Abstract
Objective:
Our aim was to compare 2 Framingham vascular risk scores with a dementia risk score in relation to 10-year cognitive decline in late middle age.
Methods:
Participants were men and women with mean age of 55.6 years at baseline, from the Whitehall II study, a longitudinal British cohort study. We compared the Framingham general cardiovascular disease risk score and the Framingham stroke risk score with the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) risk score that uses risk factors in midlife to estimate risk of late-life dementia. Cognitive tests included reasoning, memory, verbal fluency, vocabulary, and global cognition, assessed 3 times over 10 years.
Results:
Higher cardiovascular disease risk and higher stroke risk were associated with greater cognitive decline in all tests except memory; higher dementia risk was associated with greater decline in reasoning, vocabulary, and global cognitive scores. Compared with the dementia risk score, cardiovascular and stroke risk scores showed slightly stronger associations with 10-year cognitive decline; these differences were statistically significant for semantic fluency and global cognitive scores. For example, cardiovascular disease risk was associated with −0.06 SD (95% confidence interval [CI] = −0.08, −0.05) decline in the global cognitive scores over 10 years whereas dementia risk was associated with −0.03 SD (95% CI = −0.04, −0.01) decline (difference in β coefficients = 0.03; 95% CI = 0.01, 0.05).
Conclusions:
The CAIDE dementia and Framingham risk scores predict cognitive decline in late middle age but the Framingham risk scores may have an advantage over the dementia risk score for use in primary prevention for assessing risk of cognitive decline and targeting of modifiable risk factors.
Along with attempts to identify risk factors for dementia, there is increasing interest in studying predictors of cognitive decline as it is now widely accepted that dementia has a long preclinical phase. Vascular risk factors are hypothesized to be the key modifiable risk factors for dementia and adverse cognitive outcomes.1–5 Mid- rather than late-life vascular risk factors are considered to be important for late-life cognitive impairment and dementia.2,5–9 Moreover, individuals may be at higher risk of cognitive impairment from accumulation of risk, with the clustering of risk factors being associated with the risk of dementia in a cumulative manner.7,10
Recognizing the role of multiple risk factors, a number of mostly cross-sectional and prospective studies have examined the utility of risk scores to assess risk of cognitive impairment and dementia.11–18 The Framingham cardiovascular risk algorithms, in particular the Framingham stroke risk profile, initially developed to predict cerebrovascular disease, have been shown to be associated with brain pathology and cognitive dysfunction.11,13,14,16,17 A dementia risk score based on the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study that uses midlife risk factors to predict risk of late-life dementia has recently been proposed.19 However, whether it predicts cognitive decline better than the Framingham risk scores remains unknown. To our knowledge, there has been no attempt so far to compare risk scores in predicting cognitive decline in midlife.
The objective of this study was to compare 2 well-known Framingham risk scores, the Framingham stroke and general cardiovascular risk scores, with the CAIDE dementia risk score in relation to cognitive decline over 10 years.
METHODS
Study population.
Data were drawn from the Whitehall II study, an ongoing prospective cohort study established in 1985 on 6,895 men and 3,413 women, aged 35 to 55 years.20 The study design consists of a self-administered questionnaire approximately every 2.5 years and a clinical examination every 5 years. Cognitive tests were introduced at phase 5 (1997/1999) and repeated at phase 7 (2002/2004) and phase 9 (2007/2009). Phase 5 constitutes the baseline of the present study, concurrent with the first cognitive measure.
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent. Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee.
Risk scores.
Framingham risk scores.
The Framingham general cardiovascular disease (CVD) risk profile and the Framingham stroke risk profile are multivariable risk scores that provide a sex-specific absolute risk of cardiovascular events. The Framingham risk scores have been shown to be valid measures of CVD risk in the Whitehall II study population and strongly predict incidence of cardiovascular events.21
The Framingham general CVD risk score includes age, sex, systolic blood pressure, treatment for hypertension, high-density lipoprotein cholesterol, total cholesterol, smoking, and diabetes. The Framingham stroke risk score incorporates age, systolic blood pressure, treatment for hypertension, diabetes, smoking, prior CVD (myocardial infarction, angina pectoris, coronary insufficiency, intermittent claudication, or congestive heart failure), atrial fibrillation, and left-ventricular hypertrophy.
Dementia risk score.
