts of soluble Sema4D 48 hours after transfection. However, compared with the wild-type, the P761Δ construct, which lacks the calmodulin-binding domain, showed both an increase in soluble Sema4D and a decrease in Sema4D surface expression. The D781Δ construct, which retains the calmodulin-binding domain, did not.
Figure 6.
Deletion of the Sema4D calmodulin-binding domain causes constitutive shedding of the Sema4D exodomain. (A) Illustration of the Sema4D constructs that were transfected into Chinese hamster ovary (CHO) cells. D781Δ retains the calmodulin-binding domain; P761Δ does not. (B, top) Western blot of CHO cell supernate showing constitutive shedding of Sema4D. (B, middle) Relative surface expression of Sema4D in CHO cells detected by flow cytometry and normalized relative to wild-type (WT) controls. (B, bottom) A summary of three experiments. The relative fragment:Sema4D ratio is the ratio of the Sema4D fragment level versus surface Sema4D expression level normalized to that of the wild-type, which was set as 1.0. N.S., not significant; TM, transmembrane. *P < .05.
Discussion
Sema4D is a transmembrane glycoprotein that is of interest to platelet biology because of its ability to amplify thrombus formation by binding to receptors on adjacent platelets, allowing it to serve as a source of contact-dependent signaling when activated platelets come into stable proximity with each other.24 Our previous studies have shown that Sema4D is cleaved in an ADAM17-dependent manner from activated platelets, producing a 120-kDa exodomain fragment at a rate that lags well behind markers of platelet activation such as aggregation and storage granule exocytosis.23 Potential mechanisms for regulating the cleavage of Sema4D include both an agonist-driven increase in ADAM17 activity and an agonist-dependent increase in the accessibility of Sema4D to the protease, perhaps involving a conformational change in Sema4D.
Here we have asked whether the regulation involves an interaction between the Sema4D cytoplasmic domain and calmodulin. The results show that in resting platelets, calmodulin is bound to Sema4D, forming a complex that dissociates when platelets are activated. Addition of W7, a molecule that is thought to interfere with the binding of calmodulin to its targets,45 triggers cleavage of Sema4D, as does a membrane-permeable peptide corresponding to the putative calmodulin-binding site in Sema4D. Separate experiments show that this peptide can bind to calmodulin and, when added in a membrane permeable form, can enter intact platelets and cause dissociation of the Sema4D–calmodulin complex. A variant peptide lacking key basic amino acid residues has no apparent effect. Finally, deletion of the putative calmodulin-binding domain induces constitutive shedding of Sema4D.
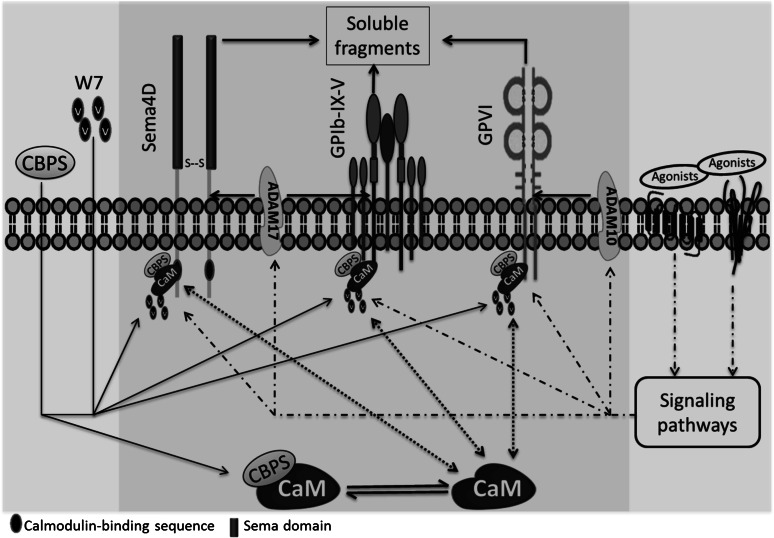
A model for Sema4D cleavage that incorporates these observations is shown in Figure 7. We propose that the release of calmodulin from Sema4D causes a conformational change that promotes access to ADAM17. Consistent with this conclusion, addition of the synthetic Sema4D cytoplasmic peptide causes shedding without a measurable increase in ADAM17 activity. Precisely how platelet activation causes dissociation of the calmodulin–Sema4D complex and how dissociation of the complex results in cleavage of the Sema4D extracellular domain is not yet entirely clear.