The CAIDE risk score was developed to predict late-life dementia based on midlife risk factors. Its components are age, education, sex, systolic blood pressure, body mass index, total cholesterol, physical activity, and APOE ε4 genotype. There are 2 versions of the dementia risk score; the difference is the inclusion of APOE in one version.19 In this study, both versions of this dementia risk score were examined.
We used standard operating protocols to measure risk factors for the risk scores (see appendix e-1 on the Neurology® Web site at www.neurology.org). Components for the 3 risk scores were drawn from questionnaire and clinical examination data at phase 5 (1997/1999); risk scores were calculated according to the original equations, and scoring methods reported by the authors of these risk scores.19,22–24
Cognitive function.
Cognitive function was assessed 3 times over 10 years. The cognitive test battery consisted of 5 standard cognitive tasks as follows.
The Alice Heim 4-I tests inductive reasoning, measuring the ability to identify patterns and infer principles and rules.25 It is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty. Participants had 10 minutes to complete this test.
Short-term verbal memory was assessed with a 20-word free-recall test. Participants were presented with a list of 20 one- or two-syllable words at 2-second intervals and were asked to recall in writing as many of the words as possible in any order. They had 2 minutes to do this test.
Two measures of verbal fluency were used: phonemic and semantic. Phonemic fluency was assessed via “S” words and semantic fluency via “animal” words.26 Participants were asked to recall in writing as many words beginning with “S” and as many animal names as they could. One minute was allowed for each test.
Vocabulary was assessed using the Mill Hill Vocabulary test in its multiple-choice format, consisting of a list of 33 stimulus words ordered by increasing difficulty and 6 response choices.27
A global cognitive score was created using all 5 tests described above by first standardizing the raw scores on each test to z scores (mean = 0; SD = 1) using the baseline mean and SD values in the entire cohort at baseline for each test. The z scores were then averaged to yield the global cognitive score. To allow comparability across the tests, standardized scores were used in the analysis.
Statistical analysis.
The analyses involve 2 analytic samples. The first is a comparison of the Framingham CVD risk score with the dementia risk score and is based on participants free of CVD (or stroke) at baseline, with data on all components of risk scores. The second is a comparison of the Framingham stroke risk score with the dementia risk score based on individuals without a history of stroke or TIA who had data on all components of the risk scores.
Using linear mixed-effects models, we examined longitudinal associations of the risk scores with cognitive change over 10 years. Mixed-effects models take into account intraindividual correlation inherent in repeated measures and have the advantage of using all available data over the 10-year follow-up period. The models included terms for risk (3 sets of analyses for CVD, stroke, and dementia risk score), time, and an interaction term between risk and time. Both the slope and intercept were fitted as random effects, allowing them to vary between individuals. Risk scores were modeled in 2 forms: in continuous form, they were standardized after natural logarithmic (loge) transformation to correct the skewed distributions. In categorical form, 3 groups with comparable numbers were constructed with categories taken to represent low, intermediate, and high risk for CVD (<7, 7 to <13, and ≥13), stroke (<4, 4 to <6, and ≥6), and dementia (<7, 7–8, and ≥9) risk scores. These risk groups are based on the risk distributions in our study samples. We compared the Framingham cardiovascular and stroke risk scores with the dementia risk score using the β estimates associated with each pair of standardized risk scores by subtracting βFramingham CVD/stroke from βCAIDE dementia. To test whether this difference was statistically significant, a 95% confidence interval (CI) around the difference was calculated using a bootstrapping technique with 2,000 resamplings.
Although our focus was on risk scores as measures of aggregate risk, in subsidiary analyses, we examined the associations of individual components to determine whether the associations with 10-year cognitive change were driven by a few risk factors. Additionally, we examined whether the association between the risk score and 10-year cognitive change remained after adjusting separately for each component of the risk score. Although the β coefficient in this case would not be meaningful, the corresponding p values can provide an indication of whether the associations may be attributable to a single risk factor. Analyses were performed using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
A total of 7,830 (75.9%) of the original 10,308 participants of the Whitehall II study participated in phase 5 (1997/1999) when cognitive tests were introduced to the study. Comparison of the Framingham CVD score and CAIDE dementia risk score was based on 4,374 participants (3,162 men, 1,212 women); comparison of the Framingham stroke and CAIDE dementia risk scores included 5,157 individuals (3,651 men, 1,506 women) (table 1). Mean dementia risk was 6.8 (SD = 2.3). Mean CVD and stroke risk (%) were 12.4 (SD = 8.8) and 4.5 (SD = 3.6), respectively. The correlation between CVD and dementia risk was 0.51, and between stroke and dementia risk was 0.38 (p < 0.05). Approximately 74% of participants had cognitive data at all 3 phases and 18% at 2 phases. Compared with individuals not included in these analyses, the analytic samples consisted of younger and more educated individuals. For example, in the first comparison sample, mean age was 55.2 years vs 56.9 years at phase 5 (p < 0.001); 28% vs 24.1% had a university degree (p < 0.001).