Figure 7.
A proposed model of Sema4D exodomain shedding regulated by the interaction of calmodulin with the Sema4D cytoplasmic domain. Sema4D was shown to be a calmodulin-binding protein with a site of interaction in the membrane-proximal cytoplasmic domain that regulates the cleavage of the membrane-proximal exodomain of Sema4D by ADAM17. It was proposed that releasing the association of calmodulin with Sema4D by incubation with a peptide CBPS or a calmodulin inhibitor W7 induces the conformational change that favors to the accessibility of its sheddases, mediating Sema4D shedding. This peptide also induces cleavage of GPVI and GPIbα, implying a generality of this mechanism that calmodulin regulates shedding of platelet membrane receptors regardless of what their sheddases are.
It is interesting to note that the peptide that triggered the shedding of Sema4D also induced cleavage of GPVI and GPIbα, despite the fact that these are structurally different proteins and, at least in the case of GPVI, a different metalloprotease (ADAM10) is thought to be responsible for cleavage. This implies a generality of the mechanism by which calmodulin regulates the shedding of platelet membrane proteins. Presumably, there is a dynamic balance between bound and unbound calmodulin. The relatively higher concentration of the Sema4D peptide needed to cause cleavage of GPVI and GPIbα compared with Sema4D could reflect differences in the sequences of each of these proteins at the calmodulin-binding site, differences in the affinity of calmodulin for the 3 proteins,32,35 or differences in their accessibility to the peptide.
The role of calmodulin in Sema4D shedding bears some resemblance to its role in shedding GPIbα31 and GPVI.31,48 The net effect is not the same, however. Shedding of GPIbα and GPVI removes molecules that are essential for the binding of platelets to collagen and for platelet activation. Sema4D also has an indirect role in amplifying platelet responses to collagen. However, the Sema4D exodomain fragment retains biological activity at its target receptors, some of which are expressed on nearby platelets, while others are expressed by endothelial cells.13,49 Thus, shedding of Sema4D can potentially have both local and remote effects.
The finding that Sema4D exodomain cleavage is regulated by calmodulin is helpful in understanding the impact of agonist-induced shedding of platelet surface molecules during thrombus formation. Sema4D on the platelet surface plays a role in platelet activation by amplifying signaling downstream of the platelet collagen receptor GPVI.23,24 However, Sema4D cleavage and shedding from platelets lags well behind platelet aggregation,23 causing us to conclude that soluble Sema4D may be most relevant for events other than hemostasis. Given that Sema4D receptors are expressed by endothelial cells, B cells, dendritic cells, and some cancer cells, such events might include wound healing, angiogenesis, tumor extension, and the immune response. Although there are data that support each of these possibilities, much of that data has been obtained in studies on mice with a global defect in Sema4D expression or on isolated cells in which recombinant soluble Sema4D has been shown to elicit receptor-mediated responses. Whether mechanistic information about the regulation of Sema4D shedding offers new strategies for manipulating hemostasis or thrombosis is under investigation. For now, it is clear that Sema4D cleavage and shedding from platelets is an actively regulated event in which the dissociation of calmodulin from Sema4D triggers shedding by allowing ADAM17 access to its cleavage site. The subsequent fate and the biological impact of soluble Sema4D derived from platelets is an intriguing question that remains to be fully addressed.
Acknowledgments
The authors thank Xi Mo, Shanghai Children’s Medical Center, and Jianping Ding, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China, for their helpful discussions.
This work was supported in part by grants from the National Science Foundation of China (NSFC81071410, NSFC81170132), Jiangsu Province’s Key Discipline of Medicine (XK201118), the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (L.Z.), and NIH HL40387, HL81012, and T32 HL07439 (L.F.B.).
Footnotes
Presented in abstract form at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 10, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M., Z.Z., and Q.L. designed and performed experiments, analyzed data, and wrote the manuscript; X.L. and X.X. conducted confocal microscopy; K.M.W., C.R., R.L., and L.F.B. supplied reagents and revised the manuscript; and L.Z. designed the research and wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li Zhu, Soochow University, Rm 509, Bldg 703, 199 Ren’ai Rd, Suzhou Industrial Park, Suzhou, Jiangsu 215123, China; e-mail: lizhusong@yahoo.com.