Table 1.
Characteristics of the study sample at baseline (phase 5)a
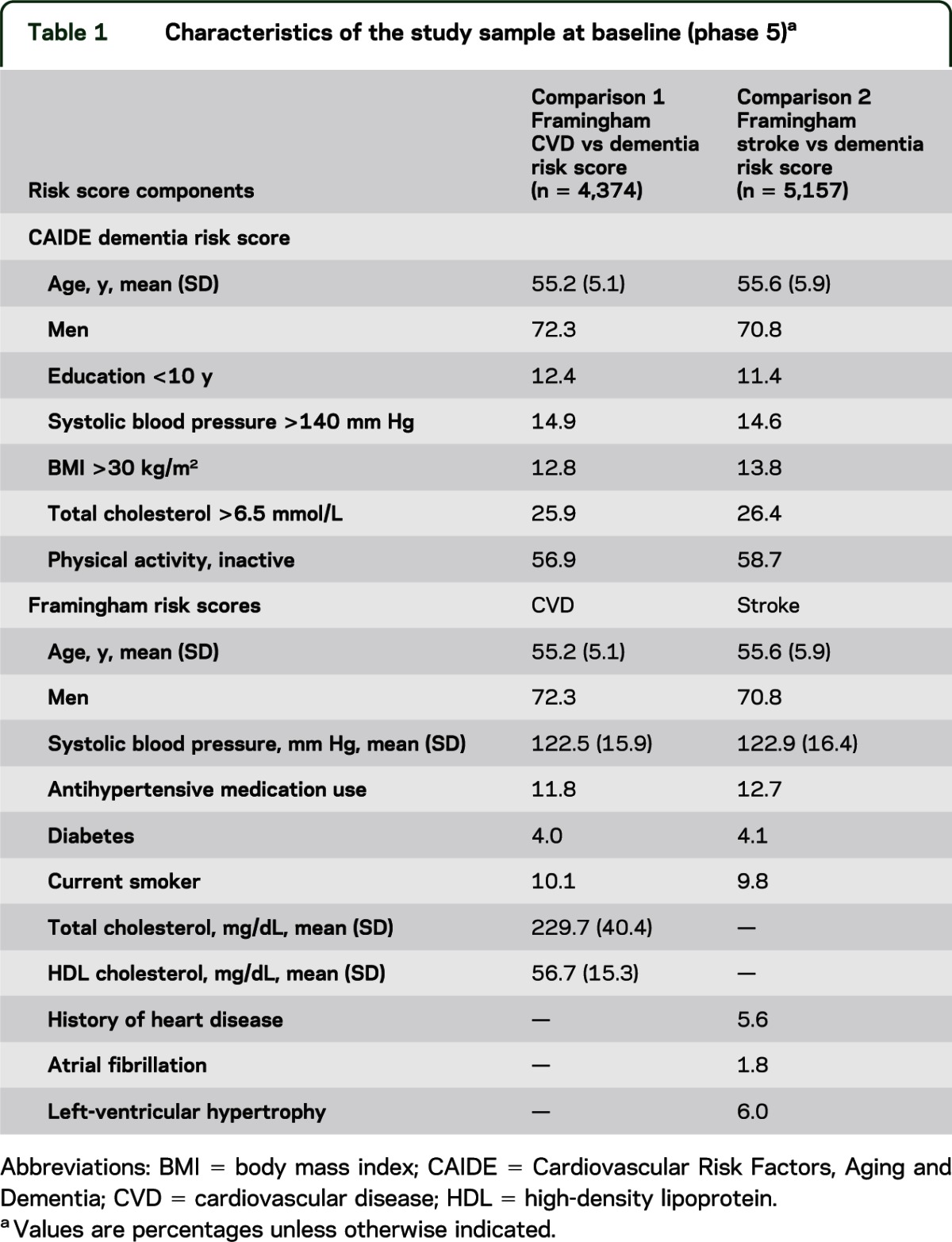
Table 2 presents 10-year cognitive change associated with dementia and CVD risk. Higher CVD risk was associated with faster cognitive decline in global cognitive score and all tests except memory; dementia risk was associated with faster decline in reasoning, vocabulary, and global cognitive score. For dementia risk, mean 10-year decline in global cognitive score was −0.35 SD (95% CI = −0.39, −0.32) in the high-risk group compared with −0.31 SD (95% CI = −0.33, −0.28) in the low-risk group. Similarly, those in the high CVD risk group had greater 10-year decline in global cognition (−0.40 SD; 95% CI = −0.43, −0.37) compared with those in the low-risk group (−0.26 SD; 95% CI = −0.28, −0.23). Compared with dementia risk, CVD risk was associated with faster decline in semantic fluency (difference in β coefficients = 0.05; 95% CI = 0.02, 0.08) and global cognitive score (difference in β coefficients = 0.03; 95% CI = 0.01, 0.05).