References
- 1.Delaire S, Elhabazi A, Bensussan A, Boumsell L. CD100 is a leukocyte semaphorin. Cell Mol Life Sci. 1998;54(11):1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci. 2003;116(Pt 17):3463–3470. doi: 10.1242/jcs.00674. [DOI] [PubMed] [Google Scholar]
- 3.Hall KT, Boumsell L, Schultze JL, et al. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93(21):11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10(10):843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 5.Esnouf RM, Love CA, Harlos K, Stuart DI, Jones EY. Structure determination of human semaphorin 4D as an example of the use of MAD in non-optimal cases. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 1):108–115. doi: 10.1107/S0907444905034992. [DOI] [PubMed] [Google Scholar]
- 6.Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13(5):621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 7.Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148(2):318–323. [PubMed] [Google Scholar]
- 8.Furuyama T, Inagaki S, Kosugi A, et al. Identification of a novel transmembrane semaphorin expressed on lymphocytes. J Biol Chem. 1996;271(52):33376–33381. doi: 10.1074/jbc.271.52.33376. [DOI] [PubMed] [Google Scholar]
- 9.Oinuma I, Ito Y, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem. 2010;285(36):28200–28209. doi: 10.1074/jbc.M110.147546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M. Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7(7):704–709. doi: 10.1038/sj.embor.7400737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oinuma I, Katoh H, Negishi M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci. 2004;24(50):11473–11480. doi: 10.1523/JNEUROSCI.3257-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305(5685):862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64(15):5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 14.Conrotto P, Valdembri D, Corso S, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105(11):4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 15.Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205(7):1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binmadi NO, Proia P, Zhou H, Yang YH, Basile JR. Rho-mediated activation of PI(4)P5K and lipid second messengers is necessary for promotion of angiogenesis by Semaphorin 4D. Angiogenesis. 2011;14(3):309–319. doi: 10.1007/s10456-011-9214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XG, Zhu Y, Liu XS, et al. Preparation and identification of monoclonal antibodies against CD100 molecule. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19(1):80–82. [PubMed] [Google Scholar]
- 18.Kato S, Kubota K, Shimamura T, et al. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci. 2011;102(11):2029–2037. doi: 10.1111/j.1349-7006.2011.02053.x. [DOI] [PubMed] [Google Scholar]
- 19.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23(30):5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 20.Ch’ng ES, Kumanogoh A. Roles of Sema4D and plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedel RH, Kerjan G, Rayburn H, et al. Plexin-B2 controls the development of cerebellar granule cells. J Neurosci. 2007;27(14):3921-3932. [DOI] [PMC free article] [PubMed]
- 22.Hirschberg A, Deng S, Korostylev A, et al. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30(3):764–780. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Bergmeier W, Wu J, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA. 2007;104(5):1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wannemacher KM, Zhu L, Jiang H, et al. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood. 2010;116(25):5707–5715. doi: 10.1182/blood-2010-04-279943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Stalker TJ, Fong KP, et al. Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(7):1039–1045. doi: 10.1161/ATVBAHA.109.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yukawa K, Tanaka T, Kishino M, et al. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med. 2010;26(1):39–44. doi: 10.3892/ijmm_00000432. [DOI] [PubMed] [Google Scholar]
- 27.Fong KP, Barry C, Tran AN, et al. Deciphering the human platelet sheddome. Blood. 2011;117(1):e15–e26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, Sickmann A. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114(1):e10–e19. doi: 10.1182/blood-2009-02-203828. [DOI] [PubMed] [Google Scholar]
- 29.Qiao JL, Shen Y, Gardiner EE, Andrews RK. Proteolysis of platelet receptors in humans and other species. Biol Chem. 2010;391(8):893–900. doi: 10.1515/BC.2010.081. [DOI] [PubMed] [Google Scholar]