Table 2.
Associations of dementia and CVD risk (1997/1999) with 10-year cognitive change (1997/1999, 2002/2004, 2007/2009) (n = 4,374)
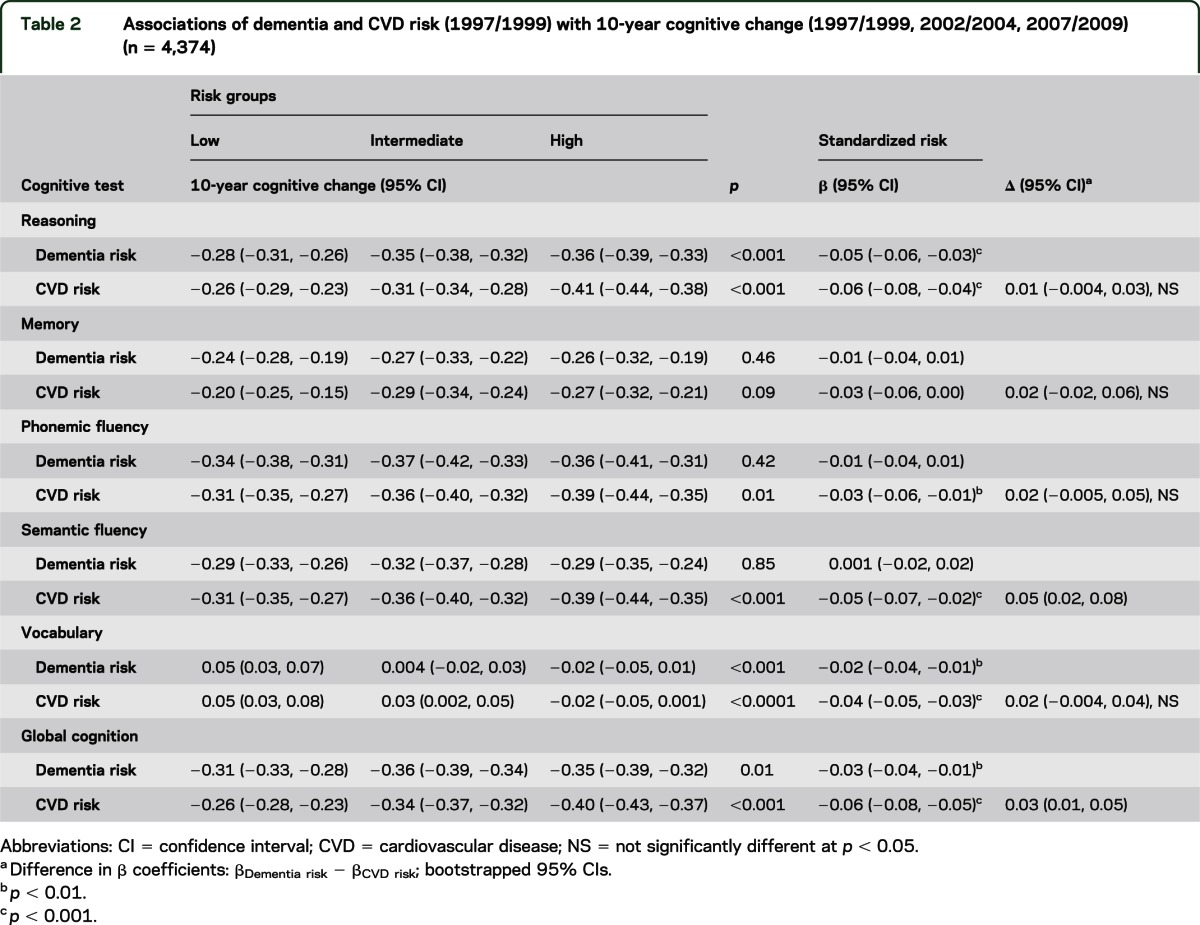
Comparison of dementia and stroke risk with 10-year cognitive change revealed similar results (table 3). Higher stroke risk was associated with cognitive decline in all tests except memory; higher dementia risk was associated with greater decline in reasoning, vocabulary, and global cognitive score. For dementia risk, mean 10-year decline in global cognitive score was −0.27 SD (95% CI = −0.29, −0.24) in the high-risk group compared with −0.22 SD (95% CI = −0.24, −0.21) in the low-risk group. For stroke risk, the corresponding high-risk group had greater mean 10-year decline in global cognitive score (−0.31 SD; 95% CI = −0.34, −0.29) compared with the low-risk group (−0.21 SD; 95% CI = −0.23, −0.19). There were slightly stronger associations between stroke risk compared with dementia risk with decline in semantic fluency (difference in β coefficients = 0.04; 95% CI = 0.02, 0.06) and global cognitive scores (difference in β coefficients = 0.02; 95% CI = 0.01, 0.04). Similar associations were observed using model 2 of the CAIDE risk score that incorporates APOE genotype (see tables e-1 to e-3).
Table 3.
Associations of dementia and stroke risk (1997/1999) with 10-year cognitive change (1997/1999, 2002/2004, 2007/2009) (n = 5,157)
Our subsidiary analyses revealed multiple components of the risk scores to be associated independently with 10-year cognitive decline. These included diabetes, total cholesterol, left-ventricular hypertrophy, and APOE ε4 (tables e-4 to e-7). In addition, all associations between risk measures and 10-year decline in global cognitive scores remained after adjustment for each risk score component, suggesting that multiple components of the risk scores were involved in these associations.
DISCUSSION
In this longitudinal study, we found all 3 risk scores examined to be associated with 10-year decline in multiple cognitive tests. However, CVD and stroke risk displayed stronger associations with cognitive decline than dementia risk. Both CVD and stroke risk were associated with decline in all cognitive tests except memory; dementia risk was not associated with decline in memory and phonemic and semantic fluency.
Notable strengths of this study include its cohort of middle-aged individuals and its longitudinal design with repeated cognitive measurements over a 10-year follow-up period as well as assessment of multiple cognitive domains. In this comparative analysis, we could not test the relative discrimination and calibration of the risk scores because the outcome did not consist of a categorical event. However, we adopted an alternative approach to compare associations of the risk scores with 10-year cognitive decline using bootstrapped CIs.
Limitations of our study include the occupational nature of the cohort of office-based employees that may not be entirely representative of the general population. In addition, because our analytic samples consisted of participants with a more favorable demographic and risk profile, reported associations between risk scores and 10-year cognitive decline may underestimate the strength of associations in the general population. However, this is unlikely to affect comparability of the risk scores.
The differences between the dementia and Framingham risk scores may be related to several factors. Because they were developed to predict different outcomes, differences in the development and validation processes of the 3 risk scores are of importance. The inclusion of education in the dementia risk score also differentiates this risk score from the 2 vascular risk scores. Education, a marker of cognitive reserve, is associated with cognitive performance and risk of dementia28–30 but not the rate of cognitive decline.31,32 Indeed, in our study, of all components of the dementia risk score, education had the strongest association with cognitive performance at baseline (results not reported) even though it was not associated with 10-year cognitive decline. The dementia risk score was developed to detect clinically diagnosable dementia and it is possible that the education component in the risk score has a major influence in driving the prediction of dementia. In contrast, the Framingham cardiovascular and stroke risk scores are composed mainly of vascular risk factors that may make them more sensitive at assessing subclinical cognitive decline.
Vascular risk factors in midlife have been consistently linked to structural brain aging, cerebral pathology such as brain atrophy and white matter abnormalities, as well as cognitive decline in processing speed and executive function.5,16,33–35 Our findings of an independent association of several components of the risk scores (diabetes, total cholesterol, left-ventricular hypertrophy) with cognitive decline suggest a cumulative effect of these risk factors on cognition. Notably, diabetes, which is a component of the 2 Framingham risk scores, showed the strongest independent association with 10-year cognitive decline. Therefore, inclusion of this and other important vascular risk factors in the Framingham risk scores also distinguishes these risk scores from the dementia risk score.