- 30.Gardiner EE, Al-Tamimi M, Andrews RK, Berndt MC. Platelet receptor shedding. Methods Mol Biol. 2012;788:321–339. doi: 10.1007/978-1-61779-307-3_22. [DOI] [PubMed] [Google Scholar]
- 31.Berndt MC, Karunakaran D, Gardiner EE, Andrews RK. Programmed autologous cleavage of platelet receptors. J Thromb Haemost. 2007;5(Suppl 1):212–219. doi: 10.1111/j.1538-7836.2007.02484.x. [DOI] [PubMed] [Google Scholar]
- 32.Andrews RK, Munday AD, Mitchell CA, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Blood. 2001;98(3):681–687. doi: 10.1182/blood.v98.3.681. [DOI] [PubMed] [Google Scholar]
- 33.Mo X, Nguyen NX, Mu FT, et al. Transmembrane and trans-subunit regulation of ectodomain shedding of platelet glycoprotein Ibalpha. J Biol Chem. 2010;285(42):32096–32104. doi: 10.1074/jbc.M110.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locke D, Liu C, Peng X, Chen H, Kahn ML. Fc Rgamma -independent signaling by the platelet collagen receptor glycoprotein VI. J Biol Chem. 2003;278(17):15441–15448. doi: 10.1074/jbc.M212338200. [DOI] [PubMed] [Google Scholar]
- 35.Andrews RK, Suzuki-Inoue K, Shen Y, Tulasne D, Watson SP, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of platelet glycoprotein VI. Blood. 2002;99(11):4219–4221. doi: 10.1182/blood-2001-11-0008. [DOI] [PubMed] [Google Scholar]
- 36.Wong MX, Harbour SN, Wee JL, Lau LM, Andrews RK, Jackson DE. Proteolytic cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is regulated by a calmodulin-binding motif. FEBS Lett. 2004;568(1-3):70–78. doi: 10.1016/j.febslet.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 37.Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166(7):4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- 38.Ruan CG, Du XP, Xi XD, Castaldi PA, Berndt MC. A murine antiglycoprotein Ib complex monoclonal antibody, SZ 2, inhibits platelet aggregation induced by both ristocetin and collagen. Blood. 1987;69(2):570–577. [PubMed] [Google Scholar]
- 39.Chen H, Locke D, Liu Y, Liu C, Kahn ML. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem. 2002;277(4):3011–3019. doi: 10.1074/jbc.M109714200. [DOI] [PubMed] [Google Scholar]
- 40.Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci USA. 2002;99(14):9219–9224. doi: 10.1073/pnas.142053899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng W, Srinivasan S, Zheng X, Putkey JA, Li R. Interaction of calmodulin with L-selectin at the membrane interface: implication on the regulation of L-selectin shedding. J Mol Biol. 2011;411(1):220–233. doi: 10.1016/j.jmb.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J Struct Funct Genomics. 2000;1(1):8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
- 43.Rabie T, Strehl A, Ludwig A, Nieswandt B. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J Biol Chem. 2005;280(15):14462–14468. doi: 10.1074/jbc.M500041200. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner EE, Arthur JF, Berndt MC, Andrews RK. Role of calmodulin in platelet receptor function. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(4):283–287. doi: 10.2174/156801605774322283. [DOI] [PubMed] [Google Scholar]
- 45.Osawa M, Kuwamoto S, Izumi Y, et al. Evidence for calmodulin inter-domain compaction in solution induced by W-7 binding. FEBS Lett. 1999;442(2-3):173–177. doi: 10.1016/s0014-5793(98)01637-8. [DOI] [PubMed] [Google Scholar]
- 46.Osawa M, Swindells MB, Tanikawa J, Tanaka T, Mase T, Furuya T, Ikura M. Solution structure of calmodulin-W-7 complex: the basis of diversity in molecular recognition. J Mol Biol. 1998;276(1):165–176. doi: 10.1006/jmbi.1997.1524. [DOI] [PubMed] [Google Scholar]
- 47.Andrews RK, Karunakaran D, Gardiner EE, Berndt MC. Platelet receptor proteolysis: a mechanism for downregulating platelet reactivity. Arterioscler Thromb Vasc Biol. 2007;27(7):1511–1520. doi: 10.1161/ATVBAHA.107.141390. [DOI] [PubMed] [Google Scholar]
- 48.Gardiner EE, Arthur JF, Kahn ML, Berndt MC, Andrews RK. Regulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinase. Blood. 2004;104(12):3611–3617. doi: 10.1182/blood-2004-04-1549. [DOI] [PubMed] [Google Scholar]
- 49.Zielonka M, Xia J, Friedel RH, Offermanns S, Worzfeld T. A systematic expression analysis implicates Plexin-B2 and its ligand Sema4C in the regulation of the vascular and endocrine system. Exp Cell Res. 2010;316(15):2477–2486. doi: 10.1016/j.yexcr.2010.05.007. [DOI] [PubMed] [Google Scholar]