Moreover, vascular risk factors as scored by the Framingham risk scores represent a wider range of categories. For example, systolic blood pressure has 5 categories in the Framingham cardiovascular risk score (office-based version) (<120, 120–129, 130–139, 140–159, ≥160 mm Hg) but only 2 categories (≤140 and >140 mm Hg) in the dementia risk score. The wider range of risk factor categories in the Framingham risk scores may better capture the continuous nature of risk, distinguishing moderately elevated levels of the risk factor as well as the higher risk imparted by multiple marginal risk factors, which is especially pertinent at younger ages when risk factor levels are generally lower.
The majority of dementia risk scores are for use in the elderly population, require a clinical assessment, and have low to moderate predictive validity.36 The CAIDE risk score addresses many constraints of previous dementia risk scores by including easily measurable risk factors in middle age. However, it is rarely used and has not been validated in other populations, perhaps because of the dearth of studies on dementia that have also assessed midlife risk factors. In practice, integration of a dementia risk score especially in primary care settings may not be realistic or practical at present. First, although this dementia risk score is not intended to state whether an individual will or will not have dementia in the future, the potential for individuals to perceive their dementia risk estimation as such still exists. Therefore, acceptability of dementia risk evaluation would expectedly be low because of the anxiety associated with cognitive impairment and dementia. Furthermore, in an already overtaxed general practice setting, it would be unrealistic to expect clinicians to add yet another screening tool to their practice and patient care.
The Framingham Heart Study has devised many risk assessment tools with good to excellent performance in relation to cardiovascular outcomes. Subsequently, great effort has been invested both to improve these risk scores and to validate them in diverse populations, some very different from the Framingham population. The good performance of Framingham primary event cardiovascular risk scores in different populations has indicated universality in the assessment of cardiovascular risk across nations.37 Framingham risk scores have been used in clinical practice guidelines and are among the most recognized and utilized risk scores both in research and primary care where various office-based and online risk calculators are widely accessible.
There are currently no effective treatments for dementia, and population screening is not advocated because in the absence of disease-modifying treatments, there is no evidence that benefit of screening outweighs potential harm. However, with a shift from dementia as an outcome to earlier stages of cognitive decline, there is great potential to affect cognitive outcomes and prevent or delay cognitive decline with early targeting of modifiable vascular risk factors.38,39 Although both the dementia and Framingham risk scores were developed with the aim of addressing multiple risk factors simultaneously and providing an estimate of risk that is easy to understand, Framingham vascular risk scores (and other vascular risk scores used in primary care) may have a dual advantage over a dementia risk score both in terms of feasibility of use and potential for real benefit from vascular risk factor modification. At present, patients are told that their cardiovascular risk predisposes them to heart disease and stroke; in the future, they could also be told that they may be at higher risk of cognitive decline.40
Although future research on cognitive impairment and dementia will likely identify additional risk factors and biomarkers to improve prediction models for cognitive impairment and dementia, there is compelling evidence at present for the role of vascular risk factors in affecting cognitive aging trajectories starting in midlife. Our study advocates the use of cardiovascular risk scores in primary care adding incentive for early identification and treatment of vascular risk factors.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants, and data entry staff, who make the study possible.
GLOSSARY
- CAIDE
Cardiovascular Risk Factors, Aging and Dementia
- CI
confidence interval
- CVD
cardiovascular disease
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Kaffashian developed the analytic plan, performed statistical analyses, and drafted the manuscript. Ms. Dugravot provided ongoing methodologic support and assisted in interpretation of results. Dr. Elbaz provided methodologic expertise and edited the manuscript. Mr. Shipley provided statistical expertise. Dr. Sabia and Dr. Kivimäki edited the manuscript. Dr. Singh-Manoux secured funding, provided ongoing guidance, codeveloped the analytic plan, and provided input on all versions of the manuscript. All authors edited and approved the final version of the manuscript.
STUDY FUNDING
The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart, Lung, and Blood institute (R01HL36310), US NIH National Institute on Aging (R01AG013196; R01AG034454); Agency for Health Care Policy Research (HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health.
DISCLOSURE
S. Kaffashian is supported by the Region Île de France. A. Dugravot and A. Elbaz report no disclosures. M. Shipley is supported by the British Heart Foundation. S. Sabia reports no disclosures. M. Kivimäki is supported by the Academy of Finland, the BUPA Foundation, the NIH (R01HL036310; R01AG034454), and the MRC. A. Singh-Manoux is supported by a European Young Investigator Award from the European Science Foundation and the National Institute on Aging, NIH (R01AG013196; R01AG034454). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–281 [DOI] [PubMed] [Google Scholar]
- 2.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48 [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology 2005;65:876–881 [DOI] [PubMed] [Google Scholar]
- 4.Nash DT, Fillit H. Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol 2006;97:1262–1265 [DOI] [PubMed] [Google Scholar]
- 5.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001;56:1683–1689 [DOI] [PubMed] [Google Scholar]
- 7.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560 [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851 [PubMed] [Google Scholar]
- 9.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 2011;76:1568–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady CB, Spiro A, III, McGlinchey-Berroth R, Milberg W, Gaziano JM. Stroke risk predicts verbal fluency decline in healthy older men: evidence from the normative aging study. J Gerontol B Psychol Sci Soc Sci 2001;56:340–346 [DOI] [PubMed] [Google Scholar]
- 12.Brandt J, Rogerson M. Preliminary findings from an internet-based dementia risk assessment. Alzheimers Dement 2011;7:e94–100 [DOI] [PubMed] [Google Scholar]
- 13.Elias MF, Sullivan LM, D'Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke 2004;35:404–409 [DOI] [PubMed] [Google Scholar]
- 14.Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham stroke risk profile and poor cognitive function: a population-based study. BMC Neurol 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reijmer YD, van den Berg E, van Sonsbeek S, et al. Dementia risk score predicts cognitive impairment after a period of 15 years in a nondemented population. Dement Geriatr Cogn Disord 2011;31:152–157 [DOI] [PubMed] [Google Scholar]
- 16.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology 2004;63:1591–1599 [DOI] [PubMed] [Google Scholar]
- 17.Unverzagt FW, McClure LA, Wadley VG, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology 2011;77:1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaffashian S, Dugravot A, Nabi H, et al. Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: evidence from the Whitehall II study. Eur Heart J 2011;32:2326–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 2006;5:735–741 [DOI] [PubMed] [Google Scholar]
- 20.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 21.Kivimaki M, Shipley MJ, Allan CL, et al. Vascular risk status as a predictor of later-life depressive symptoms: a cohort study. Biol Psychiatry 2012;72:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 1994;25:40–43 [DOI] [PubMed] [Google Scholar]
- 23.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 24.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318 [DOI] [PubMed] [Google Scholar]
- 25.Heim AW. AH4: Group Test of General Intelligence. Windsor, UK: NFER-Nelson Publishing Company, Ltd.; 1970 [Google Scholar]
- 26.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologica 1967;5:135–140 [Google Scholar]
- 27.Raven JC. Guide to Using the Mill Hill Vocabulary Test with Progressive Matrices. London: H.K. Lewis; 1965 [Google Scholar]
- 28.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol 1997;54:1399–1405 [DOI] [PubMed] [Google Scholar]
- 29.Ngandu T, von Strauss E, Helkala EL, et al. Education and dementia: what lies behind the association? Neurology 2007;69:1442–1450 [DOI] [PubMed] [Google Scholar]
- 30.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010 [PubMed] [Google Scholar]
- 31.Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol 2009;170:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol 2011;70:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010;41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 36.Stephan BC, Kurth T, Matthews FE, Brayne C, Dufouil C. Dementia risk prediction in the population: are screening models accurate? Nat Rev Neurol 2010;6:318–326 [DOI] [PubMed] [Google Scholar]
- 37.Khalili D, Hadaegh F, Soori H, Steyerberg EW, Bozorgmanesh M, Azizi F. Clinical usefulness of the Framingham cardiovascular risk profile beyond its statistical performance: the Tehran Lipid and Glucose Study. Am J Epidemiol 2012;176:177–186 [DOI] [PubMed] [Google Scholar]
- 38.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de la Torre JC. Alzheimer's disease is incurable but preventable. J Alzheimers Dis 2010;20:861–870 [DOI] [PubMed] [Google Scholar]
- 40.Diener HC. Prevention of dementia should start 20 years before symptoms become apparent. Eur Heart J 2011;32:2228–2230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


